Abstract
An influential theory of anterior cingulate cortex (ACC) function argues that this brain region plays a crucial role in the affective evaluation of performance monitoring and control demands. Specifically, control-demanding processes such as response conflict, are thought to be registered as aversive signals by the ACC, which in turn triggers processing adjustments to support avoidance-learning. In support of conflict being treated as an aversive event, recent behavioural studies demonstrated that incongruent (i.e., conflict-inducing) relative to congruent stimuli can speed up subsequent negative relative to positive affective picture processing. Here, we used functional magnetic resonance imaging (fMRI) to investigate directly whether ACC activity in response to negative versus positive pictures is modulated by preceding control demands, consisting of conflict and task-switching conditions. The results show that negative relative to positive pictures elicited higher ACC activation following congruent relative to incongruent trials, suggesting that the ACC’s response to negative (positive) pictures was indeed affectively primed by incongruent (congruent) trials. Interestingly, this pattern of results was observed on task repetitions, but disappeared on task alternations. Our findings support the proposal that conflict induces negative affect, and are the first to show that this affective signal is reflected in ACC activation.
Introduction
The anterior cingulate cortex (ACC) has been implicated in a variety of psychological processes such as emotion regulation (Etkin, Egner, & Kalisch, 2011), pain perception (Tracey & Mantyh, 2007), and cognitive control (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). In an integrative review on the ACC, Shackman and colleagues (2011) concluded that its domain-general role must be one of driving aversively motivated behavior. Consistent with this proposal, Botvinick (2007) suggested that the ACC generally signals suboptimal outcomes that drive avoidance learning. In doing so, Botvinick (2007) reinterpreted the ACC’s role in one of its most studied cognitive control functions: the monitoring of cognitive conflict (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004). Namely, Botvinick (2007) hypothesized that the ACC’s response to cognitive conflict (i.e., the simultaneous activation of mutually incompatible stimulus, task, or response representations) is registered as an aversive signal, much like pain or punishment. Although this idea has gained widespread attention and inspired many behavioral studies to test if conflict is indeed experienced as an aversive event (for reviews, see Dreisbach & Fischer, 2015; Inzlicht, Bartholow, & Hirsch, 2015; van Steenbergen, 2015), to the best of our knowledge, no imaging study to date has investigated directly whether the ACC’s response to cognitive conflict does in fact also register as an affectively aversive signal (Botvinick, 2007; see also Shenhav, Botvinick, & Cohen, 2013).
In the wake of Botvinick (2007)’s proposal, a growing number of behavioral studies confirmed its first assumption, namely that cognitive conflicts appear to be experienced as aversive events (Brouillet, Ferrier, Grosselin, & Brouillet, 2011; Dignath & Eder, 2015; Dreisbach & Fischer, 2012; Fritz & Dreisbach, 2013, 2015; Schouppe et al., 2012; 2015; for a review, see Dreisbach & Fischer, 2015). For example, Dreisbach and Fischer (2012) investigated this by combining a classic color Stroop task (Stroop, 1935) with an affective priming paradigm (Fazio, 2001). In an affective priming paradigm, participants are asked to categorize affective words (e.g., “flower” or “bomb”) based on their valence (i.e., positive or negative) as fast as possible. These affective probes are preceded by task-irrelevant stimuli (i.e., the prime). If the prime elicits an affective response it is thought to speed up the subsequent processing (i.e., categorisation) of similarly valenced stimuli. For example, a prime that elicits negative affect would speed up the categorization of negative words but slow down positive word categorization. By using congruent (e.g., the word RED in red ink) or incongruent (e.g., the word RED in blue ink) Stroop stimuli as primes, Dreisbach & Fischer (2012) demonstrated how perceiving incongruent (i.e., conflict-inducing) stimuli can speed up valence categorisation of negative, relative to positive, pictures or words (see also Fritz & Dreisbach, 2013, 2015; Schouppe et al., 2015).
This work demonstrated the value of assessing affective processing following conflict stimuli, as this shines a new light on the motivational dimension of cognitive control. However, although these behavioral studies often link back their findings to theories of the ACC, this region’s involvement in congruency-dependent affective processes has not yet been studied directly. Inspired by these affective priming paradigms, we here used an fMRI study with post-conflict affective picture probe presentation as a gauge on the affective concomitants of conflict processing in the ACC. In line with the logic of behavioral priming paradigms, it has also been shown that when a brain region is involved in processing a particular class of stimuli or signals, it will show a diminished response when two signals of the same class occur in succession, compared to when different signals or stimuli are processed – a phenomenon known as repetition suppression (for review, see Grill-Spector, Henson, & Martin, 2006). Therefore, following Botvinick (2007)’s hypothesis that the ACC registers conflict as an aversive signal, we can expect that the presentation of negative pictures following incongruent trials and positive pictures following congruent trials will result in diminished ACC activation, due to affect-based repetition suppression. By contrast, “non-matching” negative pictures following congruent trials and positive pictures following incongruent trials should result in relatively higher ACC activity, due to a lack of a repetition suppression effect.
Interestingly, these predictions are also compatible with recent reinforcement learning models of ACC function that emphasize a more general role for the ACC in signalling unexpected (performance) outcomes (Alexander & Brown, 2011; Silvetti, Alexander, Brown, & Verguts, 2014; Silvetti, Seurinck, & Verguts, 2011). For example, because people learn that the more difficult incongruent trials have a lower likelihood of being followed by a correct and fast response, incongruent trials may become associated with a poorer outcome expectancy. Therefore, when interpreting ACC activation as a surprise reaction to the affective content of the picture, participants may plausibly be more surprised to experience positive affect following an incongruent trial or negative affect following a congruent trial, due to their differences in outcome expectancies (Alexander & Brown, 2011; Silvetti et al., 2011).
Although much of the behavioral and computational work discussed above has focused on conflict processing specifically, Botvinick (2007)’s hypothesis can easily be extended to other effortful control processes, such as task switching. Therefore, we examined affective picture probe processing following trials (acting as primes) of a conflict task-switching paradigm. These analyses were applied to a subset of participants from a larger (multi-group) dataset that we previously reported on in a different context (Braem et al., 2013). None of the imaging analyses between the current and previous paper overlap. In this paradigm, trials could be defined by both their task-rule congruency (i.e., a stimulus could require either the same or a different response in both tasks) and whether or not the current task was the same (i.e., task repetition) or different (i.e., task alternation) to the preceding trial. Besides the well-known observation that people react slower and less accurately on task alternations as opposed to task repetitions (i.e., the task-switch cost, Monsell, 2003), task-rule congruency effects are also found to be larger on task alternations than task repetitions (Meiran, 2000). The latter observation is assumed to reflect an automatic priming effect from the task rules that were active on the previous trial (Meiran & Kessler, 2008). Namely, the competing task rules are more active on task alternation than task repetition trials because they were just executed on the preceding trial, and therefore interfere more with the required task rules on the present trial (i.e., larger task-rule congruency effects).
According to Botvinick (2007) both conflict processing and task switching can be considered effortful processes, characterised by their higher reaction times and error rates on incongruent trials and task alternations, respectively. Consistent with their aversive task demands, people will actively avoid tasks that require a higher amount of task switching (Kool, Mcguire, Rosen, & Botvinick, 2010) or conflict processing (Schouppe, Ridderinkhof, Verguts, & Notebaert, 2014). Hence, task alternations can be considered similar to cognitive conflict in terms of cognitive effort and therefore we could expect a similar effect on affective picture processing following task alternations versus task repetitions, as we do following incongruent versus congruent trials. Second, we can expect an enhanced effect of congruency-dependent affective processing in the ACC during task alternations – given the observation that congruency effects are enhanced during task alternations.
In sum, our primary goal was to provide a first direct test of the proposal that conflict is registered as an aversive event by the ACC, by adopting the logic of the affective priming paradigm from a purely behavioural context (Dreisbach & Fischer, 2012) to a neuroimaging setting. As a secondary goal, our task protocol also allowed us to explore the affective signature of task switching, and how the relationship between conflict processing and affective responses in the ACC may be modulated by task switching.
Materials and Methods
Participants
Seventeen subjects participated in this study. One participant was not included in the analyses because 30% of his/her responses exceeded the response registration deadline. The remaining 16 participants (eight women and eight men, mean age = 27, SD = 7%) had a mean of 3.1% unregistered responses (SD = 3%). Every participant had normal or corrected to normal vision, was right-handed as assessed by the Edinburgh Handedness Inventory, and reported no current or history of neurological, psychiatric or major medical disorder. Every participant gave their informed written consent before the experiment, and was paid $35 for participating afterwards, as well as an extra $16 as part of the experiment’s reinforcement schedule (which will be explained below). The study was completed with the approval of the Duke University Health System Institutional Review Board.
Stimuli
As affective probe stimuli, we used fifty positive and fifty negative pictures from the International Affective Pictures System (IAPS; Lang, Bradley, & Cuthbert, 2008) data-base. The pictures were matched on their semantic content (e.g. cute animal vs. dangerous animal, crying baby vs. smiling baby, sunset vs. thunderstorm) to avoid differences in picture processing that could be attributable to other non-affect related features (e.g., living versus non-living). The imperative task stimuli, which served as “primes” in this affective priming protocol, consisted of the (affectively neutral) numerals 1 to 9, excluding 5; these stimuli were always presented in isoluminant green or blue. All pictures and task stimuli were centrally presented on a black background. The experiment was projected on a back-projection screen, which participants viewed in a mirror mounted to the head coil. This arrangement resulted in picture sizes of approximately 10° width and 7.4° height and task stimuli of approximately 0.4° width and 0.8° height. Responses were made via a MR-compatible response box (Current Designs) positioned on the participant’s abdomen (perpendicular to the length of their body). Participants had to use their left or right hand to press the left- or rightmost button (out of four horizontally aligned response buttons), respectively. The stimuli were presented with Presentation software (Neurobehavioral Systems).
Procedure
To assess the effects of conflict processing during task repetitions or task alternations on affect processing (positive or negative IAPS pictures), we presented an affective picture probe (positive or negative) following each trial (acting as prime) of a standard conflict task-switching paradigm. Importantly, picture valence was always selected at random, and thus independent of the congruency or task switching conditions. However, whether or not a picture would be presented (independent of its valence) was dependent on performance speed and accuracy. Specifically, participants were informed that task stimuli would be followed by a randomly chosen positive or negative picture and that positive pictures were associated with 10 cents monetary gain (to assure picture valence processing), but there would be no picture presentation after incorrect or too slow responses (exceeding the 1500 ms deadline).
During the task, participants had to respond to the magnitude or parity of the task stimulus, depending on its color. For example, when the number was presented in green, subjects had to press left when it was smaller than five, and right when it was larger than five. Alternatively, when the number was presented in blue, subjects had to respond to its parity by indicating whether it was odd with a left-hand response, or even with a right-hand response. The color cuing the magnitude versus parity task was counterbalanced across participants. Importantly, each number was associated with one response for each task-set. Because these task-sets were assigned to overlapping response sets, each number could either produce the same (i.e., congruent) or different (i.e., incongruent) responses in the two tasks. Moreover, the stimuli were presented in a random order (excluding number repetitions), which could result in task repetitions and task alternations. Overall, these manipulations produced a factorial design with three within-subject factors of interest: prime trial congruency (congruent vs. incongruent trial) and task sequence (task-repetition vs. task-switch), and probe valence (positive vs. negative). For the behavioral data analysis, the former two factors allowed us to assess the classic congruency effect, task-switching effect, and the predicted enhancement of congruency effects on task alternations (Meiran, 2000). By contrast, our fMRI analyses exploited the full 3-way design, by focusing on the neural activity elicited by affective picture probe stimuli as a function of picture valence and the congruency and task sequence status of the preceding prime stimulus.
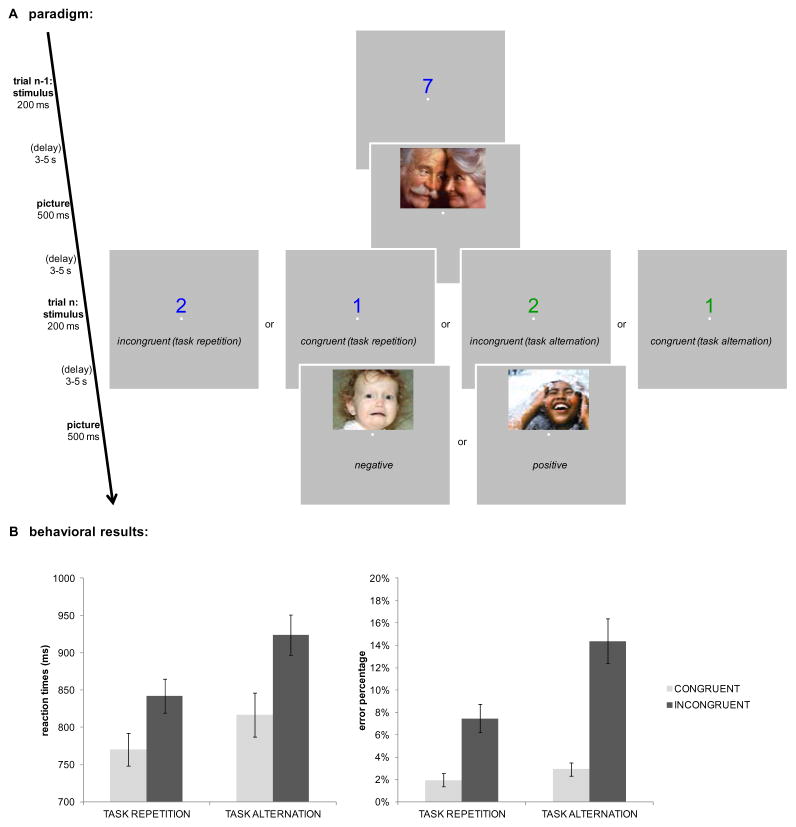
As can be seen in Figure 1A, each trial started with the presentation of a colored number stimulus 0.6° above a central fixation dot for 200 ms. The central fixation dot remained on-screen throughout the task. Subjects were required to respond within 1500 ms after stimulus onset. After a variable stimulus-picture interval, an IAPS picture was presented for 500 ms, followed by another variable picture-stimulus interval until the next digit stimulus was presented. Importantly, the stimulus-picture interval was time-locked to the stimulus onset and thus independent of response speed, ensuring that differences in picture processing following different task conditions could not be attributed to differences in the stimulus-picture interval. Both the stimulus-picture interval and picture-stimulus interval were independently randomized and drawn from a pseudo-exponential distribution (50 % lasted 3 seconds (s), 25 % 3.5 s, 12 % 4 s, 7 % 4.5 s, and 6 % 5 s), resulting in a mean time interval of ~ 3.5 s. This way, picture presentation can be studied independently from task stimulus presentations (e.g., Ollinger et al., 2001). When the subject’s response was incorrect or exceeded the response deadline, picture presentation was replaced by the presentation of the fixation dot for 500 ms.
Figure 1.
A. Trial procedure (two subsequent trials) and different task conditions of interest are denoted. Participants were presented with randomly chosen positive or negative picture after each task stimulus, except when the preceding response to the task stimulus was incorrect or too slow (> 1500 ms). Participants had to respond to the digit stimuli’s parity or magnitude, depending on stimulus color. Examples of the different possible task conditions of interest are denoted: picture processing was investigated depending on its valence (negative or positive), and the congruency (incongruent or congruent) and task sequence (repetition or alternation) identity of the immediately preceding stimulus. The words in italic were not presented during the experiment and the background color in the experiment was black. B. The behavioral results demonstrate how both response times and error rates were higher for incongruent relative to congruent trials. Moreover, this difference between both congruency conditions was smaller on task repetitions relative to task alternations. All error bars are ± 1 standard error of the mean (SEM).
Participants performed 5 experimental blocks of 64 trials each during scanning, after a short practice block of 32 trials outside the scanner. In between blocks, there was a short break during which the participants could see their updated score. Each of the 32 stimulus-picture combinations (eight numbers × two task colors × picture valence) were presented ten times in a randomized order. IAPS pictures were randomly chosen from the appropriate valence group, but never reoccurred within a block. Although there was some variation in the amount of money subjects obtained during the experiment (due to differences in performance accuracy), all participants were given the same maximum amount of money possible at the end of the experiment.
Behavioral data analyses
All trials following an error and the first trial of each block were removed from the analyses. We carried out an ANOVA with the within-subject factors trial congruency (congruent vs. incongruent) and task sequence (repetition vs. switch) on correct RTs and error rates.
fMRI data acquisition
Imaging was conducted on a GE Discovery MR750 system at 3.0 tesla using a standard head coil. We acquired functional images parallel to the AC-PC plane with a T2*-weighted single-shot gradient EPI sequence of 36 contiguous axial slices [repetition time, 2000 ms; echo time, 28 ms; flip angle, 90°; field of view, 192mm; array size, 64 × 64] with 3 mm thickness and 3 × 3mm in-plane resolution. Structural images were acquired with a T1-weighted FSPGR axial scan using a 3D inversion recovery prepared sequence, recording 120 slices of 1 mm thickness and in-plane resolution of 1 × 1 mm.
fMRI data analysis
The preprocessing steps and statistical analyses were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Functional data were slice-time corrected and spatially realigned to the first volume of the task. The structural image was normalized to the Montreal Neurological Institute (MNI) template brain (resampled voxel size 2 mm3). These normalization parameters were then applied to the functional images to ensure an anatomically informed normalization. The first 5 volumes of each run in which no stimulation occurred were discarded before estimating statistical models and a 128 s temporal high-pass filter was applied to remove low-frequency drifts. Temporal autocorrelations were estimated using restricted maximum likelihood estimates of variance components with a first-order autoregressive model, and the resulting non-sphericity was used to form maximum likelihood estimates of activations. A spatial smoothing filter of 8 mm FWHM (full-width at half maximum) was applied.
Event-related regressors convolved with a canonical hemodynamic response function (HRF) were created corresponding to the picture onsets and stimulus onsets of each trial. Importantly, the picture-locked regressors were defined by the valence of the picture (positive or negative), as well as the congruency and the task sequence identity of the preceding stimulus (congruent or incongruent, and task repetition or task alternation). We also modelled the stimulus-locked regressors as a function of their congruency and task sequence features, as well as error trials, trials following an error, and the first trial of each run as separate nuisance variables. Last, both picture- and stimulus-locked regressors were further defined by the picture valence of the preceding trial to control for possible carry-over effects of previous affective picture presentations. Single-subjects contrasts on picture-locked regressors were calculated to establish the hemodynamic correlates of picture valence, depending on the preceding congruency and task-sequence conditions. Specifically, we first investigated the contrast between negative versus positive picture presentation following congruent versus incongruent trials, for task repetitions only. This contrast allows us to evaluate our main hypothesis that the ACC is sensitive to the aversiveness of cognitive conflict (Botvinick, 2007) in a way that is most similar to earlier behavioral studies which used single conflict tasks only (for a review, see Dreisbach & Fischer, 2015). In a second step, we also looked at the picture-locked contrast that includes the factors picture valence and task sequence, to study the affective evaluation of task switching conditions. Finally, we analyzed the contrast between picture valence, congruency, and task sequence, to further explore the role of task sequences in the hypothesized congruency-dependent affective picture processing of the ACC.
To control for false-positive rates, we determined contrast-specific combined voxel activation intensities and cluster extent thresholds that are corrected for multiple comparisons by using 3dClustSim (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). This software runs 10,000 Monte Carlo simulations that take into account the whole-brain search volume and estimated smoothness of each axis of the respective group SPMs and thereby generates probability estimates of a random field of noise producing a cluster of voxels of a given extent for a set of voxels passing a specific voxelwise p-value threshold, which we set at 0.005 for all analyses. Given this threshold, the 3dClustSim simulations determined that cluster sizes of 301 to 334 voxels, depending on the specific contrast, corresponded to a combined threshold of p < 0.05 (corrected).
ROI analyses
We extracted mean β estimates (i.e., mean cluster activation) from empirically defined ROIs with Marsbar software (http://marsbar.sourceforge.net/) to evaluate interaction effects.
Results
Behavioural data
Mean accuracy on this task was 89.9 % and mean RT was 834 ms. As expected, there was a typical congruency effect which was expressed in higher RTs, F(1,15) = 54.1, p < .001, and higher error rates, F(1,15) = 48.9, p < .001, for incongruent trials (883 ms, 10.9 %) as opposed to congruent trials (793 ms, 2.4 %). We also observed a significant task-switch cost, in both RTs, F(1,15) = 44.0, p < .001, and error rates, F(1,15) = 28.3, p < .001, reflecting higher RTs and error rates when a task alternated (870 ms, 8.6 %) as opposed to when the task repeated (806 ms, 4.7 %). Furthermore, there was a significant interaction between congruency and task sequence in both RTs, F(1,15) = 7.3, p < .05, and error rates, F(1,15) = 11.7, p < .01, due to a greater congruency effect for task-switch, than task-repeat trials or, conversely, a larger switch effect for incongruent than for congruent trials (see Figure 1B). No other main effects or interactions reached significance (all p > .1).
Thus, in line with expectations, these data confirm that incongruent trials were more difficult than congruent trials, and task alternations more demanding than task repetitions. Moreover, consistent with the previous literature, the congruency effect was more pronounced during task alternations than task repetitions (Meiran, 2000). As outlined above, both observations are important in setting up our predictions regarding the influence of conflict conditions on affective processing.
fMRI data: the effect of conflict on affective picture processing
Next, we analyzed the picture-locked interaction contrast that includes both congruency and picture valence, for task repetitions only. We focused on task repetitions, as these most closely resemble the task conditions under which the affective signatures of conflict processing have previously been investigated (Brouillet, Ferrier, Grosselin, & Brouillet, 2011; Dreisbach & Fischer, 2012; Fritz & Dreisbach, 2013; Fritz & Dreisbach, 2014; Schouppe et al., 2015). If incongruent (relative to congruent) trials are indeed experienced as more negative, due to either their higher control demands (Botvinick, 2007) or lower outcome expectancy (Alexander & Brown, 2011; Silvetti et al., 2011), we expected these trials to elicit lower ACC activation during subsequent affectively matching negative picture presentations, but higher ACC activations during the presentation of affectively non-matching positive picture presentations, and vice versa for congruent trials.
The contrast for the picture valence by congruency interaction on task repetitions resulted in the whole-brain corrected activations of the (dorsal) ACC (Figure 2A), as well as the dorsomedial prefrontal cortex (dmPFC), left inferior frontal sulcus, left insula, left precuneus, and the right thalamus (see Table 1). Consistent with earlier reports of conflict-related ACC activation, our ACC activation was restricted to more dorsal portions of the ACC, seemingly extending into the supplementary motor area and other more dorsal or prefrontal medial regions. Alternatively, this could also reflect activity in the paracingulate sulcus, a portion of the dorsal ACC that is known to sometimes expand dorsally (as also discussed in this review on ACC function; Shackman et al., 2011). The interaction effect in the activation of the ACC was in line with our expectations regarding its role in the affective evaluation of congruency conditions (Alexander & Brown, 2011; Botvinick, 2007; Silvetti et al., 2011). In order to determine the source of this interaction, we extracted mean ROI activation values and submitted them to planned comparisons. Specifically, we extracted the mean ROI activation of the cluster activation in the ACC/dmPFC (Figure 2C) to evaluate the more anterior cluster. The more posterior ACC cluster, however, was connected to a larger cluster whose peak activation was in the right thalamus (see Table 1). Therefore, this ACC activation was evaluated by analyzing the mean activation around this cluster’s third local maximum, which was closest to the ACC (a 5 mm sphere around x = 6, y = 20, z = 38). Besides the significant interaction between congruency and valance (on which the ROI was selected), the ACC/dmPFC cluster also showed a main effect of valence with overall higher activation during negative as opposed to positive picture presentations, F(1,15) = 10.34, p < .01, replicating a host of previous imaging studies (reviewed in Etkin, Egner, & Kalisch, 2011; Shackman et al., 2011). The more posterior ACC activation did not show this main effect, F(1,15) = 2.09, p = .17. More importantly, as can be seen on Figures 2B and 2C, the interaction between congruency and picture valence in the ACC/dmPFC originated from a significantly lower activation for negative pictures following incongruent as opposed to congruent trials, t(15) = 2.971, p = .01, and positive pictures following congruent relative to incongruent trials, t(15) = 3.989, p = .001. A similar pattern could be observed in the more posterior ACC cluster (t(15) = 2.098, p = .053, and t(15) = 1.925, p = .073, respectively). These results are concordant with the idea that the higher control demands associated with incongruent, relative to congruent, trials triggered negative evaluation (Botvinick, 2007) that attenuated the ACC response to negative, but not positive, picture probes. Alternatively, when interpreting the ACC activation as violations of outcome expectancies (Alexander & Brown, 2011; Silvetti et al., 2011), participants might have been more surprised to encounter negative pictures following a congruent trial, or positive pictures following an incongruent trial. Both interpretations are in line with our predictions and the available literature arguing that incongruent trials are experienced as aversive events (Dreisbach & Fischer, 2015; Inzlicht, Bartholow, & Hirsch, 2015). Similar to the ACC/dmPFC, the interaction in the three other regions (one of which includes the sphere of the more posterior ACC activation) was also driven by both a significantly higher activation for negative pictures following congruent as opposed to incongruent trials, ts(15) > 2.890, ps < .05, and positive pictures following incongruent relative to congruent trials, ts(15) > 3.054, ps < .01. Different to the ACC/dmPFC, however, the other three regions did not show a main effect of valence, F(1,15) < 1.15, p > .3.
Figure 2.
A. The anterior cingulate cortex (ACC) as identified by the picture-locked contrast for the congruency-dependent valence effect on task repetitions (valence effect after congruent trials > valence effect after incongruent trials) regression analysis, plotted at p < .05 (corrected) on sagittal (x 0) of an individual brain in MNI space. B, C. Group mean activations (β estimates ± SEM) in the ACC are plotted for each valence (green is positive, red is negative) and congruency condition, for task repetitions only. The mean activations were based on either the mean cluster activation in the ACC/dorsomedial prefrontal cortex (C; abbreviated by ACC/dmPFC), or a 5 mm sphere around a more posterior ACC cluster’s peak activation (B; x 6 y 20 z 38), because this peak was part of a cluster extending into other regions as well (see results). The error bars are displayed for the purpose of visual presentation (not statistical inference) of the patterns of activation means and variance observed in the ROIs that were identified based on the statistical inference drawn by the whole-brain corrected group analysis.
Table 1.
Picture onset locked activations revealed by the picture valence × congruency condition contrast for task repetitions only, and the picture valence × congruency condition × task sequence contrast.
| Anatomical area | X | Y | Z | Voxels | Tmax |
|---|---|---|---|---|---|
| Picture valence as a function of preceding stimulus congruency, on task repetitions only (valence effect after congruent stimuli > valence effect after incongruent stimuli) | |||||
| Anterior cingulate cortex/Dorsomedial prefrontal cortex | −6 | 42 | 30 | 618 | 5.18 |
| Left inferior frontal sulcus/Left insula | −30 | 6 | 18 | 640 | 5.83 |
| Left superior parietal lobe/Left precuneus | −34 | −56 | 58 | 1320 | 4.64 |
| Right thalamus/Anterior cingulate cortex | 14 | −26 | 16 | 2202 | 5.14 |
| Picture valence as a function of preceding task sequence, on congruent trials only (valence effect after task repetitions > valence effect after task alternations) | |||||
| Anterior cingulate cortex/Supplementary motor area | 4 | 16 | 60 | 800 | 4.95 |
| Cerebellum | 8 | −52 | −6 | 450 | 5.39 |
| Picture valence as a function of preceding stimulus congruency and task sequence | |||||
| Dorsal anterior cingulate cortex | −2 | 20 | 54 | 362 | 3.94 |
| Left inferior frontal sulcus/junction | −28 | 8 | 44 | 572 | 6.11 |
| Left superior parietal lobe/Left precuneus | −40 | −24 | 32 | 5258 | 5.50 |
| Left superior temporal sulcus | −38 | −34 | 0 | 979 | 5.39 |
| Thalamus/Cerebellum | −4 | −52 | −6 | 1652 | 5.04 |
| Right superior temporal gyrus/Hippocampus | 44 | 0 | −26 | 379 | 5.44 |
fMRI data: the effect of task switching on affective picture processing
Second, we studied the picture-locked interaction contrast that includes both task sequence conditions and picture valence. The behavioral affective signatures of cognitive conflict are well documented (e.g., Dreisbach & Fischer, 2012). However, similar automatic affective evaluations of task switching conditions remain to be tested. Still, although the framework of Botvinick (2007) mainly focuses on conflict processing, its predictions can easily be extended to task switching. Similar to conflict processing, task switching can be considered an effortful mental process that could also register as an aversive signal in the ACC. Consistently, people have been found to actively avoid choice decks that are associated to a higher amount of task switching (Kool et al., 2010).
Our first brain analyses did not support this hypothesis. The contrast for picture valence by task sequence interaction did not reveal any significant whole-brain corrected activations. Also when testing in the opposite direction, no effects of task switching conditions on affective picture processing were observed. However, in close analogy to our analysis into the effects of congruency on task repetitions only, one could also look at the effects of task switching on congruent trials only, cancelling out the arguably interfering or obfuscating effects of incongruent trials. Indeed, the contrast for the picture valence by task sequence interaction on congruent trials only revealed whole-brain corrected activations of the supplementary motor area (SMA) and (dorsal) ACC (Figure 3A), as well as the cerebellum (see Table 1). Similar to the congruency-dependent analysis, follow-up analyses on the mean ROI activation values showed that this ACC/SMA region showed an overall higher activation to negative relative to positive picture presentations, F(1,15) = 6.79, p = .02. Moreover, the interaction between task sequence and picture valence was again driven by both a significantly lower activation to negative pictures following task alternation as opposed to task repetition trials, t(15) = 3.266, p = .005, and positive pictures following task repetition relative to task alternation trials, t(15) = 2.599, p = .02 (Figure 3B).
Figure 3.
A. The anterior cingulate cortex (ACC)/Supplementary motor area (SMA) as identified by the picture-locked contrast for the task sequence-dependent valence effect on congruent trials only (valence effect after task repetitions > valence effect after task alternations) regression analysis, plotted at p < .05 (corrected) on sagittal (x −4) of an individual brain in MNI space. B. Group mean activations (β estimates ± SEM) in the ACC/SMA are plotted for each valence (green is positive, red is negative) and task sequence condition, for congruent trials only. The mean activations were based on the mean cluster activation in the ACC/SMA. The error bars are displayed for the purpose of visual presentation (not statistical inference) of the patterns of activation means and variance observed in the ROIs that were identified based on the statistical inference drawn by the whole-brain corrected group analysis.
fMRI data: the role of task sequence in congruency-dependent affective picture processing
Next, we turned to task alternations to see if task switching either enhanced or counteracted the affective evaluation of congruency. On task alternations, the congruency effect is enhanced as compared to task repetitions, as evidenced by both the reaction and error rate analyses (see Fig. 1B). Therefore, if the reported effects of congruency on affect processing are indeed a consequence of their associated cognitive effort (Botvinick, 2007), we would expect these aversive effects to be further enhanced during task alternations.
As a first test of this hypothesis, we reanalyzed the mean ROI activation values from the ACC/dmPFC region uncovered by the congruency-dependent valence contrast on task repetitions (Figure 2A), but now focused on its activation pattern during task alternations instead. Intriguingly, in contrast to its modulations during task repetitions, the ACC/dmPFC was no longer responsive to picture valence, F(1,15) = 1.376, p > .25, nor its interaction with congruency, F(1,15) < 1, during task alternations. Similarly, a separate whole-brain analysis of the congruency by picture valence contrast on task alternations only did not reveal any significant cluster activations (in neither direction). These analyses were further confirmed by a whole-brain analysis of the three-way interaction between task sequence, picture valence, and congruency which documented activations consistent with these above-mentioned results (Figure 4A). Namely, the analysis again uncovered the (dorsal) ACC, as well as the left inferior frontal sulcus, left precuneus, left superior parietal areas, the left superior temporal sulcus, and right superior temporal gyrus. Although the peak ACC activation in this contrast (Fig. 4A) lay a little more posterior (x −2 y 20 z 54) than the peak of the cluster displayed in Fig. 2A, its activation pattern remained qualitatively unchanged (trend-level analyses at corrected p < .1 revealed more anterior ACC activation as well). Thus, similar to the previous ROI, the ACC showed a main effect of valence, F(1,15) = 8.170, p < .05. Moreover, the interaction between task sequence, picture valence, and congruency (on which the ROI was selected) was again driven by a congruency-dependent processing of picture valence on task repetitions, F(1,15) = 11.020, p = .005 (Figure 4B), but not on task alternations, F(1,15) = 2.870, p > .1 (Figure 4C). Planned comparisons on the task repetition trials again showed lower activation for positive pictures following congruent as opposed to incongruent trials, t(15) = 2.646, p = .018, and negative pictures following incongruent relative to congruent trials, t(15) = 1.944, p = .071. The reverse contrast (e.g., regions showing a larger congruency-dependent valence effect on task alternations) did not garner any significant (or trend-level) above-threshold clusters. Together, these analyses clearly suggest that the affective priming effect from conflict processing was absent on task alternations. These findings argue against the idea that the ACC only signals for increased, inherently negative, cognitive demand (Botvinick, 2007), because based on congruency effects being larger on task alternations, one would have expected their affective consequences to also be enhanced. In hindsight, this can be considered consistent with the reinforcement learning models of ACC function which stipulate that outcome expectancies are monitored for each task (or context) and congruency condition separately (Alexander & Brown, 2011; Silvetti et al., 2011). Given that empirical studies have shown that task-switching can interfere with task-specific conflict processes (e.g., Braem, Abrahamse, Duthoo, & Notebaert, 2014; Brown, Reynolds, & Braver, 2007; Egner, 2008; Goschke, 2000), this raises the possibility that task switching can also disrupt the task-specific evaluation of congruency outcome expectancies, which would lead to a diminished responsiveness of the ACC to the affective signatures of conflict processing on switch trials.
Figure 4.
A. The regions as identified by the picture-locked contrast for the congruency-dependent valence effect on task repetitions versus alternations regression analysis, plotted at p < .05 (corrected) on sagittal (x −4), sagittal (x −40), coronal (y −34), and axial (z 0) of an individual brain in MNI space. B, C. Group mean activations (β estimates ± SEM) in the ACC are plotted for each valence (green is positive, red is negative), congruency, for task repetitions (B) and task alternations (C) separately. The error bars are displayed for the purpose of visual presentation (not statistical inference) of the patterns of activation means and variance observed in the ROIs that were identified based on the statistical inference drawn by the whole-brain corrected group analysis.
Discussion
In this study, we investigated the effect of different control demands on subsequent affective picture processing. We report for the first time that affective pictures were differentially responded to by the ACC dependent on preceding conflict and task switching conditions. Consistent with the behavioural literature on the affective value of conflict processing (Dreisbach & Fischer, 2015), we observed diminished ACC activation for negative picture probes following incongruent prime trials or positive picture probes following congruent prime trials. Interestingly, however, this pattern was completely absent on task alternations.
Our main finding is consistent with Botvinick (2007)’s hypothesis that the ACC responds similarly to cognitive conflict and negative affect, registering both as aversive learning signals that drive certain forms of avoidance learning (see also Shackman et al., 2011; Shenhav et al., 2013). Dreisbach and Fischer (2012) already demonstrated how the presentation of incongruent compared to congruent stimuli can speed up the subsequent evaluative categorisation of negative relative to positive pictures (see also, Fritz & Dreisbach, 2013, 2015; Schouppe et al., 2015). We now document the neural correlates of this effect in the ACC. Specifically, we show diminished ACC activity in response to negative pictures following incongruent trials (relative to positive pictures and congruent trials), which, following the idea of repetition suppression (Grill-Spector, Henson, & Martin, 2006), suggests that the ACC processes incongruent trials and negatively valenced picture probe stimuli in a similar fashion (Botvinick, 2007).
Similarly, the diminished processing of positive pictures following congruent relative to incongruent trials could be interpreted as positive affect elicited by congruent conditions. Consistent with the idea people might take pleasure in encountering a congruent trial, Cannon, Hayes, & Tipper (2010) did report larger zygomaticus activity (muscle activity associated with smiling) following compatible relative to incompatible trials. Therefore, within the local context of a reaction time experiment, it seems reasonable to assume that people can experience some positive emotions during easier trials, in light of knowing that there are more difficult trials too. However, further evidence is required to determine whether congruent conditions automatically elicit positive affect.
Other studies have searched for the processing of a coding of cognitive control ‘costs’ in the ACC. For example, McGuire and Botvinick (2010) performed correlation analyses between avoidance ratings or avoidance behavior (in a subsequent test phase) with task-related brain activity during a task-switching study. Although hypothesized, they failed to find a relation between any medial frontal cortex activity and cost processing. However, their analyses focused on block-wise levels of avoidance ratings/behavior, which might be less sensitive to the ACC’s coding of costs of moment-to-moment changes in behavior (as also argued by the authors). More indirect evidence can be found in a study by Botvinick, Huffstetler, and McGuire (2009), which demonstrated that the ACC’s response to effort allocation during task performance inversely predicted the participants’ striatal response to subsequent rewards, argued to be consistent with the subjective experience of mental effort. Similarly, Vassena and colleagues (2014) demonstrated how the ACC responded more to cues indicating a difficult task or high reward (contingent on task performance), arguably signaling the need for costly and effortful cognitive control processes (see also Croxson, Walton, O’Reilly, Behrens, & Rushworth, 2009; Prévost, Pessiglione, Météreau, Cléry-Melin, & Dreher, 2010). These studies show that the ACC responds to the cost associated with the allocation of cognitive control resources. However, in our study, we focused on the affective signature of this cost and were able to demonstrate that the ACC responds to the affectively aversive signal associated with this allocation of cognitive control.
Importantly, positive pictures were also associated with the prospect of (post-study) monetary reward. On the one hand, this should serve to further enhance the positive affect elicited by the picture stimulus; on the other hand, this means we have to be careful in interpreting the exact source of the putative positive affect. Interestingly, while Shenhav and colleagues (2013) also suggest that cognitive control demands can be considered aversive in nature (as Botvinick, 2007), they added to this hypothesis that the (dorsal) ACC should only be responsive to the value of stimuli that are relevant to the allocation of control. Given that monetary gain can be considered more relevant to the participants’ goals (in contrast to the mere valence of the picture), it is possible that the present results are slightly different in nature from the automatic affective evaluation effects in the behavioral studies discussed in the introduction, where positive stimuli were not paired to monetary gains (e.g., Dreisbach and Fischer, 2012).
As outlined above, reinforcement learning models of the ACC are also consistent with these results (Alexander & Brown, 2011; Silvetti et al., 2011, 2014). These models suggest that the ACC constantly updates outcome expectancies (e.g., one’s chance to respond correctly) for multiple stimulus-response-outcome associations in parallel, thus registering separately for each task and congruency condition whenever outcomes are different than expected. Hence, if we follow this assumption that different congruency conditions automatically activate different outcome expectancies, which are affectively tagged, we can expect the same results as predicted by Botvinick (2007). First, the overall higher activation elicited by negative relative to positive pictures in the ACC/dmPFC can be related to the overall surprise to experience negative affect following a positive outcome expectancy elicited by the correct response (Aarts, De Houwer, & Pourtois, 2012; Alexander & Brown, 2011; Braem, Coenen, Bombeke, van Bochove, & Notebaert, 2015; Desmet, Deschrijver, & Brass, 2014; Silvetti et al., 2011, 2014; Wessel, Danielmeier, Morton, & Ullsperger, 2012). However, participants will also (implicitly) expect higher outcome expectancies on congruent than incongruent trials and thus show higher surprise reactions (i.e., negative prediction errors) whenever a negative event occurs during feedback presentation. Conversely, the enhanced reaction to positive pictures following incongruent relative to congruent trials can be attributed to their lower outcome expectancy, and thus higher surprise reaction to this positive outcome (i.e., positive prediction error). In line with this idea, it has been suggested that in the absence of feedback, the correct response to incongruent versus congruent trials in and of itself could be experienced as more positively surprising or inherently rewarding (Braem et al., 2012; 2015; Schouppe et al., 2015).
Our task protocol also facilitated investigating the role of task sequences on the affective evaluation of congruency conditions in the ACC. Interestingly, while we did show how task alternations relative to repetitions similarly resulted in a diminished ACC response to negative pictures (on congruent trials only), we also observed that the interaction between congruency and affect processing could only be observed on task repetitions, but not task alternations. Although the latter observation was unexpected, it can be considered concordant with the idea that outcome expectancies are task- and congruency-specific. Previous studies have demonstrated how task switching can disrupt task-specific conflict processing (Brown et al., 2007; Goschke, 2000; for reviews, see Braem et al., 2014; Egner, 2008). Therefore, task switches might have hindered an efficient affective evaluation of congruency conditions. Although this observation seems largely consistent with our interpretation of the reinforcement learning models (Alexander & Brown, 2011; Silvetti et al., 2011, 2014) of the ACC, our experiment cannot, and was not set up to, dissociate between these models and the ideas put forward by Botvinick (2007). We hypothesized that the aversive nature of cognitive demand (Botvinick, 2007) as reflected in the affective evaluation of congruency conditions would be enhanced during task alternations – which are also thought to be high in cognitive demand (Kool et al., 2010) and are known to enhance congruency effects (Meiran, 2000; our data). However, while we hypothesized otherwise, it is still possible that the affective evaluation of cognitive demand is also task-specific: our data can only suggest that the affective concomitants of conflict processing are task-specific, irrespective of whether these index the affective evaluation of cognitive demand (Botvinick, 2007) or outcome expectancies (Alexander & Brown, 2011; Silvetti et al., 2011, 2014).
More generally, this task-specificity of conflict processing is also consistent with the observation that adaptations to conflict are often found to be task-specific (for reviews, see Braem et al., 2014; Egner, 2008). According to a computational model by Brown, Reynolds, and Braver (2007), conflict and task alternations evoke opposing learning signals: whereas the former induces exploitation of task-relevant information, the latter promotes exploration for selecting the appropriate task-set. This was recently supported by two independent surprise recognition memory studies demonstrating that memory for task-relevant information was improved under conflict conditions (Krebs, Boehler, De Belder, & Egner, 2015), while it was impaired under task-switching conditions (Richter & Yeung, 2012). Also in studies on affective modulations of cognitive control, a distinction is often made between both types of cognitive effort as they are differentially affected by valence manipulations. While task switching performance has frequently been reported as improved following positive mood induction (e.g., Dreisbach & Goschke, 2004), conflict processing has often been found to be impaired under positive mood (e.g., van Steenbergen, Band, & Hommel, 2010). These findings further suggest that cognitive conflict and task switches elicit opposing processes or strategies and, on a more general level, are in line with our findings that both differentially influenced affective feedback processing.
Notably, we were able to demonstrate the affective signatures of both control processes – cognitive conflict and task switching, but only after cancelling out the other (i.e., focus on task repetition trials, or congruent trials only, respectively). Importantly, in the present study, both factors were manipulated orthogonally. Therefore, participants were required to both switch back and forth between different task sets, while simultaneously shielding the task relevant information from conflicting stimulus-response associations. This way, both processes, thought to reflect opposing forces of cognitive control (Brown, Reynolds, and Braver, 2007) might have been in constant competition. If we had used two separate designs instead (i.e., a conflict task without task switching conditions and a task switching study without conflicting stimuli), we might have been able to find a similar effect on affective picture processing without having to exclude task alternation or incongruent trials. Future studies, including behavioral, are necessary to further investigate the automatic affective evaluation of congruency and task switching conditions.
Last, the present findings also fit with recent observations that emotional distraction by negative pictures (relative to neutral pictures) appears to be reduced following incongruent trials (Cohen, Henik, & Mor, 2011; Cohen, Henik, & Moyal, 2012; Cohen, Moyal, & Henik, 2015; for a review, see Okon-Singer, Lichetenstein-Vidne, & Cohen, 2013), concordant with our findings that incongruent stimuli reduce the subsequent neural response to negative pictures, which could similarly suggest reduced negative emotion processing following incongruent trials (although note that we lack behavioral measurements to support this claim). For example, Cohen and colleagues (2015) demonstrated how both the pupillary light reflex and the pupil dilation response to negative pictures was attenuated following incongruent trials. The authors interpreted these findings as evidence for the hypothesis that emotional processing is reduced following the recruitment of executive control (Cohen et al., in press; Okon-Singer et al., 2013). Interestingly, as stated in the discussion of their findings (Cohen et al., 2015), this hypothesis would also predict reduced sensitivity to positive picture processing. The present results document a reduced response to negative, but enhanced response to positive pictures, following incongruent trials (see also, Dreisbach & Fischer, 2012; Fritz & Dreisbach, 2013, 2015; Schouppe et al., 2015). Therefore, it remains to be determined whether studies would find reduced emotional distraction when using positive pictures (as the reduced distraction reported in Cohen et al., 2011; 2012; 2015), or whether such studies would show the opposite pattern instead. The latter would suggest that the results of Cohen and colleagues (2011; 2012; 2015) could have been influenced by the aversive nature of conflict processing (Botvinick, 2007).
In conclusion, our results demonstrated how congruency conditions influence affective processing in the ACC. While the ACC responded less to negative pictures following incongruent than congruent trials, it showed more activation to positive pictures after incongruent compared to congruent trials. These results not only add to the growing number of observations that conflict is aversive (for a review, see Dreisbach & Fischer, 2015), but crucially demonstrate for the first time that the ACC can indeed be considered a prime candidate for registering the affective signature of conflict processing, consistent with recent models that emphasize its integrative role in cognition and emotion (Alexander & Brown, 2011; Botvinick, 2007; Silvetti et al., 2011, 2014). Future studies should extend these findings to other conflict paradigms to determine the robustness and generalisability of our results. Last, we demonstrated how this automatic affective evaluation of conflict could be observed on task repetitions only, suggesting that the affective concomitants of cognitive conflicts are processed in a task-specific manner.
Acknowledgments
S.B. is supported by FWO - Research Foundation Flanders (FWO15/PDO/029). This research was funded by National Institute of Mental Health Award R01MH087610 (T.E.) and FWO Travel Grant V418212N (S.B.). The authors declare no competing financial interests.
References
- Aarts K, De Houwer J, Pourtois G. Evidence for the automatic evaluation of self-generated actions. Cognition. 2012;124(2):117–127. doi: 10.1016/j.cognition.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature neuroscience. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(1):16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, Abrahamse EL, Duthoo W, Notebaert W. What determines the specificity of conflict adaptation? A review, critical analysis, and proposed synthesis. Frontiers in psychology. 2014;5:1134. doi: 10.3389/fpsyg.2014.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, Coenen E, Bombeke K, van Bochove ME, Notebaert W. Open your eyes for prediction errors. Cognitive, Affective, & Behavioral Neuroscience. 2015;15(2):374–380. doi: 10.3758/s13415-014-0333-4. [DOI] [PubMed] [Google Scholar]
- Braem S, King JA, Korb FM, Krebs RM, Notebaert W, Egner T. Affective modulation of cognitive control is determined by performance-contingency and mediated by ventromedial prefrontal and cingulate cortex. The Journal of Neuroscience. 2013;33(43):16961–16970. doi: 10.1523/JNEUROSCI.1208-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, Verguts T, Roggeman C, Notebaert W. Reward modulates adaptations to conflict. Cognition. 2012;125(2):324–332. doi: 10.1016/j.cognition.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Brouillet T, Ferrier LP, Grosselin A, Brouillet D. Action compatibility effects are hedonically marked and have incidental consequences on affective judgment. Emotion. 2011;11(5):1202–1205. doi: 10.1037/a0024742. [DOI] [PubMed] [Google Scholar]
- Brown JW, Reynolds JR, Braver TS. A computational model of fractioned conflict-control mechanisms in task-switching. Cognitive Psychology. 2007;55:37–85. doi: 10.1016/j.cogpsych.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Cannon PR, Hayes AE, Tipper SP. Sensorimotor fluency influences affect: Evidence from electromyography. Cognition & Emotion. 2010;24(4):681–691. [Google Scholar]
- Cohen N, Henik A, Mor N. Can emotion modulate attention? Evidence for reciprocal links in the attentional network test. Experimental psychology. 2015;58:171–179. doi: 10.1027/1618-3169/a000083. [DOI] [PubMed] [Google Scholar]
- Cohen N, Henik A, Moyal N. Executive control attenuates emotional effects—For high reappraisers only? Emotion. 2012;12(5):970–979. doi: 10.1037/a0026890. [DOI] [PubMed] [Google Scholar]
- Cohen N, Margulies DS, Ashkenazi S, Schaefer A, Taubert M, Henik A, Villringer A, Okon-Singer H. Using executive control training to suppress amygdala reactivity to aversive information. NeuroImage. doi: 10.1016/j.neuroimage.2015.10.069. (in press) [DOI] [PubMed] [Google Scholar]
- Cohen N, Moyal N, Henik A. Executive control suppresses pupillary responses to aversive stimuli. Biological psychology. 2015;112:1–11. doi: 10.1016/j.biopsycho.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost–benefit valuation and the human brain. The Journal of Neuroscience. 2009;29(14):4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet C, Deschrijver E, Brass M. How social is error observation? The neural mechanisms underlying the observation of human and machine errors. Social Cognitive and Affective Neuroscience. 2013;9:427–435. doi: 10.1093/scan/nst002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignath D, Eder AB. Stimulus conflict triggers behavioral avoidance. Cognitive, Affective, & Behavioral Neuroscience. 2015;15:822–836. doi: 10.3758/s13415-015-0355-6. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R. Conflicts as aversive signals. Brain and cognition. 2012;78(2):94–98. doi: 10.1016/j.bandc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R. Conflicts as Aversive Signals for Control Adaptation. Current Directions in Psychological Science. 2015;24(4):255–260. [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(2):343–353. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends in cognitive sciences. 2008;12(10):374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio RH. On the automatic activation of associated evaluations: An overview. Cognition & Emotion. 2001;15(2):115–141. [Google Scholar]
- Fritz J, Dreisbach G. Conflicts as aversive signals: Conflict priming increases negative judgments for neutral stimuli. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(2):311–317. doi: 10.3758/s13415-012-0147-1. [DOI] [PubMed] [Google Scholar]
- Fritz J, Dreisbach G. The time course of the aversive conflict signal. Experimental psychology. 2015;62:30–39. doi: 10.1027/1618-3169/a000271. [DOI] [PubMed] [Google Scholar]
- Goschke T. I A Intentional Reconfiguration and J-TI Involuntary Persistence in Task Set Switching. Control of cognitive processes: Attention and performance XVIII. 2000:331–355. [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in cognitive sciences. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB. Emotional foundations of cognitive control. Trends in cognitive sciences. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, Botvinick MM. Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology: General. 2010;139(4):665–682. doi: 10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, De Belder M, Egner T. Neural Conflict–Control Mechanisms Improve Memory for Target Stimuli. Cerebral Cortex. 2015;25:833–843. doi: 10.1093/cercor/bht283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville, FL: University of Florida; 2008. Technical Report A-8. [Google Scholar]
- McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proceedings of the National Academy of Sciences. 2010;107(17):7922–7926. doi: 10.1073/pnas.0910662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of stimulus task sets and response task sets during task switching. Control of cognitive processes: Attention and performance XVIII. 2000:377–399. [Google Scholar]
- Meiran N, Kessler Y. The task rule congruency effect in task switching reflects activated long-term memory. Journal of Experimental Psychology: Human Perception and Performance. 2008;34(1):137–157. doi: 10.1037/0096-1523.34.1.137. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in cognitive sciences. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H, Lichtenstein-Vidne L, Cohen N. Dynamic modulation of emotional processing. Biological psychology. 2013;92(3):480–491. doi: 10.1016/j.biopsycho.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI: I. The method. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. The Journal of Neuroscience. 2010;30(42):14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter FR, Yeung N. Memory and cognitive control in task switching. Psychological Science. 2012;23(10):1256–1263. doi: 10.1177/0956797612444613. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouppe N, Braem S, De Houwer J, Silvetti M, Verguts T, Ridderinkhof KR, Notebaert W. No pain, no gain: the affective valence of congruency conditions changes following a successful response. Cognitive, Affective, & Behavioral Neuroscience. 2015;15(1):251–261. doi: 10.3758/s13415-014-0318-3. [DOI] [PubMed] [Google Scholar]
- Schouppe N, Ridderinkhof KR, Verguts T, Notebaert W. Context-specific control and context selection in conflict tasks. Acta psychologica. 2014;146:63–66. doi: 10.1016/j.actpsy.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvetti M, Alexander W, Verguts T, Brown JW. From conflict management to reward-based decision making: actors and critics in primate medial frontal cortex. Neuroscience & Biobehavioral Reviews. 2014;46:44–57. doi: 10.1016/j.neubiorev.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Silvetti M, Seurinck R, Verguts T. Value and prediction error in medial frontal cortex: integrating the single-unit and systems levels of analysis. Frontiers in Human Neuroscience. 2011;5:75. doi: 10.3389/fnhum.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18(6):643–662. [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- van Steenbergen H. In Handbook of Biobehavioral Approaches to Self-Regulation. Springer; New York: 2015. Affective modulation of cognitive control: a biobehavioral perspective; pp. 89–107. [Google Scholar]
- van Steenbergen H, Band GP, Hommel B. In the mood for adaptation how affect regulates conflict-driven control. Psychological Science. 2010;21(11):1629–1634. doi: 10.1177/0956797610385951. [DOI] [PubMed] [Google Scholar]
- Vassena E, Silvetti M, Boehler CN, Achten E, Fias W, Verguts T. Overlapping neural systems represent cognitive effort and reward anticipation. PLOS ONE. 2014;9(3):e91008. doi: 10.1371/journal.pone.0091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and error: common neuronal architecture for the processing of errors and novelty. The Journal of Neuroscience. 2012;32(22):7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]