ABSTRACT
The tooth root is an integral, functionally important part of our dentition. The formation of a functional root depends on epithelial-mesenchymal interactions and integration of the root with the jaw bone, blood supply and nerve innervations. The root development process therefore offers an attractive model for investigating organogenesis. Understanding how roots develop and how they can be bioengineered is also of great interest in the field of regenerative medicine. Here, we discuss recent advances in understanding the cellular and molecular mechanisms underlying tooth root formation. We review the function of cellular structure and components such as Hertwig's epithelial root sheath, cranial neural crest cells and stem cells residing in developing and adult teeth. We also highlight how complex signaling networks together with multiple transcription factors mediate tissue-tissue interactions that guide root development. Finally, we discuss the possible role of stem cells in establishing the crown-to-root transition, and provide an overview of root malformations and diseases in humans.
KEY WORDS: Tooth, Root, Odontogenesis, Signaling network, Stem cells and tissue regeneration
Summary: This Review discusses the factors involved in tooth root development and in human root developmental defects, and highlights the role of stem cells in establishing the crown-to-root transition.
Introduction
Teeth perform important physiological functions in our daily life, playing roles in mastication and speech. Each tooth has two main anatomical components: the crown and the root (Fig. 1). Whereas the crown is mostly made up of highly vascularized dental pulp surrounded by dentin and enamel on the outer surface (for anatomical terms, see the Glossary, Box 1), the dentin of the root is covered by cementum, rather than enamel. The root is also surrounded by the periodontal ligament (PDL; see Glossary, Box 1), a fibrous connective tissue structure that connects the root cementum to the alveolar bone (Fig. 1). Importantly, the tooth root is an essential element in the function of the dentition because it anchors teeth to the maxilla or mandible. Accordingly, the loss of roots leads to diminished bone support and, hence, perturbed tooth function. In addition, during mastication and resting states, the root helps transmit and balance occlusal forces through PDLs to the jaw bones and serves as a passageway for the neurovascular bundle (see Glossary, Box 1) that supplies blood flow, nutrition and sensation to our teeth (Fig. 1) (Orban, 1980).
Fig. 1.
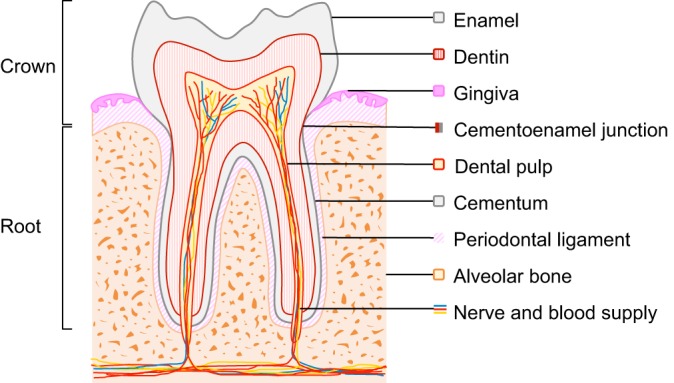
Schematic of a molar. The crown portion of the tooth is covered with enamel, whereas the root surface is covered with cementum; the junction between these components is referred to as the cementoenamel junction. The roots are attached to the alveolar bone by periodontal ligaments. Nerves and blood vessels enter the apical foramen of the tooth to provide innervation and a blood supply. Dentin surrounds the pulp chamber, which contains odontoblasts, pulp cells, nerves and blood vessels.
Box 1. Glossary.
Cementoenamel junction: The point at which the enamel and cementum meet, which defines the anatomical boundary between the crown and the root.
Cementum: Specialized hard tissue covering the root that provides attachment for periodontal ligaments.
Dental follicle: A population of cells around both the enamel organ and dental papilla that gives rise to the cementum, periodontal ligament and alveolar bone.
Dental papilla: A group of cranial neural crest-derived cells under the enamel organ. It gives rise to dental pulp, including odontoblasts.
Dental pulp: Soft tissue comprising the central part of the tooth.
Dentin: Mineralized tissue secreted by odontoblasts adjacent to the enamel.
Enamel: Highly mineralized tissue covering the crown.
Enamel knot: A signaling center during tooth development. It secretes signaling molecules to guide tooth morphogenesis.
Enamel organ: A complex epithelial structure located above the dental papilla that produces enamel for the developing tooth.
Epithelial cell rests of Malassez (ERM): Residual cells from HERS, found within the periodontal space.
Hertwig's epithelial root sheath (HERS): A bilayered epithelial structure extending from the apical region of the enamel organ, growing apically, and guiding tooth root formation.
Neurovascular bundle: Anatomical structure consisting of nerves, arteries and veins in close proximity.
Periodontal ligament (PDL): Connective tissue between the tooth root and the alveolar bone.
As with most ectodermal organs, tooth development occurs through a series of reciprocal interactions between epithelial and mesenchymal cells (Thesleff and Sharpe, 1997). Tooth development initiates with a thickening of the oral epithelium, which eventually becomes the dental lamina. At this stage, the epithelium exhibits so-called odontogenic inductive potential and is able to induce the initiation of tooth development when recombined with non-odontogenic neural crest derived-mesenchyme (Lumsden, 1988; Mina and Kollar, 1987). The dental lamina then invaginates into the underlying cranial neural crest (CNC)-derived mesenchyme to form the tooth bud. The mesenchyme condenses around the epithelial tooth bud and, via the expression of a particular set of transcription factors and signaling molecules, gains the ability to instruct tooth morphogenesis (Kollar and Baird, 1970a,b; Thesleff and Sharpe, 1997). Subsequently, the epithelium undergoes folding that determines the shape and number of cusps, with additional factors secreted by the enamel knot (see Glossary, Box 1) regulating these events (Jernvall and Thesleff, 2000; Thesleff and Sharpe, 1997). Finally, the epithelium differentiates into ameloblasts and the mesenchyme differentiates into odontoblasts; ameloblasts deposit enamel, which is the hard outermost layer of the tooth crown, whereas odontoblasts secrete dentin matrix, which hardens into dentin surrounding the dental pulp (Fig. 1) (Thesleff and Sharpe, 1997). Together, these cells help to build the crown portion of the tooth.
The molecular regulation of early tooth morphogenesis leading to the formation of the crown has been studied extensively. Indeed, a large body of work has shown that the network controlling crown development encompasses the action of major signaling pathways, including the Tgfβ, Bmp, Fgf, Wnt and Shh pathways, which act recurrently at various stages (Chai and Maxson, 2006; Tucker and Sharpe, 2004). However, the precise molecular network controlling late stages of tooth development, including the crown-to-root transition and root formation, is yet to be determined. Indeed, most of our knowledge about root development is based on histology and 3D imaging analyses, and although some studies have shown that many of the same signaling molecules involved in regulating crown formation are also involved in controlling root formation, how these molecules achieve their signaling specificity during root formation is only just beginning to be understood. In addition, although multiple mutant animal models have been shown to exhibit root development defects, most studies of these animals have simply highlighted root developmental defect phenotypes, but have yet to elucidate the molecular regulatory network for root development. There is thus a need to integrate these studies to gain a more comprehensive understanding of root development. Furthermore, it is not yet known what determines the number of roots per tooth and the direction of root formation, although they are likely to be influenced by the Hertwig's epithelial root sheath (HERS; see Glossary, Box 1), CNC-derived mesenchyme and adjacent anatomical structures. Future studies will help us to gain a better understanding of the integration between dental roots and jaw bones required for proper function.
In this Review, we explore some of the unique transcriptional regulatory and signaling networks that may play key roles in regulating root formation. Based on advances in our understanding of how these networks function during tooth morphogenesis, we review what is currently known about the cellular and molecular mechanisms involved in the formation of tooth roots and their potential implications in stem cell-mediated tissue regeneration, as well as their relevance in human disease. Finally, we suggest some future directions for investigating the molecular and cellular regulatory mechanism of root development and stem cell-mediated tissue regeneration.
An overview of the tooth root and its morphogenesis
Development of the tooth root starts following crown formation, once the enamel tissue has reached the future cementoenamel junction (see Glossary, Box 1), which is the point at which the enamel and cementum meet and that defines the anatomical boundary between the crown and the root (Fig. 1). The apical region of the enamel organ (see Glossary, Box 1) elongates and gives rise to the HERS (Fig. 2), a bilayer epithelial structure between the dental papilla (see Glossary, Box 1) and dental follicle (Orban, 1980) (see Glossary, Box 1). The HERS then grows apically and guides root formation (Fig. 2), determining the size, shape and number of tooth roots (Cate, 1996). Any disturbance in the formation of the HERS leads to malformations affecting root structure, shape, number, length and other features (reviewed by Luder, 2015). The CNC-derived mesenchyme condenses around and continuously interacts with the HERS and, subsequently, the mesenchyme of the apical papilla comes into contact with the inner layer of the HERS and undergoes differentiation into odontoblasts, which secrete radicular (i.e. root-covering) dentin. If the continuity of the HERS is disturbed prematurely, the differentiation of root odontoblasts is compromised (Kim et al., 2013). This function of the HERS in regulating odontoblast differentiation requires direct contact; laminin 5 secreted by the HERS induces the growth, migration and differentiation of mesenchymal cells in the dental papilla (Mullen et al., 1999). Moreover, the HERS produces growth factors that contribute to the induction of odontoblast differentiation, suggesting that the HERS functions as a signaling center to guide root formation (Huang et al., 2009, 2010).
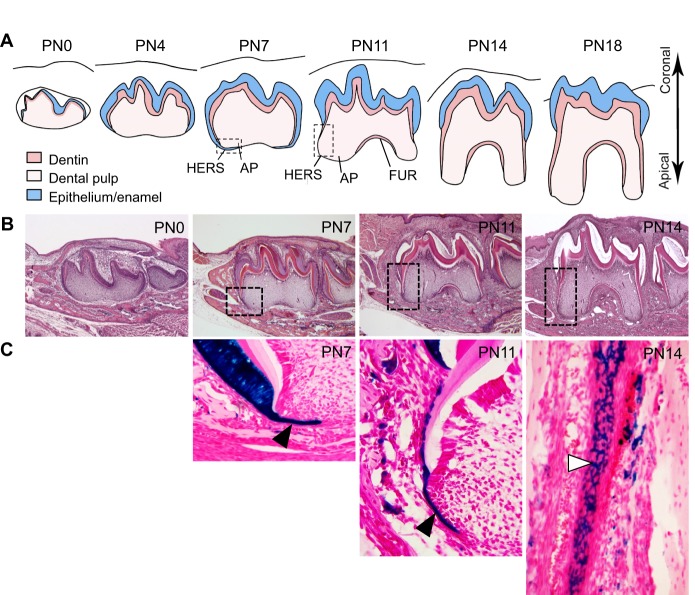
Fig. 2.
Tooth root development in mice. (A) Schematics of root development in mice from postnatal day (PN) 0 to 18. Epithelium-derived tissues, which include the ameloblast layer and enamel in the crown and the Hertwig's epithelial root sheath (HERS) in the root region, are depicted in blue. Dentin is depicted in pink, and the pulp is shown in light pink. The furcation (FUR) – a bridge that marks the point at which the root is about to divide – appears as the transition from crown to root growth occurs, at around PN11. By PN18, root development is complete and the tooth has erupted, as indicated by its position relative to the gum level (black line). AP, apical papilla. (B) Histology sections of molars at different developmental stages corresponding to the schematic drawings in A. Boxed areas indicate regions that are shown in detail in C. (C) Selected sections of molars from K14-Cre;R26R mice at developmental stages corresponding to those of the histological sections in B, highlighting the growth and structure of the HERS. Black arrowheads, HERS cells; white arrowhead, network of HERS cells on the developing root surface. lacZ-positive (i.e. epithelial-derived cells) appear in blue.
The HERS also plays a role in controlling cementum formation. After its dynamic movement towards the apical region of the tooth, the HERS becomes perforated via localized apoptosis or epithelial-to-mesenchymal transition (EMT), giving rise to a mesh network structure (Fig. 2C) (Huang et al., 2009; Luan et al., 2006). This network might facilitate the continuous interaction among epithelial cells and their collective interaction with the CNC-derived mesenchyme, as well as contact between dental follicle cells and newly formed dentin. After they initiate contact with dentin, cells in the dental follicle differentiate into cementoblasts, which produce cementum-specific extracellular matrix proteins, including collagen fibers (Zeichner-David, 2006). In the apical region of the root, cementoblasts remain embedded in the matrix, giving rise to cellular cementum. By contrast, the rest of the root is covered by acellular cementum. Both cementoblasts and the HERS are indispensable for the formation of cementum. For example, if the HERS is not perforated at the correct developmental stage, dental follicle cells cannot contact dentin and, consequently, cementoblast differentiation is altered and cementum formation is abnormal (Luan et al., 2006).
The dental follicle is not the only source of cementoblasts; the HERS expresses collagen I, bone sialoprotein and ALPase, all of which are typical cementoblast markers (Huang et al., 2009), and it is now clear that the HERS contributes directly to the pool of cementoblasts in the root via EMT (Bosshardt et al., 2015; Huang et al., 2009; Xiong et al., 2013). Indeed, the HERS does not completely degenerate during root formation; besides undergoing EMT, some fragments of the HERS become the epithelial cell rests of Malassez (ERM; see Glossary, Box 1), which are quiescent residues that contribute to cementum regeneration and repair (Xiong et al., 2013).
In addition to contributing to cell differentiation, the HERS helps to determine the number of tooth roots that form (Fig. 3) (Cate, 1996). As it forms, the HERS develops tongue-shaped epithelial protrusions that join horizontally to form a bridge, called the furcation, where the root becomes divided, constituting the base of the pulp cavity (Orban, 1980). After the furcation forms, root development in multi-rooted teeth is driven by the apical growth of the HERS, in the same way as occurs in single-rooted teeth (Orban, 1980). Thus, the growth of the HERS in different orientations contributes to the formation of multi-rooted teeth, including two-rooted lower molars and three-rooted upper molars (Fig. 3) (Orban, 1980). The differential proliferation of mesenchymal cells in furcation-forming and root-forming regions may also be involved in determining root number through regulation of the directionality of HERS growth (Sohn et al., 2014).
Fig. 3.
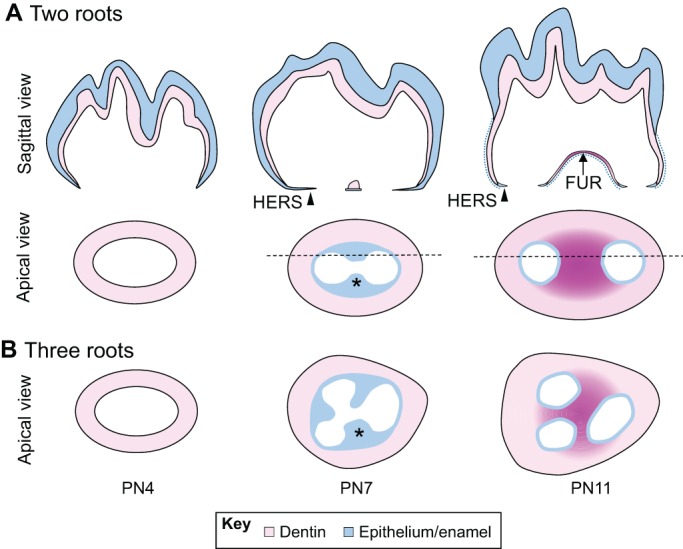
Development of the tooth root furcation in mice. (A) Schematics (sagittal view) of root furcation development in mice from PN4 to PN11. Epithelium-derived tissues, which include the ameloblast layer and enamel in the crown and the HERS in the root region, are depicted in blue. Dentin is depicted in pink. Arrowhead, HERS cells; blue dotted line, dissociated HERS cells. (B,C) Schematics (apical views) of root furcation development in two-rooted (B) and three-rooted (C) teeth in mice from PN4 to PN11. The HERS (blue) develops tongue-shaped epithelial protrusions (asterisks) that eventually fuse together to form the furcation (dark pink). Black horizontal dashed lines indicate the locations of the sagittal views shown in A.
The correct formation and degeneration of the HERS are also crucial for PDL development (Cho and Garant, 2000). PDL formation starts with the migration of dental follicle cells in contact with the HERS in between the root and alveolar bone. This event coincides with the beginning of HERS perforation. During migration, a number of cytoplasmic processes project from the leading edges of the CNC-derived dental follicle cells and begin secreting collagen fibers. Initially, these collagen fibers are disorganized, but as development progresses they thicken and become arranged in a structured manner. The proper secretion and distribution of these collagen fibers contribute to the correct orientation and attachment of the PDL, which is crucial for its ability to connect the root and alveolar bone, stabilizing and preparing the tooth for mastication (Cho and Garant, 2000; Palmer and Lumsden, 1987).
The CNC-derived mesenchyme also contributes to the formation of many cell types during root formation, including odontoblasts, dental pulp cells, cementoblasts and PDL cells. Previous studies have indicated that CNC-derived cells contribute to mesenchymal tissues during early development (Chai et al., 2000), although there have been limited studies using genetic cell lineage tracing to demonstrate the dynamic contribution of CNC-derived cells specifically during root formation. Recently, however, using an inducible Cre line, researchers have found that osterix (Sp7)-positive mesenchymal cells contain progenitor cells that contribute to different mesenchymal cell types during root development (Ono et al., 2016). We have also recently generated a Pax9-CreER line that can specifically target the CNC-derived mesenchyme in order to follow its contribution to different mesenchymal cell types during root formation (Feng et al., 2016). These genetic tools will be highly useful for targeting the CNC-derived cell population and will offer the opportunity to investigate how these CNC-derived cells may interact with the HERS to contribute to root formation.
Signaling networks that regulate tooth root development
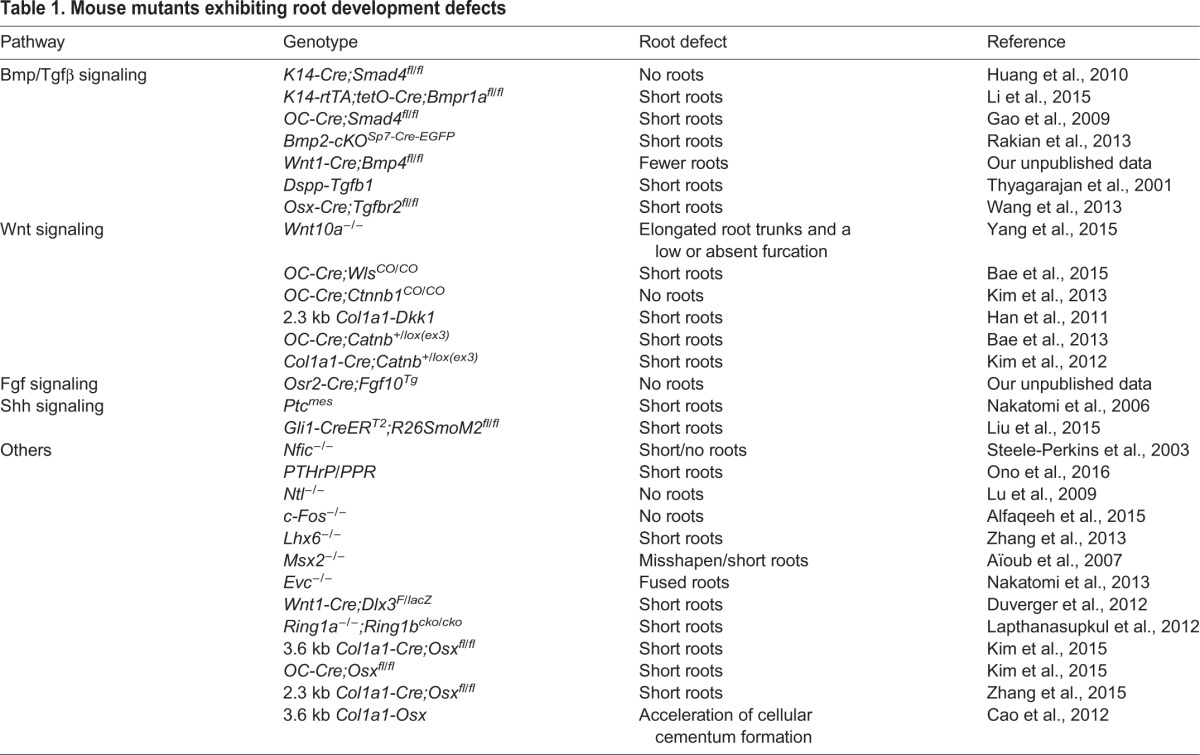
An array of growth and transcription factors is expressed during the initiation of root formation, suggesting that these factors might perform crucial functions in regulating the epithelial-mesenchymal interactions that are involved in all steps of tooth development. For example, Bmp, Tgfβ, and their mediator Smad4, as well as Shh, Msx2 and Dlx2, are expressed in HERS cells (Åberg et al., 1997; Huang et al., 2010; Lezot et al., 2000; Nakatomi et al., 2006; Yamashiro et al., 2003). In the CNC-derived dental mesenchyme adjacent to the HERS, there is expression of Gli1, Nfic, Fgf, Tgfβ, Bmp, Wnt and its inhibitors, as well as PTHrP/PPR (Pthlh/Pth1r) (Huang et al., 2010; Ono et al., 2016; Steele-Perkins et al., 2003; Wang et al., 2013). In addition, some signaling molecules are expressed in both the HERS and the CNC-derived dental mesenchyme (Liu et al., 2015). To date, multiple mutant animal models have been generated to elucidate the importance of these signaling molecules and their downstream target genes in regulating root formation (summarized in Table 1). Below, we discuss these approaches and highlight our current knowledge of how these key signaling molecules and their networks regulate tooth root development.
Table 1.
Mouse mutants exhibiting root development defects
Bmp/Tgfβ signaling
In root development, Bmp signaling is actively involved in regulating cell fate decisions during the formation of the HERS and the differentiation of odontoblasts (Fig. 4). Bmp2, 3, 4 and 7 are expressed during the initiation of tooth root development (Yamashiro et al., 2003). Although most of the Bmp ligands are detected in the CNC-derived dental mesenchyme, Bmp signaling is clearly required in the HERS to control root development. The Bmp-Smad4-Shh-Gli1-Sox2 signaling cascade controls the fate of the HERS during root formation (Li et al., 2015). Specifically, loss of Bmp signaling in the HERS leads to compromised Bmp-Shh signaling interactions, alters the epithelial stem cell (Sox2-positive) niche environment, and affects HERS formation during root development, leading to root developmental defects (Li et al., 2015). Furthermore, Msx2, which is a direct downstream target of Smad-mediated Bmp signaling (Brugger et al., 2004), encodes a transcription factor that is expressed in the HERS and is involved in regulating root formation (Aïoub et al., 2007; Yamashiro et al., 2003). Bmp signaling in the HERS also controls the patterning of root development: inhibiting Bmp activity by overexpression of the Bmp antagonist noggin in epithelial cells leads to delayed root formation and root patterning defects (Plikus et al., 2005). The presence of Bmp ligands (Bmp2, 3, 4 and 7) (Yamashiro et al., 2003) and Bmp signaling activation (phospho-Smad1/5/8; our unpublished data) in the dental mesenchyme suggest that Bmp signaling in this tissue might also play an important role in regulating root development (Fig. 4). Specifically, deletion of Bmp2 in osterix-positive mesenchymal progenitor cells causes abnormal odontoblast differentiation and short roots (Rakian et al., 2013). Moreover, restriction of Bmp4 expression in the dental mesenchyme by Ring proteins, which contain a characteristic zinc-finger domain that mediates protein-protein interactions, affects odontoblast differentiation and root formation (Lapthanasupkul et al., 2012). To date, however, the precise regulatory function of mesenchymal Bmp signaling during root development remains unclear.
Fig. 4.
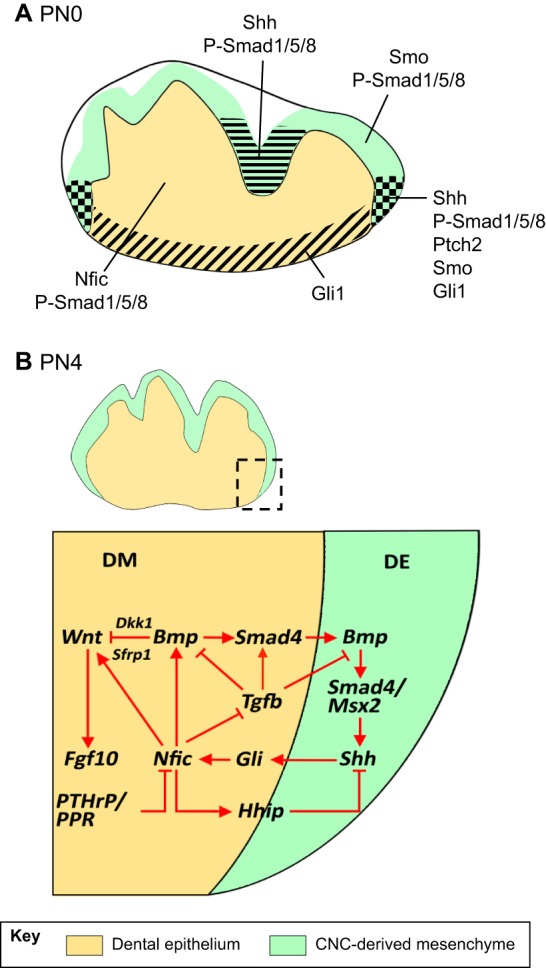
Molecular regulation of early tooth root development. Schematics highlighting the expression domains of diverse signaling molecules and transcription factors in developing teeth. (A) At birth (PN0), some of the key regulators of the initiation of root development, such as Bmp, Shh and Nfic, are present in the inner enamel epithelium and the cranial neural crest (CNC)-derived mesenchyme. The different domains are indicated by the various patterns. (B) At PN4, when root development initiates, a complex signaling network regulates epithelial-mesenchymal interactions to control root formation. DE, dental epithelium; DM, dental mesenchyme.
Although Tgfβ is expressed in both the HERS and dental mesenchyme, it appears that Tgfβ signaling plays a more important role in the mesenchyme during root development because ablation of Tgfbr2 in the HERS does not affect root formation (Li et al., 2015), whereas loss of Tgfbr2 in the CNC-derived mesenchyme leads to a root development defect (Oka et al., 2007). More recently, it was shown that Tgfβ signaling within the CNC-derived dental mesenchyme can indirectly regulate HERS cells and control root development through tissue-tissue interactions (Wang et al., 2013). Specifically, loss of Tgfβ signaling in odontoblasts and bone-producing mesenchyme results in failure of root elongation, reduced radicular dentin matrix density, and delayed molar eruption (Wang et al., 2013). Furthermore, Smad4, a central mediator of the canonical Bmp/Tgfβ signaling pathway, controls the size of roots; ablation of Smad4 in odontoblasts results in short roots and defects in odontoblast differentiation and dentin formation (Gao et al., 2009). Moreover, loss of Smad4 in odontoblasts leads to the failure of HERS dissociation through mesenchymal-epithelial interaction (Gao et al., 2009). This phenotype is similar to that of Tgfbr2 mutant mice, suggesting that one of the important functions of Smad4 is to mediate Tgfβ signaling in the dental mesenchyme in the regulation of root formation (Fig. 4).
Smad-mediated Tgfβ1 signaling appears to act together with Nfic to regulate both the early and late stages of odontoblast differentiation. Nfic is a member of the nuclear factor I family and functions as a key regulator of root dentin formation. In humans and mice, Nfic expression is restricted to odontoblasts and preodontoblasts in developing molars (Gao et al., 2014). In Nfic knockout mice, molars develop a normal crown but root development is affected due to alterations in patterning, growth and dentin formation (Steele-Perkins et al., 2003). Nfic signaling modulates Tgfβ signaling via dephosphorylation of phospho-Smad2/3 during late odontoblast differentiation, maturation and mineralization (Lee et al., 2009). Similarly, Nfic antagonizes the effects of Tgfβ1 on stem cells from the apical papilla (SCAPs) in vitro (He et al., 2014).
Wnt signaling
Wnt signaling is also important in regulating root formation (Lohi et al., 2010). Although it is well known that Wnt ligands are expressed during the early stages of tooth development and that dental mesenchyme-specific deletion of the Wnt signaling mediator β-catenin leads to tooth development arrest at the bud stage (Chen et al., 2009; Sarkar and Sharpe, 1999), the precise functional role of Wnt signaling in regulating tooth root development is only now emerging. The canonical Wnt pathway is highly active in developing molar roots, based on the expression of Axin2 in root odontoblasts and preodontoblasts (Lohi et al., 2010) and the expression pattern of Wnt10a during root development (Yamashiro et al., 2007). Moreover, Wnt10a can induce the expression of Dspp, a gene associated with dentinogenesis, in odontoblasts (Yamashiro et al., 2007). These studies suggest that canonical Wnt activity might play a role in the regulation of root dentinogenesis. Furthermore, Wnt10a null mice exhibit taurodontism, which is characterized by elongated root trunks and a low or absent furcation, as do many patients with WNT10A mutations (Yang et al., 2015). Strikingly, odontoblast differentiation and root dentin formation are not affected in Wnt10a null mice (Yang et al., 2015), indicating that other Wnt ligands are likely to contribute to root dentinogenesis and that Wnt10a must be specifically involved in the formation of the root furcation. Moreover, deletion of the wntless (Wls) gene in odontoblasts reduces canonical Wnt activity. This gene encodes a chaperone protein that regulates all Wnt ligands. Its loss leads to inhibition of odontoblast maturation and therefore to root elongation (Bae et al., 2015). Consistently, Wnt10a and Axin2 expression levels are also dramatically decreased in the odontoblasts of Wls mutant roots (Bae et al., 2015). Additionally, β-catenin-mediated Wnt signaling is required to regulate the differentiation of CNC-derived odontoblast cells during tooth formation, because tissue-specific inactivation of β-catenin in developing odontoblasts results in molars that completely lack roots (Kim et al., 2013). This study also shows that compromised Wnt signaling in the CNC-derived mesenchyme can adversely affect the formation of HERS cells and may interrupt the epithelial-mesenchymal interactions that are crucial for root formation (Fig. 4). Similarly, tissue-specific overexpression of Dkk1, an inhibitor of Wnt/β-catenin signaling, in odontoblasts leads to short roots and dentin defects in mandibular molars (Han et al., 2011). Together, these studies suggest that Wnt signaling must be tightly controlled during root formation, and it is thus not surprising that the overactivation of Wnt signaling can also lead to root development defects (Bae et al., 2013; Kim et al., 2012).
Wnt signaling also interacts with other signaling pathways to control root formation. For example, canonical Bmp signaling is required for maintaining the expression of Wnt signaling inhibitors, such as Dkk1 and Sfrp1, in odontoblasts to regulate dentin formation; accordingly, loss of Bmp signaling in the dental mesenchyme leads to elevated Wnt signaling, resulting from downregulation of Dkk1 and Sfrp1, and the formation of ectopic bone-like structures in the dentin region (Fig. 4) (Li et al., 2011). HERS cells also require β-catenin-mediated Wnt signaling to initiate root cementum formation following the cessation of Bmp signaling as tooth crown formation concludes (Yang et al., 2013). When Bmp signaling is blocked via tissue-specific depletion of Bmpr1a in the crown epithelia at the end of tooth crown formation, tooth root development initiates earlier than normal, as indicated by the premature switch in cell fate of crown epithelia from ameloblasts to cementoblasts, caused by the upregulation of Wnt/β-catenin signaling (Yang et al., 2013). This study suggests that the interaction between Bmp and Wnt signaling determines the transition between crown and root formation during tooth development.
Fgf and Hh signaling
The Fgf and Hh signaling pathways are involved in mediating epithelial-mesenchymal interactions that are crucial for organogenesis. For example, Fgf10 signaling in the dental mesenchyme plays a key role in controlling the crown-to-root transition via the regulation of HERS formation during molar development. Indeed, the disappearance of Fgf10 expression in the dental mesenchyme near the epithelial cervical loop is required for the initiation of root formation in mouse, rat and human molars, and the persistence of Fgf10 expression is required for the continuous growth of crowns in vole molars (Tummers and Thesleff, 2003; Yokohama-Tamaki et al., 2006). Furthermore, treatment of molar tooth germs with exogenous Fgf10 at the crown-to-root transition stage leads to the inhibition of HERS formation in vitro (Yokohama-Tamaki et al., 2006). These studies suggest that Fgf10 controls the switch between crown and root formation during molar tooth development by regulating epithelial-mesenchymal interactions (Fig. 4). In the developing tooth, Fgf2 is expressed in differentiating odontoblasts at the apical end and in the furcation zone of the root, as well as in cementoblasts and fibroblasts of the PDL, suggesting that Fgf2 might be involved in regulating various aspects of root development (Gao et al., 1996; Madan and Kramer, 2005).
Similarly, the Shh signaling pathway is clearly involved in regulating the interactions between the HERS and the CNC-derived mesenchyme during root formation (Fig. 4). Shh ligand secreted by dental epithelial cells in the apical region of molar roots functions in both the HERS and nearby dental mesenchymal cells during root formation, as indicated by the expression patterns of genes encoding the Shh ligand, the Hh receptors Ptch1 and Ptch2, the transcriptional regulator Smo, and the transcription factor Gli1 (Nakatomi et al., 2006). As root development proceeds, Shh signaling activity is gradually reduced in both the epithelium and mesenchyme of the molar root (Li et al., 2015; Nakatomi et al., 2006). Overactivation of the Shh pathway before the initiation of the molar root, either by disruption of Ptch1 or constitutive activation of Smo, results in reduced proliferation in dental mesenchymal cells near the HERS and shortened roots (Liu et al., 2015; Nakatomi et al., 2006). Furthermore, loss of Bmp signaling in the dental epithelium results in persistence of Shh-Gli1 signaling activity in the molar root, leading to the inhibition of HERS formation and shorter roots (Li et al., 2015). Intriguingly, persistence of Shh-Gli1 activity in the molar root can be corrected by deleting Shh ligand in the Smad4 mutant model, leading to rescue of the shorter root phenotype (Li et al., 2015). On the other hand, inhibition of the Shh pathway before molar root initiation, via treatment with Hh inhibitor, also leads to decreased mesenchymal cell proliferation around the HERS and reduced root length (Liu et al., 2015). Taken together, these data suggest a dose-dependent role for Shh signaling activity in the regulation of molar root development (Li et al., 2015; Liu et al., 2015).
As mentioned above, Nfic interacts with Tgfβ signaling during root development, but it is also an important downstream target of Gli1-mediated Hh signaling in the root (Huang et al., 2010). Reduction of Shh signaling activity at the early stage of molar tooth germ development leads to decreased Nfic expression in the dental mesenchyme and an eventual lack of roots, which can be partially rescued by restoring Nfic expression by addition of exogenous Shh protein (Huang et al., 2010). Significantly, Nfic also regulates the expression of Hh-interacting protein (Hhip) to provide negative feedback to Shh signaling (Fig. 4) through mesenchymal-epithelial interactions during root formation (Liu et al., 2015). A recent study has shown that PTHrP/PPR inhibits Nfic expression in the CNC-derived mesenchyme during root formation and that loss of this inhibition leads to a root development defect (Ono et al., 2016), suggesting that Nfic is another factor that must be regulated precisely in order to ensure normal root formation.
In addition to the signaling molecules mentioned above, studies have shown that IGF1 and HGF may also be involved in regulating root formation (Fujiwara et al., 2005; Sakuraba et al., 2012), although their precise function in regulating epithelial or mesenchymal cells is yet to be elucidated. Nonetheless, taking all of these studies together, we are beginning to understand the complex signaling network that regulates epithelial-mesenchymal interactions during root formation (summarized in Fig. 4). These studies suggest that there are multiple activators and inhibitors that appear to work together to achieve a balanced signaling outcome and produce the proper patterning, number and length of dental roots during later stages of tooth morphogenesis. Clearly, studies of mutant animal models have provided important insights into the regulatory mechanisms of tooth root development. However, in parallel, we have also learned that tooth root development defects are part of monogenic, multifactorial inheritance and chromosomal disorders (discussed below). Moving forward, it will be important to explore how well these mutant animal models reflect the tooth developmental defects seen in humans, and whether these animal models can be used to discover potential therapeutic solutions for patients.
Dental stem cells and the transition from crown to root development
The crown/root ratio is one of the parameters used to classify teeth among different species. Teeth are classified into three categories based on this parameter: (1) brachydonts, in which the root is longer than the crown; (2) hypsodonts, where the crown is longer than the root; and (3) hypselodonts, which grow continuously during the lifetime of the animal and typically do not include a classic root (Tummers and Thesleff, 2003, 2008). Recent studies suggest that modulation of stem cell populations might explain, at least in part, the differences between these tooth types.
Members of the third group, especially the mouse incisor, are used to study stem cell biology because they harbor both epithelial and mesenchymal stem cells in the proximal region of the tooth that allow them to grow continuously (Fig. 5A) (Kuang-Hsien Hu et al., 2014; Zhao et al., 2014). In the mouse incisor, continuous growth via formation of enamel by the epithelium only occurs on the labial side. The lingual side of the incisor exhibits characteristics distinct from the labial side and is thought to function as a root, although because no classic root formation takes place this region is known as the root analog. Study of the root analog, by investigation of the labial versus the lingual side of the mouse incisor, might provide insight into the development of classic roots.
Fig. 5.
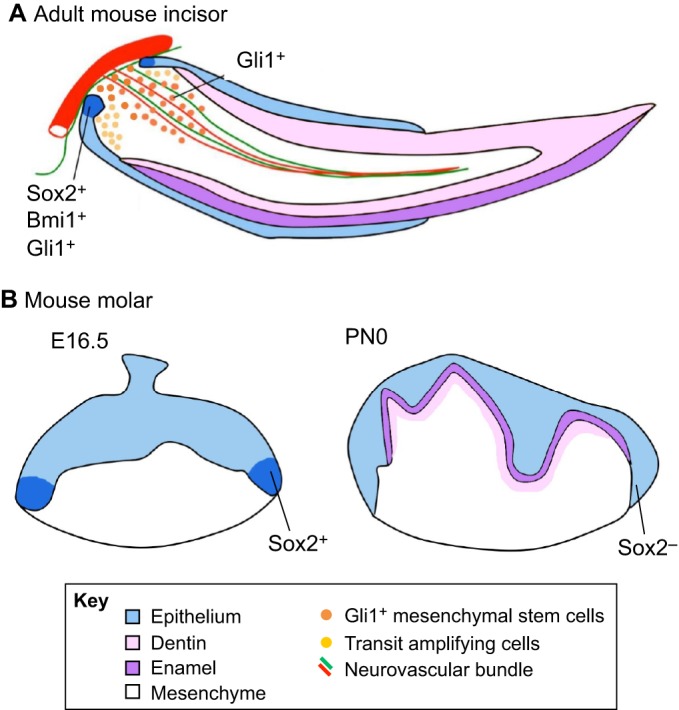
Stem cells in developing and adult teeth. (A) Schematic of an adult mouse incisor. Epithelial stem cells (dark blue) expressing Sox2, Bmi1 and/or Gli1 are located in the proximalmost region of the cervical loop. Mesenchymal stem cells (orange) expressing Gli1 reside in the proximalmost region of the incisor pulp, in a periarterial niche that responds to signals from the adjacent sensory nerve. Together, these stem cells fuel the continuous growth of the mouse incisor. (B) Schematics of embryonic day (E) 16.5 and a PN0 mouse molars. Epithelial stem cells (dark blue) expressing Sox2 are detectable in the apicalmost region of the cervical loop at E16.5 but not at PN0.
In the mouse incisor epithelium, the epithelial stem cells express Sox2, Bmi1 and Gli1 (Fig. 5A), all of which have recently been identified as epithelial stem cell markers (Zhao et al., 2014; Biehs et al., 2013; Juuri et al., 2012; Seidel et al., 2010). Sox2-positive stem cells migrate from the cervical loop to the inner enamel epithelium and give rise to a group of transit amplifying (TA) cells (Juuri et al., 2012). These cells proliferate at high rates and then move towards the distal tip. As they migrate, they differentiate into ameloblasts, which produce and deposit enamel matrix proteins. However, the size of the tooth remains constant through abrasion at the distal tip of the tooth. Notably, Fgf10 from the mesenchyme and Notch signaling from the epithelium support the epithelial stem cell niche (reviewed by Tummers and Thesleff, 2003), whereas Shh controls the differentiation of epithelial progenitors into ameloblasts (Kuang-Hsien Hu et al., 2014; Seidel et al., 2010). In turn, Shh function is regulated by Bmp and, accordingly, the disruption of Bmp signaling in the incisor leads to an expansion of Shh-Gli1 signaling and epithelial stem cell defects (Li et al., 2015). The biology of mesenchymal stem cells in the proximal region of the incisor is less well known but recent studies have begun to provide some insights. Mesenchymal stem cells (Gli1-positive) in the incisor pulp of the proximal region originate from CNC cells and reside in a periarterial niche (Fig. 5A) that responds to Shh signals from the adjacent sensory nerve (Chai et al., 2000; Zhao et al., 2014). They differentiate into the pulp cells and odontoblasts that form dentin as the tooth grows, in coordination with the epithelial cells. Previous studies have shown that peripheral nerve-associated glial cells can also serve as mesenchymal stem cells for maintaining incisor pulp tissue homeostasis and repair (Kaukua et al., 2014), with these dental mesenchymal stem cells offering potential clinical applications (reviewed by Sharpe, 2016).
Human teeth and mouse molars, by contrast, are brachydonts and do not grow continuously. Molars of adult mice lack adult epithelial stem cells and possibly mesenchymal stem cells, and thus lose the capacity for renewal and regeneration once their development has been completed. However, a recent study has shown that Sox2-positive dental epithelial stem cells do exist in the cervical loop during molar crown formation (Fig. 5B) but only for a brief period of time (Li et al., 2015). Importantly, these Sox2-positive cells give rise to all epithelial cell lineages of the molar, but then disappear before root formation begins. Along with the disappearance of Sox2-positive dental epithelial stem cells, the cervical loop structure also disappears following crown formation, giving rise to the HERS, which directs root formation (Tummers and Thesleff, 2003). At the molecular level, also like in incisors, a molecular network including the Shh and Bmp pathways appears to control the fate of Sox2-positive dental epithelial stem cells, which is likely to be related to the crown-to-root transition. Specifically, it has been shown that Smad4 mutant mice exhibit a significant change in the molar crown/root ratio such that the crown seems to grow continuously and the roots appear smaller than those of control molars (Li et al., 2015). Furthermore, loss of Bmp-Smad4 signaling in the dental epithelium results in persistent Shh-Gli1 signaling activity in the dental epithelial cells near the cervical loop, leading to the maintenance of Sox2-positive cells in the cervical loop postnatally. Based on these findings, Shh-expressing cells were proposed to act as a niche for Sox2-positive epithelial stem cells (Li et al., 2015). Interestingly, restoring the correct dosage of Shh in Smad4 mutant mice is enough to rescue the abnormal crown/root ratio (Li et al., 2015). This study suggests that a Bmp/Smad4/Shh signaling network might control the crown/root switch via the regulation of dental epithelial stem cells and their niche during tooth development.
From an evo-devo perspective, the phenotype of Smad4 mutant mice is reminiscent of vole, rabbit and guinea pig molars, which also grow continuously and harbor a population of epithelial stem cells at their apical ends. Interestingly, the expression patterns of genes that control epithelial stem cell behavior are similar in mouse incisors and vole molars. For instance, Notch genes and Fgf10 are expressed in both mouse incisors and vole molars but are absent in mouse molars (Harada et al., 2002; Tummers and Thesleff, 2003). These genes, together with the Shh and Bmp pathways, are likely to constitute a molecular network that regulates the niche and epithelial stem cell compartments in mammals. Consequently, this network differentially controls the transition from crown to root during tooth development, depending on the species, producing the observed variety of different tooth types.
Root developmental defects in humans
As highlighted above, a number of signaling pathways and transcriptional regulators have been identified as important for tooth root development in animal models. However, how well these animal models reflect the causes of root development defects in humans remains to be determined. Notably, human dentition is more complex than that in any of the animal models available and includes single-rooted incisors, canines and bicuspids, as well as multi-rooted molars. Furthermore, the direction and number of dental roots are different in the upper and lower molars; these properties together represent an important anatomical feature supporting the functions performed by human dentition.
In humans, certain genetic mutations have been linked to root development defects (Dong et al., 2005; Yang et al., 2015). However, the majority of root defects observed in humans are associated with complex genetic disorders that lead to multiple developmental defects (reviewed by Luder, 2015). For example, BCOR mutations are responsible for causing oculofaciocardiodental (OFCD) syndrome, in which patients suffer from multiple craniofacial and cardiac anomalies but also have canines with extremely long roots (Fan et al., 2009; Gorlin, 1998). In these patients, BCOR mutations cause abnormal activation of AP-2α (TFAP2A), which in turn promotes the osteodentinogenic capacity of mesenchymal stem cells and hence root malformation (Fan et al., 2009). Studies of OFCD syndrome serve as a perfect example of how studying a rare genetic disorder can help reveal the molecular mechanisms of congenital malformation.
Among the disorders that only affect root development, the premature arrest of root development is common. These disorders are usually associated with trauma that affects the HERS and neighboring neurovascular structures, which disturbs elongation of the root and dentin formation (Andreasen and Kahler, 2015). A similar phenotype is observed in patients exposed to radiation or chemotherapy (Pedersen et al., 2012). This finding is consistent with recent work demonstrating that the neurovascular bundle secretes factors that contribute to stem cell maintenance and homeostasis (Zhao et al., 2014). Dilaceration is another malformation of the tooth root, typically observed as part of an eruption disorder (Topouzelis et al., 2010). It is characterized by a sharp curvature in the apex of the tooth that is frequently the consequence of indirect trauma to the primary teeth.
Taurodontism, another disorder affecting roots only, is specific to primary and permanent multirooted teeth and is characterized by apical displacement of the bi- or trifurcation (Dineshshankar et al., 2014). It is often associated with reduced constriction at the cementoenamel junction and an increased occluso-apical height of the crown and pulp cavity due to a delay in the development of the epithelial bridges in the presumptive area of the furcation (Dineshshankar et al., 2014). Taurodontism may have a genetic component; for example, in tricho-dento-osseous syndrome, which is associated with DLX3 mutations (Wright et al., 2008). Mutations in DLX3 are also associated with amelogenesis imperfecta hypoplastic-hypomaturation with taurodontism in humans (Dong et al., 2005). Of note, the ablation of Smad4, Nfic or Wnt10a in mice leads to similar phenotypes (Li et al., 2015; Liu et al., 2015; Yang et al., 2015).
There are also a number of tooth development defects that affect both the crown and root. These include double teeth, regional odontodysplasia, hypophosphatasia, dentin dysplasia type I, dentinogenesis imperfecta types I, II and III, and X-linked hypophosphatemia. Double teeth result from the merging of two adjacent tooth germs during odontogenesis (Hattab, 2014). A related but distinct disorder is concrescence, in which two adjacent teeth are joined by means of only radicular cementum (Romito, 2004). In regional odontodysplasia, root formation often ends prematurely, leaving a wide-open apex (Hamdan et al., 2004). Hypophosphatasia, which is characterized by defective mineralization and sometimes skeletal abnormalities, is caused by loss-of-function mutations in the ALPL gene, which encodes tissue-nonspecific alkaline phosphatase in humans and mice (Foster et al., 2013). Dentin dysplasia type I (DDI) is an infrequent disorder that affects the formation of the dentin in both the crown and root; in the root, dysplastic hard tissue and scattered soft tissue fill the central space. DDI is transmitted as an autosomal dominant trait but its specific genetic cause is unknown (Kim and Simmer, 2007). The teeth of dentinogenesis imperfecta type I and II patients exhibit early and complete obliteration of the pulp cavity, which is instead filled with dentin. By contrast, dentinogenesis imperfecta type III patients exhibit excessively large pulp cavities (Kim and Simmer, 2007). Again, the genetic causes of these dentinogenesis imperfecta types are unclear, although some studies have linked certain genes to particular types. For example, dentinogenesis imperfecta type I, which is associated with osteogenesis imperfecta, is attributed to mutations in COL1A1 and COL1A2, whereas mutations in DSPP are responsible for dentinogenesis imperfecta type II. Finally, X-linked hypophosphatemia, which is characterized by hypomineralized dentin and enlarged pulp cavities, similar to taurodontism except that the furcation is not displaced apically (Fong et al., 2009), is linked to mutations in PHEX.
Overall, disruptions of root development are rather frequent and are commonly associated with the early loss of teeth, with deleterious consequences for oral health. Importantly, we need to link animal models with human tooth root development defects in order to gain a better understanding of the etiology of these disorders as well as of the normal development of roots.
Conclusions and future directions
Despite the significant recent progress that we have discussed above, the overall regulatory mechanisms of dental root formation remain poorly understood. It is clear that, during early tooth development, reciprocal and sequential interactions between the epithelium and mesenchyme eventually lead to the formation of root dentin, cementum and periodontal tissues. The major signaling pathways involved in these processes are the Tgfβ/Bmp, Wnt, Fgf and Shh pathways, which work together with multiple transcription factors to mediate tissue-tissue interactions that guide root development. However, there are still several important unanswered questions concerning root development, including how root patterning is established. Among the features that are decided during development are the number, size, direction and fine morphology of the roots. Intrinsic elements such as combinatorial gene expression and the activity of complex molecular networks in the root epithelium and mesenchyme have been shown to control tissue-tissue interactions during root development (Fig. 4). However, several anatomical features suggest that other craniofacial structures can also influence the root pattern. For instance, in humans and mice, maxillary first molars have three roots whereas mandibular first molars only have two. The density of the bone in the maxilla and mandible differs significantly, being lower in the maxilla (Devlin et al., 1998), and researchers and clinicians have thus speculated that maxillary molars possess three roots to increase the stability of these teeth in a bone of low density. Other factors that may potentially affect molar root development are vascularization and nerve innervation. Indeed, recent studies have shown that the neurovascular bundle serves as a niche environment for mesenchymal stem cells in mouse incisors (Kaukua et al., 2014; Zhao et al., 2014). However, the mouse incisor does not have a traditional root, making it difficult to study how the neurovascular bundle may influence root development. Future studies might shed light on whether molar mesenchymal stem cells are also influenced by the neurovascular bundle during root development. Understanding all these aspects of root development will improve our knowledge of the factors that regulate tooth development. From a clinical perspective, understanding how roots develop, how they might be affected by periodontal diseases and how they could be bioengineered using regenerative approaches (see Box 2) is also crucial for the oral health field.
Box 2. Bio-roots: engineered tooth roots and their applications.
Although studies using animal models have shown that it is indeed possible to regenerate a whole tooth (Ikeda et al., 2009), clinicians have questioned whether such a newly formed tooth will have the proper size, shape, color and occlusion necessary to function with the rest of the natural dentition, especially in humans. By contrast, the regeneration of a ‘bio-root' using stem cells and a scaffold is more feasible and, hence, a more attractive approach. Furthermore, if restored with a porcelain crown, a bio-root functions as a natural tooth (Wei et al., 2013). Two general approaches have been explored in the tooth regeneration field: (1) recapitulating embryonic development to produce a tooth/root; and (2) using adult stem cells combined with scaffolding biomaterials to produce a bio-root (reviewed by Dadu, 2009). Taking the first approach, several studies have shown that dental cells obtained from dissociated porcine or rat tooth buds can generate multiple small, organized tooth crowns with root-like structures (e.g. Young et al., 2005). More recently, a study reported that functional teeth with roots could be generated after the transplantation of tooth buds formed from reaggregated mouse tooth bud cells (Ikeda et al., 2009). However, the regeneration of functional human teeth by utilizing cells from human tooth germs is not clinically feasible or practical. With regard to the second approach, the assembly of stem cell-derived tissues into a functional root has proven to be challenging. To date, PDL, cementum and dentin have been bioengineered using different sources of adult stem cells, including human dental pulp stem cells (DPSCs), human periodontal ligament stem cells (PDLSCs), and stem cells from human exfoliated deciduous teeth (SHED) (Gronthos et al., 2000; Miura et al., 2003; Seo et al., 2004). Most recently, using a combination of scaffold, DPSCs and PDLSCs, a bio-root has been developed in miniature pigs (Wei et al., 2013). Notably, the regenerated bio-root restored with a porcelain crown exhibited characteristics of a normal tooth after 6 months of use, including dentinal tubule-like and functional PDL-like structures (Wei et al., 2013). Although the success rate of bio-root-supported restorations is less than that of restorations supported by implants (Gao et al., 2016), we are confident that bio-root-supported restoration will ultimately offer an important biological solution for patients as tissue engineering procedures improve.
Acknowledgements
We thank Julie Mayo and Bridget Samuels for critical reading of the manuscript and Jill Harunaga for expert assistance with artwork.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Y.C. is supported by grants from the National Institutes of Health/National Institute of Dental and Craniofacial Research (DE022503, DE020065 and DE012711). Deposited in PMC for release after 12 months.
References
- Åberg T., Wozney J. and Thesleff I. (1997). Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev. Dyn. 210, 383-396. [DOI] [PubMed] [Google Scholar]
- Aïoub M., Lézot F., Molla M., Castaneda B., Robert B., Goubin G., Néfussi J. R. and Berdal A. (2007). Msx2−/− transgenic mice develop compound amelogenesis imperfecta, dentinogenesis imperfecta and periodental osteopetrosis. Bone 41, 851-859. 10.1016/j.bone.2007.07.023 [DOI] [PubMed] [Google Scholar]
- Alfaqeeh S., Oralova V., Foxworthy M., Matalova E., Grigoriadis A. E. and Tucker A. S. (2015). Root and eruption defects in c-Fos mice are driven by loss of osteoclasts. J. Dent. Res. 94, 1724-1731. 10.1177/0022034515608828 [DOI] [PubMed] [Google Scholar]
- Andreasen F. M. and Kahler B. (2015). Diagnosis of acute dental trauma: the importance of standardized documentation: a review. Dent. Traumatol. 31, 340-349. 10.1111/edt.12187 [DOI] [PubMed] [Google Scholar]
- Bae C. H., Lee J. Y., Kim T. H., Baek J. A., Lee J. C., Yang X., Taketo M. M., Jiang R. and Cho E. S. (2013). Excessive Wnt/β-catenin signaling disturbs tooth-root formation. J. Periodontal Res. 48, 405-410. 10.1111/jre.12018 [DOI] [PubMed] [Google Scholar]
- Bae C. H., Kim T. H., Ko S. O., Lee J. C., Yang X. and Cho E. S. (2015). Wntless regulates dentin apposition and root elongation in the mandibular molar. J. Dent. Res. 94, 439-445. 10.1177/0022034514567198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehs B., Hu J. K.-H., Strauli N. B., Sangiorgi E., Jung H., Heber R.-P., Ho S., Goodwin A. F., Dasen J. S., Capecchi M. R. et al. (2013). BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat. Cell Biol. 15, 846-852. 10.1038/ncb2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt D. D., Stadlinger B. and Terheyden H. (2015). Cell-to-cell communication–periodontal regeneration. Clin. Oral Implants Res. 26, 229-239. 10.1111/clr.12543 [DOI] [PubMed] [Google Scholar]
- Brugger S. M., Merrill A. E., Torres-Vazquez J., Wu N., Ting M.-C., Cho J. Y.-M., Dobias S. L., Yi S. E., Lyons K., Bell J. R. et al. (2004). A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development 131, 5153-5165. 10.1242/dev.01390 [DOI] [PubMed] [Google Scholar]
- Cao Z., Zhang H., Zhou X., Han X., Ren Y., Gao T., Xiao Y., de Crombrugghe B., Somerman M. J. and Feng J. Q. (2012). Genetic evidence for the vital function of Osterix in cementogenesis. J. Bone Miner. Res. 27, 1080-1092. 10.1002/jbmr.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate A. R. T. (1996). The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Dis. 2, 55-62. 10.1111/j.1601-0825.1996.tb00204.x [DOI] [PubMed] [Google Scholar]
- Chai Y. and Maxson R. E. (2006). Recent advances in craniofacial morphogenesis. Dev. Dyn. 235, 2353-2375. 10.1002/dvdy.20833 [DOI] [PubMed] [Google Scholar]
- Chai Y., Jiang X., Ito Y., Bringas P., Han J., Rowitch D. H., Soriano P., McMahon A. P. and Sucov H. M. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671-1679. [DOI] [PubMed] [Google Scholar]
- Chen J., Lan Y., Baek J.-A., Gao Y. and Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev. Biol. 334, 174-185. 10.1016/j.ydbio.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. I. and Garant P. R. (2000). Development and general structure of the periodontium. Periodontol. 2000 24, 9-27. 10.1034/j.1600-0757.2000.2240102.x [DOI] [PubMed] [Google Scholar]
- Dadu S. S. (2009). Tooth regeneration: current status. Indian J. Dent. Res. 20, 506-507. 10.4103/0970-9290.59444 [DOI] [PubMed] [Google Scholar]
- Devlin H., Horner K. and Ledgerton D. (1998). A comparison of maxillary and mandibular bone mineral densities. J. Prosthet. Dent. 79, 323-327. 10.1016/S0022-3913(98)70245-8 [DOI] [PubMed] [Google Scholar]
- Dineshshankar J., Sivakumar M., Balasubramanium A. M., Kesavan G., Karthikeyan M. and Prasad V. S. (2014). Taurodontism. J. Pharm. Bioallied Sci. 6, 13-15. 10.4103/0975-7406.137252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Amor D., Aldred M. J., Gu T., Escamilla M. and MacDougall M. (2005). DLX3 mutation associated with autosomal dominant amelogenesis imperfecta with taurodontism. Am. J. Med. Genet. A 133A, 138-141. 10.1002/ajmg.a.30521 [DOI] [PubMed] [Google Scholar]
- Duverger O., Zah A., Isaac J., Sun H.-W., Bartels A. K., Lian J. B., Berdal A., Hwang J. and Morasso M. I. (2012). Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J. Biol. Chem. 287, 12230-12240. 10.1074/jbc.M111.326900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Yamaza T., Lee J. S., Yu J., Wang S., Fan G., Shi S. and Wang C.-Y. (2009). BCOR regulates mesenchymal stem cell function by epigenetic mechanisms. Nat. Cell Biol. 11, 1002-1009. 10.1038/ncb1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Jing J., Sanchez-Lara P. A., Bootwalla M. S., Buckley J., Wu N., Yan Y. and Chai Y. (2016). Generation and characterization of tamoxifen-inducible Pax9-CreER knock-in mice using CrispR/Cas9. Genesis 54, 490-496. 10.1002/dvg.22956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H., Chu E. Y., Tompkins K. A., Foster B. L., Sitara D., Lanske B. and Somerman M. J. (2009). Aberrant cementum phenotype associated with the hypophosphatemic hyp mouse. J. Periodontol. 80, 1348-1354. 10.1902/jop.2009.090129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster B. L., Nagatomo K. J., Tso H. W., Tran A. B., Nociti F. H. Jr., Narisawa S., Yadav M. C., McKee M. D., Millán J. I. and Somerman M. J. (2013). Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J. Bone Miner. Res. 28, 271-282. 10.1002/jbmr.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N., Tabata M. J., Endoh M., Ishizeki K. and Nawa T. (2005). Insulin-like growth factor-I stimulates cell proliferation in the outer layer of Hertwig's epithelial root sheath and elongation of the tooth root in mouse molars in vitro. Cell Tissue Res. 320, 69-75. 10.1007/s00441-004-1065-5 [DOI] [PubMed] [Google Scholar]
- Gao J., Jordan T. W. and Cutress T. W. (1996). Immunolocalization of basic fibroblast growth factor (bFGF) in human periodontal ligament (PDL) tissue. J. Periodontal Res. 31, 260-264. 10.1111/j.1600-0765.1996.tb00491.x [DOI] [PubMed] [Google Scholar]
- Gao Y., Yang G., Weng T., Du J., Wang X., Zhou J., Wang S. and Yang X. (2009). Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice. Mol. Cell. Biol. 29, 5941-5951. 10.1128/MCB.00706-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Zhao Y.-M. and Ge L.-H. (2014). Nuclear factor I-C expression pattern in developing teeth and its important role in odontogenic differentiation of human molar stem cells from the apical papilla. Eur. J. Oral Sci. 122, 382-390. 10.1111/eos.12151 [DOI] [PubMed] [Google Scholar]
- Gao Z. H., Hu L., Liu G. L., Wei F. L., Liu Y., Liu Z. H., Fan Z. P., Zhang C. M., Wang J. S. and Wang S. L. (2016). Bio-root and implant-based restoration as a tooth replacement alternative. J. Dent. Res. 95, 642-649. 10.1177/0022034516639260 [DOI] [PubMed] [Google Scholar]
- Gorlin R. J. (1998). Otodental syndrome, oculo-facio-cardio-dental (OFCD) syndrome, and lobodontia: dental disorders of interest to the pediatric radiologist. Pediatr. Radiol. 28, 802-804. 10.1007/s002470050469 [DOI] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P. G. and Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 97, 13625-13630. 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan M. A., Sawair F. A., Rajab L. D., Hamdan A. M. and Al-Omari I. K. (2004). Regional odontodysplasia: a review of the literature and report of a case. Int. J. Paediatr. Dent. 14, 363-370. 10.1111/j.1365-263X.2004.00548.x [DOI] [PubMed] [Google Scholar]
- Han X. L., Liu M., Voisey A., Ren Y. S., Kurimoto P., Gao T., Tefera L., Dechow P., Ke H. Z. and Feng J. Q. (2011). Post-natal effect of overexpressed DKK1 on mandibular molar formation. J. Dent. Res. 90, 1312-1317. 10.1177/0022034511421926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Toyono T., Toyoshima K., Yamasaki M., Itoh N., Kato S., Sekine K. and Ohuchi H. (2002). FGF10 maintains stem cell compartment in developing mouse incisors. Development 129, 1533-1541. [DOI] [PubMed] [Google Scholar]
- Hattab F. N. (2014). Double talon cusps on supernumerary tooth fused to maxillary central incisor: review of literature and report of case. J. Clin. Exp. Dent. 6, e400-e407. 10.4317/jced.51428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Zhang J., Niu Z., Yu Q., Wang Z., Zhang R., Su L., Fu L., Smith A. J. and Cooper P. R. (2014). Regulatory interplay between NFIC and TGF-β1 in apical papilla-derived stem cells. J. Dent. Res. 93, 496-501. 10.1177/0022034514525200 [DOI] [PubMed] [Google Scholar]
- Huang X., Bringas P. Jr, Slavkin H. C. and Chai Y. (2009). Fate of HERS during tooth root development. Dev. Biol. 334, 22-30. 10.1016/j.ydbio.2009.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Xu X., Bringas P., Hung Y. P. and Chai Y. (2010). Smad4-Shh-Nfic signaling cascade–mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J. Bone Miner. Res. 25, 1167-1178. 10.1359/jbmr.091103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda E., Morita R., Nakao K., Ishida K., Nakamura T., Takano-Yamamoto T., Ogawa M., Mizuno M., Kasugai S. and Tsuji T. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 106, 13475-13480. 10.1073/pnas.0902944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J. and Thesleff I. (2000). Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 92, 19-29. 10.1016/S0925-4773(99)00322-6 [DOI] [PubMed] [Google Scholar]
- Juuri E., Saito K., Ahtiainen L., Seidel K., Tummers M., Hochedlinger K., Klein O. D., Thesleff I. and Michon F. (2012). Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev. Cell 23, 317-328. 10.1016/j.devcel.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua N., Shahidi M. K., Konstantinidou C., Dyachuk V., Kaucka M., Furlan A., An Z., Wang L., Hultman I., Ährlund-Richter L. et al. (2014). Glial origin of mesenchymal stem cells in a tooth model system. Nature 513, 551-554. 10.1038/nature13536 [DOI] [PubMed] [Google Scholar]
- Kim J.-W. and Simmer J. P. (2007). Hereditary dentin defects. J. Dent. Res. 86, 392-399. 10.1177/154405910708600502 [DOI] [PubMed] [Google Scholar]
- Kim T.-H., Bae C.-H., Jang E.-H., Yoon C.-Y., Bae Y., Ko S.-O., Taketo M. M. and Cho E.-S. (2012). Col1a1-cre mediated activation of β-catenin leads to aberrant dento-alveolar complex formation. Anat. Cell Biol. 45, 193-202. 10.5115/acb.2012.45.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. H., Bae C. H., Lee J. C., Ko S. O., Yang X., Jiang R. and Cho E. S. (2013). β-catenin is required in odontoblasts for tooth root formation. J. Dent. Res. 92, 215-221. 10.1177/0022034512470137 [DOI] [PubMed] [Google Scholar]
- Kim T. H., Bae C. H., Lee J. C., Kim J. E., Yang X., de Crombrugghe B. and Cho E. S. (2015). Osterix regulates tooth root formation in a site-specific manner. J. Dent. Res. 94, 430-438. 10.1177/0022034514565647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar E. J. and Baird G. R. (1970a). Tissue interactions in embryonic mouse tooth germs. I. Reorganization of the dental epithelium during tooth-germ reconstruction. J. Embryol. Exp. Morphol. 24, 159-171. [PubMed] [Google Scholar]
- Kollar E. J. and Baird G. R. (1970b). Tissue interactions in embryonic mouse tooth germs. II. The inductive role of the dental papilla. J. Embryol. Exp. Morphol. 24, 173-186. [PubMed] [Google Scholar]
- Kuang-Hsien Hu J., Mushegyan V. and Klein O. D. (2014). On the cutting edge of organ renewal: identification, regulation, and evolution of incisor stem cells. Genesis 52, 79-92. 10.1002/dvg.22732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapthanasupkul P., Feng J., Mantesso A., Takada-Horisawa Y., Vidal M., Koseki H., Wang L., An Z., Miletich I. and Sharpe P. T. (2012). Ring1a/b polycomb proteins regulate the mesenchymal stem cell niche in continuously growing incisors. Dev. Biol. 367, 140-153. 10.1016/j.ydbio.2012.04.029 [DOI] [PubMed] [Google Scholar]
- Lee D.-S., Park J.-T., Kim H.-M., Ko J. S., Son H.-H., Gronostajski R. M., Cho M.-I., Choung P.-H. and Park J.-C. (2009). Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J. Biol. Chem. 284, 17293-17303. 10.1074/jbc.M109.009084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezot F., Davideau J.-L., Thomas B., Sharpe P., Forest N. and Berdal A. (2000). Epithelial Dlx-2 homeogene expression and cementogenesis. J. Histochem. Cytochem. 48, 277-284. 10.1177/002215540004800213 [DOI] [PubMed] [Google Scholar]
- Li J., Huang X., Xu X., Mayo J., Bringas P., Jiang R., Wang S. and Chai Y. (2011). SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development 138, 1977-1989. 10.1242/dev.061341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Feng J., Liu Y., Ho T.-V., Grimes W., Ho H. A., Park S., Wang S. and Chai Y. (2015). BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell 33, 125-135. 10.1016/j.devcel.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Feng J., Li J., Zhao H., Ho T.-V. and Chai Y. (2015). An Nfic-hedgehog signaling cascade regulates tooth root development. Development 142, 3374-3382. 10.1242/dev.127068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi M., Tucker A. S. and Sharpe P. T. (2010). Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev. Dyn. 239, 160-167. 10.1002/dvdy.22047 [DOI] [PubMed] [Google Scholar]
- Lu X., Rios H. F., Jiang B., Xing L., Kadlcek R., Greenfield E. M., Luo G. and Feng J. Q. (2009). A new osteopetrosis mutant mouse strain (ntl) with odontoma-like proliferations and lack of tooth roots. Eur. J. Oral Sci. 117, 625-635. 10.1111/j.1600-0722.2009.00690.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Ito Y. and Diekwisch T. G. H. (2006). Evolution and development of Hertwig's epithelial root sheath. Dev. Dyn. 235, 1167-1180. 10.1002/dvdy.20674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luder H. U. (2015). Malformations of the tooth root in humans. Front. Physiol. 6, 307 10.3389/fphys.2015.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A. G. (1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103 Suppl, 155-169. [DOI] [PubMed] [Google Scholar]
- Madan A. K. and Kramer B. (2005). Immunolocalization of fibroblast growth factor-2 (FGF-2) in the developing root and supporting structures of the murine tooth. J. Mol. Histol. 36, 171-178. 10.1007/s10735-005-2684-1 [DOI] [PubMed] [Google Scholar]
- Mina M. and Kollar E. J. (1987). The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 32, 123-127. 10.1016/0003-9969(87)90055-0 [DOI] [PubMed] [Google Scholar]
- Miura M., Gronthos S., Zhao M., Lu B., Fisher L. W., Robey P. G. and Shi S. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 100, 5807-5812. 10.1073/pnas.0937635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen L. M., Richards D. W. and Quaranta V. (1999). Evidence that laminin-5 is a component of the tooth surface internal basal lamina, supporting epithelial cell adhesion. J. Periodontal Res. 34, 16-24. 10.1111/j.1600-0765.1999.tb02217.x [DOI] [PubMed] [Google Scholar]
- Nakatomi M., Morita I., Eto K. and Ota M. S. (2006). Sonic hedgehog signaling is important in tooth root development. J. Dent. Res. 85, 427-431. 10.1177/154405910608500506 [DOI] [PubMed] [Google Scholar]
- Nakatomi M., Hovorakova M., Gritli-Linde A., Blair H. J., MacArthur K., Peterka M., Lesot H., Peterkova R., Ruiz-Perez V. L., Goodship J. A. et al. (2013). Evc regulates a symmetrical response to Shh signaling in molar development. J. Dent. Res. 92, 222-228. 10.1177/0022034512471826 [DOI] [PubMed] [Google Scholar]
- Oka S., Oka K., Xu X., Sasaki T., Bringas P. Jr and Chai Y. (2007). Cell autonomous requirement for TGF-β signaling during odontoblast differentiation and dentin matrix formation. Mech. Dev. 124, 409-415. 10.1016/j.mod.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono W., Sakagami N., Nishimori S., Ono N. and Kronenberg H. M. (2016). Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 7, 11277 10.1038/ncomms11277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban B. J. (1980). Orban's Oral histology and embryology. St. Louis: Mosby. [Google Scholar]
- Palmer R. M. and Lumsden A. G. S. (1987). Development of periodontal ligament and alveolar bone in homografted recombinations of enamel organs and papillary, pulpal and follicular mesenchyme in the mouse. Arch. Oral Biol. 32, 281-289. 10.1016/0003-9969(87)90022-7 [DOI] [PubMed] [Google Scholar]
- Pedersen L. B., Clausen N., Schrøder H., Schmidt M. and Poulsen S. (2012). Microdontia and hypodontia of premolars and permanent molars in childhood cancer survivors after chemotherapy. Int. J. Paediatr. Dent. 22, 239-243. 10.1111/j.1365-263X.2011.01199.x [DOI] [PubMed] [Google Scholar]
- Plikus M. V., Zeichner-David M., Mayer J.-A., Reyna J., Bringas P., Thewissen J. G. M., Snead M. L., Chai Y. and Chuong C.-M. (2005). Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol. Dev. 7, 440-457. 10.1111/j.1525-142X.2005.05048.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakian A., Yang W.-C., Gluhak-Heinrich J., Cui Y., Harris M. A., Villarreal D., Feng J. Q., MacDougall M. and Harris S. E. (2013). Bone morphogenetic protein-2 gene controls tooth root development in coordination with formation of the periodontium. Int. J. Oral. Sci. 5, 75-84. 10.1038/ijos.2013.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romito L. M. (2004). Concrescence: report of a rare case. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 97, 325-327. 10.1016/j.tripleo.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Sakuraba H., Fujiwara N., Sasaki-Oikawa A., Sakano M., Tabata Y., Otsu K., Ishizeki K. and Harada H. (2012). Hepatocyte growth factor stimulates root growth during the development of mouse molar teeth. J. Periodontal Res. 47, 81-88. 10.1111/j.1600-0765.2011.01407.x [DOI] [PubMed] [Google Scholar]
- Sarkar L. and Sharpe P. T. (1999). Expression of Wnt signalling pathway genes during tooth development. Mech. Dev. 85, 197-200. 10.1016/S0925-4773(99)00095-7 [DOI] [PubMed] [Google Scholar]
- Seidel K., Ahn C. P., Lyons D., Nee A., Ting K., Brownell I., Cao T., Carano R. A. D., Curran T., Schober M. et al. (2010). Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development 137, 3753-3761. 10.1242/dev.056358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B.-M., Miura M., Gronthos S., Bartold P. M., Batouli S., Brahim J., Young M., Robey P. G., Wang C. Y. and Shi S. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149-155. 10.1016/S0140-6736(04)16627-0 [DOI] [PubMed] [Google Scholar]
- Sharpe P. T. (2016). Dental mesenchymal stem cells. Development 143, 2273-2280. 10.1242/dev.134189 [DOI] [PubMed] [Google Scholar]
- Sohn W.-J., Choi M.-A., Yamamoto H., Lee S., Lee Y., Jung J.-K., Jin M.-U., An C.-H., Jung H.-S., Suh J.-Y. et al. (2014). Contribution of mesenchymal proliferation in tooth root morphogenesis. J. Dent. Res. 93, 78-83. 10.1177/0022034513511247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Perkins G., Butz K. G., Lyons G. E., Zeichner-David M., Kim H.-J., Cho M.-I. and Gronostajski R. M. (2003). Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol. Cell. Biol. 23, 1075-1084. 10.1128/MCB.23.3.1075-1084.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I. and Sharpe P. (1997). Signalling networks regulating dental development. Mech. Dev. 67, 111-123. 10.1016/S0925-4773(97)00115-9 [DOI] [PubMed] [Google Scholar]
- Thyagarajan T., Sreenath T., Cho A., Wright J. T. and Kulkarni A. B. (2001). Reduced expression of dentin sialophosphoprotein is associated with dysplastic dentin in mice overexpressing transforming growth factor-β1 in teeth. J. Biol. Chem. 276, 11016-11020. 10.1074/jbc.M010502200 [DOI] [PubMed] [Google Scholar]
- Topouzelis N., Tsaousoglou P., Pisoka V. and Zouloumis L. (2010). Dilaceration of maxillary central incisor: a literature review. Dent. Traumatol. 26, 427-433. 10.1111/j.1600-9657.2010.00915.x [DOI] [PubMed] [Google Scholar]
- Tucker A. and Sharpe P. (2004). The cutting-edge of mammalian development; how the embryo makes teeth. Nat. Rev. Genet. 5, 499-508. 10.1038/nrg1380 [DOI] [PubMed] [Google Scholar]
- Tummers M. and Thesleff I. (2003). Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development 130, 1049-1057. 10.1242/dev.00332 [DOI] [PubMed] [Google Scholar]
- Tummers M. and Thesleff I. (2008). Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns. Evol. Dev. 10, 187-195. 10.1111/j.1525-142X.2008.00226.x [DOI] [PubMed] [Google Scholar]
- Wang Y., Cox M. K., Coricor G., MacDougall M. and Serra R. (2013). Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation. Dev. Biol. 382, 27-37. 10.1016/j.ydbio.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Song T., Ding G., Xu J., Liu Y., Liu D., Fan Z., Zhang C., Shi S. and Wang S. (2013). Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev. 22, 1752-1762. 10.1089/scd.2012.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. T., Hong S. P., Simmons D., Daly B., Uebelhart D. and Luder H. U. (2008). DLX3 c.561_562delCT mutation causes attenuated phenotype of tricho-dento-osseous syndrome. Am. J. Med. Genet. A 146A, 343-349. 10.1002/ajmg.a.32132 [DOI] [PubMed] [Google Scholar]
- Xiong J., Gronthos S. and Bartold P. M. (2013). Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontol. 2000 63, 217-233. 10.1111/prd.12023 [DOI] [PubMed] [Google Scholar]
- Yamashiro T., Tummers M. and Thesleff I. (2003). Expression of bone morphogenetic proteins and Msx genes during root formation. J. Dent. Res. 82, 172-176. 10.1177/154405910308200305 [DOI] [PubMed] [Google Scholar]
- Yamashiro T., Zheng L., Shitaku Y., Saito M., Tsubakimoto T., Takada K., Takano-Yamamoto T. and Thesleff I. (2007). Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation 75, 452-462. 10.1111/j.1432-0436.2006.00150.x [DOI] [PubMed] [Google Scholar]
- Yang Z., Hai B., Qin L., Ti X., Shangguan L., Zhao Y., Wiggins L., Liu Y., Feng J. Q., Chang J. Y. F. et al. (2013). Cessation of epithelial bmp signaling switches the differentiation of crown epithelia to the root lineage in a β-catenin-dependent manner. Mol. Cell. Biol. 33, 4732-4744. 10.1128/MCB.00456-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wang S.-K., Choi M., Reid B. M., Hu Y., Lee Y.-L., Herzog C. R., Kim-Berman H., Lee M., Benke P. J. et al. (2015). Taurodontism, variations in tooth number, and misshapened crowns in Wnt10a null mice and human kindreds. Mol. Genet. Genomic Med. 3, 40-58. 10.1002/mgg3.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokohama-Tamaki T., Ohshima H., Fujiwara N., Takada Y., Ichimori Y., Wakisaka S., Ohuchi H. and Harada H. (2006). Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development 133, 1359-1366. 10.1242/dev.02307 [DOI] [PubMed] [Google Scholar]
- Young C. S., Abukawa H., Asrican R., Ravens M., Troulis M. J., Kaban L. B., Vacanti J. P. and Yelick P. C. (2005). Tissue-engineered hybrid tooth and bone. Tissue Eng. 11, 1599-1610. 10.1089/ten.2005.11.1599 [DOI] [PubMed] [Google Scholar]
- Zeichner-David M. (2006). Regeneration of periodontal tissues: cementogenesis revisited. Periodontol. 2000 41, 196-217. 10.1111/j.1600-0757.2006.00162.x [DOI] [PubMed] [Google Scholar]
- Zhang Z., Gutierrez D., Li X., Bidlack F., Cao H., Wang J., Andrade K., Margolis H. C. and Amendt B. A. (2013). The LIM homeodomain transcription factor LHX6: a transcriptional repressor that interacts with pituitary homeobox 2 (PITX2) to regulate odontogenesis. J. Biol. Chem. 288, 2485-2500. 10.1074/jbc.M112.402933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Jiang Y., Qin C., Liu Y., Ho S. P. and Feng J. Q. (2015). Essential role of osterix for tooth root but not crown dentin formation. J. Bone Miner. Res. 30, 742-746. 10.1002/jbmr.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Feng J., Seidel K., Shi S., Klein O., Sharpe P. and Chai Y. (2014). Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 14, 160-173. 10.1016/j.stem.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]