Abstract
Mammalian spermatogenesis is a complex process in which spermatogonial stem cells of the testis (SSCs) develop to ultimately form spermatozoa. In the seminiferous epithelium, SSCs self-renew to maintain the pool of stem cells throughout life, or they differentiate to generate a large number of germ cells. A balance between SSC self-renewal and differentiation is therefore essential to maintain normal spermatogenesis and fertility. Stem cell homeostasis is tightly regulated by signals from the surrounding microenvironment, or SSC niche. By physically supporting the SSCs and providing them with these extrinsic molecules, the Sertoli cell is the main component of the niche. Earlier studies have demonstrated that GDNF and CYP26B1, produced by Sertoli cells, are crucial for self-renewal of the SSC pool and maintenance of the undifferentiated state. Down-regulating the production of these molecules is therefore equally important to allow germ cell differentiation. We propose that NOTCH signaling in Sertoli cells is a crucial regulator of germ cell fate by counteracting these stimulatory factors to maintain stem cell homeostasis. Dysregulation of this essential niche component can lead by itself to sterility or facilitate testicular cancer development.
Keywords: germ cell homeostasis, NOTCH signaling, Sertoli cells, spermatogonial stem cell niche, spermatogonial stem cells
Introduction
Stem cells are immature, undifferentiated cells that are able to self-renew to maintain their own pool, but also to differentiate into specialized cell types that will ultimately perform a biological function. In the embryo and fetus, stem cell activity is critical for organ development and growth. In adults, stem cells are essential for the maintenance, repair and regeneration of many tissues. In particular, a pool of actively self-renewing stem cells is necessary to sustain hematopoiesis in the bone marrow, to regenerate the intestinal epithelium and to maintain spermatogenesis in the seminiferous tubules, since in all these tissues differentiated cells are eliminated and must be replenished throughout life (Oatley and Brinster, 2008; Blanpain and Fuchs, 2009; Rossi et al.., 2011; Arwert et al.., 2012). Proper regulation of stem cell fate is therefore critical to maintain adequate cell numbers in health and diseases. Accumulating evidence suggests that stem cells behavior is regulated by both extracellular signals from their microenvironment, or niche, and intrinsic signals within the cells. Using diverse model organisms, much work has been done to understand how the niche controls stem cell self-renewal and differentiation and how in turn stem cells influence their environment. This mini-review focuses on recent findings pertaining to germ cell development and the relationship between spermatogonial stem cells of the testis and their niche.
Development of the male germ line
In the mouse, the germ line originates from a small population of primordial germ cells (PGCs), which arise from the pluripotent epiblast during gastrulation and are specified by BMP signaling at embryonic day 6.5 (E6.5; Ewen and Koopman, 2010). PGCs proliferate, migrate through the hindgut and colonize the genital ridges between E7.5 and E11. Once in the developing gonads, PGCs stop proliferating and initially develop according to cues based on the surrounding somatic cells. For example, studies have shown that irrespectively of their sex, germ cells will enter meiosis if they reside in a developing ovary, but not in a developing testis (Evans et al., 1977; Adams and McLaren, 2002), and that only subsequent gamete differentiation depends on the sex of the germ cells. In the developing ovary, between E12.5 and E14.5, meiosis entry is signaled by retinoic acid (RA), a powerful morphogen provided by the mesonephros (Rhinn and Dolle, 2012; Feng et al., 2014). In the developing testis, meiosis cannot proceed because of the activity of the enzyme CYP26B1, produced by Sertoli and interstitial cells, which catalyzes the oxidization of RA into inactive metabolites (White et al., 2000). Therefore once in the gonads, male PGCs, now called prospermatogonia, or gonocytes, do not further differentiate due to depleted levels of RA and instead undergo mitotic arrest. They re-enter the cell cycle at postnatal day 3 (P3), then migrate to the basement membrane of the seminiferous epithelium to become spermatogonial stem cells (SSCs).
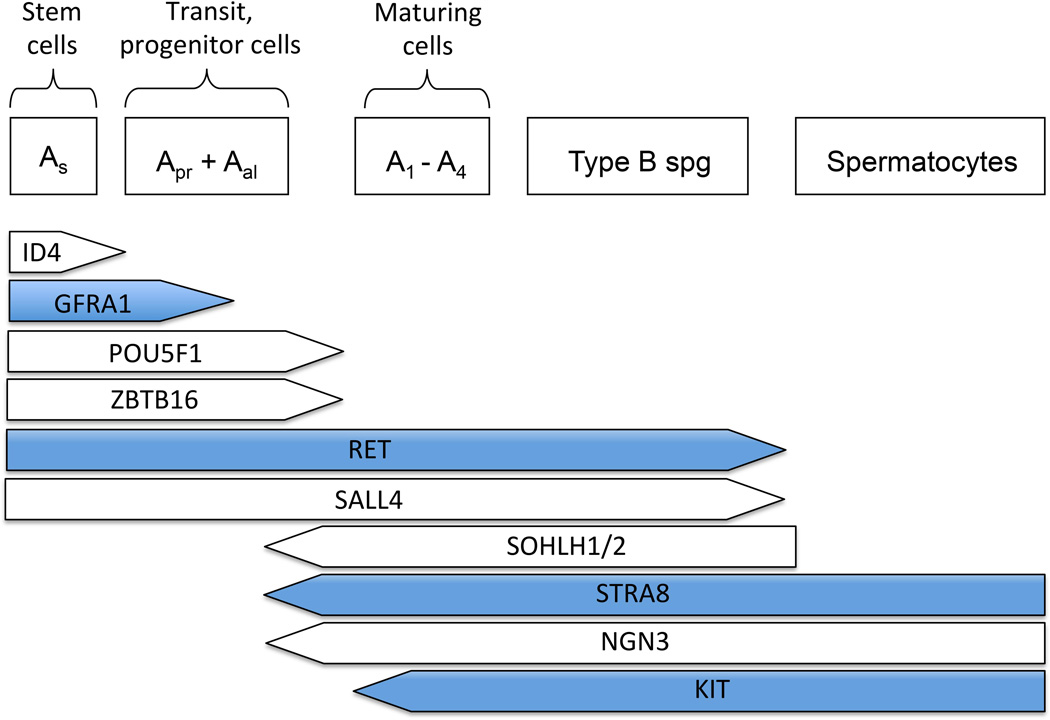
SSCs self-renew throughout life to maintain a pool of undifferentiated cells that are at the origin of spermatogenesis and ensure the permanent production of sperm (de Rooij and Griswold, 2012; Fig. 1). SSCs are single cells (Asingle spermatogonia, As) that are not connected by intercellular bridges like the other more mature germ cells (Dym and Fawcett, 1971; Huckins, 1971; de Rooij and Russell, 2000). SSCs self-renew under the influence of glial cell line-derived neurotrophic factor (GDNF), which is produced by Sertoli cells and binds to the membrane receptor complex GFRA1/RET (Meng et al., 2000; Kubota et al., 2004; Hofmann et al.., 2005; Naughton et al., 2006). When rodent SSCs differentiate, they divide into two daughter cells called Apaired spermatogonia (Apr), which will in turn divide to form chains of 4, 8 or 16 Aaligned spermatogonia (Aal). This step is considered an amplification step that increases the number of progenitors, and Asingle, Apaired and Aaligned are often referred to as undifferentiated spermatogonia (de Rooij and Russell, 2000). Aaligned cells will further differentiate into type A1-A4 spermatogonia and B spermatogonia, which finally divide to form primary spermatocytes. Throughout this postnatal germ cell development period, the levels of CYP26B1 in Sertoli cells gradually decrease (Gaido and Lehmann, 2006), allowing the levels of retinoic acid to rise, which drives Stra8 expression in germ cells, meiosis entry and spermatocyte differentiation into haploid spermatids and spermatozoa (Anderson et al., 2008). The molecular events underlying the regulation of CYP26B1 are not completely understood, nor are the cause and function of mitotic arrest in prospermatogonia.
Figure 1.
Intrinsic markers essential for SSC self-renewal and differentiation. The transcription factor ID4 identifies a small population of Asingle spermatogonia that are believed to be the true stem cells (Chan et al., 2014). The membrane receptors GFRA1 and RET bind the ligand GDNF, which is crucial for stem cell self-renewal (Meng et al., 2000; Hofmann et al., 2005; Naughton et al., 2006). ZBTB16 and POU5F1 are transcription factors expressed by SSCs and undifferentiated spermatogonia (Ohbo et al., 2003; Buaas et al., 2004; Costoya et al., 2004; Dann et al., 2008). Upon downregulation of CYP26B1, increased retinoic acid levels will stimulate germ cell expression of STRA8, which will induce them to differentiate and enter meiosis (Anderson et al., 2008). SOHLH1/2 and NGN3 are expressed at the boundary between Aaligned and A1-A4 differentiating spermatogonia, while KIT is a known marker of differentiating spermatogonia (Schrans-Stassen et al., 1999; Yoshida et al., 2006; Suzuki et al., 2012). ZBTB16 is an inhibitor of KIT, which therefore contributes in maintaining the undifferentiated state (Filipponi et al., 2007). Molecules discussed more in depth in the present review are marked in blue.
The male germ line stem cell microenvironment
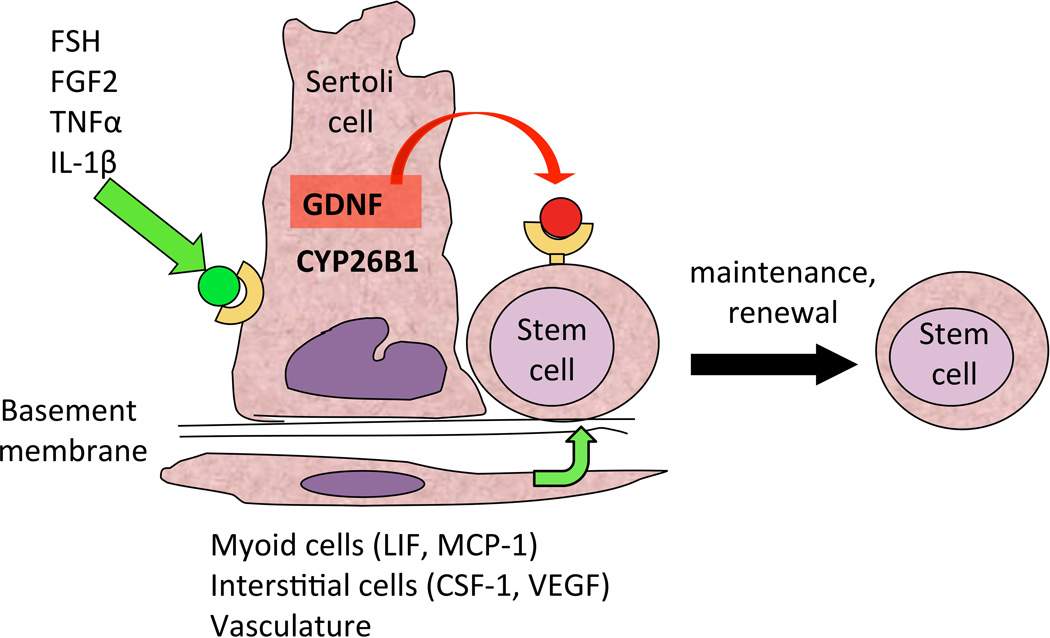
Stem cell behavior is regulated by specialized microenvironments, or niches, which maintain the size and homeostasis of the stem cell population. The role of the niche has been demonstrated in several tissues, in particular intestinal epithelium, bone marrow and epidermis (Singh, 2012). Many of the events determining germ cell development in the perinatal testis, including development of prospermatogonia into stem cells, and spermatogonial stem cell fate throughout life, are also orchestrated by their microenvironment, which is composed of several types of somatic cells (Fig. 2). Within the seminiferous epithelium, the Sertoli cell is an important cellular component of the testicular niche and is responsible for the production of several growth factors that influence the development of prospermatogonia and the fate of SSCs as described in the next section (Li et al., 1997; Meng et al., 2000; Miles et al., 2012; Garcia et al., 2013). Sertoli cells also contribute to the architecture of the seminiferous tubules before and after birth (van der Wee and Hofmann, 1999; Kim et al., 2007), provide structural support to the germ cells and ensure their proper differentiation along the basal-apical axis of the seminiferous epithelium (Su et al., 2013). Peritubular myoid cells, which surround the seminiferous tubules, also contribute to the niche by producing growth factors such as LIF (Piquet-Pellorce et al., 2000), and the CC-chemokine MCP-1 (Aubry et al., 2000). Together with Sertoli cells, peritubular myoid cells produce components of the basement membrane onto which they reside together with undifferentiated spermatogonia (Richardson et al., 1995; Siu and Cheng, 2008).
Figure 2.
The spermatogonial stem cell niche. Depicted are the positive effects of FSH, FGF2 and other molecules on GDNF expression by Sertoli cells, which ensure SSC self-renewal (Tadokoro et al., 2002; Simon et al., 2007). Simultaneously, CYP26B1, by degrading retinoic acid, ensures maintenance of the undifferentiated state (Feng et al., 2014). Other cells belonging to the niche produce growth factors such as CSF-1, VEGF, LIF and MCP-1, which also contribute to positive induction of self-renewal (Aubry et al., 2000; Piquet-Pellorce et al., 2000; Oatley et al., 2009; Caires et al., 2012).
Other cellular components of the testicular niche are found in the interstitial space between the seminiferous tubules. Leydig cells, which are known to synthesize testosterone, also produce growth factors crucial for SSC self-renewal such as CSF-1 (Dirami et al., 1999; Kokkinaki et al., 2009; Oatley et al., 2009) and VEGF (Collin and Bergh, 1996; Ergun et al., 1997; Korpelainen et al., 1998; Anand et al., 2003; Caires et al., 2012; Lu et al., 2013). Lymph and blood vessels also occupy the interstitial space and must contribute to SSC maintenance and self-renewal since these cells are always located at the proximity of the testicular capillaries (Yoshida et al., 2007). Little is known about the factors that emanate from the vasculature, but it is thought that circulating hormones and VEGF may play an important role. All these components contribute together to create the SSC microenvironment, which regulates many aspects of stem cell function.
Niche factors produced by Sertoli cells
The Sertoli cell is arguably the most important component of the testis niche. In the past decade, a number of factors produced by Sertoli cells have been found that are crucial for gonocyte maintenance in the fetus and stem cell renewal after birth.
Low levels of the growth factor glial cell line-derived neurotrophic factor (GDNF) are detectable in the bipotential gonads as early as E11 (Beverdam and Koopman, 2006). Glial cell line-derived neurotrophic factor expression by Sertoli cells increases steadily during testis development and reaches a maximum at P3. Subsequently, GDNF expression decreases without completely disappearing since it is used by SSC to self-renew throughout life (Meng et al., 2000; Tadokoro et al., 2002; Shima et al., 2004; Miles et al., 2012). Therefore GDNF might be used by fetal prospermatogonia to ensure their survival (Miles et al., 2012; Garcia et al., 2013) in addition to be used by SSCs to maintain their pool after birth. In older animals, GDNF expression also varies across the stages of the seminiferous epithelium, and is highest at stages XIII–I, when undifferentiated spermatogonia proliferate (Johnston et al., 2011). It is lowest at stages VII and VIII, when most of the cells are quiescent and the majority of Aaligned spermatogonia transition to the differentiating A1-A4 cells. These observations indicate that GDNF levels in the developing testis vary over time and are cyclic, and that its dosage is crucial for the regulation of perinatal germ cell fate and stage-specific proliferation of undifferentiated spermatogonia. However the mechanisms responsible for this control are not understood.
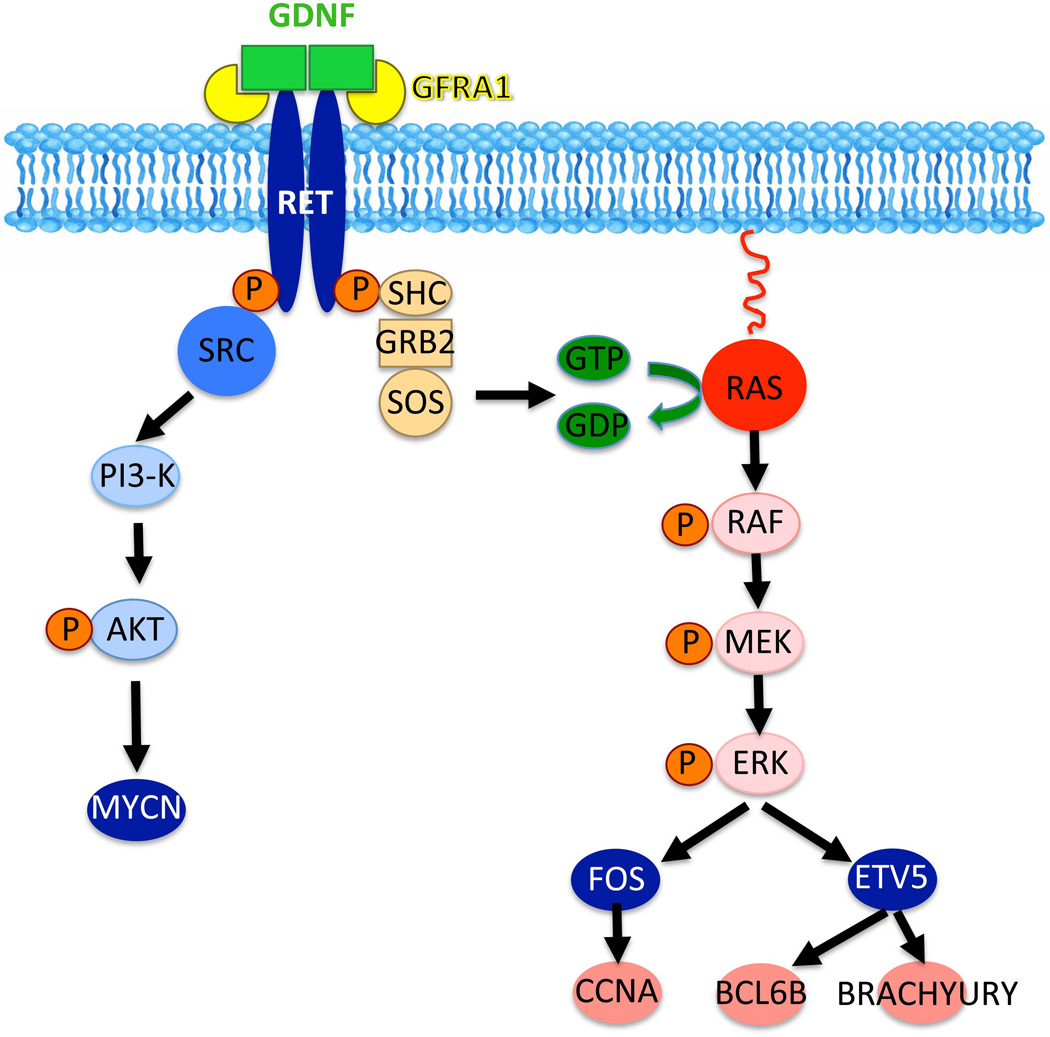
When SSCs are established, GDNF binds to the RET/GFRA1 receptor complex to induce their self-renewal (Meng et al., 2000; Hofmann et al., 2005; Hofmann, 2008; Oatley and Brinster, 2008; Kanatsu-Shinohara and Shinohara, 2013). Absence of GDNF or its receptors RET and GFRA1 leads to a lack of SSCs (Naughton et al., 2006). Two signaling pathways triggered by GDNF binding to its receptors have been so far identified in SSCs (Fig. 3). In one scenario, RET phosphorylation induces SRC-family kinases (SFKs) and PI3K/AKT activation (Braydich-Stolle et al., 2007; Oatley et al., 2007) and ultimately the upregulation of Mycn (Braydich-Stolle et al., 2007; Lucas et al., 2012). Further analysis indicated that FYN kinase is probably the major SFK used by undifferentiated spermatogonia to proliferate (Braydich-Stolle et al., 2010). A recent study using spermatogonial transplantation confirmed that MYC family transcription factors are crucial for SSC self-renewal (Kanatsu-Shinohara et al., 2014). Another pathway activated by the binding of GFNF to the RET/GFRA1 complex is the canonical RAS pathway (He et al., 2008; Lee et al., 2009), which upregulates the expression of the transcription factor Fos (He et al., 2008). In turn, FOS increases the levels of the cyclin Ccna2, which is crucial for spermatogonial proliferation (Sunters et al., 2004; He et al., 2008; Wolgemuth et al., 2013). More recently the cyclin Ccne1 as a target of RAS signaling in germ cells has received some interest since its downregulation through siRNA inhibits SSC self-renewal (Kanatsu-Shinohara et al., 2014). However, it is not known whether both SRC and RAS, or only one of these pathways, specifically triggers self-renewal.
Figure 3.
Signaling pathways triggered by GDNF activation of the RET transmembrane receptor. GDNF promotes cell cycle progression via binding to RET and its co-receptor GFRA1. RET phosphorylation activates SRC kinase family members (SFKs), in particular FYN kinase (Braydich-Stolle et al., 2010). This in turn triggers the PI3K/AKT pathway to increase N-myc gene expression (Braydich-Stolle et al., 2007). Another pathway initiated by the interaction of GDNF with RET/GFRA1 is the RAS-dependent canonical signaling pathway. This pathway ultimately upregulates the transcription factors Fos and ETV5, which themselves induce the expression of cyclins and stem cell-specific genes (He et al., 2008; Wu et al., 2011).
A molecule produced by fetal and neonatal Sertoli cells is the enzyme CYP26B1 as mentioned above. Levels of this enzyme are high enough in the male gonads before birth to degrade retinoic acid and prevent germ cell differentiation. Therefore, like GDNF, CYP26B1 contributes to the maintenance of the undifferentiated state of prospermatogonia in the perinatal testis. CYP26B1 expression starts decreasing around birth (Gaido and Lehmann, 2006), which allows retinoic acid production and activity. When retinoic acid reaches sufficient levels, it will induce the expression of the gene Stra8 (stimulated by retinoic acid 8), which increases in spermatogonia and eventually triggers meiosis in spermatocytes. Although the mode of action of STRA8 is still unknown, we know from knockout experiments that it is required for induction of the meiotic prophase (Anderson et al., 2008).
Control of germ cell development by Sertoli cells also requires the production of FGF2, which, together with GDNF, is crucial for the proliferation of prospermatogonia and SSC in cultures (Van Dissel-Emiliani et al., 1996; Kubota et al., 2004; Kubota and Brinster, 2008). PDGF, another factor produced by Sertoli cells, induces prospermatogonia proliferation after birth (Loveland et al., 1995; Li et al., 1997), probably in cooperation with estrogen (Thuillier et al., 2010). Disruption of cross-talks between PDGF and estrogen-triggered signaling pathways has been suggested to take place upon exposure to xenoestrogens in the environment, which could lead to alteration of prospermatogonial behavior and preneoplastic states (Thuillier et al., 2003).
Control of growth factor production
Maintenance of spermatogenesis depends on SSCs and is sustained throughout life in a highly ordered manner. Despite the essential role of Sertoli cells within the niche, little is known about how the production of growth factors that influence the fate of SSCs is regulated. It is however evident that homeostasis within the stem cell compartment must be governed by positive and negative feedback regulation of these extrinsic signals.
E.1. Positive regulation
In 2002, the group of Nishimune demonstrated that follicle-stimulating hormone (FSH) was one of the major regulators of GDNF production by Sertoli cells and increased proliferation of undifferentiated spermatogonia in vivo (Tadokoro et al., 2002). They treated steel/steel Dickie (Sl/Sld) mutant mice, which cannot produce KIT ligand (KITL) and lack A1-A4 differentiating spermatogonia, with a GnRH antagonist (Nal-Glu) for 5 days. This treatment significantly reduced GDNF expression and decreased the rate of proliferation of undifferentiated spermatogonia in the testes of these mice. Also, FSH stimulation induced GDNF expression in primary cultures of Sertoli cells. More recently, Simon and colleagues demonstrated that FGF2, TNFα and IL-1β also stimulated Gdnf expression in cultured Sertoli cells (Simon et al., 2007; Fig. 2).
Positive regulation of CYP26B1 in fetal Sertoli cells has been well documented. Transcription factor genes steroidogenic factor-1 (SF1) and Sry-related HMG box 9 (SOX9) are co-expressed with CYP26B1 in fetal Sertoli cells, and exogenous expression of SOX9 or SF1 increases Cyp26b1 mRNA levels (Kashimada et al., 2011). Conversely, in embryonic gonads deficient in Sox9 or Sf1, Cyp26b1 expression is decreased relative to wild-type controls (Kashimada et al., 2011). These results strongly suggest that SF1 and SOX9 are transcription factors that positively influence CYP26B1 production, ultimately downregulating retinoic acid under a critical level in the developing male gonad and blocking meiosis before birth.
E.2. Negative regulation
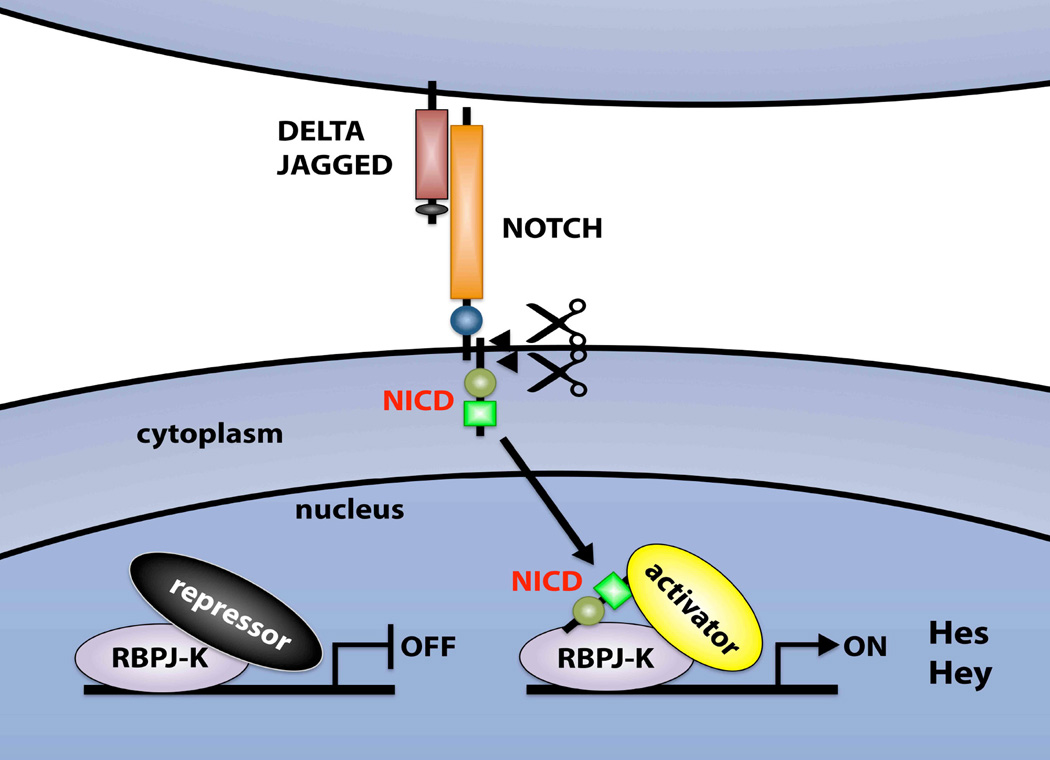
In order for the number of germ cells or stem cells to be properly regulated, negative regulators that antagonize growth factors and molecules essential for self-renewal and the undifferentiated state must exist. We recently demonstrated that NOTCH signaling within the SSC niche fulfills this function (Garcia and Hofmann, 2013; Garcia et al., 2013). NOTCH signaling is a highly conserved juxtacrine signaling pathway involved in a variety of cell fate decisions key to the development and maintenance of multiple organ and tissue systems (Bray and Bernard, 2010; Fig. 4). The NOTCH proteins (NOTCH1-4) are large cell-surface receptors that are activated by contact with membrane-bound ligands on neighboring cells, such as JAGGED (JAG1 and JAG2) and DELTA-like (DLL1, DLL3 and DLL4; Wang, 2011). Upon activation of the canonical pathway, the NOTCH intracellular domain (NICD) is cleaved and translocates to the nucleus where it associates with a DNA-binding protein called “recombining binding protein suppressor of hairless” (RBPJ), also called CBF1, (Tanigaki and Honjo, 2010). This displaces a repressor complex from the RBPJ protein and triggers the conversion of RBPJ from a transcriptional repressor to a transcriptional activator (Shao et al., 2012). In the canonical pathway, the Hes/Hey family of transcriptional repressors are targets of RBPJ (Iso et al., 2003; Bray and Bernard, 2010). HES/HEY proteins are bHLH transcription factors that inhibit the expression of other genes by forming complexes with corepressors (Iso et al., 2003; Fischer and Gessler, 2007). Mammalian HES/HEY proteins fulfill important roles during development and adulthood, but the result of their activation (proliferation, differentiation or lateral inhibition) depends on the cell type.
Figure 4.
Basic components of the NOTCH signaling pathway. Ligands of the JAGGED and DELTA-like families interact with NOTCH family receptors on an adjacent cell. Receptor-ligand interaction releases the NOTCH intracellular domain (NICD), which translocates to the nucleus, where it forms a complex with the RBPJ protein. This leads to the transcriptional activation of Notch target genes (Hey/Hes). Hey/Hes proteins are transcriptional repressors.
A number of NOTCH receptors, ligands and targets are expressed in the pre- and postnatal testis (Dirami et al., 2001; Hahn et al., 2009). Over the past two decades, constitutively active forms of the NOTCH pathway have been used to uncover its role in many model systems including Drosophila, C. elegans, and vertebrates (Coffman et al., 1993; Fortini et al., 1993; Rebay et al., 1993; Roehl and Kimble, 1993; Kopan et al., 1994). Indeed our gain-of-function mouse model that constitutively activates NOTCH1 signaling only in Sertoli cells led to a complete loss of germ cells around birth (Garcia et al., 2013). Interestingly, in this model E15.5 prospermatogonia, which are normally quiescent at this stage of development, re-entered the cell cycle and differentiated, as demonstrated by their de novo expression of the cyclins CCND1 and CCND3, and the markers STRA8 and cKIT (Garcia and Hofmann, 2013; Garcia et al., 2013). Further investigations demonstrated significant downregulation of Gdnf and Cyp26b1 in the mutant testes, indicating that germ cell differentiation occurred possibly because of a lack of niche molecules maintaining the undifferentiated state.
Functional studies of the NOTCH pathway in many tissues have been hampered by the fact that the NOTCH receptors are redundant. Therefore to ablate NOTCH signaling specifically in Sertoli cells, we disrupted the DNA-binding sequence of the RBPJ protein, which is at the intersection of the NOTCH1-4 pathways (Fig. 4). Loss-of-function of RBPJ in Sertoli cells led to an increase in Gdnf and Cyp26b1 expression resulting in a significant increase in SSCs and overall germ cell numbers (hyperplasia). In 20% of testes, meiotic arrest and bilateral testicular failure occurred, which we attribute to the inability of Sertoli cells to process all germ cells during spermiogenesis. This deficiency results in an increase of the number and size of residual bodies, which can be observed in unusual stages of the seminiferous epithelium, and epididymal occlusion. This phenotype of the NOTCH signaling knockout is in stark contrast - and in many ways opposite - to the phenotype of the NOTCH signaling overactivation mutant.
Altogether, our results indicate that activation of NOTCH signaling in Sertoli cells upregulates the transcriptional repressors HES1 and HEYL, which inhibit GDNF and CYP26B1 production. This in turn could potentially be used as a mechanism to dampen germ cell proliferation when necessary and favor differentiation.
E3. Control of NOTCH signaling by germ cells
To identify which cells provide the ligand for NOTCH signaling in Sertoli cells, we specifically isolated pure populations of germ cells and Sertoli cells by FACS and demonstrated that the NOTCH ligand JAG1 was specifically expressed in undifferentiated spermatogonia. Further, only JAG1 induced an increase in Hes1, Hey1, and Heyl expression by Sertoli cells in an in vitro assay, with concomitant decreased expression of Cyp26b1. However, Gdnf expression did not change in this context, indicating that the control of GDNF expression might be only indirectly dependent on the canonical NOTCH pathway. To demonstrate that germ cells are the signal–presenting cells, we isolated and cultured Sertoli cells from a Transgenic Notch Reporter GFP (TNR–GFP) mouse model (Duncan et al., 2005). In these mice, GFP expression depends on an active NOTCH signaling pathway, and in the testis GFP is exclusively expressed in Sertoli cells (Garcia et al., 2013). When Sertoli cells were isolated from TNR-GFP testes, their expression of GFP disappeared within 24 h. After 5 days of in vitro culture, we recombined these Sertoli cells with germ cells isolated from CD1 mice that are not expressing GFP. Addition of germ cells induced GFP expression in Sertoli cells within 24 h, demonstrating that germ cells are the ligand-presenting cells activating NOTCH signaling in Sertoli cells. Altogether, these results indicate that germ cells might use JAG1 to activate the NOTCH pathway in Sertoli cells and control their own numbers, ensuring proper homeostasis and sperm output.
Conclusion
In the testis, as in other tissues belonging to multicellular organisms, stem cell homeostasis is governed by endocrine and paracrine communication via soluble factors including hormones, growth factors, and cytokines. Our laboratory is investigating spermatogonial stem cells homeostasis in the normal testis to gain a better understanding of deregulated growth patterns. Many characteristics of solid tumors, i.e., uncontrolled proliferation, deregulation of cellular and morphological differentiation, invasion, and colonization to distant organs, can be attributed, at least in part, to alterations in intercellular communication between neoplastic cells and normal cells in their microenvironment (Hanahan and Weinberg, 2000; Park et al., 2000). For example Sertoli cell-only syndrome and growth of testicular cancer cells following a genetic mutation might be viewed as a result of the disruption of homeostatic regulations, which determine whether prospermatogonia and SSCs remain quiescent, proliferate, differentiate, or die. Interestingly, in two studies that examined only patients with germ cell maturation arrest, all were found to bear alterations in either the NOTCH1 receptor or its ligand JAG (Hayashi et al., 2001). In addition, alterations of NOTCH signaling have been observed in testicular tumors as well, and lack of NOTCH receptor expression seems associated to seminoma (Hayashi et al., 2004), a lesion developing from prospermatogonia (Rajpert-De Meyts, 2006). Insights into the causes of germ cell maturation arrest and testicular cancer will only arise with a better understanding of the molecular mechanisms driving germ cell proliferation and differentiation in the perinatal testis. Indeed we do not yet fully understand what drives a prospermatogonia into quiescence once it reaches the gonads, and what determines resumption of mitosis and differentiation after birth.
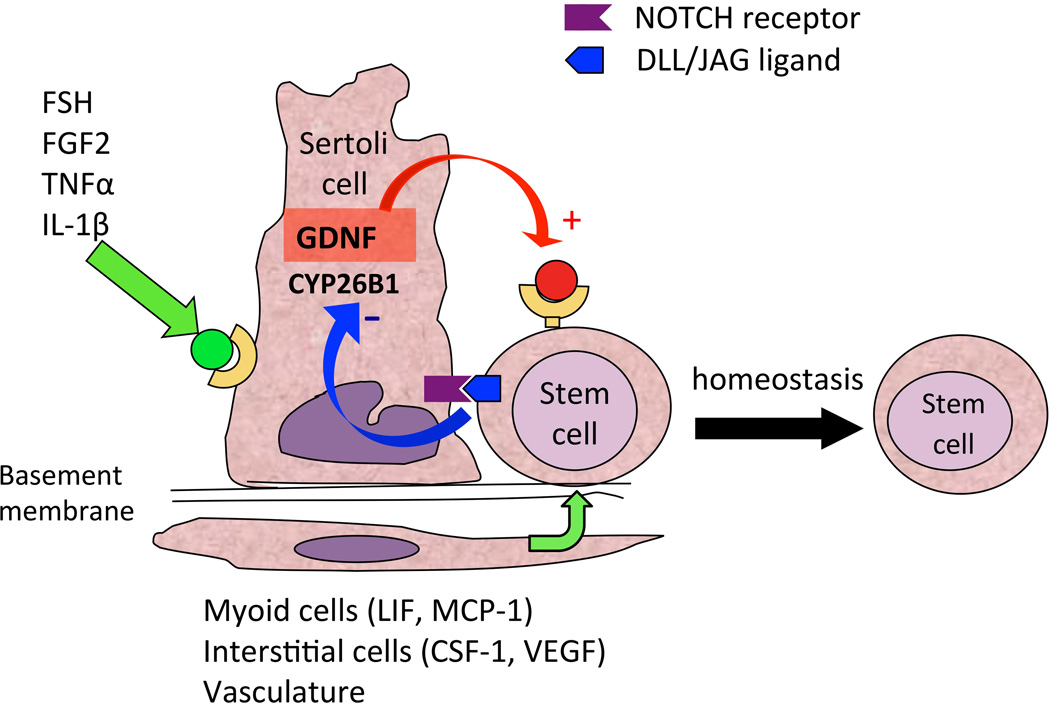
Our results indicate that Sertoli cells, the main component of the SSC niche, are able to regulate the levels of GDNF and CYP26B1 through FSH, FGF2 and NOTCH signaling. FSH and FGF2 upregulate GDNF production, while JAG1, a ligand for NOTCH, downregulates GDNF and CYP26B1 levels (Fig. 5). Interestingly, the signals activating the NOTCH pathway are probably produced by germ cells. An excess of prospermatogonia/SSC will therefore increase NOTCH signaling in Sertoli cells, which will downregulate germ cell proliferation and promote differentiation through a negative feedback loop. Therefore, dysregulation of NOTCH signaling, by modifying components of the germ cell niche, will lead to maturation arrest and might possibly contribute to the growth of testicular cancer cells.
Figure 5.
Integrated model showing how the effects of FSH/FGF2 are balanced by NOTCH activation and might regulate GDNF and CYP26B1 production in Sertoli cells to ensure stem cell homeostasis.
Acknowledgments
This work was funded by NIH HD044543 and HD068989 to MCH, and NIH F32 HD058433 fellowship to TXG.
Abbreviations
- AKT/Akt
Thymoma viral proto-oncogene 1, or protein kinase B
- BMP/Bmp
Bone morphogenetic protein
- CBF1/Cbf1
C promoter-binding factor 1(synonym for RBPJ)
- CCNA2/Ccna2
Cyclin A2
- CCND1/Ccnd1
Cyclin D1
- CCND3/Ccnd3
Cyclin D3
- CCNE1/Ccne1
Cyclin E1
- CSF-1/Csf1
Colony stimulating factor-1
- CYP26B1/Cyp26b1
Cytochrome P450, family 26, subfamily b, polypeptide 1
- DLL1/Dll1
Delta-like 1
- DLL3/Dll2
Delta-like 2
- DLL4/Dll4
Delta-like 4
- E6.5
Embryonic day 6.5
- FYN/Fyn
Fyn proto-oncogene, Src kinase p59
- FOS/Fos
FBJ murine osteosarcoma proto-oncogene
- FGF2/Fgf2
Fibroblast growth factor 2
- FSH/Fsh
Follicle stimulating hormone
- GDNF
Glial cell line-derived neurotrophic facor
- GFRA1
Glial cell line derived neurotrophic factor family receptor alpha-1
- GnRH
Gonadotropin releasing hormone
- GFP
Green fluorescent protein
- HES/Hes
Hairy and enhancer-of-split
- HEY/Hey
Hairy and enhancer-of-split-related with YRPW motif
- HEYL/HeyL
Hairy and enhancer-of-split-related with YRPW motif-like
- IL-1β/Il1b
Interleukin 1 beta
- JAG1/Jag1
Jagged 1
- JAG2/Jag2
Jagged 2
- KIT/Kit
Kit oncogene, Hardy-Zuckerman 4 feline sarcoma proto-oncogene homolog
- KITL/Kitl
Kit ligand, or stem cell factor
- LIF/Lif
Leukemia inhibitory factor
- MCP-1/Mcp1
Monocyte chemotactic protein 1
- MYC/Myc
Myelocytomatosis proto-oncogene
- MYCN/Mycn
Myelocytomatosis proto-oncogene neuroblastoma-derived homolog
- NOTCH/Notch
Notch (Drosophila) homolog
- PGC
Primordial germ cell
- P3
Postnatal day 3
- PI3K/Pi3k
Phosphatidylinositol 3-kinase
- PDG/Pdgf
Platelet-derived growth factor
- RA
Retinoic acid
- RET/Ret
Rearranged during transfection
- RAS/Ras
Rat sarcoma proto-oncogene
- RBPJ/Rbpj
Recombination signal binding protein for immunoglobulin kappa J region
- SSC
Spermatogonial stem cell
- STRA8/Stra8
Stimulated by retinoic acid gene 8
- SRC/Src
Rous sarcoma proto-oncogene
- SFK
Src family kinase
- Sl/Sl
Steel mutation
- Sld/Sld
Steel-Dickie mutation
- SF1/Sf1
Splicing factor 1
- SRY/Sry
Sex determining region of chromosome Y
- SOX9/Sox9
SRY (sex determining region Y)-box 9
- TNFα/Tnfa
Tumor necrosis factor alpha
- TNR-GFP
Transgenic Notch reporter-green fluorescent protein
- VEGF/Vegf
Vascular endothelial growth factor
References
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- Anand RJ, Paust HJ, Altenpohl K, Mukhopadhyay AK. Regulation of vascular endothelial growth factor production by Leydig cells in vitro: the role of protein kinase A and mitogen-activated protein kinase cascade. Biol Reprod. 2003;68:1663–1673. doi: 10.1095/biolreprod.102.009795. [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- Aubry F, Habasque C, Satie AP, Jegou B, Samson M. Expression and regulation of the CC-chemokine monocyte chemoattractant protein-1 in rat testicular cells in primary culture. Biol Reprod. 2000;62:1427–1435. doi: 10.1095/biolreprod62.5.1427. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:417–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Bernard F. Notch targets and their regulation. Curr Top Dev Biol. 2010;92:253–275. doi: 10.1016/S0070-2153(10)92008-5. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, Hussain SM, Hofmann MC. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci. 2010;116:577–589. doi: 10.1093/toxsci/kfq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Caires KC, de Avila JM, Cupp AS, McLean DJ. VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology. 2012;153:887–900. doi: 10.1210/en.2011-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F, Oatley MJ, Kaucher AV, Yang QE, Bieberich CJ, Shashikant CS, Oatley JM. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014;28:1351–1362. doi: 10.1101/gad.240465.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- Collin O, Bergh A. Leydig cells secrete factors which increase vascular permeability and endothelial cell proliferation. Int J Androl. 1996;19:221–228. doi: 10.1111/j.1365-2605.1996.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- de Rooij DG, Griswold MD. Questions about spermatogonia posed and answered since 2000. J Androl. 2012;33:1085–1095. doi: 10.2164/jandrol.112.016832. [DOI] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Pursel V, Dym M. Effects of stem cell factor and granulocyte macrophagecolony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61:225–230. doi: 10.1095/biolreprod61.1.225. [DOI] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Achi MV, Dym M. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl. 2001;22:944–952. doi: 10.1002/j.1939-4640.2001.tb03434.x. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Ergun S, Kilic N, Fiedler W, Mukhopadhyay AK. Vascular endothelial growth factor and its receptors in normal human testicular tissue. Mol Cell Endocrinol. 1997;131:9–20. doi: 10.1016/s0303-7207(97)00082-8. [DOI] [PubMed] [Google Scholar]
- Evans EP, Ford CE, Lyon MF. Direct evidence of the capacity of the XY germ cell in the mouse to become an oocyte. Nature. 1977;267:430–431. doi: 10.1038/267430a0. [DOI] [PubMed] [Google Scholar]
- Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323:76–93. doi: 10.1016/j.mce.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Feng CW, Bowles J, Koopman P. Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol. 2014;382:488–497. doi: 10.1016/j.mce.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP, Dolci S. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Gessler M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- Gaido K, Lehmann K. Gene expression profiling of whole testes in embryonic and postnatal day 2 mice. NCBI GEO:GSE4818. 2006 [Google Scholar]
- Garcia TX, DeFalco T, Capel B, Hofmann MC. Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence. Dev Biol. 2013;377:188–201. doi: 10.1016/j.ydbio.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia TX, Hofmann MC. NOTCH signaling in Sertoli cells regulates gonocyte fate. Cell Cycle. 2013;12:2538–2545. doi: 10.4161/cc.25627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn KL, Beres B, Rowton MJ, Skinner MK, Chang Y, Rawls A, Wilson-Rawls J. A deficiency of lunatic fringe is associated with cystic dilation of the rete testis. Reproduction. 2009;137:79–93. doi: 10.1530/REP-08-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl. 2001;22:999–1011. doi: 10.1002/j.1939-4640.2001.tb03441.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada T, Kageyama Y, Kihara K. Expression failure of the notch signaling system is associated with the pathogenesis of testicular germ cell tumor. Tumour Biol. 2004;25:99–105. doi: 10.1159/000079140. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288:95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult ratsITheir morphology, proliferation and maturation. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Olivas E, DiCandeloro P, Wright WW. Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia. Biol Reprod. 2011;85:763–769. doi: 10.1095/biolreprod.110.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–187. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Onoyama I, Nakayama KI, Shinohara T. Skp1-Cullin-F-box SCF.-type ubiquitin ligase FBXW7 negatively regulates spermatogonial stem cell self-renewal. Proc Natl Acad Sci USA. 2014;111:8826–8831. doi: 10.1073/pnas.1401837111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimada K, Svingen T, Feng CW, Pelosi E, Bagheri-Fam S, Harley VR, Schlessinger D, Bowles J, Koopman P. Antagonistic regulation of Cyp26b1 by transcription factors SOX9/SF1 and FOXL2 during gonadal development in mice. FASEB J. 2011;25:3561–3569. doi: 10.1096/fj.11-184333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci USA. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, Chan WY, Dym M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol Reprod. 2009;80:707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- Korpelainen EI, Karkkainen MJ, Tenhunen A, Lakso M, Rauvala H, Vierula M, Parvinen M, Alitalo K. Overexpression of VEGF in testis and epididymis causes infertility in transgenic mice: evidence for nonendothelial targets for VEGF. J Cell Biol. 1998;143:1705–1712. doi: 10.1083/jcb.143.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Brinster RL. Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 2008;86:59–84. doi: 10.1016/S0091-679X(08)00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, Toyokuni S, Shinohara T. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138:1289–1298. doi: 10.1210/endo.138.3.5021. [DOI] [PubMed] [Google Scholar]
- Loveland KL, Zlatic K, Stein-Oakley A, Risbridger G, deKretser DM. Platelet-derived growth factor ligand and receptor subunit mRNA in the Sertoli and Leydig cells of the rat testis. Mol Cell Endocrinol. 1995;108:155–159. doi: 10.1016/0303-7207(94)03471-5. [DOI] [PubMed] [Google Scholar]
- Lu N, Sargent KM, Clopton DT, Pohlmeier WE, Brauer VM, McFee RM, Weber JS, Ferrara N, Silversides DW, Cupp AS. Loss of vascular endothelial growth factor A VEGFA) isoforms in the testes of male mice causes subfertility, reduces sperm numbers, and alters expression of genes that regulate undifferentiated spermatogonia. Endocrinology. 2013;154:4790–4802. doi: 10.1210/en.2013-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas BE, Fields C, Joshi N, Hofmann MC. Mono-2-ethylhexyl)-phthalate MEHP) affects ERK-dependent GDNF signalling in mouse stem-progenitor spermatogonia. Toxicology. 2012;299:10–19. doi: 10.1016/j.tox.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Miles DC, van den Bergen JA, Wakeling SI, Anderson RB, Sinclair AH, Western PS. The protooncogene Ret is required for male foetal germ cell survival. Dev Biol. 2012;365:101–109. doi: 10.1016/j.ydbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factormediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Scholer HR, Suda T. Identification and characterization of stem cells in prepubertal spermatogenesis in mice. Dev Biol. 2003;258:209–225. doi: 10.1016/s0012-1606(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000;6:324–329. doi: 10.1016/s1357-4310(00)01756-1. [DOI] [PubMed] [Google Scholar]
- Piquet-Pellorce C, Dorval-Coiffec I, Pham MD, Jegou B. Leukemia inhibitory factor expression and regulation within the testis. Endocrinology. 2000;141:1136–1141. doi: 10.1210/endo.141.3.7399. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fehon RG, Artavanis-Tsakonas S. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993;74:319–329. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- Richardson LL, Kleinman HK, Dym M. Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod. 1995;52:320–330. doi: 10.1095/biolreprod52.2.320. [DOI] [PubMed] [Google Scholar]
- Roehl H, Kimble J. Control of cell fate in C. elegans by a GLP-1 peptide consisting primarily of ankyrin repeats. Nature. 1993;364:632–635. doi: 10.1038/364632a0. [DOI] [PubMed] [Google Scholar]
- Rossi L, Challen GA, Sirin O, Lin KK, Goodell MA. Hematopoietic stem cell characterization and isolation. Methods Mol Biol. 2011;750:47–59. doi: 10.1007/978-1-61779-145-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- Shao H, Huang Q, Liu ZJ. Targeting Notch signaling for cancer therapeutic intervention. Adv Pharmacol. 2012;65:191–234. doi: 10.1016/B978-0-12-397927-8.00007-5. [DOI] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]
- Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, Cooke PS. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313:3090–3099. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Singh SR. Stem cell niche in tissue homeostasis, aging and cancer. Curr Med Chem. 2012;19:5965–5974. doi: 10.2174/092986712804485917. [DOI] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Extracellular matrix and its role in spermatogenesis. Adv Exp Med Biol. 2008;636:74–91. doi: 10.1007/978-0-387-09597-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Mruk DD, Cheng CY. Regulation of actin dynamics and protein trafficking during spermatogenesis-- insights into a complex process. Crit Rev Biochem Mol Biol. 2013;48:153–172. doi: 10.3109/10409238.2012.758084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunters A, Thomas DP, Yeudall WA, Grigoriadis AE. Accelerated cell cycle progression in osteoblasts overexpressing the c-fos proto-oncogene: induction of cyclin A and enhanced CDK2 activity. J Biol Chem. 2004;279:9882–9891. doi: 10.1074/jbc.M310184200. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, Rajkovic A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol. 2012;361:301–312. doi: 10.1016/j.ydbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Honjo T. Two opposing roles of RBP-J in Notch signaling. Curr Top Dev Biol. 2010;92:231–252. doi: 10.1016/S0070-2153(10)92007-3. [DOI] [PubMed] [Google Scholar]
- Thuillier R, Wang Y, Culty M. Prenatal exposure to estrogenic compounds alters the expression pattern of platelet-derived growth factor receptors alpha and beta in neonatal rat testis: identification of gonocytes as targets of estrogen exposure. Biol Reprod. 2003;68:867–880. doi: 10.1095/biolreprod.102.009605. [DOI] [PubMed] [Google Scholar]
- Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, Culty M. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod. 2010;82:825–836. doi: 10.1095/biolreprod.109.081729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wee K, Hofmann MC. An in vitro tubule assay identifies HGF as a morphogen for the formation of seminiferous tubules in the postnatal mouse testis. Exp Cell Res. 1999;252:175–185. doi: 10.1006/excr.1999.4630. [DOI] [PubMed] [Google Scholar]
- Van Dissel-Emiliani FM, De Boer-Brouwer M, De Rooij DG. Effect of fibroblast growth factor-2 on Sertoli cells and gonocytes in coculture during the perinatal period. Endocrinology. 1996;137:647–654. doi: 10.1210/endo.137.2.8593814. [DOI] [PubMed] [Google Scholar]
- Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol. 2011;43:1550–1562. doi: 10.1016/j.biocel.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, Creighton S, Tam SP, Jones G, Petkovich M. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci USA. 2000;97:6403–6408. doi: 10.1073/pnas.120161397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth DJ, Manterola M, Vasileva A. Role of cyclins in controlling progression of mammalian spermatogenesis. Int J Dev Biol. 2013;57:159–168. doi: 10.1387/ijdb.130047av. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Goodyear SM, Tobias JW, Avarbock MR, Brinster RL. Spermatogonial stem cell self-renewal requires ETV5-mediated downstream activation of Brachyury in mice. Biol Reprod. 2011;85:1114–1123. doi: 10.1095/biolreprod.111.091793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]