Abstract
T-cell exhaustion due to persistent antigen stimulation is a key feature of chronic viral infections and cancer. Programmed cell death-1 (PD-1) is a major regulator of T-cell exhaustion, and blocking the PD-1 pathway restores T-cell function and improves pathogen control and tumor eradication. Immunotherapy targeting the PD-1 inhibitory receptor pathway has demonstrated significant antitumor activity. Recently, antibodies blocking PD-1 have been approved for use in cancer patients. In this review, we summarize the role of the PD-1 pathway in chronic infection and cancer and the therapeutic potential of PD-1-directed immunotherapy in patients with chronic infection or cancer.
Keywords: cancer, chronic infection, immunotherapy, programmed cell death-1, T-cell exhaustion
I. PERSPECTIVES
Memory T cells are generated when acute infections are cleared by the immune system. These cells rapidly reactivate effector functions upon antigen re-encounter and persist long term via homeostatic proliferation, independently of antigen.1, 2 These key properties of memory T cells allow them to provide long-term protective immunity. In contrast, chronic infections where the virus persists result in exhaustion of the T cells, which are then unable to bring the infection under control.
Prolonged antigen stimulation and inflammation lead to loss of effector functions of virus-specific CD8 T cells in a progressive and hierarchical manner, even resulting in clonal deletion.3, 4 This process, originally found in chronic viral infections, was termed T-cell exhaustion and has since been demonstrated to be a common feature of many chronic infections and cancer.1, 5 Exhausted T cells, characterized by defects in effector functions and elevated and sustained expression of inhibitory receptors, are distinctly different from functional effector or memory cells.6, 7 Although complex immunosuppressive mechanisms, including both intrinsic and extrinsic factors, can contribute to the establishment and maintenance of the persistent infection and T-cell dysfunction, PD-1 (CD279),8 an inhibitory receptor of CD28 family, is well known to play a major role in regulating T-cell exhaustion. In this review, we summarize the role of the PD-1 pathway in regulating T-cell exhaustion in chronic infection and cancer and discuss the therapeutic potential of PD-1-directed immunotherapy to treat patients who are chronically infected or have cancer.
II. THE ROLE OF THE PD-1 PATHWAY IN T-CELL EXHAUSTION
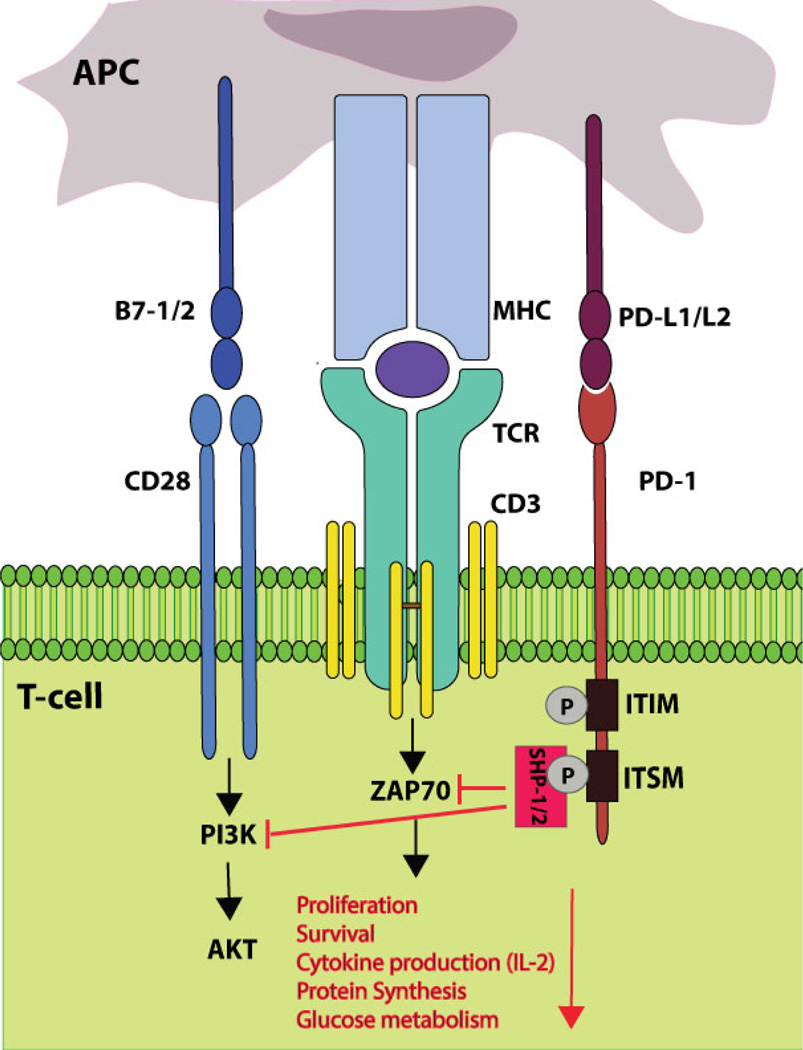
PD-1 is expressed in various hematopoietic cells including T cells, B cells, natural killer (NK) cells, NK T (NKT) cells, monocytes, macrophages, and dendritic cells (DCs) following their activation.9 PD-1 binds to its two ligands: programmed cell death 1 ligand-1 (PD-L1; B7-H1; CD274)10, 11 and PD-L2 (B7-DC; CD273),12, 13 both of which are B7 family members. PD-L1 is constitutively expressed in a wide range of cells including hematopoietic and nonhematopoietic cells. In contrast, PD-L2 expression is restricted to professional antigen presenting cells (APCs; monocytes, macrophages, and DCs) and a certain subset of B cells. Inflammatory cytokines such as interferons (IFNs; α, β, and γ) are potent regulators of both PD-L1 and PD-L2 expression.9, 14 The function of PD-1 is best characterized in T cells. Its expression is induced by activation-driven T-cell receptor (TCR) signaling and further up-regulated by cytokines.14 Upon engagement of PD-1 with its ligands, the SH2-domain containing tyrosine phosphatase 1 (SHP-1) and SHP-2 are recruited to the phosphorylated immunoreceptor tyrosine-based switch motif (ITSM) in the cytoplasmic domain of PD-1. Recruitment of SHP-1 and SHP-2 inactivates proximal effector molecules such as ZAP70 and phosphatidylinositol-3-kinase (PI3K), attenuating TCR- and CD28-mediated signaling (Figure 1).15–17
FIG. 1.
PD-1 signaling. PD-1 contains two tyrosine-based signaling motifs in the cytoplasmic domain: an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an ITSM. Upon engagement by PD-L1 or PD-L2, PD-1 is phosphorylated at both tyrosine residues. Phosphorylated ITSM recruits SHP-1 and SHP-2 that dephosphorylate effector molecules such as ZAP70 and PI3K activated by TCR and CD28 signaling. As a result, PD-1 signaling inhibits T-cell proliferation, survival, cytokine production, protein synthesis, and glucose metabolism.
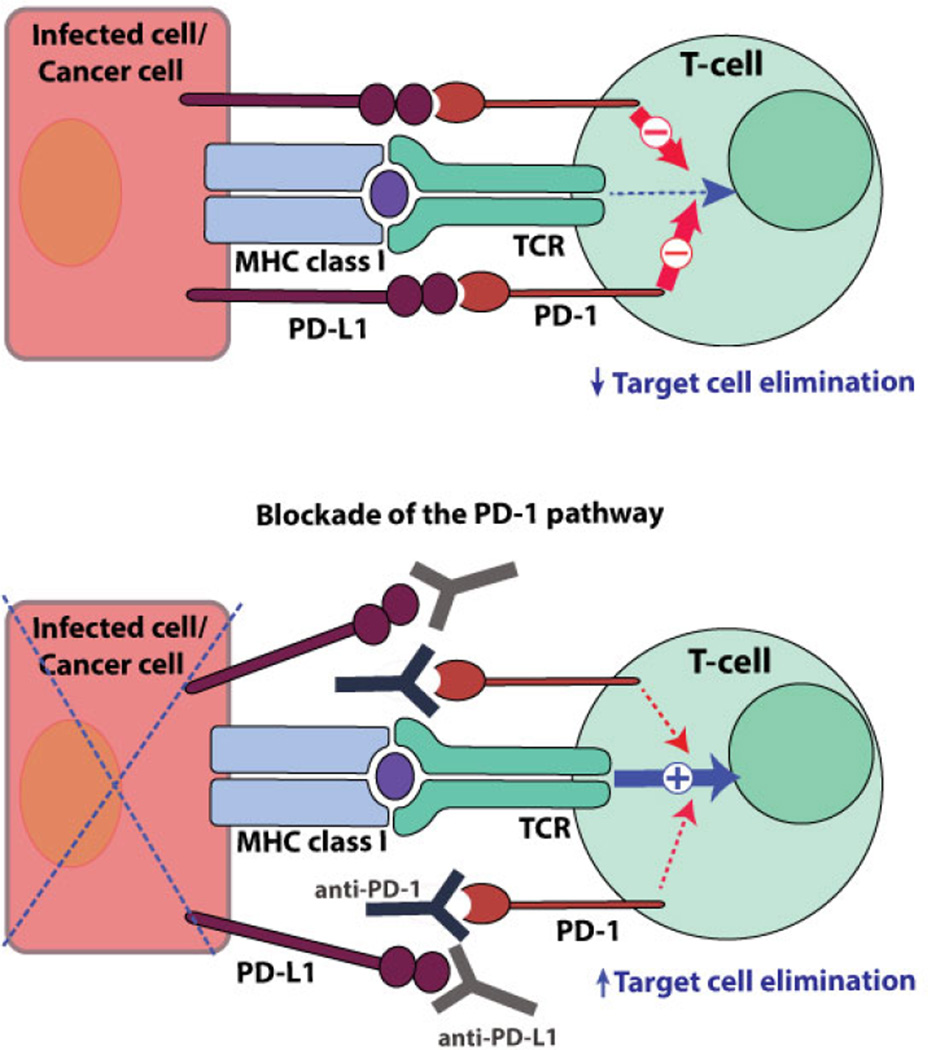
Our previous finding that PD-L1 has a differential role in hematopoietic cells and nonhematopoietic cells in regulating the T-cell response suggests a model for which PD-1/PD-1 ligand (PD-L) interaction operates.18 In chronic LCMV infection, PD-L1 deficiency in hematopoietic cells enhanced the T-cell response in terms of both magnitude and function. In comparison, PD-L1 deficiency in nonhematopoietic cells had no effect on the T-cell response but resulted in better virus control. This indicates that the PD-1 pathway restrains T cells from killing virus-infected cells as well as attenuating T-cell activation. The PD-1/PD-L1 interaction between T cells and infected cells (or cancer cells) inhibits target cell elimination by T cells. Abrogating this interaction releases the brake on T cells and promotes their effector functions, killing of target cells (Figure 2). Therefore, the PD-1 pathway negatively regulates T cells during priming and also the effector phase when T cells act on the target cells. This presumably results in more profound “rescue” effects by the blockade of PD-1 than do other inhibitory receptor blockades.
FIG. 2.
Blockade of PD-1/PD-L1 interactions between CD8 T cells and target cells. Antibody-mediated blockade of the PD-1 pathway promotes T cell-mediated elimination of target cells.
The immunoregulatory roles of PD-1 are responsible for limiting excessive T-cell activation to prevent immune-mediated tissue damage. However, prolonged TCR stimulation and PD-1 expression lead to T-cell dysfunction, and pathogens or cancer cells exploit the PD-1 pathway to persist and resist immune response. Therefore, the PD-1 pathway is an important determinant of the outcome of the T-cell response, regulating the balance between effective host defense and immunopathology, implicating the potential for manipulating the PD-1 pathway against various human diseases.
During chronic infection and cancer, expression of both PD-1 and PD-1 ligands is abundant; continuous antigen stimulation maintains high levels of PD-1 expression on antigen-specific T cells and the expression of PD-1 ligands is also up-regulated by inflammatory stimulation. PD-1–mediated T-cell dysfunction strongly dampens antiviral or antitumor immune response. The effect of interfering with the PD-1 pathway on the restoration of T-cell function has been shown in many animal models and human diseases. Recently, clinical trials targeting the PD-1 pathway have revealed very promising results. Many preclinical studies of PD-1 pathway blockade in chronic viral infections and clinical trials in many different cancers are currently ongoing.
III. THE THERAPEUTIC POTENTIAL OF INHIBITING PD-1 SIGNALING IN CHRONIC VIRAL INFECTION
The dominant role of PD-1 in regulating T-cell exhaustion was first described by our group in a mouse model of chronic LCMV infection. In this model, we found that exhausted CD8 T cells had increased PD-1 expression. Furthermore, blockade of the PD-1 pathway restored effector functions of LCMV-specific CD8 T cells and significantly reduced viral load.19 This finding has been further extended to other types of chronic infections in mice, nonhuman primates, and humans.
In HIV infection, PD-1 expression on HIV-specific CD8 T cells was correlated with impairment of CD8 T-cell function, high viral load, disease progression, and reduced CD4 count. In vitro blockade of PD-1 on HIV-specific CD8 and CD4 T cells enhanced proliferation, cytokine production, and survival.20, 21 Recently, the effect of blocking PD-1/PD-L interactions on HIV disease progression has been shown in vivo using the humanized mouse model of chronic HIV infection. In vivo administration of anti-PD-L1 antibody increased both CD4 and CD8 T cells that could suppress viral replication in HIV-1 chronically infected mice, showing a reduction in the HIV plasma viral load.22 In addition to HIV, the role of the PD-1 pathway has been investigated in other chronic viral infections such as HCV. In the initial stage of HCV infection, most HCV-specific T cells expressed PD-1. In patients that resolve this disease, PD-1 expression on these cells was reduced, whereas chronically infected patients maintained a high level of PD-1 expression and HCV-specific CD8 T cells remained dysfunctional. In vitro blockade of the PD-1 and PD-L interaction enhanced the proliferation and function of HCV-specific CD8 T cells.23, 24 One recent report demonstrated the impact of interrupting PD-1 signals in chronically HCV-infected chimpanzees. Following PD-1 blockade, one of the three animals had significantly reduced HCV viremia that was associated with restored intrahepatic CD4 and CD8 T-cell response. It has been suggested that preexisting virus-specific T cells are likely to be essential for the success of PD-1 blockade therapy in this model.25 Blocking the PD-1 pathway was also found to promote an antiviral immune response in simian immunodeficiency virus (SIV) infection of rhesus macaques. Proliferation and polyfunctionality of SIV-specific CD8 T cells were augmented upon PD-1 blockade, and improved antiviral immunity was followed by viral load reduction and prolonged survival of chronically infected rhesus macaques.26 Together, these preclinical studies show that PD-1 expression on virus-specific T cells is correlated with their functional defects, and interrupting PD-1 signaling can reverse this decline. The fact that exhaustion is reversible and not an untreatable state indicates powerful therapeutic potential for manipulating the PD-1 axis to reinvigorate dysfunctional T cells in chronic viral infections.
Currently, one clinical trial has been reported on PD-1 blockade in chronic viral infection. Anti-PD-1 antibody (BMS-936558, a fully human monoclonal antibody targeting PD-1) was used to treat patients chronically infected with HCV. Following a single infusion, suppression of HCV replication was observed in 11.1% of patients (5/45).27 Also in this trial, one patient who previously did not respond to IFN-α therapy had undetectable viral load for at least 1 year following administration of the anti-PD-1 antibody. This promising result warrants further exploration of PD-1 blocking agents for therapeutic use in human chronic viral infection.
IV. THE PD-1 PATHWAY IN ANTITUMOR IMMUNITY AND PD-1-DIRECTED CANCER IMMUNOTHERAPY
PD-1 and PD-L1 interaction in the tumor environment is a mechanism used by the tumor to resist destruction by the immune system. PD-L1 is expressed by many types of cancer cells and up-regulated by various inflammatory stimuli in the tumor environ-ment.28, 29 Myeloid cells in tumors were shown to express PD-L1 and mediate inhibition of T cells.30 Tumor-infiltrating T cells express high levels of PD-1 due to prolonged exposure to the tumor antigen and immunosuppressive environment and exhibit similar functional and phenotypic properties as the exhausted T cells in chronic infection. This includes defects in effector cytokine production and up-regulated expression of inhibitory receptors.31–33 Currently, the prevailing mechanism underlying the PD-1/PD-L1 axis in tumor sites is that the interaction of PD-L1 on tumor cells with PD-1 on tumor-infiltrating lymphocytes (TILs) delivers negative signals and inhibits antitumor T-cell response, facilitating tumorigenesis.
The role of PD-1 in tumor immune evasion was first shown when P815 tumor cells were transfected with PD-L1 and they became less susceptible to cytotoxic T-cell-mediated killing. This report also showed that the growth of PD-L1+ myeloma cells was completely suppressed in syngeneic PD-1–deficient mice, whereas rapid tumor growth was observed in wild-type littermates.34 Multiple in vivo mouse studies have shown that the PD-1/PD-L1 interaction inhibits antitumor immunity, and abrogating this interaction enhances the T-cell-mediated antitumor response, leading to tumor regression.29, 34, 35 Encouraging results from preclinical studies and the therapeutic potential of blocking the PD-1 pathway have led to clinical development of several blocking antibodies against PD-1 or PD-L1. Currently, the results of clinical trials targeting the PD-1 pathway are very promising. Blockade of the PD-1 pathway using either anti-PD-1 or anti-PD-L1 antibodies has revealed high clinical response rates and was effective in patients with advanced cancer including metastatic melanoma, non-small cell lung cancer (NSCLC), renal cell cancer (RCC), bladder cancer, Hodgkin’s lymphoma, head and neck cancer, and breast cancer36–60 (Table 1). Clinical responses tended to be durable and were accompanied by less adverse effects than those seen with ipilimumab, a CTLA-4 blocking antibody used for treating metastatic melanoma. Recently, the Food and Drug Administration (FDA) approved two anti-PD-1 antibodies, pembrolizumab (Merck) and nivolumab (Bristol-Myers Squibb), for the treatment of unresectable or metastatic melanoma.
TABLE 1.
Published clinical trials targeting PD-1 pathway in cancer patients.
| Cancer types | Sponsor/Company | Target | References |
|---|---|---|---|
| Melanoma | BMS | PD-1 | 38, 40, 42, 43, 44, 49, 51, 60 |
| PD-L1 | 39 | ||
| Merck | PD-1 | 41, 48 | |
| Roche/Genentech | PD-L1 | 45, 61 | |
| Non small-cell lung
cancer (NSCLC) |
BMS | PD-1 | 40 49 |
| PD-L1 | 39 | ||
| Merck | PD-1 | 58, 59 | |
| Roche/Genentech | PD-L1 | 45 | |
| AstraZeneca/MedImmune | PD-L1 | 54, 57 | |
| Renal cell cancer (RCC) | BMS | PD-1 | 38, 40, 42, 46, 49 |
| PD-L1 | 39 | ||
| Roche/Genentech | PD-L1 | 45 | |
| Urothelial bladder
cancer (UBC) |
Merck | PD-1 | 56 |
| Roche/Genentech | PD-L1 | 47 | |
| Hodgkin’s lymphoma | BMS | PD-1 | 50 |
| Head and neck cancer | Merck | PD-1 | 55 |
| Roche/Genentech | PD-L1 | 45, 53 | |
| AstraZeneca/MedImmune | PD-L1 | 54 | |
| Triple negative breast cancer | Merck | PD-1 | 52 |
| Roche/Genentech | PD-L1 | 62 |
BMS, Bristol-Myers Squibb
Consistent with the concept that the tumor evades host immune response through engagement of PD-L1 with PD-1 on T cells, early studies suggested a correlation between PD-L1 expressed by the tumor and poor prognosis. However, several studies indicated a lack of correlation or even a positive association of PD-L1 expression on tumor cells with lymphocyte infiltration and better prognosis.28 A recent study reported a negative feedback loop, whereby activated T cells infiltrating the tumor environment produce proinflammatory cytokines, such as IFNγ, that induce the up-regulation of PD-L1 on tumor cells. PD-L1 interaction with PD-1 on tumor-infiltrating T cells suppresses T-cell functions.61 Therefore, PD-L1 expression in tumor cells possibly indicates preexisting immune responses.
Based on the mechanism of PD-1/PD-L1 expression, PD-L1 expression by tumor cells has been suggested as a biomarker for predicting the clinical response to PD-1 blockade therapy. Several clinical studies evaluated a correlation between tumor-associated PD-L1 expression and the clinical response to PD-1 blocking agents, and there seemed to be a trend of positive association. However, tumor expression of PD-L1 is apparently not an absolute biomarker because not all patients with PD-L1+ tumors respond to PD-1 blockade, and some patients with PD-L1-(PD-L1 negative) tumors are still responsive to PD-1 therapy.62, 63 Considering the inducible nature of PD-L1 and the fact that many other PD-1/PD-L interactions are possibly affected by PD-1 pathway blockade along with tumor cells and TIL interactions, tumor PD-L1 expression as a single marker is not an optimal biomarker of the response to PD-1-targeted immunotherapy. Therefore, it is imperative to identify reliable biomarkers to select patients who can benefit from this therapy.
V. COMBINATION THERAPY WITH PD-1 PATHWAY BLOCKADE
Because PD-1 plays a critical role in T-cell exhaustion, the efficacy of other immunotherapies attempting to restore the function of exhausted T cells might be enhanced by simultaneously blocking PD-1 signaling. In addition, combinations with other therapeutic strategies may enhance treatments targeting PD-1. It has been shown that PD-1 blockade rescues the less exhausted CD8 T cells expressing intermediate levels of PD-1, whereas exhausted cells with high levels of PD-1 respond poorly and are unlikely to be reversed by the treatment.64 Several studies have shown that a certain level of preexisting antigen-specific T cells is essential to better respond to blockade of the PD-1 pathway. Therefore, combining PD-1 pathway blockade with other therapies that possibly stimulate T-cell responses or interrupt other negative signaling pathways could generate a synergistic effect.
Therapeutic vaccination in chronic infection or cancer has been shown to have limited efficacy due to T-cell exhaustion.65 In chronic viral infection, immunization with recombinant vaccinia virus vectors encoding LCMV glycoprotein (rVV-LCMV GP33) had a minimal effect on enhancing CD8 T-cell response, but combined PD-L1 blockade significantly improved LCMV-specific CD8 T-cell response and virus control.66 Furthermore, PD-L1 blockade markedly enhanced antitumor T-cell response driven by granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting tumor cell immunotherapy in mouse models of melanoma and colon carcinoma.67 This result indicates that blocking PD-1 signaling can enhance the efficacy of therapeutic vaccination. Currently, clinical trials assessing the efficacy of multipeptide melanoma vaccines in combination with PD-1 blockade are ongoing (NCT01176474, NCT01176461) and the effect of combining dendritic cell-based tumor vaccines with PD-1 blockade is being tested in several types of cancer including RCC and multiple myeloma (NCT01067287, NCT01441765, NCT01096602).
The severity of T-cell exhaustion has been shown to be correlated with coexpression of multiple inhibitory receptors including PD-1, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3), CD160, and 2B4.68 During chronic LCMV infection, Tim-3 or LAG-3 blockade alone had a minimal effect on rescuing virus-specific CD8 T cells, but combining with PD-1 pathway blockade synergistically improved LCMV-specific CD8 T-cell response and virus control.68, 69 In addition, in murine cancer models, PD-1 pathway blockade in combination with blocking Tim-3, LAG-3, or CTLA-4 was more effective in restoring antitumor immunity and promoting tumor regression than targeting either pathway alone.70–72 An additive or synergistic effect on rescuing T cells by combined blockade of different inhibitory receptors indicates their nonredundant roles and the complex regulatory mechanisms underlying T-cell dysfunction. Recently, a clinical evaluation of PD-1 and CTLA-4 combination blockade reported a higher rate of clinical response than single therapy in patients with advanced melanoma.42 Dual blockade of PD-1 and LAG-3 is being tested in solid tumors (NCT01968109).
The effect of manipulating stimulatory or inhibitory cytokines can be enhanced when combined with PD-1 pathway blockade. IL-10 is an immunosuppressive cytokine involved in T-cell exhaustion and blocking the IL-10 signal leads to restoration of T-cell function and viral clearance in chronic LCMV infection.73 Combined blockade of the IL-10 receptor and PD-1 pathway further enhanced virus-specific T-cell response and virus control during chronic LCMV infection.74 Previously, we found that administration of IL-2, an immunostimulatory cytokine, during chronic infection resulted in the rescue of exhausted T cells and better viral control.75 Combined IL-2 treatment and PD-1 pathway blockade had a synergistic effect on augmenting virus-specific CD8 T-cell response and reducing viral load in chronic LCMV infection.76 IL-21 enhances cytolytic activity of CD8 T cells and NK cells and recombinant IL-21 (rIL-21) administration has demonstrated potent antitumor activity.77 In preclinical murine tumor models, rIL-21 administration combined with PD-1 blockade further enhanced antitumor responses.78 Clinical evaluation of combination treatment with rIL-21 and the anti-PD-1 antibody has recently been performed in advanced or metastatic solid tumors.79
Adoptive transfer of T cells is an effective immunotherapeutic approach to restore the antiviral or antitumor immune response. However, under the influence of continuous antigen exposure, transferred cells become dysfunctional. Therefore, blocking the PD-1/PD-L interaction can further augment the therapeutic efficacy of adoptively transferred cells. Our recent work has shown that in chronic LCMV infection, the therapeutic effects of naïve antigen-specific CD4 T-cell transfer were further enhanced by blocking the PD-1 pathway, resulting in greater functionality of LCMV-specific CD8 T cells and in a further reduction of viral load.80 In tumor mouse models, combined therapy of adoptive cell transfer and PD-1 pathway blockade has shown a synergistic effect on tumor regression than single treatment. Blocking PD-1 increased the number of transferred cells at the tumor site, and this was associated with greater T-cell proliferation and increased expression of IFNγ and the IFNγ-inducible chemokine at the tumor site, facilitating immune cell infiltration.81
IFNα has potent antitumor effects, but at the same time it can induce expression of PD-1 and its ligands. Preclinical studies have shown that the combination of IFNα therapy or IFNα-transduced cancer vaccines with PD-1 pathway blockade further enhances antitumor immunity in tumor-bearing mice.82, 83 This combination therapy suggests a promising candidate for cancer treatment, and combined treatment with IFNα-2b and the anti-PD-1 antibody is being tested or planned in patients with melanoma or RCC (NCT02089685, NCT02112032, NCT02339324). Furthermore, the combination of PD-1 blockade and the treatment with an antiangiogenic agent blocking the vascular endothelial growth factor (VEGF)-VEGF receptor 2 (VEGFR2) interaction has revealed a synergistic antitumor effect in a murine cancer model. 84 Based on the synergistic effects found from preclinical studies, many combination therapies are being evaluated for cancer patients.
VI. CONCLUSION
Cancer clinical trials targeting the PD-1 pathway have achieved a very high rate of antitumor response. Currently, monotherapies targeting PD-1 or PD-L1 and combination therapies with various immunotherapeutic strategies, including checkpoint inhibitors, tumor vaccines, chemotherapy, and antiangiogenic agents, are being evaluated in different types of cancer. It is important to assess different combination therapies for those who do not respond to PD-1 blockade therapy. The clinical evaluation of the PD-1 blocking agents is currently focused on cancer treatment, but the therapies targeting the PD-1 pathway also have potential for treating chronic infections. Still, the molecular mechanisms associated with the PD-1 pathway regulating T-cell exhaustion and the way in which PD-1 signaling is altered upon blocking the PD-1/PD-L interaction to restore exhausted T cells remain to be determined. It is also essential to identify the predictive biomarkers to personalize the therapy.
Acknowledgments
This work was supported by the National Institutes of Health grants AI30048 and AI56299 to R.A.
ABBREVIATIONS
- HCV
Hepatitis C virus
- HIV
human immunodeficiency virus
- LAG-3
lymphocyte-activation gene-3
- LCMV
lymphocytic choriomeningitis virus
- PD-1
programmed cell death-1
- PD-L
programmed cell death-1 ligand
- PD-L1
programmed cell death-1 ligand-1
REFERENCES
- 1.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific-cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187(9):1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19(4):408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 7.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193(7):839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfeld EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 14.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 15.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 16.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaud-hary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1–3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 18.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120(7):2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 21.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 22.Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, Remling-Mulder L, Korman AJ, Fontenot AP, Akkina R. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J Immunol. 2013;190(1):211–219. doi: 10.4049/jimmunol.1201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81(17):9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A, Ahmed R, Walker CM. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc Natl Acad Sci U S A. 2013;110(37):15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8(5):e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(5):1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 30.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, Rufer N, Lubenow N, Speiser D, Cerottini JC, Romero P, Pittet MJ. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64(8):2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 33.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 36.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(2):462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(34):4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agun-wamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Lambert AM, Waxman IM, Hammers HJ. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(13):1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in meta-static bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 46.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elas-saiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 47.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 50.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Dolled-Filhart M, Emancipator K, Gonzalez EJ, Houp J, Pathiraja K, Karantza V, Iannone R, Gause CK, Cheng JD. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Presented at 2014 San Antonio Breast Cancer Symposium; San Antonio, TX: 2014. Dec 9–13, (Abstract S1–09.) [Google Scholar]
- 51.Tabernero J, Powderly JD, Hamid O, Gordon MS, Fisher GA, Braiteh FS, Garbo LE, Fine GD, Kowanetz M, McCall B, Shen X, Chen DS, Kohrt HE. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic CRC, gastric cancer (GC), SCCHN, or other tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31 (suppl; abstr 3622) [Google Scholar]
- 52.Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, Vasselli J, Ibrahim RA, Lutzky J, Khleif S. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5s suppl) abstr 3002) [Google Scholar]
- 53.Seiwert TY, Burtness B, Weiss J, Gluck I, Eder JP, Pai SI, Dolled-Filhart M, Emancipator K, Pathiraja K, Gause C, Iannone R, Brown H, Houp J, Cheng JD, Chow LQM. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV–associated head and neck (H/N) cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5s suppl) abstr 6001) [Google Scholar]
- 54.Plimack ER, Gupta S, Bellmunt J, Berger R, Montgomery B, Gonzalez EJ, Pulini J, Dolled-Filhart M, Emancipator K, Pathiraja K, Gause C, Perini R, Cheng JD, O’donnell PH. A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with advanced urothelial tract cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(5):1–41. [Google Scholar]
- 55.Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li X, Robbins PB, Vasselli J, Ibrahim RA, Antonia SJ. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5s suppl) abstr 8021. [Google Scholar]
- 56.Garon EB, Leighl NB, Rizvi NA, Blumenschein GP, Balmanoukian AS, Eder JP, Goldman JW, Hui R, Soria JC, Gangadhar TC, Sun JM, Patnaik A, Gubens MA, Lubiniecki GM, Zhang J, Niewood M, Emancipator K, Dolled-Filhart M, Hanson ME, Gandhi L. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5s suppl) abstr 8020. [Google Scholar]
- 57.Rizvi NA, Garon EB, Patnaik A, Gandhi L, Leighl NB, Balmanoukian AS, Goldman JW, Eder JP, Johnson E, Blumenschein GR, Gubens MA, Papadopoulos KP, Lubiniecki GM, Zhang J, Niewood M, Emancipator K, Dolled-Filhart M, Hanson ME, Hui R. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5s suppl) abstr 8007. [Google Scholar]
- 58.Weber JS, Minor D, D’Angelo SP, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Grob JJ, Lao C, Linette G, Grossmann K, Hassel JC, Lorigan P, Maio M, Sznol M, Lambert A, Yang A, Larkin J. A phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma with prior anti-CTLA-4 therapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(suppl 4) [Google Scholar]
- 59.Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, Boasberg PD, Flaherty K, Hwu P, Ballinger M, Mokatrin A, Kowanetz M, Chen DS, Hodi FS. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(suppl) abstr 9010. [Google Scholar]
- 60.Emens LA, Braiteh FS, Cassier P, DeLord JP, Eder JP, Shen X, Xiao Y, Wang Y, Hegde PS, Chen DS, Krop I. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer; Presented at 2014 San Antonio Breast Cancer Symposium; San Antonio, TX: 2014. Dec 9–13, (Abstract PD1–6) [Google Scholar]
- 61.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 63.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2(5):662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105(39):15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79(14):8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205(3):543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(5):1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 68.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks DG, Triflo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105(51):20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9(5):540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 76.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123(6):2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13(5):379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 78.Jure-Kunkel M, Selby M, Lewis K, Masters G, Valle J, Grosso J, Dito G, Curtis W, Garcia R, Holdren M, Korman AJ, Dillon S. Nonclinical evaluation of the combination of mouse IL-21 and anti- mouse CTLA-4 or PD-1 blocking antibodies in mouse tumor models. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(suppl) abstr 3019. [Google Scholar]
- 79.Chow LQM, Gordon MS, Logan TF, Antonia SJ, Bhatia S, Thompson JA, Brahmer JR, Solberg G, Bittner R, Fontana D, Grosso J, Cohen LJ, Ahlers CM, Wigginton JM, Drake CG. Phase I dose escalation study of recombinant interleukin-21 (rIL-21; BMS-982470) in combination with nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients (pts) with advanced or metastatic solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(suppl) abstr TPS3112. [Google Scholar]
- 80.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108(52):21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizee G, Radvanyi L, Hwu P. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72(20):5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omori R, Eguchi J, Hiroishi K, Ishii S, Hiraide A, Sakaki M, Doi H, Kajiwara A, Ito T, Kogo M, Imawari M. Effects of interferon-alpha-transduced tumor cell vaccines and blockade of programmed cell death-1 on the growth of established tumors. Cancer Gene Ther. 2012;19(9):637–643. doi: 10.1038/cgt.2012.42. [DOI] [PubMed] [Google Scholar]
- 83.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186(5):2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 84.Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, Yagita H, Nakajima Y. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172(3):500–506. doi: 10.1111/cei.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]