Abstract
Background
Although patients with diabetes mellitus experience high rates of adverse events after acute myocardial infarction (AMI), including death and recurrent ischemia, some diabetic patients are likely at low risk, while others are at very high risk. We sought to develop prediction models to stratify risk after AMI in patients with diabetes.
Methods and Results
We developed prediction models for long-term mortality and angina among 1613 patients with diabetes discharged alive after AMI from 24 US hospitals and then validated the models in a separate, multi-center registry of 786 patients with diabetes. Event rates in the derivation cohort were 27% for 5-year mortality and 27% for 1-year angina. Parsimonious prediction models demonstrated good discrimination (c-indices=0.78 and 0.69, respectively) and excellent calibration. Within the context of the predictors we estimated, the strongest predictors for mortality were higher creatinine, not working at the time of the AMI, older age, lower hemoglobin, left ventricular dysfunction, and chronic heart failure. The strongest predictors for angina were angina burden in the 4 weeks prior to the AMI, younger age, history of prior coronary bypass graft surgery, and non-Caucasian race. The lowest and highest deciles of predicted risk ranged from 4–80% for mortality and 12–59% for angina. The models also performed well in external validation (c-indices=0.78 and 0.73, respectively).
Conclusion
We found a wide range of risk for adverse outcomes after AMI in diabetic patients. Predictive models can identify patients with diabetes for whom closer follow-up and aggressive secondary prevention strategies should be considered.
Keywords: diabetes mellitus, myocardial infarction, angina
Recent advances in the management of acute myocardial infarction (AMI) have contributed to improved short- and long-term outcomes.1 However, patients with diabetes mellitus (DM) continue to experience a higher risk of adverse events after AMI, including death and recurrent ischemia compared with patients without DM.2, 3 Despite this association of diabetes with adverse outcomes, patients with DM are not a homogenous group, with important differences in demographics and comorbidities, glucose metabolism, insulin resistance, and other clinical factors. Such differences can markedly alter the risks for individual patients with DM and their potential benefit from various treatment strategies.
Illuminating which patient and treatment factors are associated with subsequent adverse outcomes could help identify those diabetic patients at highest risk who are most likely to benefit from intensive secondary prevention strategies and closer follow-up after AMI. Conversely, lower-risk patients may prefer more conservative treatments. Currently, no prediction models exist that specifically risk stratify AMI patients with DM. Given the growing prevalence of diabetes,4 we used a contemporary, representative population of AMI patients to build risk prediction models for 2 important long-term outcomes among patients with DM: mortality and angina.
METHODS
Study Population and Protocol
Between June 2005 and December 2008, 4340 patients from 24 US hospitals were enrolled into the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study, which was used to develop the prediction models.5 Similarly, between January 2003 and June 2004, 2498 patients with AMI were recruited from 19 US hospitals into the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) study, which was used to externally validate the models.6 Both registries employed identical inclusion and exclusion criteria and were coordinated by the Saint Luke’s Mid America Heart Institute. To be eligible for either registry, patients were required to have biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI, including prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes during the initial 24 hours of admission.
Baseline data were obtained through chart abstraction and a structured interview by trained research staff within 24 to 72 hours of admission. Consenting patients had fasting blood specimens collected prior to discharge. Fasting blood was drawn from each subject, which was processed, refrigerated and sent by overnight mail to the core laboratory (Clinical Reference Laboratory, Lenexa, KS) on a daily basis. Blood was analyzed for glucose, HbA1c, and lipid levels. Chart data on laboratory values drawn for clinical purposes were also recorded, which included up to 3 fasting plasma glucose levels and a random plasma glucose on admission. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent for baseline and follow-up assessments. Spanish-speaking patients were provided with translations of informed consent documents and interviews, which were conducted by Spanish-speaking staff or medical interpreters.
DM Diagnoses
Both patients with known DM and with incident DM (i.e., diagnosed during the AMI) were included. Known DM was defined as patients with a chart-documented diagnosis of DM or those on glucose-lowering medications at the time of admission (except for metformin or thiazolidinedione used as monotherapy without a documented DM diagnosis, as these may be used for DM prevention [n=2 patients]). Incident DM was defined as HbA1c≥6.5% by core lab or chart abstraction. If HbA1c level was missing, DM could be additionally diagnosed as 1) ≥2 fasting blood glucose levels ≥126 mg/dL or 2) ≥1 fasting blood glucose ≥126 mg/dL and random blood glucose (at presentation) ≥200 mg/dL.7
Outcomes Assessment
Detailed follow-up interviews were attempted on all patients at 1 month, 6 months, and 1 year after AMI. Mortality was assessed through a combination of follow-up interviews (through 1 year) and a query of the Social Security Death Masterfile. Angina and disease-specific health status was assessed using the Seattle Angina Questionnaire (SAQ)8 during each follow-up interview after AMI. The SAQ is a reliable and valid 19-item questionnaire comprised of 5 clinically important domains in patients with coronary artery disease. The scores for all SAQ domains range from 0 to 100, with higher scores indicating less disease burden. For the outcome of this study, we focused on the SAQ angina frequency domain (categorized as none vs. any [score 100 vs. <100]).9
Statistical Analysis
We constructed two models: 1) a Cox proportional hazards model for time to death of any cause over 5 years and 2) a logistic regression model for prevalence of angina (SAQ Angina Frequency score <100) at the 1-year assessment. Using clinical judgment, we selected candidate predictors available at the time of the AMI hospitalization from larger conceptual domains: demographics, socioeconomics, lifestyle, medical comorbidities, health status at time of AMI, clinical status at presentation, treatments during AMI hospitalization, and metabolic factors (Supplemental Table 1 for full candidate variable list). We avoided including highly correlated variables, and collinearity diagnostics on the selected variables were excellent (condition index=3.0, largest variance inflation factor=1.8). These variables comprised 30 total degrees of freedom, which was within the 10–20 events/degrees of freedom threshold for minimizing the risk of overfitting for mortality (436 events) but exceeded the threshold for angina (263 events). We therefore evaluated the potential for overfitting using bootstrap validation of the model calibration slopes of observed versus predicted outcomes. The calibration slope is always equal to 1 in the data on which the model was fit, but will be less than 1 on subsequent data sets if overfitting is present. The bootstrapped calibration slopes were 0.92 for mortality but 0.86 for angina, suggesting moderate overfitting in the latter model. To correct for this, we employed penalized maximum likelihood estimation, which shrinks parameter estimates to account for overfitting with the goal of improved predictive accuracy in future data.10
Because of the constraints on model degrees of freedom, continuous variables were entered as linear effects to avoid further increasing the risk of overfitting. We assessed the potential for misspecification by adding nonlinear terms for all continuous variables using 3-knot restricted cubic splines and conducting a global test for any nonlinearity. This test was non-significant for the angina model (p=0.98) but significant for mortality (p=0.04) due to a strong non-linear association with admission creatinine (p=0.001). However, the association with log creatinine was linear (p=0.33), so creatinine was log-transformed for the analyses. No other effects deviated from linearity. We also conducted a global test of the proportional hazards assumption for the mortality model, which was non-significant (p=0.51). Harrell’s backward selection strategy was used to select a parsimonious set of variables for a simplified prediction tool.11 The contribution of each covariate to the predicted values from the full model was ranked by F-value, and variables with the smallest contribution were sequentially eliminated until further variable elimination led to a greater than 5% loss in model prediction, as compared with the full model. The remaining covariates comprised the parsimonious model and explained over 95% of the variance of the full model. The performance of the reduced model (model discrimination and predicted risks) was compared with the full model to ensure that variable elimination did not degrade model performance.
Approximately 31% of patients had missing data for various predictor variables; the highest missing rate for any one variable was 10% (hemoglobin A1c) and only 8% of patients were missing data on more than one variable. In addition, 30% of patients who were alive at 1 year after AMI were missing 1-year angina assessments due to incomplete follow-up. However, nearly 60% of these patients had assessments at either 1 or 6 months (or both), which were fairly strongly correlated with 1-year assessments (r=0.41 and 0.50, respectively). We used multiple imputation to account for missing predictor values and 1-year angina scores, using sequential regression imputation on all available covariates and outcome variables as well as 1- and 6-month angina scores.12 Model development was repeated on each of the 50 imputed data sets, and final model estimates were obtained by pooling across data sets. Missing angina assessments due to death (10% of patients) was not imputed; results for the angina model are conditional on survival through 1 year. Survival status through 5 years was obtained on 100% of patients.
Internal validation of c-statistics and calibration curves for both the full and reduced models was conducted using bootstrap methods: the above modelling process was repeated on each of 100 bootstrap samples, and optimism in model performance was calculated as the difference between apparent performance (on the bootstrapped data set) and honest performance from applying the model to the original data set. The average optimism over all replications was calculated and subtracted from the original model performance. In addition, models were also validated externally using an independent data set (PREMIER). When we used the coefficient estimates from the TRIUMPH prediction model to calculate the predicted risk of angina in PREMIER, the intercept for the model was re-centered using the global mean for risk of angina for PREMIER, congruent with approaches used to risk-standardize 30-day outcomes by the Center for Medicare and Medicaid Services.1, 13 Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.2.1,14 and statistical significance was determined by a 2-sided p-value of <0.05.
RESULTS
Study Population
Among 4340 patients enrolled in TRIUMPH, 1626 were confirmed as having diabetes (2567 did not have diabetes and 147 had insufficient data to determine diabetes status). Of these, 13 patients died in-hospital, leaving 1613 patients in the primary cohort for these analyses. There were 143 patients who died prior to 1 year and were therefore ineligible to be in the angina cohort. Among 1470 surviving patients, 976 (66%) had a 1-year angina assessment, 285 (19%) had an angina assessment at 1- and/or 6-months, and 209 (14%) were lost to follow-up (all surviving patients were included in the angina cohort, using multiply imputed scores for those missing angina assessments). The demographic, clinical, and treatment characteristics of the 2 analytic cohorts are shown in Table 1. Mean age of the population was 60 years, 60% were male, 58% were white, and 38% were currently working. Thirty-four percent of patients presented with an ST-elevation AMI, 59% had multivessel disease, and 22% had moderate-to-severe left ventricular dysfunction. Mean HbA1c was 8.0% and mean fasting glucose level was 174 mg/dL.
Table 1.
Baseline demographic and clinical characteristics of the analytic cohorts
| Mortality Cohort n=1613 | Angina Cohort n=1470 | |
|---|---|---|
| Sociodemographics | ||
| Age (years) | 60.3 ± 11.6 | 59.7 ± 11.4 |
| Male | 60.3% | 60.7% |
| White race | 58.2% | 58.0% |
| Married | 48.6% | 49.5% |
| Lives alone | 26.7% | 25.3% |
| Insurance | 77.7% | 76.6% |
| High school education | 75.3% | 76.2% |
| Avoids care due to cost | 28.0% | 28.2% |
| Lifestyle factors | ||
| Currently working | 38.1% | 40.5% |
| Active during leisure time | 19.0% | 19.9% |
| Current smoker | 31.6% | 32.9% |
| Medical History | ||
| Body mass index (kg/m2) | 31.4 ± 7.0 | 31.5 ± 7.0 |
| Dyslipidemia | 58.1% | 58.3% |
| Hypertension | 80.4% | 79.8% |
| Prior myocardial infarction | 26.3% | 24.6% |
| Prior coronary angioplasty | 24.1% | 23.9% |
| Prior coronary bypass surgery | 16.8% | 15.6% |
| Peripheral vascular disease | 6.7% | 6.3% |
| Prior stroke | 6.6% | 6.0% |
| Chronic heart failure | 14.4% | 12.5% |
| Atrial fibrillation/flutter | 5.9% | 5.2% |
| Chronic lung disease | 8.4% | 7.7% |
| Depression | 22.1% | 21.4% |
| High emotional stress | 16.1% | 15.8% |
| Acute Presentation | ||
| ST-elevation myocardial infarction | 33.8% | 35.2% |
| Peak troponin (ng/dL) | 23.8 ± 77.9 | 23.5 ± 76.9 |
| Creatinine (mg/dL) | 1.4 ± 1.3 | 1.4 ± 1.2 |
| Hemoglobin (mg/dL) | 13.5 ± 2.2 | 13.7 ± 2.1 |
| Multivessel disease | 59.0% | 58.2% |
| Left ventricular systolic dysfunction | 21.7% | 20.6% |
| GRACE risk score | 106.7 ± 29.9 | 104.2 ± 28.7 |
| Systolic blood pressure (mmHg) | 146.4 ± 30.7 | 146.7 ± 30.6 |
| Diastolic blood pressure (mmHg) | 83.3 ± 19.3 | 83.7 ± 19.3 |
| Metabolic Factors | ||
| Taking insulin prior to admission | 26.7% | 25.3% |
| Hemoglobin A1c (%) | 8.0 ± 2.0 | 8.0 ± 2.1 |
| Fasting glucose (mg/dL) | 174.3 ± 75.3 | 173.3 ± 75.0 |
| Total cholesterol (mg/dL) | 175.3 ± 59.0 | 177.3 ± 59.3 |
| Triglycerides (mg/dL) | 180.1 ± 239.9 | 183.9 ± 247.9 |
| High density lipoprotein (mg/dL) | 40.1 ± 12.8 | 40.2 ± 12.8 |
| Low density lipoprotein (mg/dL) | 101.4 ± 43.8 | 102.6 ± 44.1 |
| Health Status | ||
| SAQ Angina Frequency score | 83.5 ± 22.9 | 83.8 ± 22.4 |
| SAQ Quality of Life score | 62.0 ± 24.8 | 62.1 ± 24.2 |
| In-Hospital Treatments | ||
| In-hospital coronary angioplasty | 56.9% | 58.5% |
| In-hospital coronary bypass surgery | 10.5% | 10.7% |
GFR, glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; SAQ, Seattle Angina Questionnaire
A comparison between patients with and without data on 1-year angina is shown in Supplemental Table 2. Patients missing 1-year angina data were more likely younger, non-white, unmarried, lower socio-economic status, current smokers, had more hypertension, atrial fibrillation/flutter and left ventricular dysfunction, and had lower baseline health status compared with those with angina data available.
Mortality Model
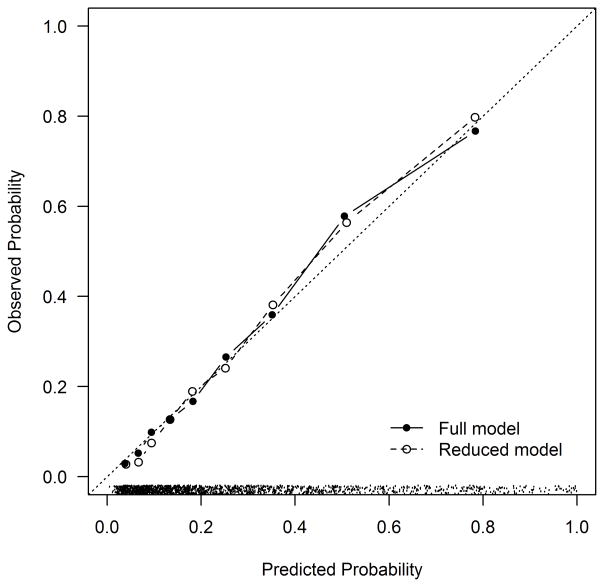
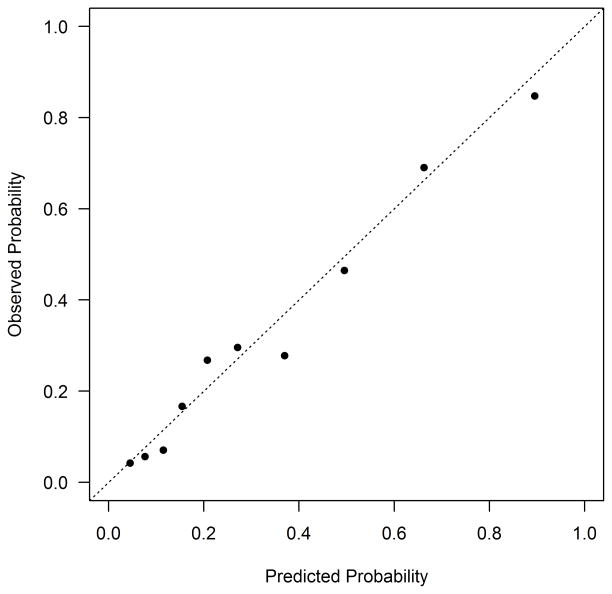
The Kaplan-Meier estimated rate of mortality among DM patients discharged alive was 8.9% at 1 year and 27.1% at 5 years. Estimated predictor effects from the full model are shown in Table 2. The most important predictors (in terms of chi square value) of long-term mortality after AMI were higher serum creatinine (HR 1.27 per 50% increase, 95% CI 1.18–1.36), not working at the time of the AMI (HR 1.95, 95% CI 1.47–2.58), older age (HR 1.29 per 10 years, 95% CI 1.16–1.44), and lower admission hemoglobin (HR 0.90 per 1 g/dL increase, 95% CI 0.85–0.94). The global test for any deviation from the proportional hazards assumption over 5 years was non-significant (p=0.51), indicating that the effect of patient characteristics on mortality risk does not change appreciably between 1 and 5 years. The simplified model demonstrated good discrimination (c-index=0.79, bootstrap validated 0.78) and excellent calibration (Figure 1a). The lowest decile of predicted risk had a 5-year mortality event rate of 4%, whereas 80% of the patients in the highest decile of predicted risk died at 5 years. The simplified model with integer scoring and risk categorization is shown in Supplemental Table 3. Among patients with DM in PREMIER, the model continued to demonstrate good discrimination (c-index=0.78) and excellent calibration (Figure 1b).
Table 2.
Factors Associated with the Mortality over the 5-years After AMI
| HR (95% CI) | Chi-Square Value | p-value | |
|---|---|---|---|
| Admission creatinine (+50%) | 1.27 (1.18–1.36) | 45.3 | <0.001 |
| Not currently working | 1.95 (1.47–2.58) | 22.0 | <0.001 |
| Age (+10 years) | 1.29 (1.16–1.44) | 20.8 | <0.001 |
| Admission hemoglobin (+1 mg/dL) | 0.90 (0.85–0.94) | 16.5 | <0.001 |
| Left ventricular systolic dysfunction | 1.50 (1.20–1.86) | 13.2 | <0.001 |
| Chronic heart failure | 1.51 (1.19–1.92) | 11.6 | 0.001 |
| Active during leisure time | 1.83 (1.29–2.59) | 11.5 | 0.001 |
| Chronic lung disease | 1.55 (1.17–2.05) | 9.6 | 0.002 |
| In-hospital revascularization | 0.72 (0.59–0.89) | 9.0 | 0.003 |
| Body mass index (+5 kg/m2) | 0.90 (0.83–0.97) | 7.9 | 0.005 |
| Currently on insulin | 1.34 (1.08–1.68) | 6.9 | 0.009 |
| Fasting glucose (+100 mg/dL) | 1.19 (1.04–1.35) | 6.4 | 0.012 |
| Prior myocardial infarction | 1.28 (1.03–1.60) | 4.9 | 0.027 |
|
| |||
| SAQ Angina Frequency (−10 points) | 0.95 (0.91–1.00) | 4.2 | 0.041 |
| Prior bypass graft surgery | 1.24 (0.98–1.57) | 3.3 | 0.067 |
| Peak troponin (+100 ng/mL) | 1.17 (0.99–1.39) | 3.3 | 0.068 |
| Prior stroke/TIA | 1.23 (0.94–1.61) | 2.2 | 0.134 |
| ST-elevation myocardial infarction | 0.83 (0.64–1.09) | 1.8 | 0.181 |
| Depression (PHQ-9 ≥ 10) | 1.19 (0.92–1.53) | 1.7 | 0.187 |
| Health insurance | 1.19 (0.88–1.61) | 1.2 | 0.264 |
| Avoids care due to cost | 1.13 (0.89–1.44) | 1.1 | 0.305 |
| Peripheral vascular disease | 1.13 (0.84–1.53) | 0.7 | 0.418 |
| Non-Caucasian race | 0.92 (0.74–1.14) | 0.6 | 0.440 |
| Current smoker | 1.10 (0.86–1.42) | 0.6 | 0.447 |
| Female | 0.93 (0.75–1.16) | 0.4 | 0.537 |
| High emotional stress (PSS-4 ≥ 9) | 1.11 (0.82–1.50) | 0.4 | 0.509 |
| Prior coronary stenting | 1.04 (0.82–1.31) | 0.1 | 0.746 |
| Atrial fibrillation/flutter | 1.01 (0.73–1.41) | 0.0 | 0.935 |
| Hemoglobin A1c (+1%) | 1.00 (0.94–1.07) | 0.0 | 0.990 |
| SAQ Quality of Life (−10 points) | 1.00 (0.95–1.04) | 0.0 | 0.859 |
Variables above the line comprise the reduced model (Supplemental Table 4)
Full model (apparent): c-index=0.80, calibration slope=1.00
Full model (bootstrap validated): c-index=0.79, calibration slope=0.92
Reduced model (apparent): c-index=0.79, calibration slope=1.00
Reduced model (bootstrap validated): c-index=0.78, calibration slope=0.92
Reduced model (externally validated): c-index=0.78, calibration slope=0.92
Figure 1. Calibration Plot for the Mortality Prediction Model in TRIUMPH (a) and PREMIER (b).
(a) TRIUMPH Internal Validation: Calibration slope 0.92 (full model) and 0.92 (reduced model). The dots at the bottom of the figure indicate the distribution of predicted risks. (b) PREMIER External Validation: Calibration slope 0.92.
Angina Model
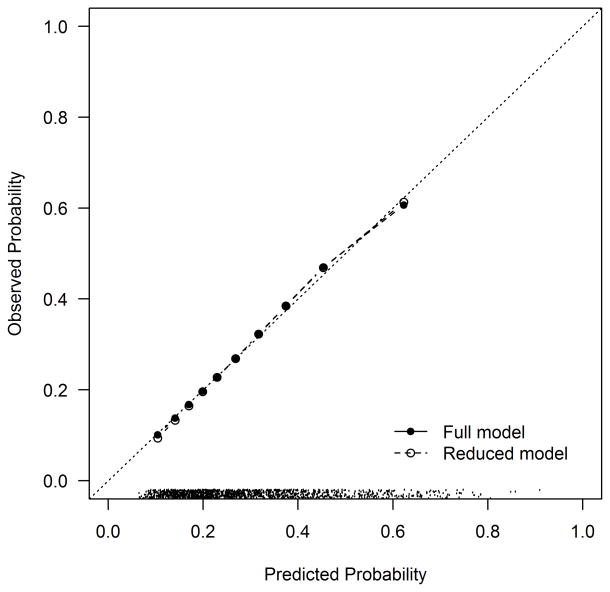
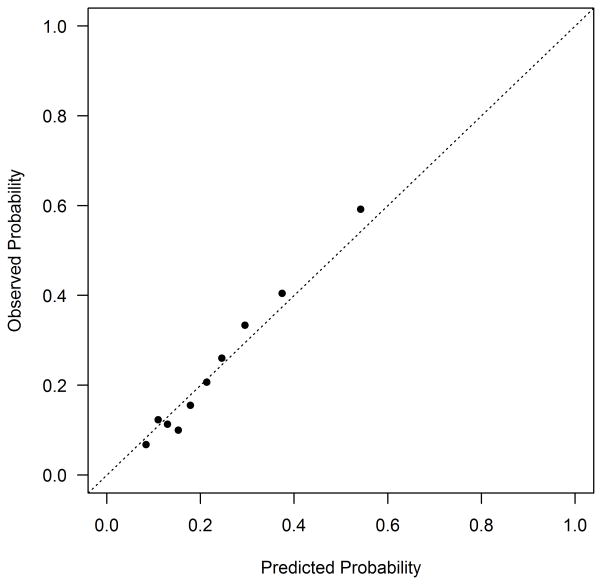
At 1 year after AMI, 27.0% of DM patients reported having angina. The variables included in the final model are shown in Table 3. The most important predictor of angina after AMI (in terms of chi-square value) was the burden of angina present prior to the AMI (OR 1.18 per 10 point decrease in baseline SAQ Angina Frequency score, 95% CI 1.11–1.26). Other important predictors of having angina at 1 year after the AMI were younger age (OR 0.79 per 10 year increase, 95% CI 0.60–0.90) and a history of prior coronary bypass graft surgery (OR 1.67, 95% CI 1.16–2.40). The simplified model demonstrated moderate discrimination (c-index=0.71, bootstrap validated 0.69) and excellent calibration (Figure 2a). Twelve percent of the patients in the lowest decile of risk reported angina at 1 year, compared with 59% of patients in the highest decile of risk. The simplified model with integer scoring and risk categorization is shown in Supplemental Table 4. Among patients with DM in PREMIER, the model continued to demonstrate moderate discrimination (c-index=0.73) and excellent calibration (Figure 2b).
Table 3.
Factors Associated with the Presence of Angina 1-year After AMI
| OR (95% CI) | Chi-square value | p-value | |
|---|---|---|---|
| SAQ Angina Frequency (−10 pts) | 1.18 (1.11–1.26) | 25.6 | <0.001 |
| Age (+10 years) | 0.79 (0.69–0.90) | 11.8 | 0.001 |
| Prior bypass graft surgery | 1.67 (1.16–2.40) | 7.5 | 0.006 |
| Non-Caucasian race | 1.47 (1.08–1.99) | 6.2 | 0.013 |
| Chronic heart failure | 1.58 (1.03–2.42) | 4.5 | 0.035 |
| Currently working | 1.26 (0.94–1.69) | 2.4 | 0.125 |
| Current smoker | 1.26 (0.94–1.70) | 2.4 | 0.119 |
| Female | 1.26 (0.93–1.72) | 2.2 | 0.135 |
|
| |||
| Left ventricular systolic dysfunction | 0.76 (0.53–1.09) | 2.3 | 0.134 |
| High emotional stress (PSS-4 ≥ 9) | 1.34 (0.91–1.98) | 2.2 | 0.135 |
| Admission creatinine (+50%) | 0.91 (0.80–1.04) | 2.0 | 0.155 |
| Chronic lung disease | 1.40 (0.87–2.24) | 1.9 | 0.169 |
| Depression (PHQ-9 ≥ 10) | 1.21 (0.87–1.69) | 1.3 | 0.248 |
| Peripheral vascular disease | 1.34 (0.79–2.26) | 1.2 | 0.275 |
| Avoids care due to cost | 1.19 (0.86–1.66) | 1.1 | 0.292 |
| Body mass index (+5 kg/m2) | 0.95 (0.86–1.05) | 1.1 | 0.291 |
| In-hospital revascularization | 0.86 (0.64–1.18) | 0.9 | 0.351 |
| Hemoglobin A1c (+1%) | 0.97 (0.90–1.04) | 0.7 | 0.398 |
| Peak troponin (+100 ng/mL) | 0.92 (0.70–1.21) | 0.4 | 0.544 |
| Atrial fibrillation/flutter | 0.86 (0.48–1.54) | 0.3 | 0.615 |
| SAQ Quality of Life (−10 pts) | 1.02 (0.96–1.08) | 0.3 | 0.563 |
| Fasting glucose (+100 mg/dL) | 0.96 (0.79–1.16) | 0.2 | 0.669 |
| Health insurance | 0.95 (0.68–1.34) | 0.1 | 0.776 |
| Active during leisure time | 1.05 (0.75–1.48) | 0.1 | 0.774 |
| Prior myocardial infarction | 1.05 (0.75–1.45) | 0.1 | 0.790 |
| Prior coronary stenting | 1.04 (0.73–1.46) | 0.0 | 0.840 |
| Prior stroke/TIA | 1.03 (0.64–1.65) | 0.0 | 0.897 |
| ST-elevation myocardial infarction | 1.02 (0.74–1.38) | 0.0 | 0.923 |
| Admission hemoglobin (+1 mg/dL) | 0.99 (0.92–1.07) | 0.0 | 0.852 |
| Currently on insulin | 1.03 (0.75–1.43) | 0.0 | 0.841 |
Variables above the line comprise the reduced model (Supplemental Table 5)
Full model (apparent): c-index=0.74, calibration slope=1.00
Full model (bootstrap validated): c-index=0.71, calibration slope=1.00
Reduced model (apparent): c-index=0.71, calibration slope=1.00
Reduced model (bootstrap validated): c-index=0.69, calibration slope=1.02
Reduced model (externally validated): c-index=0.73, calibration slope=1.22
Figure 2. Calibration Plot for the Angina Prediction Model in TRIUMPH (a) and PREMIER (b).
(a) TRIUMPH Internal Validation: Calibration slope 1.00 (full model) and 1.02 (reduced model). The dots at the bottom of the figure indicate the distribution of predicted risks. (b) PREMIER External Validation: Calibration slope 1.22.
DISCUSSION
Due to the rising prevalence of DM among patients admitted with an AMI, which is now nearly 40%,15 understanding the effect of DM on outcomes after an AMI is becoming increasingly important. Patients with DM have long been known to be at high risk for morbidity and mortality after an AMI;2, 3 due, in part, to more extensive coronary artery disease,16 additional cardiovascular risk factors and higher burden of comorbidities. In our analyses, we were able to build and externally validate prediction models to identify patients with DM who are at particularly high risk for post-AMI morbidity and mortality. Use of these models at the time of hospital discharge after AMI may permit identification of patients with DM and AMI for whom closer follow-up and more intensive secondary prevention strategies may be warranted, including careful discharge education and counseling provided for patients and their families.
DM-Specific Predictors
Interestingly, while we identified several unique predictors for poor outcomes among AMI patients with DM that are not in traditional risk models (e.g., not employed at time of AMI, low hemoglobin, low activity during leisure time),17 most of these predictors were not specific to the underlying DM. Specifically, HbA1c level was not a significant predictor for either of the outcomes studied. In the mortality models, use of insulin prior to admission (often considered a proxy for longer duration of DM and worse glycemic control) was associated with increased risk of death but not subsequent angina. For angina, there were no DM-specific predictors in the reduced model. However, there were some predictors of angina among patients with DM that had not been identified in prior models examining predictors of angina after AMI. For example, chronic heart failure has not been identified as a predictor of 1-month angina among all patients with AMI18 or 6-month angina among all patients after percutaneous coronary intervention.19 While the time frame of analysis may partially explain this finding, it may also be that patients with DM and heart failure, in combination (a growing and very high-risk patient group), are more likely to have residual angina after AMI. Furthermore, we found that certain factors that had been predictive of poor outcomes in models including all AMI patients, such as peak troponin, were not identified as significant predictors among only patients with DM. Other identified predictors of angina among patients with DM—such as depression, younger age, prior bypass graft surgery, smoking, and lower socioeconomic status18–21—and of mortality among patients with DM—older age, renal dysfunction, anemia, heart failure, prior AMI, and low body weight22–24—are consistent with prior studies in patients with and without DM.
Clinical Implications
Patients with DM who are hospitalized with an AMI are typically considered high risk for subsequent ischemic outcomes. However, our findings suggest that there is a wide distribution of risk in this patient population. For example, the overall 5-year mortality rate was 27%; however, 30% of the patients had a predicted risk of mortality of <10% while 16% of the population had a 5-year risk of death of >50%. Similar relationships were observed for angina. Given this broad distribution of risk among those with DM and AMI, our risk models would allow for better identification of patients’ risk so that follow-up and treatment strategies can be triaged and allocated accordingly, rather than just on the basis of having DM. Individualized risk-stratification at the time of discharge may allow better-informed counseling for patients and families and a more objective approach to earlier vs. later follow-up after hospital discharge. Given the fact that angina is a powerful driver of repeat hospitalizations and healthcare costs,25 such an approach is especially relevant in the era when hospitals are under increased scrutiny to reduce hospital readmissions after AMI.
Furthermore, while some treatments during and after an AMI should be applied to all patients—such as intensive statins, cardiac rehabilitation, etc.—there remain other therapies that may be selected in the case of higher risk features. For example, the risk-benefit ratio for prasugrel or ticagrelor may be more favorable in patients at higher ischemic risk and could be targeted to patients at high risk for post-AMI mortality. In addition, staged revascularization of non-culprit coronary arteries or adjunctive antianginal therapies may be useful in patients at high risk for angina after AMI. As these treatments are associated with higher costs, targeting them in the higher risk patients may be an effective and efficient strategy for improving outcomes in patients with DM and AMI. Whether such an approach can improve patient outcomes and reduce costs remains to be determined and should be evaluated in future studies.
Limitations
There are potential limitations to our study that merit further discussion. The discrimination of our models was moderate with c-index ranges of ~0.7–0.8. While this performance is similar or better than most existing prediction models in the general AMI population,22, 26 further exploration to identify factors that contain incremental prognostic information, such as genetic factors or more detailed metabolic factors, will be important to improve these models over time. Second, given the number of events in our analytic population, we had to limit our number of candidate variables to avoid over-fitting. In addition, we explicitly accepted over-fitting for the angina model (which we subsequently corrected for) in order to examine a broader number of candidate variables. As such, it is possible that there were some predictors that we did not consider. Third, missing data, both predictor and outcomes (angina), could have influenced our results. However, our inclusion of a broad range of predictors and use of multiple imputation, including 1- and 6-month angina assessments in the imputation model, mitigate selection biases associated with observed factors and appropriately adjust statistical inferences to account for uncertainty due to missingness. Finally, patients who died prior to 1 year were not included in the angina model, potentially introducing a survival bias in this analysis. Importantly, these two models should be used to inform patients in combination, to estimate risk of long-term mortality and (if alive) risk of angina.
Conclusion
In 2 large multicenter AMI registries, we built and validated models for predicting long-term outcomes specifically among AMI patients with DM. We demonstrated that while patients with DM and AMI are high risk on average, there was a wide distribution of risk within this population, with some patients being at extremely high risk for adverse events during follow up, while others being at low risk. Our models could be used for an individualized approach to risk-stratification at the time of discharge and identification of patients for whom closer follow-up and secondary prevention strategies can be targeted most aggressively.
Supplementary Material
Acknowledgments
Sources of Funding: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): SCCOR Grant #P50HL077113-01. This study was sponsored by a research grant from Genentech, South San Francisco, CA. The funding organizations did not play a role in the collection, management, and analysis of the data. YX is an employee of Genentech and did participate, along with the other coauthors, in the design of the study, the interpretation of the analyses, and writing of the manuscript. The decision to submit the manuscript for publication was made independent of the study sponsor.
Footnotes
Disclosures: DKM: Consultant honoraria: Takeda; Janssen; Merck; Regeneron; Boehringer Ingelheim. Clinical trial leadership honoraria: Boehringer Ingelheim, Takeda, Orexigen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Daiichi Sankyo, Merck Schering Plough; Eisai; Omthera; Lexicon. JAS: Research grants: NHLBI, AHA, PCORI, ACCF, Gilead, Lilly, EvaHeart, Amorcyte. Consultation: United Healthcare, Genentech, Amgen, Janssen, Novartis. YX: Employee: Genentech. JMS: consultation for Cordis Corp. and serves on the speaker’s bureau for Astra Zeneca, Astellas, and InfraReDx LLC. MK: Research grants: AstraZeneca, Genetech, Sanofi-Aventis, Gilead; Consultant honoraria: AstraZeneca, Amgen, Eli Lilly, GSK, Sanofi, Boehringer Ingelheim, Takeda, Glytec; Speakers’ Bureau: Amgen. The other authors report no conflicts of interest.
References
- 1.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995–2006. JAMA. 2009;302:767–73. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–75. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 4.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–97. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Arnold SV, Lipska KJ, Inzucchi SE, Li Y, Jones PG, McGuire DK, Goyal A, Stolker JM, Lind M, Spertus JA, Kosiborod M. The reliability of in-hospital diagnoses of diabetes mellitus in the setting of an acute myocardial infarction. BMJ Open Diabetes Res Care. 2014;2:e000046. doi: 10.1136/bmjdrc-2014-000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 9.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789–94. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 10.Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer; 2008. [Google Scholar]
- 11.Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 12.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- 13.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–13. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2015. [Google Scholar]
- 15.Wallander M, Malmberg K, Norhammar A, Ryden L, Tenerz A. Oral glucose tolerance test: a reliable tool for early detection of glucose abnormalities in patients with acute myocardial infarction in clinical practice: a report on repeated oral glucose tolerance tests from the GAMI study. Diabetes Care. 2008;31:36–8. doi: 10.2337/dc07-1552. [DOI] [PubMed] [Google Scholar]
- 16.Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ, Ross AM. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996;28:1661–9. doi: 10.1016/s0735-1097(96)00397-x. [DOI] [PubMed] [Google Scholar]
- 17.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 18.Kureshi F, Arnold SV, Li Y, Masoudi FA, Jones PG, Cresci S, Maddox TM, Ho PM, Spertus JA. Development of a 30-Day Angina Risk Standardization Model Following an Acute Myocardial Infarction. Circulation. 2013;128:A14960. [Google Scholar]
- 19.Arnold SV, Jang JS, Tang F, Graham G, Cohen DJ, Spertus JA. Prediction of Residual Angina After Percutaneous Coronary Intervention. Eur Heart J Qual Care Clin Outcomes. 2015;1:23–30. doi: 10.1093/ehjqcco/qcv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchanan DM, Arnold SV, Gosch KL, Jones PG, Longmore LS, Spertus JA, Cresci S. Association of Smoking Status With Angina and Health-Related Quality of Life After Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2015;8:493–500. doi: 10.1161/CIRCOUTCOMES.114.001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maddox TM, Reid KJ, Spertus JA, Mittleman M, Krumholz HM, Parashar S, Ho PM, Rumsfeld JS. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med. 2008;168:1310–6. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 22.Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, Jr, Granger CB. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roe MT, Chen AY, Thomas L, Wang TY, Alexander KP, Hammill BG, Gibler WB, Ohman EM, Peterson ED. Predicting long-term mortality in older patients after non-ST-segment elevation myocardial infarction: the CRUSADE long-term mortality model and risk score. Am Heart J. 2011;162:875–883. e1. doi: 10.1016/j.ahj.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Vaglio J, Safley DM, Rahman M, Kosiborod M, Jones P, Thompson R, Krumholz HM, Spertus JA. Relation of anemia at discharge to survival after acute coronary syndromes. Am J Cardiol. 2005;96:496–9. doi: 10.1016/j.amjcard.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–53. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 26.de Araujo Goncalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26:865–72. doi: 10.1093/eurheartj/ehi187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.