ABSTRACT
Urothelium is the protective lining of the urinary tract. The mechanisms underlying urothelial formation and maintenance are largely unknown. Here, we report the stage-specific roles of PRC2 epigenetic regulators in embryonic and adult urothelial progenitors. Without Eed, the obligatory subunit of PRC2, embryonic urothelial progenitors demonstrate reduced proliferation with concomitant dysregulation of genes including Cdkn2a (p16), Cdkn2b (p15) and Shh. These mutants display premature differentiation of keratin 5-positive (Krt5+) basal cells and ectopic expression of squamous-like differentiation markers. Deletion of Ezh2, the major enzymatic component of PRC2, causes upregulation of Upk3a+ superficial cells. Unexpectedly, Eed and Eed/Ezh2 double mutants exhibit delayed superficial cell differentiation. Furthermore, Eed regulates the proliferative and regenerative capacity of adult urothelial progenitors and prevents precocious differentiation. Collectively, these findings uncover the epigenetic mechanism by which PRC2 controls urothelial progenitor cell fate and the timing of differentiation, and further suggest an epigenetic basis of urothelial maintenance and regeneration.
KEY WORDS: Mouse, Urothelium, Squamous differentiation, Bladder, Regeneration, Injury, Epigenetics, PRC2, Shh, Ezh2, Eed, Krt5, Krt14, Krt17
Summary: In mouse, mutations in different subunits of the PRC2 complex have specific effects on urothelial progenitor cell proliferation and differentiation.
INTRODUCTION
The urinary bladder is an anterior extension of the urogenital sinus, the ventral region of the cloaca or the embryonic hindgut (Huang et al., 2016). Unlike gut epithelium, mature bladder urothelium is essentially quiescent with a low turnover rate (Jost, 1989). In response to injury, however, urothelium readily switches to a proliferative and regenerative mode to repair the damaged site. The mechanisms underlying urothelium formation and regeneration remain poorly understood.
Definitive bladder urothelium consists of three major cell types, keratin 5-positive (Krt5+) basal cells, Krt20+ superficial or umbrella cells, and Krt5- and Krt20-double negative intermediate cells (Georgas et al., 2015). Basal and intermediate cells represent ∼68% and ∼29%, respectively, of the entire population of mature bladder urothelium in adult mice (Jost, 1989). Superficial cells, which face directly to bladder lumen, are terminally differentiated large polyploid cells consisting of less than 3% of adult urothelium. During the early stages of bladder organogenesis between embryonic days (E)9.5 to E13.5 of murine embryos (Huang et al., 2016), the primitive bladder and gut epithelia originate from embryonic hindgut, as known as the cloaca, and share several common molecular signatures, including Shh, Trp63 and Foxa2. Expression of uroplakin 1b (Upk1b) at the ventral subdomain of the cloaca marks the initial sign of bladder urothelial specification. These cells are the proposed P-cells (Gandhi et al., 2013). Krt5 expression is detected much later at E15.0. Ectopic expression of RaraDN, the dominant negative form of retinoic acid (RA) receptor α, in P-cells results in the persistent expression of Foxa2 and loss of intermediate and superficial cells (Gandhi et al., 2013). Conversely, RA treatment of cultured embryonic stem cells induces expression of uroplakin genes (Mauney et al., 2010), suggesting that the RA signaling pathway directs urothelial specification, particularly formation of the Upk+ intermediate and superficial cell lineages.
Mature bladder urothelium has a very low turnover rate. However, injuries such as urinary tract infection (UTI) or cyclophosphamide (CPP) treatment trigger a rapid proliferative response of the basal and intermediate cells to regenerate superficial cells (Colopy et al., 2014; Gandhi et al., 2013; Kunze et al., 1980; Mysorekar et al., 2009; Papafotiou et al., 2016; Shin et al., 2011). While the molecular mechanisms underlying the urothelial quiescent state and regenerative capacity remain largely unknown, these studies begin to uncover the cellular basis of urothelial regeneration. Shh is expressed in primitive urothelial cells, P-cells and adult urothelium. Beachy and colleagues suggest that adult Shh expression is restricted to Krt5+ basal cells (Shin et al., 2011). However, Mendelsohn and colleagues show that Shh is expressed in both basal and intermediate cell layers (Gandhi et al., 2013). Using ShhCreER, a Cre-ERT2 fusion gene inserted into the Shh genetic locus, to indelibly label Shh+ progenitors and their daughter cells, both groups demonstrated that the genetically labeled Shh+ cells proliferate and differentiate into superficial cells during urothelial injury and repair, indicating that Shh+ cells function as adult urothelial progenitors (Gandhi et al., 2013; Shin et al., 2011). When Krt5+ basal cells were indelibly labeled using a Cre transgenic line (Indra et al., 1999), no labeled cells were detected in the superficial cell layer after CPP treatment (Gandhi et al., 2013). Krt14 marks a small subpopulation of Krt5+ basal cells. Surprisingly, Krt14+ cells contribute extensively to superficial cells after repeated CPP-induced bladder injury (Papafotiou et al., 2016). By comparing the urothelial response to different types of injuries, Mysorekar et al. (2009) noted that UTI and protamine sulfate preferentially induced basal and intermediate cell proliferation, respectively, suggesting that the cellular basis of urothelial regeneration may differ depending on the type and/or severity of injury. Collectively, mature urothelial cells are largely quiescent, but both basal and intermediate cells maintain proliferative potential and possible regenerative capacity.
By regulating the chromatin state, epigenetic mechanisms are central to the establishment and maintenance of gene expression patterns in progenitors and differentiated cells (Margueron and Reinberg, 2011). The polycomb repressive complex 2 (PRC2) is responsible for trimethylation of histone H3 lysine 27 (H3K27me3), which is associated with transcription silencing (Cao et al., 2002; Czermin et al., 2002; Kuzmichev et al., 2002; Müller et al., 2002). Here, we used Cre/loxP technology to conditionally inactivate Eed and Ezh2, which encode the obligatory structural component (Montgomery et al., 2005) and the catalytic subunit of PRC2 (Cao et al., 2002; Kuzmichev et al., 2002), respectively, in embryonic or adult urothelial progenitors. Deletion of Eed but not Ezh2 from embryonic urothelial progenitors caused premature differentiation of Krt5+ basal cells and ectopic expression of squamous cell markers. Deletion of Eed from adult urothelial progenitors resulted in precocious superficial cell differentiation and reduced regenerative capacity. Collectively, our findings demonstrate the stage- and subunit-specific roles of PRC2 epigenetic regulators in bladder urothelial progenitors during development and further suggest the epigenetic basis of urothelial regeneration after injury.
RESULTS
PRC2 activity is enriched in bladder urothelium
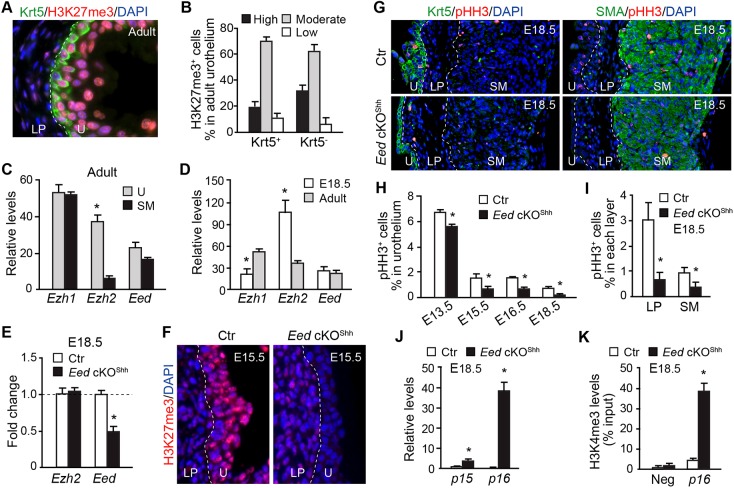
We analyzed H3K27me3 distribution to determine tissue-specific PRC2 activity in both embryonic and adult murine bladders. Strong H3K27me3 staining was detected in the urothelium with reduced expression in the lamina propria (LP) or smooth muscle cell (SMC) layers (Fig. 1A and Fig. S1A,B). Variable levels of H3K27me3 were observed among urothelial cells within the Krt5+ basal as well as Krt5− intermediate and superficial cells (Fig. 1B). Expression of key PRC2 complex genes was further analyzed using microdissected bladder tissues. Eed expression was distributed uniformly within the urothelium and smooth muscle tissue at both adult and embryonic stages (Fig. 1C,D). In contrast, Ezh2 was significantly enriched in the urothelium (Fig. 1C), with higher Ezh2 expression in embryonic urothelium than adult urothelium (Fig. 1D). To study the role of the PRC2-dependent epigenetic program in the urothelium, Eed was conditionally deleted from embryonic urothelial progenitors by crossing a ShhGC Cre driver, which expresses eGFP and Cre fusion gene (GC) in the primitive urothelium at E9.5 (Harfe et al., 2004; Seifert et al., 2008), with a conditional floxed allele of Eedf/f (Xie et al., 2014). The resulting urothelium-specific Eed conditional knockout embryos, namely Eed cKOShh (Eedf/f;ShhGC/+), exhibited a significant reduction in Eed transcripts from microdissected urothelium compared with compound heterozygous controls (Eedf/+;ShhGC/+) (Fig. 1E). More importantly, Eed cKOShh mice lacked any detectable H3K27me3 in the urothelium (Fig. 1F), confirming the central role of Eed in establishing the PRC2-dependent epigenetic program in vivo.
Fig. 1.
Urothelium-specific PRC2 activity regulates bladder cell proliferation during development. (A) Immunofluorescence microscopy of bladder sections from P45 wild-type mice stained with Krt5 (green), H3K27me3 (red) and DAPI (blue). (B) Quantification of the relative levels of H3K27me3 expression in Krt5+ and Krt5− urothelial cells. (C,D) qRT-PCR analysis of Ezh1, Ezh2 and Eed expression relative to Gapdh in bladder urothelium (U) and smooth muscle (SM) at P45 (C) and urothelium of E18.5 and P45 bladders (D). (E) qRT-PCR analysis of control (Ctr) and Eed cKOShh microdissected urothelium at E18.5. (F) Immunofluorescence microscopy of bladder sections from E18.5 control and Eed cKOShh mice stained with H3K27me3 (red) and DAPI (blue). (G) Immunofluorescence microscopy of sections from E18.5 control and Eed cKOShh bladders stained with Krt5 (green, left), SMA (green, right), pHH3 (red) and DAPI (blue). (H) The percentage of pHH3+ cells within the urothelium was calculated relative to the total number of urothelial cells in control versus Eed cKOShh bladders from E13.5, E15.5, E16.5 and E18.5 embryos. Control (Ctr), Eedf/+;ShhGC/+. (I) The percentage of pHH3+ cells within the lamina propria (LP) and smooth muscle (SM) was calculated relative to the total number of cells in each layer in E18.5 control versus Eed cKOShh bladders. (J) qRT-PCR analysis of Cdkn2b (p15) and Cdkn2a (p16) expression relative to Gapdh in microdissected urothelium from E18.5 control versus Eed cKOShh embryos. (K) ChIP-qPCR for H3K4me3 occupancy of the p16 promoter versus an unrelated promoter (Neg) in control versus Eed cKOShh urothelium at E18.5. White dashed lines demarcate the different layers within the bladder. U, urothelium; LP, lamina propria; SM, smooth muscle. Data in C-E and H-K represent mean±s.e.m. of n=3-6. Unpaired Student's t-test, *P<0.05.
Urothelium-specific PRC2 has both cell-autonomous and non-autonomous functions
All Eed cKOShh mutants died shortly after birth, most likely because of a hypoplastic lung defect (Galvis et al., 2015; Snitow et al., 2015) (Fig. S2A,E,F). The lower urinary tract was grossly normal in these mutants (Fig. S2B). Histological analyses, however, demonstrated that the mutant urothelium was thinner (Fig. S2C,D). There was no detectable difference in levels of apoptosis at all stages analyzed (data not shown). However, cell proliferation rate as revealed by the mitotic marker phospho-histone H3 (pHH3) was significantly reduced in Eed cKOShh urothelium at all stages analyzed (Fig. 1G,H, E13.5-E18.5). Consistent with the urothelial proliferation defect, the canonical PRC2 target genes, including the cyclin-dependent kinase inhibitors 2A [Cdkn2a (p16)] and 2B [Cdkn2b (p15)] were significantly increased in the mutants (Fig. 1J). Concomitantly, the H3K4me3 epigenetic mark, which associates with active transcription, was significantly enriched at the Cdkn2a promoter region in the mutant urothelium (Fig. 1K). Unexpectedly, cell proliferation rates were also reduced in the LP and SM layers, indicating that the defect was not restricted to the urothelium (Fig. 1I). Expression of several smooth muscle differentiation genes was also upregulated (Fig. S3). Since Eed deletion is restricted to the urothelium, these findings collectively suggest that the PRC2-dependent urothelial epigenetic program has both cell-autonomous and non-autonomous functions during bladder development.
PRC2 regulates Shh expression in urothelial progenitors
The cell non-autonomous Eed activity probably depends upon downstream signaling molecules. To identify potential candidates, we focused on Shh because its activity is crucial for proliferation of the surrounding mesenchyme and differentiation of smooth muscle cells (DeSouza et al., 2013; Shin et al., 2011; Shiroyanagi et al., 2007). Shh and its receptors Ptch1 and Ptch2 were significantly reduced in Eed cKOShh mutant bladders compared with expression in bladders of littermate controls (Fig. 2A). Whole-mount RNA in situ hybridization demonstrated that Shh expression in bladder urothelium and the urethral groove of the genital tubercle was markedly reduced in the mutants (Fig. 2B). Shh expression in the preputial glands (PGs) was comparable between the mutants and controls, suggesting that PRC2 regulates Shh gene expression in a tissue-specific manner.
Fig. 2.
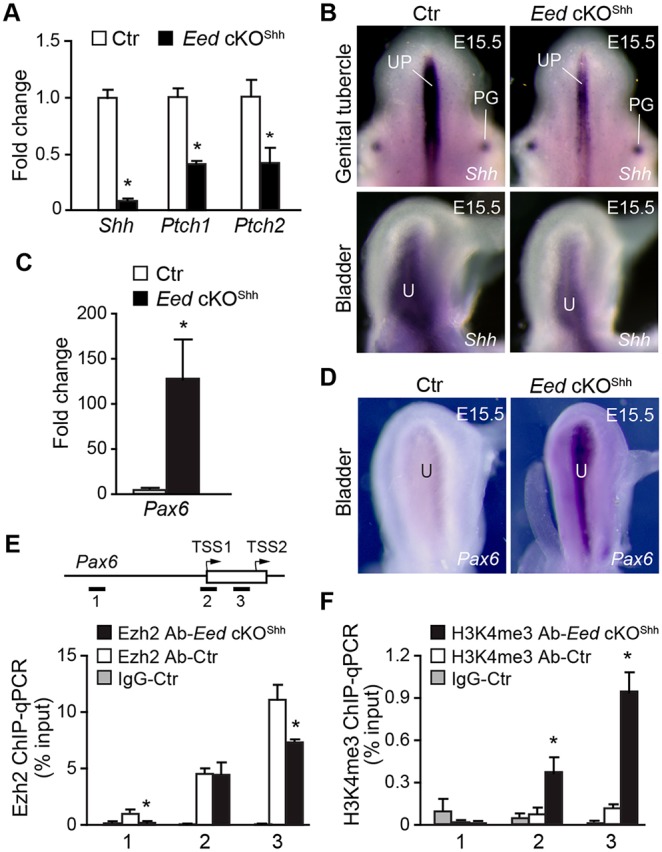
Deletion of Eed results in downregulation of Shh in the developing urothelium. (A,C) qRT-PCR analysis of Shh, Ptch1, Ptch2 and Pax6 expression relative to Gapdh in whole bladders from E18.5 control (Ctr, Eedf/+;ShhGC/+) and Eed cKOShh embryos. Control transcript levels were normalized to 1.0 and transcript levels in Eed cKOShh samples are displayed as fold change relative to control. (B,D) Whole mount in situ hybridization of control (Ctr, Eedf/+;ShhGC/+) and Eed cKOShh mutants at E15.5 using RNA probes for Shh (B) and Pax6 (D). PG, preputial glands; UP, urethral plate; U, urothelium. (E,F) ChIP-qPCR of Pax6 locus using anti-Ezh2 (E) and anti-H3K4me3 (F) antibodies. Schematic in E indicates location of PCR oligos (1-3) and transcription start sites (TSS) of Pax6. Open box, exon; arrows, direction of transcription. Data in A,C,E,F represent mean±s.e.m. of n=3-6. Unpaired Student's t-test, *P<0.05.
To understand how PRC2 may regulate Shh, we tested the hypothesis that PRC2 inhibited expression of transcriptional repressors, which negatively regulate Shh gene expression. Pax6 is a known transcriptional repressor that directly inhibits expression of Shh in vivo (Caballero et al., 2014). Pax6 is ectopically expressed in cardiomyocytes with deleted PRC2 (Delgado-Olguín et al., 2012; He et al., 2012). Pax6 was undetectable in wild-type mouse bladders. However, high levels of Pax6 were observed in mutant bladders at E15.5 and E18.5 based on both RNA in situ hybridization and quantitative RT-PCR analyses, respectively (Fig. 2C,D). Furthermore, ChIP analysis demonstrated that Ezh2 was strongly associated with the promoter regions of Pax6 in control urothelium, and Ezh2 occupancy was significantly reduced in Eed cKOShh mutant urothelium (Fig. 2E). Conversely, the active epigenetic mark H3K4me3 was significantly elevated in mutant urothelium (Fig. 2F). These findings suggest that PRC2 indirectly promotes Shh expression via repression of genes, including Pax6, in fetal mouse bladder urothelium.
PRC2 regulates the timing of urothelial differentiation
Urothelial differentiation was analyzed using known cell type-specific molecular markers. Expression of uroplakin 3a (Upk3a), a superficial cell marker, was readily detected in wild-type bladders at E15.5 (Fig. 3A). In contrast, the basal cell marker Krt5 was barely detectable at this same stage. In Eed cKOShh mutants, Upk3a expression was reduced, with fewer Upk3a+ cells (Fig. 3A and Fig. S4A). In contrast, a large number of Krt5+ basal cells were observed in the mutant urothelium (Fig. 3A and Fig. S4B). Additionally, ectopic Krt5 expression was observed occasionally in Upk3a+ superficial cells (Fig. 3B, arrowheads). Nevertheless, by E16.5, expression levels of Krt5 and Upk3a become comparable between the mutants and littermate controls. Thus, the PRC2-dependent epigenetic program regulates the timing of urothelial differentiation by attenuating basal cell formation while promoting superficial cell differentiation.
Fig. 3.
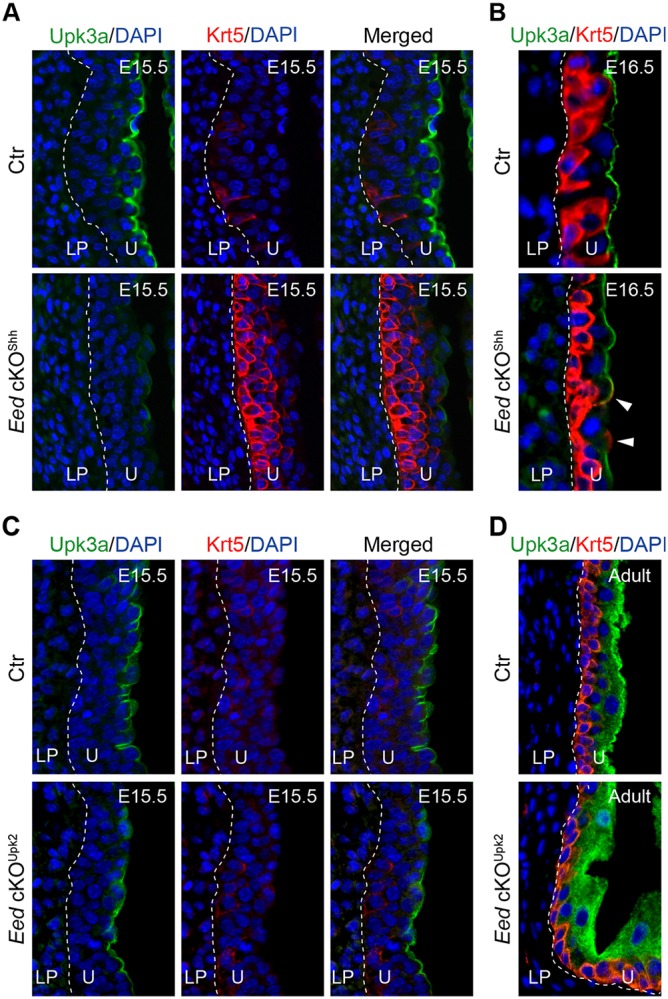
Deletion of Eed from embryonic urothelial progenitors leads to premature differentiation of Krt5+ basal cells and delayed formation of Upk3a+ superficial cells. Immunofluorescence microscopy of bladder sections stained for Upk3a (green), Krt5 (red) and DAPI (blue). (A,B) control (Ctr, Eedf/+;ShhGC/+) and Eed cKOShh bladder sections from E15.5 (A) and E16.5 (B) embryos. (C,D) Control (Ctr, Eedf/+;Upk2-Cre) and Eed cKOUpk2 (Eedf/f;Upk2-Cre) bladder sections from E15.5 (C) and adult (D) mice. White dashed lines demarcate the different layers within the bladder. Arrowheads indicate superficial cells that express both Upk3a and Krt5. U, urothelium; LP, lamina propria.
To examine whether PRC2 regulates Krt5 and Upk3a gene expression and urothelial differentiation in a stage-specific manner, we deleted Eed from bladder urothelium starting at E13.5 (Fig. S5), using an Upk2-Cre driver (Kanasaki et al., 2013). As expected, overall levels of H3K27me3 were reduced in the urothelium of UpkII-Cre-mediated Eed conditional mutants, Eed cKOUpk2 (Fig. S6A). Cell proliferation rates were also significantly reduced in the Eed cKOUpk2 mutants (Fig. S6C,D). Despite these observations, the time course of basal and superficial cell differentiation in Eed cKOUpk2 mutants was comparable to wild-type littermate controls (Fig. 3C). Likewise, there was no apparent histological defect of the adult mutants (Fig. 3D, Fig. S6B). Collectively, these findings suggest a stage-specific role of PRC2 in controlling the timing of urothelial progenitor cell differentiation. Specifically, early Eed activity (from E9.5 and on) in primitive urothelium and P-cells is crucial for urothelial cell proliferation and differentiation. However, late Eed activity (after E13.5) in intermediate and basal cells is required for proliferation, but not differentiation.
PRC2 controls urothelial identity by preventing ectopic expression of squamous epithelial markers
Bladder urothelium differs significantly from other types of stratified epithelium with regards to the expression profile of cytokeratin family genes. Abnormal urothelial differentiation of Eed cKOShh mutants prompted us to examine whether PRC2 is required to establish the molecular identity of bladder urothelium. Normal murine urothelium expressed high levels of Krt5 in the basal cell layer at E18.5 (Fig. 4A and Fig. 5A). About 14% of Krt5+ urothelial cells also expressed Krt14, but Krt17 expression was undetectable in normal urothelium (Fig. 5A,B). In Eed cKOShh mutants, both Krt14 (90%) and Krt17 (71%) were significantly elevated (Fig. 4A,B and Fig. 5A,B). These observations were confirmed independently by quantitative analysis of mRNA levels (Fig. 4C,D). In addition, Krt1, Krt6a and Krt16 were significantly upregulated in the mutants (Fig. 4C,D). Previous studies have shown that vitamin A deficiency induces keratinizing squamous metaplasia of urothelium, including ectopic expression and expansion of Krt14+ basal cells (Liang et al., 2005; Molloy and Laskin, 1988). To examine whether the RA signaling pathway was affected in the mutants, we examined expression of genes that are essential for RA metabolism. Expression of Raldh2, which catalyzes production of RA, was not affected. However, Cyp26a1 and Cyp26a2, which encode enzymes that degrade RA, were significantly increased (Fig. 4E). Consistently, Foxa1, a downstream target of the RA signaling pathway (Jacob et al., 1994, 1999), was significantly decreased (Fig. 4F). Nuclear hormone receptor Pparg binds to DNA as a heterodimer with RA receptors (Kojetin et al., 2015), and Pparg gene expression inversely correlates with squamous differentiation of urothelium (Strand et al., 2013). Pparg was significantly downregulated in Eed cKOShh mutants (Fig. 4F). Collectively, these findings suggest that PRC2 controls urothelial identity by preventing squamous-like differentiation, possibly through modulation of the RA signaling pathway.
Fig. 4.
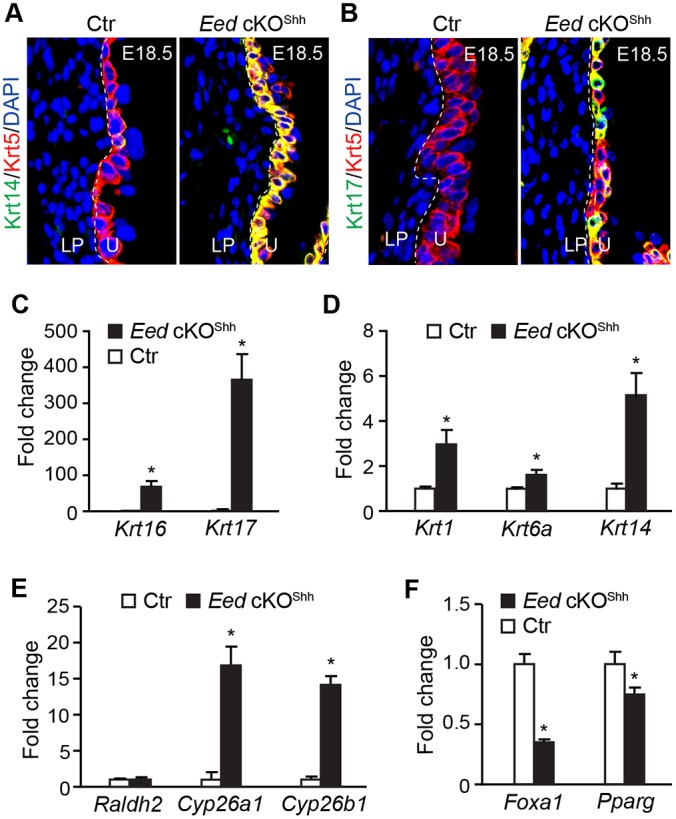
Aberrant squamous-like differentiation of Eed cKOshh mutant urothelium. (A) Immunofluorescence staining of bladder sections using antibodies against Krt5 (red) and Krt14 (green). Control (Ctr), Eedf/+;ShhGC/+. (B) Immunofluorescence staining for Krt5 (red) and Krt17 (green). Blue, DAPI counter staining. (C-F) qRT-PCR analysis of gene expression levels relative to Gapdh in microdissected bladder urothelium from E18.5 control (Ctr, Eedf/+;ShhGC/+) and Eed cKOShh embryos. Control transcript levels were normalized to 1.0 and transcript levels in Eed cKO samples are displayed as fold change relative to control. Data represent mean±s.e.m. of n=6. Unpaired Student's t-test, *P<0.05.
Fig. 5.
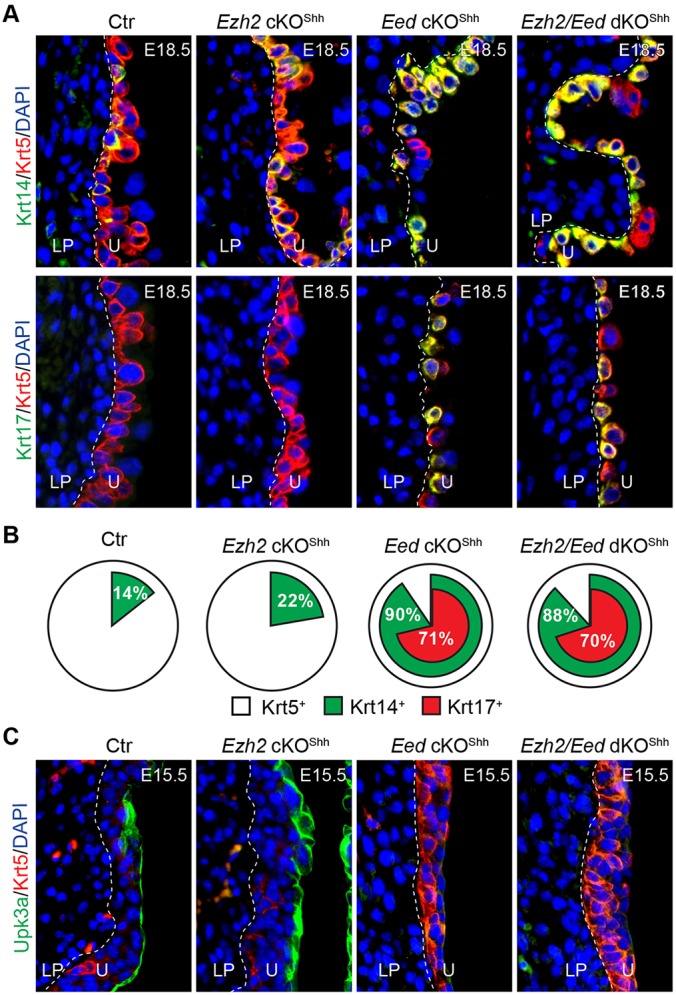
Eed and Ezh2 have distinct roles in urothelial differentiation. (A,B) Double immunofluorescence staining of bladder sections from control (Ctr, Eedf/+;ShhGC/+), Ezh2 cKOShh (Ezh2f/f;ShhGC/+), Eed cKOShh (Eedf/f;ShhGC/+) and Ezh2/Eed double cKOShh (dKOShh, Eedf/f;Ezh2f/f;ShhGC/+) embryos at E18.5 using antibodies against Krt5 (red) and Krt14 (green) or Krt5 (red) and Krt17 (green) (A). The results are summarized in B. (C) Double immunofluorescence staining of bladder sections from E15.5 embryos with indicated genotypes using antibodies against Krt5 (red) and Upk3a (green). LP, lamina propria; U, urothelium.
PRC2 subunit-specific functions in urothelial differentiation
Ezh2 is the major enzymatic subunit of the PRC2 complex and it is highly expressed in the urothelium (Fig. 1C). Consistently, H3K27me3 was undetectable from mutant urothelium in which Ezh2 was conditionally deleted using the ShhGC Cre driver (Fig. S7). Similar to the Eed mutants, Ezh2 cKOShh mutants died after birth with defective lung development (Fig. S7A-D). The Ezh2 cKOShh mutant urothelium was hypoplastic (Fig. S7F,G). P16 expression was aberrantly upregulated and, consistent with this finding, cell proliferation rates were also significantly reduced (Fig. S7H-J). Interestingly, both Eed and Ezh2 cKOShh mutants developed a polydactyly phenotype (Fig. S2G and Table S1). To examine whether Ezh2 and Eed act synergistically in vivo, we generated compound cKO mice in which copies of Eed and Ezh2 genes were deleted using ShhGC. The gross polydactyly phenotype was observed in ∼11.3% of Eed heterozygous mutants but not in Ezh2 heterozygous mutants. Penetrance of this phenotype was increased significantly to 36.4% in Ezh2 and Eed double heterozygous mutants. Penetrance was further enhanced when additional alleles of Ezh2 or Eed were deleted (Table S1). Collectively, these observations indicate that Ezh2 and Eed are two integral components of the PRC2 complex with overlapping functions in vivo.
We also analyzed the urothelial differentiation phenotype in Ezh2 cKOShh mutant bladders. While significantly more Krt14+ cells were found within the Krt5+ basal population in Ezh2 cKOShh mutants (Fig. 5A,B) compared with wild-type controls at E18.5 (22% versus 14%, P<0.01), the percentage of Krt14+ cells was more markedly increased to 90% and 88% in Eed and Eed/Ezh2 double knockout mutants, respectively (Fig. 5A,B). Additionally, ectopic Krt17 expression was only observed in the Eed single and double knockouts but not in the Ezh2 mutants (Fig. 5A,B). The dramatic difference in urothelial differentiation between Ezh2 and Eed mutants prompted us to examine whether these PRC2 subunits also have different functions in regulating the timing of urothelial differentiation. We therefore analyzed the mutants at E15.5, which corresponds to the onset of urothelial differentiation. In contrast to Eed mutants, Ezh2 conditional knockouts demonstrated a significant upregulation of Upk3a+ superficial cells at E15.5 (Fig. 5C) whereas Krt5 expression was unaffected. We next examined whether Ezh2 and Eed functionally depended on each other within the canonical PRC2 complex, or whether they act independently. Specifically, we compared expression of Upk3a and Krt5 in wild-type controls, single knockout and double knockout animals at E15.5. As shown in Fig. 5C, Upk3a was upregulated in Ezh2 mutants but was downregulated in both Eed mutants and double knockouts (Ezh2/Eed dKOShh). In addition, Krt5 was dramatically upregulated in Eed and double knockout mutants but not in Ezh2 mutants. Collectively, these findings suggest that, while the PRC2 subunits have overlapping functions in urothelial progenitor cell proliferation, the Eed and Ezh2 subunits have unique roles in urothelial differentiation – Eed suppresses premature Krt5+ basal cell differentiation but promotes Upk3a+ superficial cell differentiation; Ezh2, however, has minimal effect on Krt5+ basal cells while inhibiting premature Upk3a+ superficial cell differentiation.
Eed is required to maintain the quiescent state of mature urothelium
We next examined the role of PRC2 in adult urothelial progenitors using ShhCreER (Gandhi et al., 2013; Shen et al., 2008; Shin et al., 2011). The mutants ShhCreER;Eedf/f;R26RlacZ, termed Eed icKOShh, were healthy and fertile. Tamoxifen treatment induced Eed gene deletion and activation the genetic lineage marker lacZ in a limited population of adult urothelial progenitors. This genetic mosaic approach was used to track and monitor individual Eed mutant urothelial progenitor prospectively thereby to assess regenerative potential of urothelial progenitors. Specifically, the genetically tagged and Eed mutant progenitors (and their daughter cells) were analyzed by β-galactosidase (β-gal) staining 4-6 weeks after tamoxifen treatment (Fig. 6A, PBS). Most β-gal+ cells in both control and Eed icKOShh mutants were small Shh+ urothelial progenitor cells located at the basal or intermediate layers of urothelium (Fig. 6A,C). Very few large β-gal+ superficial cells were observed in controls, confirming that adult urothelium is largely quiescent. However, the mutant urothelium demonstrated significantly more β-gal+ superficial cells than controls (Fig. 6A,B), suggesting that Eed is required to maintain the quiescent state of mature urothelium by preventing precocious differentiation.
Fig. 6.
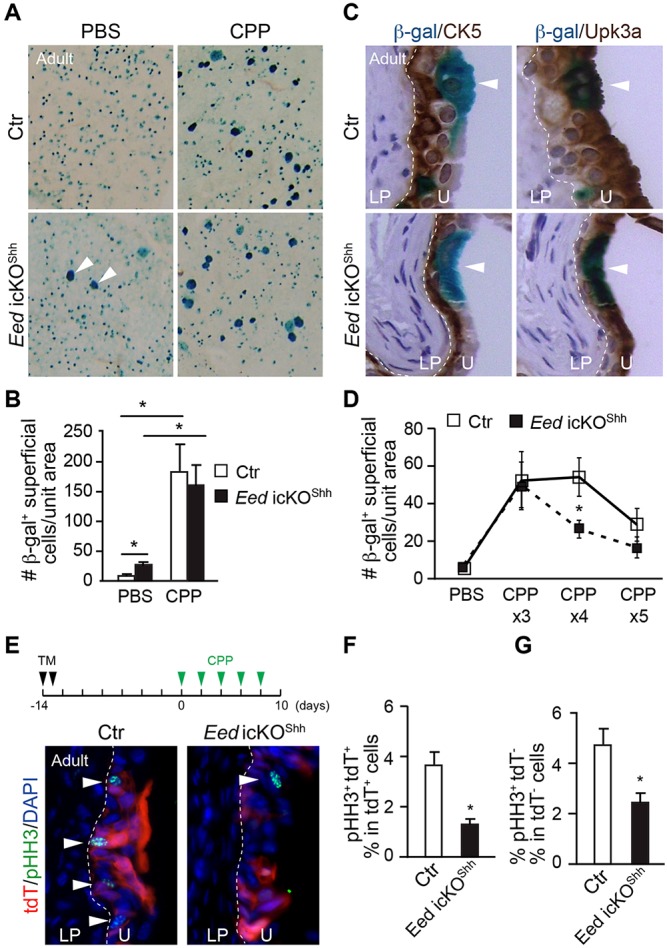
Eed is required to maintain the quiescent state and regenerative capacity of Shh+ adult urothelial progenitors. (A) Following tamoxifen treatment of ShhCreEREedf/+;R26RlacZ control (Ctr) and ShhCreER;Eedf/f;R26RlacZ mutant (Eed icKOShh) mice to induce Eed deletion and/or reporter expression from Shh+ urothelial progenitors, mice were treated with a single dose of PBS or CPP. Two weeks later, bladders were harvested, stained to detect β-gal, and whole mounts were made from urothelium. Following injury, small blue cells that include Shh+ progenitor cells will differentiate to larger blue superficial cells (arrowheads). (B) Quantification of the number of β-gal+ superficial cells per unit area in control or Eed icKOShh treated with a single dose of PBS or CPP (n=12-15 mice per group). (C) Immunohistochemical staining of adult control (Ctr) and Eed icKOShh bladders that have been tamoxifen induced, treated with a single dose of CPP, sectioned, and double stained for β-gal and Krt5 (brown; left panels) or Upk3a (brown; right panels). β-gal+ superficial cells (white arrowheads) stain for Upk3a, and basal cells (red arrowheads) stain for Krt5. (D) Control and Eed icKOShh mice were tamoxifen induced and treated with 3-5 rounds of CPP. Two weeks after the last injury, the number of β-gal+ superficial cells per unit area was calculated for each group (n=3-8 mice per group). (E) Control (ShhCEEedf/+;R26RtdT) and Eed icKOShh mice expressing the R26RtdT reporter (red) were tamoxifen (TM) induced and treated with five rounds of CPP. Forty-eight hours after the last injury, bladder sections were stained for pHH3 (green) to measure proliferation. Arrowheads indicate pHH3+ cells. (F,G) The percentage of pHH3+ cells that are tdT+ within the total population of tdT+ cells (F) and the percentage of pHH3+ cells that are tdT− within the total population of tdT− cells (G). White dashed lines demarcate the different layers within the bladder (U, urothelium; LP, lamina propria). Data in B,D,F,G represent mean±s.e.m. Unpaired Student's t-test, *P<0.05.
PRC2 regulates regenerative capacity of adult urothelial progenitors
To determine whether the PRC2-dependent epigenetic program is required for adult urothelial regeneration, we injured bladders with a single dose of the alkylating agent cyclophosphamide (CPP) 4 weeks after tamoxifen-induced deletion of Eed in Shh+ urothelial progenitor cells (Gandhi et al., 2013; Shin et al., 2011). CPP is known to cause an acute urothelial injury with damaged urothelium regenerating rapidly and recovering completely within 7-10 days (Kunze et al., 1980). There was a significant increase in number of β-gal+ large superficial cells 2 weeks post-CPP injury in control urothelium, confirming the complete cycle of urothelium injury and regeneration (Fig. 6A, top right panel, and B). Similar numbers of β-gal+ superficial cells were observed in Eed icKOShh mutants after a single dose of CPP (Fig. 6A, bottom right panel, and B). However, after repeated CPP injury, significantly fewer β-gal+ superficial cells were detected in mutant urothelium (Fig. 6D). Furthermore, cell proliferation was also reduced in the mutants during the regenerative process after repeated injury. Notably, this proliferation defect was not limited to the mutant cells (Fig. 6E-G), consistent with the notion that PRC2 has both cell-autonomous and non-autonomous functions in regulating urothelial proliferation. Collectively, these results suggest that PRC2 regulates the regenerative capacity of adult urothelium including progenitor cell proliferation and superficial cell differentiation.
DISCUSSION
In this study, we report for the first time that the PRC2-dependent epigenetic program regulates urothelial progenitor cell proliferation and timing of differentiation, and PRC2 controls the fate and regenerative potential of adult urothelial progenitors. These findings establish the crucial roles of the epigenetic program in urothelial development and adult urothelial homeostasis.
Bladder urothelium, one of the most effective epithelial barriers, is central to protecting the urinary tissue from toxic substances and pathogens in the urine. The urothelial cells change size and shape consistently during urine storage and voiding. Under physiological conditions, the urothelial cells have low turnover rate but regenerate rapidly upon injury. The bladder urothelium is thought to have three major cell types. Our observation that the PRC2-mediated H3K27me3 epigenetic mark is highly enriched and variable in the urothelium suggest the possibility that different levels of H3K27me3 may reflect diverse basal, intermediate and superficial cell types. Papafotiou et al. (2016) show that Krt14 expression marks a subpopulation of murine bladder basal cells with essential roles in regeneration and tumorigenesis. Expression of KRT14 is also linked to human bladder cancer development (Cancer Genome Atlas Research Network, 2014; Sjodahl et al., 2012; Volkmer et al., 2012). Interestingly, the Krt14+ as well as the ectopic Krt17+ sub-populations of basal cells are significantly increased in Eed mutants. In addition, Eed mutation causes downregulation of genes important for RA signaling pathways (e.g. Cyp26a1, Cyp26b1, Foxa1 and Pparg). Consistent with our observations, inactivation of the RA signaling pathway, through Vitamin A deficiency (Liang et al., 2005; Molloy and Laskin, 1988), Pparg knockdown (Strand et al., 2013) and Foxa1 downregulation (Jacob et al., 1994, 1999) results in the squamous-like phenotype of bladder urothelium. Collectively, these findings strongly suggest that there is a significant cellular diversity of bladder urothelium with unique properties and functions for each cell type. Moreover, the PRC2-dependent epigenetic program plays an important role in the formation and maintenance of diverse urothelial cells.
The observation that PRC2 regulates the timing of urothelial progenitor cell differentiation is particularly intriguing. Deletion of Eed triggers premature differentiation of Krt5+ basal cells and a delay in Upk3a+ superficial cell formation. By contrast, Ezh2 deletion increased Upk3a gene expression but had no apparent effect on Krt5. The phenotypic difference between Eed and Ezh2 cKO mice is somewhat surprising given the global loss of H3K27me3 marks in the urothelium in both mutant lines. However, we could not rule out the possibility that H3K27me3 marks at discrete gene loci are actually preserved in Ezh2 cKOshh urothelial cells because of the compensatory effect of Ezh1, as shown in other organ systems including embryonic stem cells, hematopoietic cells and skin (Ezhkova et al., 2011; Shen et al., 2008; Xie et al., 2014). Indeed, our observation that Eed/Ezh2 double cKO mice have an identical phenotype to Eed single cKO mice argues against the notion that Ezh2 functions independent of Eed to prevent superficial cell differentiation. On the contrary, it is possible that Eed may have an independent function because, in addition to PRC2, Eed may also control PRC1 activity (Cao et al., 2014). Unlike other stratified epithelia, Krt5 expression follows, rather than precedes, superficial and intermediate cell differentiation during normal bladder urothelium formation (Gandhi et al., 2013). The timing defect of urothelial differentiation seen in Eed and Ezh2 cKOShh mutants indicates that, regardless of the lineage relationship, timing of basal and superficial cell differentiation may vary depending on the genetic background and epigenetic landscape.
Consistent with the essential roles of PRC2 in progenitor cell proliferation through repression of cell cycle inhibitors such as Cdkn2a, we have observed a significant increase in Cdkn2a gene expression with a reduction in urothelial cell proliferation of Eed cKOShh embryos. Moreover, we provide evidence that PRC2 indirectly promotes Shh expression in the urothelium by repressing Pax6, a known transcriptional repressor of Shh (Caballero et al., 2014). Feedback regulation between Shh and Wnt signals is essential for the regenerative proliferation of urothelial progenitors (Shin et al., 2011). In addition, in vitro and in vivo studies have underscored the importance of urothelial Shh in patterning of the bladder wall and in the proliferation and differentiation of SMCs (Cao et al., 2010; Haraguchi et al., 2007). This is consistent with our finding that Eed cKO bladders exhibit decreased LP and SMC proliferation as well as aberrant SMC differentiation. Therefore, PRC2 exerts both cell-autonomous and non-autonomous functions in bladder urothelium by regulating the expression of genes including cell cycle regulators, transcription factors and signaling molecules.
Using a chemical-induced bladder injury model, we found that PRC2 regulates the regenerative capacity of Shh+ adult urothelial progenitors. Adult Shh+ urothelial progenitors deleted in Eed precociously differentiate into superficial cells, indicating that PRC2 is required to maintain the quiescent state of adult urothelium under normal physiological conditions. Upon chemical-induced injury, we show that the proliferation rate of Shh+ urothelial progenitors is significantly reduced in the absence of Eed. Moreover, the ability of mutant progenitors to generate superficial cells is significantly reduced after repeated injuries. Urothelial regeneration is essential for barrier function to protect the urinary tract, and aberrant regenerative responses may predispose to neoplastic transformation and, in the case of urinary tract infection, to chronic and ascending infections (Mysorekar et al., 2009; Shin et al., 2011). Our findings highlight the essential roles of epigenetic regulation of urothelial formation and regeneration (Fig. 7), which may provide a new framework for understanding the molecular basis of urinary tract infection and tumorigenesis.
Fig. 7.
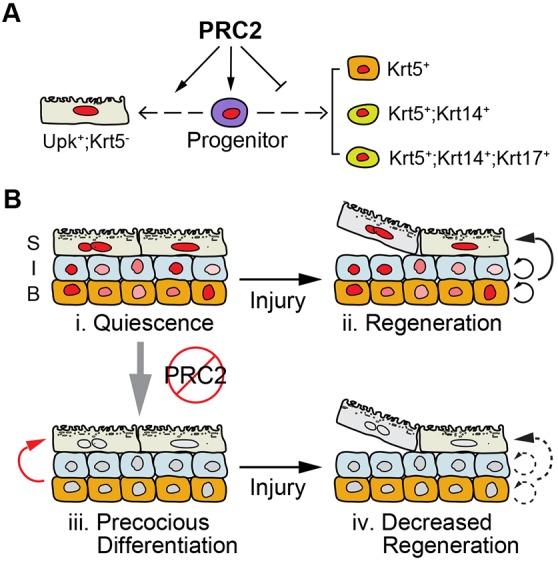
PRC2 regulates bladder urothelium development, maintenance and regeneration. (A) PRC2 promotes proliferation of embryonic urothelial progenitor cells. Moreover, PRC2 controls urothelial differentiation by inhibiting premature differentiation of Krt5+ basal cells and preventing ectopic expression of Krt14 and Krt17. Conversely, PRC2 may promote differentiation of Upk3a+ superficial cells. (B) PRC2 is required to maintain the quiescent state (i) and regenerative potential (ii) of adult urothelial progenitors. Without PRC2, adult urothelial progenitors (e.g. the Shh+ urothelial progenitors) precociously differentiate to superficial cells (iii, red arrow). However, their ability to repeatedly form superficial cells is limited (iv, dashed arrows).
MATERIALS AND METHODS
Mouse strains
The bladder urothelium-specific Ezh2 or Eed conditional knockout embryos/mice were generated by crossing either ShhGC (Harfe et al., 2004) (Jackson Laboratory, 005622) or Upk2-Cre Cre drivers (Kanasaki et al., 2013) with Ezh2f/f or Eedf/f conditional alleles (Shen et al., 2008; Xie et al., 2014) (obtained from Dr Stuart Orkin, Boston Children's Hospital, Harvard Medical School). Double cKO mice and littermate controls were generated by crossing ShhGC;Ezh2f/+;Eedf/+ mice with Ezh2f/f;Eedf/f mice. Upk2-Cre mice were crossed with Rosa26SorCAG-tdTomatoRed mice (Jackson Laboratory, 007905) to generate Upk2-Cre;R26RtdT reporter mice for lineage tracing experiments. Eed tamoxifen-inducible cKO mice (ShhCreER;Eedf/f) were crossed with mice carrying either the R26RlacZ (003309) or R26RtdT genes to generate ShhCreER;Eedf/f;R26RlacZ or ShhCreER;Eedf/f;R26RtdT reporter mice, respectively. Controls used throughout the study were compound heterozygous with one allele of the Cre driver and one conditional allele. There was no detectable gender difference in phenotype, therefore, male and female mice were used interchangeably in the study. All animal studies were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital.
Histology, immunohistochemistry and TUNEL staining
For histological examination, tissues were fixed in 4% paraformaldehyde (PFA), embedded in either paraffin or OCT medium, and sectioned at a thickness of 4-10 µm. Hematoxylin and Eosin (H&E, Thermo Fisher Scientific) staining was performed on paraffin sections according to standard protocol. Paraffin sections and/or cryostat sections were used for immunohistochemistry. After blocking sections in 10% goat serum/10% BSA for 1 h at room temperature, sections were incubated overnight at 4°C with the following primary antibodies: anti-Krt5 (Abcam, ab53121, 1:500 and Covance, SIG-3475, 1:1000), anti-Krt14 (Santa Cruz, SC-53253, 1:100), anti-Krt17 (Santa Cruz, SC-101931, 1:100), anti-Upk3a (Hu et al., 2005; Liang et al., 2001) (Clone AU1, provided by Dr Tung-Tien Sun, New York University, 1:200), anti-H3K27me3 (Millipore, 07-449, 1:200), anti-SMA (Sigma, A2547, 1:200), and anti-pHH3 (Upstate, 06-570, 1:200). Fluorescently labeled donkey anti-mouse or anti-rabbit secondary antibodies (Jackson ImmunoResearch, 1:200) were incubated for 1 h at room temperature. All sections were counterstained with DAPI. For immunohistochemical staining with DAB, an HRP-conjugated goat anti-rabbit or anti-mouse secondary antibody (Abcam, 1:500) was used followed by development with the DAB peroxidase substrate kit (Vector Laboratories) according to the manufacturer's instructions. To measure apoptosis, TUNEL staining was performed on paraffin sections using the In Situ Cell Death Detection Kit (Roche) according to manufacturer's instructions. Imaging was performed using a Zeiss fluorescence microscope.
Genetic mosaic analysis
ShhCreER;Eedf/f;R26RlacZ or ShhCreER;Eedf/f;R26RtdT mutants and compound heterozygous controls (ShhCreER;Eedf/+;R26RlacZ or ShhCreER;Eedf/+;R26RtdT) mice were intraperitoneally injected with tamoxifen (150 µg/g body weight; Sigma T5648) once daily for 2 days between ages P30-P60. Two weeks later, mice were intraperitoneally injected with either PBS or CPP (150 µg/g body weight, Sigma C7397). Analyses were performed at either 48 h for proliferation studies or at 2 weeks after CPP injection for regeneration studies, as indicated in the text. Specifically, β-gal staining was performed after bisecting bladder along the midline sagittal plane. Whole mounts of microdissected urothelium from bladder halves were then prepared and imaged. Using ImageJ software, β-gal+ superficial cells were counted based on their large size, polyhedral shape, and/or Upk3a+ staining. Each sample was normalized against total surface area of urothelium.
RNA isolation and qRT-PCR
Total RNA was extracted from either whole bladders or microdissected urothelium using the RNeasy Plus Mini Kit (Qiagen). Genomic DNA was removed using gDNA Eliminator spin columns (Qiagen). Total RNA was reverse-transcribed into cDNA using the SuperScript III Reverse Transcriptase Kit (Invitrogen). Quantitative real-time PCR (qRT-PCR) analyses were performed using SYBR Green (Roche) on an ABI-7500 detector (Applied Biosystems). Relative gene expression levels were normalized to the internal control Gapdh. Gene-specific primers are shown in Table S2.
In situ hybridization
Whole-mount in situ hybridization of control and mutant embryos was performed as previously described (Guo et al., 2014, 2011; Li et al., 2002), using digoxigenin-labeled RNA probes against Shh and Pax6.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation was performed as previously described (Guo et al., 2011). Briefly, microdissected urothelial cells from ∼15-20 bladders per group were crosslinked with 1% formaldehyde at room temperature for 15 min. Cells were then lysed and sonicated to an average size of 500-1000 base pairs. Chromatin lysates were incubated with specific antibodies (1 µg per reaction) including Ezh2 (Cell Signaling, 5246), H3K4me3 (Motif Active, 39159), or control IgG. Immunoprecipitates were collected with Protein G Dynabeads (Invitrogen) and protein/DNA crosslinks were reversed with 5 M NaCl. DNA was purified and ChIP DNA was analyzed using qPCR as described above. Each sample was normalized against total DNA input. The gene-specific primers used are listed in Table S3.
Statistical analyses
For all graphs, data are presented as the mean±s.e.m. Either the unpaired two-tailed Student's t-test or the unpaired Mann–Whitney test was used to determine significance between two groups, as indicated in the Results. P-values <0.05 were considered statistically significant.
Acknowledgements
We thank Roslyn Adam, Joshua Mauney and Satoshi Kaneko for helpful discussions and comments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: X.L.; Methodology: X.L., C.G., Z.R.B.; Formal analysis and investigation: C.G., Z.R.B.; Writing - original draft preparation: Z.R.B., C.G.; Writing - review and editing: X.L., Z.R.B., C.G.; Funding acquisition: X.L., C.G., Z.R.B.; Resources: X.L., W.G.H.; Supervision: X.L.
Funding
This work was supported by American Urological Association scholar awards (to C.G. and Z.R.B.), National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK091645-01A1 to X.L.), National Cancer Institute (1R21CA198544 to X.L.) and the American Heart Association (13GRNT16950006). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.143958.supplemental
References
- Caballero I. M., Manuel M. N., Molinek M., Quintana-Urzainqui I., Mi D., Shimogori T. and Price D. J. (2014). Cell-autonomous repression of Shh by transcription factor Pax6 regulates diencephalic patterning by controlling the central diencephalic organizer. Cell Rep. 8, 1405-1418. 10.1016/j.celrep.2014.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2014). Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315-322. 10.1038/nature12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S. and Zhang Y. (2002). Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039-1043. 10.1126/science.1076997 [DOI] [PubMed] [Google Scholar]
- Cao M., Tasian G., Wang M.-H., Liu B., Cunha G. and Baskin L. (2010). Urothelium-derived Sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation 79, 244-250. 10.1016/j.diff.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Wang X., Zhao M., Yang R., Malik R., Qiao Y., Poliakov A., Yocum A. K., Li Y., Chen W. et al. (2014). The central role of EED in the orchestration of polycomb group complexes. Nat. Commun. 5, 3127 10.1038/ncomms4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colopy S. A., Bjorling D. E., Mulligan W. A. and Bushman W. (2014). A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Dev. Dyn. 243, 988-998. 10.1002/dvdy.24143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermin B., Melfi R., McCabe D., Seitz V., Imhof A. and Pirrotta V. (2002). Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111, 185-196. 10.1016/S0092-8674(02)00975-3 [DOI] [PubMed] [Google Scholar]
- Delgado-Olguín P., Huang Y., Li X., Christodoulou D., Seidman C. E., Seidman J. G., Tarakhovsky A. and Bruneau B. G. (2012). Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat. Genet. 44, 343-347. 10.1038/ng.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza K. R., Saha M., Carpenter A. R., Scott M. and McHugh K. M. (2013). Analysis of the Sonic Hedgehog signaling pathway in normal and abnormal bladder development. PLoS ONE 8, e53675 10.1371/journal.pone.0053675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E., Lien W.-H., Stokes N., Pasolli H. A., Silva J. M. and Fuchs E. (2011). EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485-498. 10.1101/gad.2019811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvis L. A., Holik A. Z., Short K. M., Pasquet J., Lun A. T. L., Blewitt M. E., Smyth I. M., Ritchie M. E. and Asselin-Labat M.-L. (2015). Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development 142, 1458-1469. 10.1242/dev.122077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi D., Molotkov A., Batourina E., Schneider K., Dan H., Reiley M., Laufer E., Metzger D., Liang F., Liao Y. et al. (2013). Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev. Cell 26, 469-482. 10.1016/j.devcel.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgas K. M., Armstrong J., Keast J. R., Larkins C. E., McHugh K. M., Southard-Smith E. M., Cohn M. J., Batourina E., Dan H., Schneider K. et al. (2015). An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development 142, 1893-1908. 10.1242/dev.117903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Sun Y., Zhou B., Adam R. M., Li X. K., Pu W. T., Morrow B. E., Moon A. and Li X. (2011). A Tbx1-Six1/Eya1-Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. J. Clin. Invest. 121, 1585-1595. 10.1172/JCI44630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Sun Y., Guo C., MacDonald B. T., Borer J. G. and Li X. (2014). Dkk1 in the peri-cloaca mesenchyme regulates formation of anorectal and genitourinary tracts. Dev. Biol. 385, 41-51. 10.1016/j.ydbio.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R., Motoyama J., Sasaki H., Satoh Y., Miyagawa S., Nakagata N., Moon A. and Yamada G. (2007). Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134, 525-533. 10.1242/dev.02736 [DOI] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P. and Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- He A., Ma Q., Cao J., von Gise A., Zhou P., Xie H., Zhang B., Hsing M., Christodoulou D. C., Cahan P. et al. (2012). Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ. Res. 110, 406-415. 10.1161/CIRCRESAHA.111.252205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.-C. A., Liang F.-X., Zhou G., Tu L., Tang C.-H. A., Zhou J., Kreibich G. and Sun T.-T. (2005). Assembly of urothelial plaques: tetraspanin function in membrane protein trafficking. Mol. Biol. Cell 16, 3937-3950. 10.1091/mbc.E05-02-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. C., Chen F. and Li X. (2016). Clarification of mammalian cloacal morphogenesis using high-resolution episcopic microscopy. Dev. Biol. 409, 106-113. 10.1016/j.ydbio.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra A. K., Warot X., Brocard J., Bornert J.-M., Xiao J.-H., Chambon P. and Metzger D. (1999). Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 27, 4324-4327. 10.1093/nar/27.22.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Budhiraja S., Qian X., Clevidence D., Costa R. H. and Reichel R. R. (1994). Retinoic acid-mediated activation of HNF-3 alpha during EC stem cell differentiation. Nucleic Acids Res. 22, 2126-2133. 10.1093/nar/22.11.2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Budhiraja S. and Reichel R. R. (1999). The HNF-3alpha transcription factor is a primary target for retinoic acid action. Exp. Cell Res. 250, 1-9. 10.1006/excr.1999.4512 [DOI] [PubMed] [Google Scholar]
- Jost S. P. (1989). Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 57, 27-36. 10.1007/BF02899062 [DOI] [PubMed] [Google Scholar]
- Kanasaki K., Yu W., von Bodungen M., Larigakis J. D., Kanasaki M., Ayala de la Pena F., Kalluri R. and Hill W. G. (2013). Loss of beta1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J. 27, 1950-1961. 10.1096/fj.12-223404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojetin D. J., Matta-Camacho E., Hughes T. S., Srinivasan S., Nwachukwu J. C., Cavett V., Nowak J., Chalmers M. J., Marciano D. P., Kamenecka T. M. et al. (2015). Structural mechanism for signal transduction in RXR nuclear receptor heterodimers. Nat. Commun. 6, 8013 10.1038/ncomms9013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze E., Engelhardt W., Steinröder H., Wöltjen H.-H. and Schauer A. (1980). Proliferation kinetics of regenerating urothelial cells in the rat urinary bladder after administration of cyclophosphamide (author's transl). Virchows Archiv. B Cell Pathol. Incl. Mol. Pathol. 33, 47-66. 10.1007/BF02899170 [DOI] [PubMed] [Google Scholar]
- Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P. and Reinberg D. (2002). Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 16, 2893-2905. 10.1101/gad.1035902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Perissi V., Liu F., Rose D. W. and Rosenfeld M. G. (2002). Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science 297, 1180-1183. 10.1126/science.1073263 [DOI] [PubMed] [Google Scholar]
- Liang F.-X., Riedel I., Deng F.-M., Zhou G., Xu C., Wu X.-R., Kong X.-P., Moll R. and Sun T.-T. (2001). Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem. J. 355, 13-18. 10.1042/bj3550013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F.-X., Bosland M. C., Huang H., Romih R., Baptiste S., Deng F.-M., Wu X.-R., Shapiro E. and Sun T.-T. (2005). Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J. Cell Biol. 171, 835-844. 10.1083/jcb.200505035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R. and Reinberg D. (2011). The Polycomb complex PRC2 and its mark in life. Nature 469, 343-349. 10.1038/nature09784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney J. R., Ramachandran A., Yu R. N., Daley G. Q., Adam R. M. and Estrada C. R. (2010). All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PLoS ONE 5, e11513 10.1371/journal.pone.0011513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy C. J. and Laskin J. D. (1988). Effect of retinoid deficiency on keratin expression in mouse bladder. Exp. Mol. Pathol. 49, 128-140. 10.1016/0014-4800(88)90027-5 [DOI] [PubMed] [Google Scholar]
- Montgomery N. D., Yee D., Chen A., Kalantry S., Chamberlain S. J., Otte A. P. and Magnuson T. (2005). The murine polycomb group protein Eed is required for global histone H3 lysine-27 methylation. Curr. Biol. 15, 942-947. 10.1016/j.cub.2005.04.051 [DOI] [PubMed] [Google Scholar]
- Müller J., Hart C. M., Francis N. J., Vargas M. L., Sengupta A., Wild B., Miller E. L., O'Connor M. B., Kingston R. E. and Simon J. A. (2002). Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197-208. 10.1016/S0092-8674(02)00976-5 [DOI] [PubMed] [Google Scholar]
- Mysorekar I. U., Isaacson-Schmid M., Walker J. N., Mills J. C. and Hultgren S. J. (2009). Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5, 463-475. 10.1016/j.chom.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papafotiou G., Paraskevopoulou V., Vasilaki E., Kanaki Z., Paschalidis N. and Klinakis A. (2016). KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat. Commun. 7, 11914 10.1038/ncomms11914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert A. W., Harfe B. D. and Cohn M. J. (2008). Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev. Biol. 318, 143-152. 10.1016/j.ydbio.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Liu Y., Hsu Y.-J., Fujiwara Y., Kim J., Mao X., Yuan G.-C. and Orkin S. H. (2008). EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491-502. 10.1016/j.molcel.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Lee J., Guo N., Kim J., Lim A., Qu L., Mysorekar I. U. and Beachy P. A. (2011). Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472, 110-114. 10.1038/nature09851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroyanagi Y., Liu B., Cao M., Agras K., Li J., Hsieh M. H., Willingham E. J. and Baskin L. S. (2007). Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation 75, 968-977. 10.1111/j.1432-0436.2007.00187.x [DOI] [PubMed] [Google Scholar]
- Sjodahl G., Lauss M., Lovgren K., Chebil G., Gudjonsson S., Veerla S., Patschan O., Aine M., Ferno M., Ringner M. et al. (2012). A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18, 3377-3386. 10.1158/1078-0432.CCR-12-0077-T [DOI] [PubMed] [Google Scholar]
- Snitow M. E., Li S., Morley M. P., Rathi K., Lu M. M., Kadzik R. S., Stewart K. M. and Morrisey E. E. (2015). Ezh2 represses the basal cell lineage during lung endoderm development. Development 142, 108-117. 10.1242/dev.116947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand D. W., DeGraff D. J., Jiang M., Sameni M., Franco O. E., Love H. D., Hayward W. J., Lin-Tsai O., Wang A. Y., Cates J. M. M. et al. (2013). Deficiency in metabolic regulators PPARgamma and PTEN cooperates to drive keratinizing squamous metaplasia in novel models of human tissue regeneration. Am. J. Pathol. 182, 449-459. 10.1016/j.ajpath.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmer J.-P., Sahoo D., Chin R. K., Ho P. L., Tang C., Kurtova A. V., Willingham S. B., Pazhanisamy S. K., Contreras-Trujillo H., Storm T. A. et al. (2012). Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc. Natl. Acad. Sci. USA 109, 2078-2083. 10.1073/pnas.1120605109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Xu J., Hsu J. H., Nguyen M., Fujiwara Y., Peng C. and Orkin S. H. (2014). Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental-stage-specific manner. Cell Stem Cell 14, 68-80. 10.1016/j.stem.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]