Abstract
Most bladder cancer (BC) patients need life-long, invasive and expensive monitoring and treatment, making it a serious burden on the health system. Thus, there is a pressing need for an accurate test to assist diagnosis and surveillance of BC as an alternative to cystoscopy. Mutations in human TERT, FGFR3, PIK3CA, and RAS genes have been proposed as potential molecular markers in bladder tumor. Their concomitant presence in urine samples has not been fully explored.
We investigated a panel of mutations in DNA from exfoliated urinary cells of 255 BC patients at diagnosis. Forty-one mutations in TERT, FGFR3, PIK3CA, and RAS were analyzed by SNaPshot assay in relation to clinical outcome. In 81 of these patients under surveillance, the same set of mutations was screened in additional 324 samples prospectively collected.
The most common mutations detected in urine at diagnosis were in the TERT promoter. In non-invasive BC, these mutations were related to high risk and grade (p<0.0001) as well as progression to muscle-invasive disease (p=0.01), whereas FGFR3 mutations were observed in low-grade BC (p=0.02) and patients with recurrences (p=0.05). Stronger associations were observed for combined TERT and FGFR3 mutations and number of recurrences (OR: 4.54 95% CI: 1.23-16.79, p=0.02). Analyses of the area under the curve for combinations of mutations detected at diagnosis and follow-up showed an accuracy of prediction of recurrence of 0.80 (95% CI: 0.71-0.89).
Mutations in urine of BC patients may represent reliable biomarkers. In particular, TERT and FGFR3 mutations have a good accuracy of recurrence prediction.
Keywords: bladder cancer, urine mutation analyses, TERT, recurrence
INTRODUCTION
Bladder cancer (BC) is the seventh most frequently diagnosed malignancy worldwide in men [1] and the most common cancer of the urinary tract, with urothelial carcinoma being the dominant histology in more than 90% of BC cases [2]. Approximately 75% of BC patients present with non-muscle-invasive BC (NMIBC), of which 70% are confined to the mucosa (Ta), 20% to the submucosa (T1), and 10% are carcinoma in situ (CIS) [3]. According to current guidelines, these lesions are treated with transurethral resection of the bladder (TURB) followed by one intravesical instillation with mitomycin C. Patients with intermediate or high risk of progression to muscle-invasive cancer (MIBC) subsequently undergo several cycles of Bacillus Calmette-Guerin (BCG) instillations according to the guidelines of the European Association of Urology [4]. The remaining cases are MIBC for which the standard of care is radical cystectomy with pelvic lymph node dissection, with possible neoadjuvant or adjuvant chemotherapy [5].
MIBC cases exhibit a poor prognosis with a high risk of post-operative complications and an estimated five-year overall survival (OS) rate of 66% [5]. Although survival for NMIBC cases is over 90%, patients with these tumors are subject to high rates of recurrence (up to 78% at five years) and progression to muscle-invasive (up to 45% at five years) despite treatment [4]. For these patients, the goal is to detect and treat recurrences early in order to avoid the progression to MIBC. Currently, the only approach is a lifelong surveillance consisting of cystoscopy with or without biopsies and voided urine cytology [4, 5]. To improve the management of BC and at the same time decrease the costs, it is essential to find a non-invasive, highly sensitive, and specific molecular biomarker that allows detection of recurrences in urine.
BCs are derived from the urothelium involving, at least, two different genetic pathways and distinct progenitor cell types [6]. NMIBCs are characterized by frequent mutations in the fibroblast growth factor receptor 3 (FGFR3) and three of the five human RAS genes such as Harvey rat sarcoma viral oncogene homolog (HRAS), Kirsten rat sarcoma viral oncogene homolog (KRAS), and neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS) [7–9]. Also, a large proportion of human bladder tumors show alterations in some of the phosphatidylinositol 3-kinase (PI3K) pathway components; in particular, the phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) gene [10] that is involved in controlling cell growth, survival, and proliferation and has been considered a promising target for cancer therapy [11, 12]. MIBC cases, which show a propensity to progress to local and distant metastasis, often contain defects in tumor suppressor genes [13–15]. The telomerase reverse transcriptase (TERT) promoter, an important element of telomerase expression, has emerged as a target of cancer-specific mutations. Originally described in melanoma, the mutations in human TERT promoter, mainly at -124 and -146 base pair positions from the ATG start site, have been shown to be common in certain tumor types including BC of all stages with mutation frequencies of up to 80% [16–20].
The choice of a urine-based assay to analyze BC genetic profiles poses a major advantage as urine is in contact with the tumor and can be collected in an easy, non-invasive and repeatable way. Urinary exfoliated cancer cells and secreted nucleic acids mirror bladder tumors along each step of cancer development [21]. In particular, the study of relevant mutations in urine has the potential to become a reliable tool for diagnostic/prognostic purposes [22–24].
The aim of our study was to search simultaneously for mutations in TERT, FGFR3, PIK3CA, and RAS genes in the urinary exfoliated cells of a consecutive series of BC patients, both NMIBC and MIBC, and to evaluate their role as predictors of recurrence, progression, and survival. In a subgroup of 81 NMIBC patients, who have provided additional urine samples during their follow-up (for a total of 324 samples), mutations were repeatedly screened. To the best of our knowledge, this represents the largest number of mutations simultaneously investigated in urine, both at diagnosis and during clinical surveillance, for their association with BC.
RESULTS
Patient characteristics
Baseline patients’ characteristics are reported in Table 1. The cohort included 230 (90.2%) NMIBC patients most of whom were classified as high-risk (40%) and received adjuvant intravesical therapy (52.2%). Recurrence and progression were experienced by 38.3% and 4.3% of patients, respectively.
Table 1. Baseline characteristics of the patients with NMIBC or MIBC and information at follow-up.
| VARIABLES | NMIBC (n= 230) |
MIBC (n= 25) |
|---|---|---|
| N (%) | N (%) | |
| Age (years) | ||
| Mean ± SD | 63.8 ± 8.0 | 64.7 ± 8.6 |
| Range | 40.0-74.9 | 40.2-73.5 |
| Smoking Status | ||
| Never | 22 (9.6) | 3 (12.0) |
| Former | 121 (52.6) | 9 (36.0) |
| Current | 87 (37.8) | 13 (52.0) |
| T stage | ||
| Tx | 2 (0.9) | - |
| Ta | 147 (63.9) | - |
| Tis | 7 (3.0) | - |
| T1 | 74 (32.2) | - |
| T2 | - | 23 (92.0) |
| T3+T4 | - | 2 (8.0) |
| Grading (1973) | ||
| G1 | 70 (30.4) | - |
| G2 | 99 (43.0) | 1 (4.0) |
| G3 | 61 (26.6) | 24 (96.0) |
| Grading (2004) | ||
| Low grade | 125 (54.3) | - |
| High grade | 105 (45.7) | 25 (100.0) |
| Tumor size (cm) | ||
| <3 | 168 (73.0) | 7 (28.0) |
| ≥3 | 62 (27) | 18 (72.0) |
| Risk | ||
| Low-risk | 60 (26.1) | - |
| Intermediate Risk | 78 (33.9) | - |
| High-risk | 92 (40.0) | - |
| Recurrence | ||
| No | 142 (61.7) | - |
| Yes | 88 (38.3) | - |
| Number of recurrences | ||
| 1 | 56 (63.6) | - |
| 2 | 21 (23.9) | - |
| ≥3 | 11 (12.5) | - |
| Progression to MIBC | ||
| No | 220 (95.7) | - |
| Yes | 10 (4.3) | - |
| Therapy | ||
| No | 110 (47.8) | 22 (88.0) |
| Yes | 120 (52.2) | 3 (12.0) |
| Type | ||
| BCG | 67 (55.8) | - |
| Chemotherapy | 53 (44.2) | 3 (100.0) |
| Cystectomy | ||
| No | 209 (90.9) | 6 (24.0) |
| Yes | 21 (9.1) | 19 (76.0) |
| Disease relapse after cystectomy | ||
| No | 14 (66.7) | 9 (47.4) |
| Yes | 7 (33.3) | 10 (52.6) |
| Type | ||
| Local | 3 (42.8) | 3 (30.0) |
| Distal | 2 (28.6) | 1 (10.0) |
| Local+Distal | 2 (28.6) | 6 (60.0) |
| Status at follow-up | ||
| Alive | 198 (86.1) | 9 (36.0) |
| Dead of all causes | 32 (13.9) | 16 (64.0) |
| Dead of bladder cancer | 11 (4.8) | 9 (36.0) |
Twenty-five (9.8%) primary MIBC patients were also enrolled, 19 of which underwent radical cystectomy.
At a median follow-up of 5.83 years (range 1.21-8.54 years), cancer-specific and overall mortality rates in primary NMIBC patients were 4.8% (n=11) and 13.9% (n=32), respectively, and in primary MIBC cases were 36% (n=9) and 64% (n=16), respectively.
Mutation analysis in urinary exfoliated cells at diagnosis
Among the 230 urines obtained from the primary NMIBC cases, 159 samples had at least one mutation, thus sensitivity of tumor detection was 69%. TERT mutations were found in 119 (52%) urine samples while 95 (41.6%) had a FGFR3 mutation. Sensitivity of the combination of both genes was 67%. Hence mutations in the RAS and PIK3CA genes only added 2% to the sensitivity (Table 2). The most common TERT mutations were the -124C>T (n=117) and -146C>T (n=19), with two subjects presenting both mutations simultaneously, while only five patients had mutations at -124C>A and -138_139CC>TT. The most common FGFR3 mutation was S249C (46%), followed by Y375C, K652M, K652E, and R248C (45% together). In 12 subjects, two simultaneous FGFR3 mutations were detected while two subjects presented three mutations. Occurrence of more than one mutation for FGFR3 and TERT has been found before [22, 25] and is probably due to either tumor heterogeneity or the presence of multiple tumor clones in the bladder. Mutations in PIK3CA and RAS genes were detected in 13% and 4.8% of urine samples, respectively. For PIK3CA, the most frequent mutation was E545K (44%). Finally, for RAS genes, the majority of mutations were in KRAS G12V (25%). Distribution of mutations for each investigated gene is reported in Supplementary Figure S1.
Table 2. Distributions of mutations of investigated genes in urinary exfoliated cells according to clinical and demographic characteristics of NMIBC patients.
| Variables | TERT | FGFR3 | PIK3CA | Ras | ||||
|---|---|---|---|---|---|---|---|---|
| No Mutations N (%) | ≥1 Mutations N (%) | No Mutations N (%) | ≥1 Mutations N (%) | No Mutations N (%) | ≥1 Mutations N (%) | No Mutations N (%) | ≥1 Mutations N (%) | |
| All patients | 110 (48%) | 119 (52%) | 133 (58.4) | 95 (41.6) | 200 (87.0) | 30 (13.0) | 219 (95.2) | 11 (4.8) |
| Age (years) Mean ± SD | 63.1 ± 8.1 | 64.7± 7.9 | 63.7±8.3 | 63.9±7.6 | 64.1±7.9 | 61.5±8.4 | 63.7±8.0 | 64.4±8.0 |
| p= 0.13 | p= 0.85 | p= 0.10 | p= 0.78 | |||||
| Smoking status | ||||||||
| Never | 42 (38.2%) | 44 (37.0%) | 50 (37.6%) | 34 (35.8%) | 76 (38.0%) | 10 (33.3%) | 81 (37.0%) | 5 (45.5%) |
| Former | 59 (53.6%) | 62 (52.1%) | 70 (52.6) | 52 (54.7%) | 104 (52.0%) | 18 (60.0%) | 117 (53.4%) | 5 (45.5%) |
| Current | 9 (8.2%) | 13 (10.9%) | 13 (9.8%) | 9 (9.5%) | 20 (10.0%) | 2 (6.7) | 21 (9.6%) | 1 (9.0%) |
| p= 0.78 | p= 0.95 | p= 0.68 | p= 0.84 | |||||
| Grading (1973) | ||||||||
| G1 | 48 (43.7%) | 22 (18.5%) | 35 (26.3%) | 34 (35.8%) | 57 (28.5%) | 13 (43.3%) | 66 (30.1%) | 4 (36.4%) |
| G2 | 47 (42.7%) | 51 (42.9%) | 50 (37.6%) | 48 (50.5%) | 86 (43.0%) | 13 (43.3%) | 94 (42.9%) | 5 (45.4%) |
| G3 | 15 (13.6%) | 46 (38.6%) | 48 (36.1%) | 13 (13.7%) | 57 (28.5%) | 4 (13.4%) | 59 (27.0%) | 2 (18.2%) |
| p<0.0001 | p= 0.01 | p= 0.13 | p= 0.80 | |||||
| Grading (2004) | ||||||||
| Low grade | 75 (68.2%) | 50 (42.0%) | 63 (47.4%) | 60 (63.2%) | 103 (51.5%) | 22 (73.3%) | 117 (53.4%) | 8 (72.7%) |
| High grade | 35 (31.8%) | 69 (58.0%) | 70 (52.6%) | 35 (36.8%) | 97 (48.5%) | 8 (26.7%) | 102 (46.6%) | 3 (27.3%) |
| p <0.0001 | p= 0.02 | p= 0.03 | p= 0.21 | |||||
| Tumour size | ||||||||
| <3cm | 86 (78.2%) | 81 (68.1%) | 100 (75.2%) | 66 (69.5%) | 147 (73.5%) | 21 (70.0%) | 161 (73.5%) | 7 (63.6%) |
| ≥3cm | 24 (21.8%) | 38 (31.9%) | 33 (24.8%) | 29 (30.5%) | 53 (26.5%) | 9 (30.0%) | 58 (26.5%) | 4 (36.4%) |
| p= 0.09 | p= 0.34 | p= 0.69 | p= 0.47 | |||||
| Stage | ||||||||
| Ta | 82 (74.6%) | 64 (53.8%) | 78 (58.6%) | 67 (70.5%) | 126 (63.0%) | 21 (70.0%) | 139 (63.5%) | 8 (72.7%) |
| T1 | 24 (21.8%) | 50 (42.0%) | 48 (36.1%) | 26 (27.4%) | 65 (32.5%) | 9 (30.0%) | 71 (32.4%) | 3 (27.3%) |
| Tis | 2 (1.8%) | 5 (4.2%) | 5 (3.8%) | 2 (2.1%) | 7 (3.5%) | 0 | 7 (3.2%) | 0 |
| Tx | 2 (1.8%) | 0 | 2 (1.5%) | 0 | 2 (1.0%) | 0 | 2 (0.9%) | 0 |
| p= 0.01 | p= 0.22 | p= 0.66 | p= 0.88 | |||||
| Risk | ||||||||
| Low Risk | 40 (36.4%) | 20 (16.8%) | 31 (23.3%) | 28 (29.5%) | 49 (24.5%) | 11 (36.7%) | 57 (26.0%) | 3 (27.3%) |
| Intermediate Risk | 40 (36.4%) | 37 (31.1%) | 41 (30.8%) | 36 (37.9%) | 68 (34.0%) | 10 (33.3%) | 73 (33.4%) | 5 (45.4%) |
| High Risk | 30 (27.2%) | 62 (52.1) | 61 (45.9%) | 31 (32.6%) | 83 (41.5%) | 9 (30.0%) | 89 (40.6%) | 3 (27.3%) |
| p <0.0001 | p= 0.13 | p= 0.31 | p= 0.62 | |||||
| Recurrence | ||||||||
| No | 73 (66.4%) | 69 (58.0%) | 90 (67.7%) | 52 (54.7%) | 126 (63.0%) | 16 (53.3%) | 132 (60.3%) | 10 (90.9%) |
| Yes | 37 (33.6%) | 50 (42.0%) | 43 (32.3%) | 43 (45.3%) | 74 (37.0%) | 14 (46.7%) | 87 (39.7%) | 1 (9.1%) |
| p= 0.19 | p= 0.05 | p= 0.30 | p= 0.04 | |||||
| Number of recurrences | ||||||||
| 1 | 27 (73.0%) | 28 (56.0%) | 32 (74.4%) | 23 (53.5%) | 47 (63.5%) | 9 (64.3%) | 55 (63.2%) | 1 (100.0%) |
| ≥2 | 10 (27.0%) | 22 (44.0%) | 11 (25.6%) | 20 (46.5%) | 27 (36.5%) | 5 (35.7%) | 32 (36.8%) | 0 |
| p= 0.10 | p= 0.04 | p= 0.96 | p= 0.45 | |||||
| Progression to MIBC | ||||||||
| No | 109 (99.1%) | 110 (92.4%) | 125 (94.0%) | 93 (97.9%) | 191 (95.5%) | 29 (96.7%) | 209 (95.5%) | 11 (100.0%) |
| Yes | 1 (0.9%) | 9 (7.6%) | 8 (6.0%) | 2 (2.1%) | 9 (4.5%) | 1 (3.3%) | 10 (4.5%) | 0 |
| p= 0.01 | p= 0.16 | p= 0.77 | p= 0.47 | |||||
| Survival at follow-up | ||||||||
| Alive (or died due to causes other than BC) | 109 (99.1%) | 109 (91.6%) | 124 (93.2%) | 93 (97.9%) | 190 (95.0%) | 29 (96.7%) | 209 (95.5%) | 10 (90.9%) |
| Dead | 1 (0.9%) | 10 (8.4%) | 9 (6.8%) | 2 (2.1%) | 10 (5.0%) | 1 (3.3%) | 10 (4.5%) | 1 (9.1%) |
| p= 0.01 | p= 0.11 | p= 0.70 | p= 0.49 | |||||
Significant results in bold.
The relationship between mutations observed in urine and clinical factors is reported for NMIBC in Table 2. TERT mutations significantly correlated with the presence of high-risk, high-grade tumors (for both p<0.0001) and progression to muscle-invasive disease (p=0.01). FGFR3 was associated with low-grade tumors (p=0.02) and event of recurrence/number of recurrences (p=0.05, p=0.04, respectively). PIK3CA mutations were more common in urine collected from low-grade tumors (p=0.03). Despite the low frequency of RAS mutations, an association was also seen between mutated RAS and no recurrence (p=0.04). The distribution of mutations at diagnosis with respect to the different genes and relation to tumor grading, risk, and recurrence are highlighted in Figure 1. As shown, TERT, FGFR3, and PIK3CA mutations overlap to some extent.
Figure 1. Concurrent and mutually exclusive mutations in NMIBC patients at diagnosis.
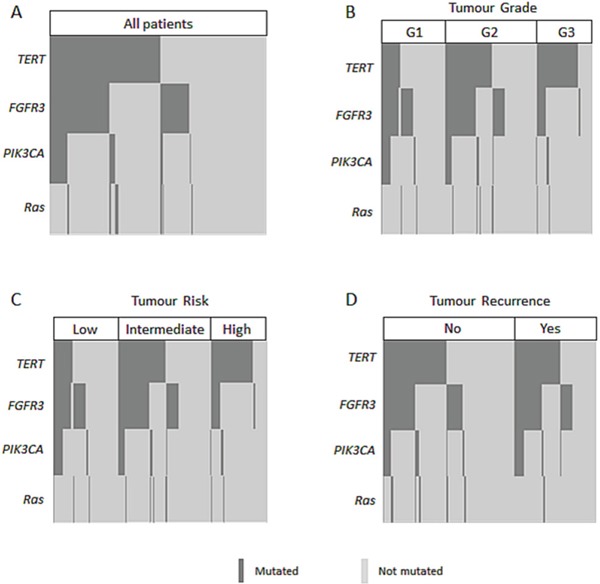
A. and stratified according to grade B., risk C., or recurrence D.
In Supplementary Table S1 the sensitivities of the assays for detection of muscle-invasive primary tumors is shown. In this group, 80% of the urine samples presented with a TERT mutation while other genes were less frequently mutated (<20%). We further investigated the possible association of the mutations and clinical parameters for MIBC patients. However, although there were some statistically significant relations, the numbers were too low to draw conclusions.
Overall and cancer survival
A higher percentage of patients who died either from any cause or cancer carried mutations in the analyzed genes at diagnosis (89.4% and 88.5%, respectively, data not shown). As presented in Supplementary Table S2, there was a significant association with OS in the adjusted model for FGFR3 mutations (p=0.04). Associations with OS were also noted with different combinations of genetic mutations. Figure 2 depicts Kaplan-Meier curves for all mutations combined (log-rank p=0.002) and TERT mutations only (log-rank p=0.003). No significant associations were observed between the mutations and cancer-specific survival.
Figure 2. Kaplan-Meier curve showing OS for.
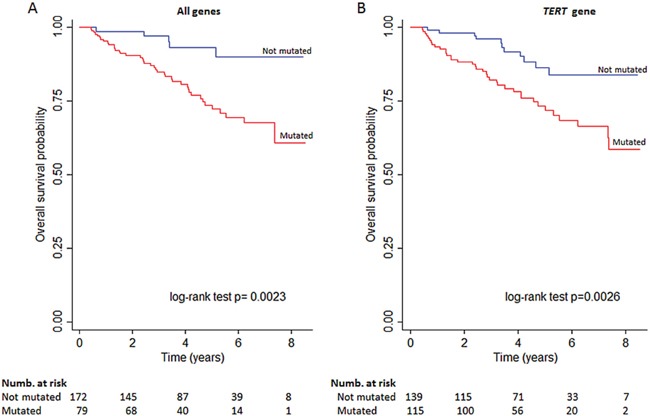
A. subjects stratified for combined genes mutational status or B. TERT mutations alone.
Association of the presence of mutations at diagnosis and the occurrence of recurrences
In the NMIBC group, 88 subjects out of 230 (38%) experienced a recurrence during the clinical follow-up; among them, 56 recurred only once while the others manifested two or more recurrences. Of all patients who recurred, 28 (32%) had one gene mutated at diagnosis while 34 (40%) presented two or more mutations. Thirty-one subjects had more than one recurrence (with a maximum of five) with 27 of them (87%) presenting one or more mutations. Among the remaining 142 non-recurring patients, 23 (16%) had at least one mutation in a gene while 21 (15%) had more genes mutated simultaneously.
There was a consistently significant increase in the number of mutations in concomitance with an increased number of recurrences when genes were analyzed in combination or individually (OR: 2.05 95% CI: 1.05-3.98, p=0.03). Although combining mutations in all genes significantly associated with recurrences, stronger associations were observed especially when mutations in the FGFR3 and TERT genes were combined (OR: 2.51 95% CI: 1.26-5.00, p=0.01, Supplementary Table S3).
Detection of recurrences in NMIBC patients under surveillance
For 81 NMIBC patients, four additional urine samples were periodically collected over approximately three years after recruitment. There were no major differences in the investigated clinical parameters between this subgroup and the whole cohort. Within this subgroup, 34 subjects underwent intravesical BCG treatment, 19 underwent intravesical chemotherapy, while the remaining 28 did not receive further treatment beyond TURB. Five of these patients died of causes other than BC during follow-up, all after recurrence and one after progression, and all of them presented mutations in their analyzed urine samples, either at diagnosis or follow-up.
In the first follow-up mutation analysis after diagnosis, the frequency of mutations dropped from 68% of subjects to 19%, still being mutations TERT mutation -124C>T the most frequent. The number of TERT mutations remained similar in the exfoliated cells from the collections that followed (data not shown).
Thirty-five subjects (43.2%) had a recurrence; of them, 27 (77%) presented one or more gene mutations. All 14 patients with multiple recurrences, except one, presented at least one mutation. Multivariate analyses (Table 3) confirmed the association between multiple gene mutations and the number of recurrences. Stronger associations were observed for TERT and FGFR3 mutations combined with number of recurrences (OR: 4.51, 95% CI: 1.27-16.06, p= 0.02 at diagnosis; OR: 4.54 95% CI: 1.23-16.79, p= 0.02 at diagnosis, and follow-up). When FGFR3 and TERT gene mutations were observed in the diagnostic and a follow-up urine sample, the sum of their mutations detected at diagnosis and follow-up were more strongly associated either with the event of recurrence or with the number of recurrences than at diagnosis alone. Of those 29 patients with a mutation at diagnosis who did not recur, 23 (79.3%) did not present any further mutations over the four follow-up samplings. The distribution of mutations in FGFR3 and TERT in respect to recurrences in repeated samples is depicted by some examples in Supplementary Figure S2.
Table 3. Mutations in repeated urine samples collected during follow-up in the subgroup of NMIBC patients (n= 81) in association with event(s) of recurrence.
| Genes | At diagnosis | At diagnosis +follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| Recurrence yes/no | Number of recurrences | Recurrence yes/no | Number of recurrences | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| All genes combined | ||||||||
| 1 gene mutated | 1.71 (0.48-6.09) | 0.41 | 1.91 (0.56-6.52) | 0.30 | 0.99 (0.19-5.14) | 0.99 | 1.02 (0.21-4.83) | 0.98 |
| 2 or more | 3.19 (0.90-11.28) | 0.07 | 4.11 (1.23-13.69) | 0.02 | 3.16 (0.81-12.23) | 0.10 | 3.80 (1.06-13.58) | 0.04 |
| TERT+FGFR3+PIK3CA | ||||||||
| 1 gene mutated | 1.78 (0.52-6.15) | 0.36 | 1.98 (0.60-1.26) | 0.26 | 2.02 (0.44-9.29) | 0.37 | 1.87 (0.45-7.72) | 0.39 |
| 2 or more | 3.30 (0.90-12.11) | 0.07 | 4.31 (1.26-14.76) | 0.02 | 3.40 (0.85-13.63) | 0.08 | 4.07 (1.11-14.84) | 0.03 |
| TERT+FGFR3+Ras | ||||||||
| 1 gene mutated | 1.86 (0.55-6.35) | 0.32 | 2.07 (0.63-6.73) | 0.23 | 0.86 (0.17-4.40) | 0.86 | 0.92 (0.20-4.31) | 0.91 |
| 2 or more | 3.17 (0.864-11.63) | 0.08 | 4.21 (1.23-14.35) | 0.02 | 3.51 (0.89-13.77) | 0.07 | 4.13 (1.15-14.84) | 0.03 |
| TERT+FGFR3 | ||||||||
| 1 gene mutated | 1.91 (0.57-6.37) | 0.29 | 2.11 (0.66-6.73) | 0.21 | 1.75 (0.39-7.89) | 0.47 | 1.67 (0.41-6.86) | 0.48 |
| 2 or more | 3.32 (0.86-12.86) | 0.08 | 4.51 (1.27-16.06) | 0.02 | 3.87 (0.94-15.87) | 0.06 | 4.54 (1.23-16.79) | 0.02 |
| TERT | 2.42 (0.79-7.39) | 0.12 | 3.01 (1.06-8.59) | 0.04 | 1.91 (1.13-3.22) | 0.01 | 1.65 (1.14-2.39) | 0.01 |
| FGFR3 | 2.01 (0.72-5.61) | 0.18 | 2.27(0.86-6.02) | 0.10 | 1.89 (1.04-3.46) | 0.04 | 1.54 (1.05-2.26) | 0.02 |
| PIK3CA | 1.30 (0.33-5.14) | 0.71 | 1.35 (0.39-4.76) | 0.64 | 1.36 (0.68-2.77) | 0.38 | 1.28 (0.75-2.21) | 0.37 |
| Ras | 1.59 (0.08-29.76) | 0.76 | 1.28(0.099-22.05) | 0.78 | 1.56 (0.52-4.70) | 0.43 | 1.38 (0.52-3.67) | 0.52 |
Adjusted for age, smoking status, grade, stage and therapy. Significant results in bold.
A prediction model for the presence of a recurrence was developed including age, smoking status, and risk of recurrence as covariates (Model A). Then, the mutational status as a predictor (Model B) and, finally, the mutational status during follow-up (Model C) were added each time to Model A. Considering all genes together, the area under curve (AUC) of the model increased from 0.72 (95% CI: 0.61-0.83, Model A) to 0.75 (95% CI: 0.65-0.86, Model B) and to 0.78 (95% CI: 0.68-0.88, Model C). Using only the genes FGFR3 and TERT, model C reached an AUC of 0.80 (95% CI: 0.71-0.89, model C). Receiver operating characteristic (ROC) curves for the different models are reported in Figure 3A and 3B, respectively, for all genes together or the FGFR3 and TERT combination. Adding the information of mutational status at diagnosis and follow-up, the predictive accuracy of the model was 8% higher when compared to that of the models usually used in clinical practice.
Figure 3.
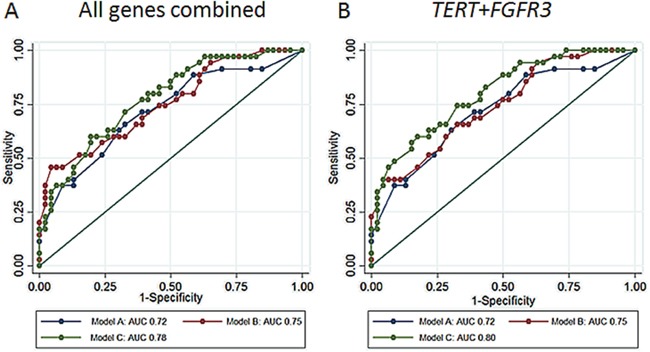
A. AUC for a set of BC risk factors (age, smoking status, and risk of recurrence, model A) and the same set of factors and mutational status of all genes at diagnosis (model B), and at diagnosis plus follow-up (model C). B. AUC for the same models but considering the mutational status of TERT and FGFR3 genes only.
DISCUSSION
In the present study, we used urine analysis to diagnose primary and recurrent bladder tumors. We also looked at associations between mutations in urine at first diagnosis and clinical parameters. We selected six genes (FGFR3, PIK3CA, RAS family genes, and TERT promoter) and determined their mutation profile in urine samples of BC patients, first at diagnosis, prior to treatment, and secondly, for NMIBC patients, during their follow-up. The tests detected 70% of the first presentation NMIBC. Sensitivity for TERT and FGFR3 were 52% and 42%, respectively. The distribution of mutations among genes is similar to other previous studies on tumor [22, 26–29] and voided urine [22, 30, 31], although TERT mutations are lower [22, 32]. Two very recent studies have reported the same TERT mutation frequencies in urine of BC patients as us by different methodologies [26, 33]. In the small group of MIBC patients, sensitivity of the TERT assay was 80% while mutations in the other genes were less represented, in line with the distribution found in other studies [22]. Both TERT and FGFR3 mutations were related to clinical parameters: TERT mutations with high-risk, high-grade NMIBC, as well as with progression to MIBC and FGFR3 with low-grade tumors. Mutations in the FGFR3 gene have been previously found in 70% of the low-grade BC and are usually associated with a favorable prognosis [25, 34]. Patients with low-grade tumors are those at lower risk of progression, and the presence of an FGFR3 mutation has been associated with such behavior earlier [34, 35]. For TERT, mutations in urine were not previously observed in association with clinical parameters.
The same set of mutations was analyzed in exfoliated cells of samples collected from NMIBC patients during clinical surveillance. To our knowledge, this is the largest series of mutation analyses in multiple genes in repeated observations over time (for an overall number of 405 analyses). As expected, after the removal of the primary tumors with TURB and subsequent intravesical therapy, the number of patients with mutations in urinary cells was considerably lower. Considering the potential of FGFR3 and TERT mutations at diagnosis and during follow-up for the prediction of recurrences, we explored if their implementation in clinical practice could improve the risk models currently used by urologists [4]. According to our results, the analysis of mutational status could improve the recurrence risk as measured by the EORTC risk tables with 8%. Similar findings were observed for FGFR3 only in repeated urine samples collected over three years by Zuiverloon and colleagues [30]. We detected an association between the presence of multiple mutations and the number of recurrences, in particular, when TERT and FGFR3 mutations were combined. Even though TERT promoter mutations were more frequent than FGFR3 ones, the alterations at the two loci occurred together more frequently than per chance.
Considering the importance of detecting recurrences in patients with a low grade NMIBC, we have repeated analyses only in those patients belonging to this category. Interestingly, the frequency of mutations detected (combining all genes or considering them individually) and the associations previously described remained fairly similar to the whole set of NMIBC patients at diagnosis, or in the subset of those who provided multiple urine samples over time. In particular, in this last category, subjects with multiple recurrences were all mutated. This is particularly important considering that low grade tumors will experience more recurrences (as we also observed in our study population, i.e. 46% vs. 38%) and in routine investigations, like cytology, have a lower sensitivity in comparison to high-grade ones.
We are aware that the present study is affected by some limitations. First, there are inherent problems with urinary testing. As volume and stage/grade of BC can vary vastly, DNA testing must be able to deal with a small amount of exfoliated cells in urine. This is particularly true in post-diagnostic repeated sample collections, where the primary tumor has been removed and treatments may have occurred. In this respect, preliminary experiments were performed to investigate the sensitivity of the mutation detection assay by single nucleotide primer extension (SNaPshot) sequencing. Serial dilutions of samples at increasing concentrations of DNA from a healthy subject and a human cell line derived from a transitional cell carcinoma of the renal pelvis (UM-UC-14) were tested to identify the optimal concentration. The SNaPshot assay has proven to be an extremely sensitive technique able to detect even 1% of mutant alleles in a given sample, either with pre-amplification or without (data not shown). Moreover, in this study, no pre-amplification of the DNA of the samples was necessary. Urine represents a very dynamic fluid, and its composition can be affected by fluid status, renal disease, infection, and urinary tract instrumentation posing a disadvantage for the identification of a good marker. However, gene-based urinary biomarkers are more sensitive and specific as they detect cancer-related changes which are less likely to be affected by inflammatory and other benign conditions compared with protein-based detection [36].
From the point of view of the study design, repeated samplings of the subgroup of patients were performed not always in concomitance with the detection of a recurrence. Thus, we could not detect the presence of a mutation before or simultaneously with an event of recurrence and establish a clear relationship. Finally, our study is also limited since it is based on males only. BC is almost three times more common in men than in women; therefore, it is relatively simple to collect male patients. Although most likely some differences exist between genders for mutation detection and clinical outcomes [37, 38], we were unable to explore this. However, performing the assay only on men greatly reduced the variability.
Nevertheless, several strengths of this study must also be acknowledged: amongst them, the prospective design, the large number of samples analyzed, and the possibility of analyzing the mutational status of patients during follow-up. The heterogeneity of the population enrolled might be seen as a drawback, but in our opinion it reflects the complexity of BC, strengthening our findings. In addition, the analyses of all mutations were performed simultaneously by multiplexing the reactions. This is a very useful approach to screen a vast number of mutations at once, allowing us to save the biological material and always using the same experimental conditions at relatively low costs [12]. Moreover, urine is in direct contact with BC and sediment cells collected from voided samples are an ideal source of DNA because they are readily available, and the sampling is not invasive [39].
Most patients diagnosed with BC have NMIBC (75%) that are amenable to TURB and have a good five-year survival rate (80-90%). However, 70% of subjects will have at least one recurrence within five years, and some will even recur after 15 years of surveillance. Although most NMIBC patients have recurrences of low-stage and grade, progression to MIBC is observed in 10-20%, leading to an entirely different scenario characterized by a worse prognosis despite radical treatments. The high recurrence rate and risk of progression to MI disease necessitates the frequent monitoring of NMIBC patients by performing periodic endoscopic procedures, with huge costs for the health system and discomfort for patients themselves [40–42]. To address this issue, in the era of the so-called “liquid biopsies”, research is focusing on the identification of molecular markers associated with the presence of BC [43, 44]. The ultimate goal would be to develop a urine-based biomarker to reduce the cystoscopy frequency and improve the patients' quality of life without jeopardizing their safety. Such tests should be easy-to-perform, reproducible, and simple to interpret. In this respect, from our study the panel of investigated gene mutations in voided urine samples may have the potential to be helpful in the diagnosis and surveillance of BC patients. In particular, mutations in the TERT promoter and FGFR3 are the most frequently detected, with rates comparable to those of other studies and reflecting the situation in primary tumor tissue. Larger prospective studies from international consortia will provide the adequate study population size, including specific subgroups of patients with specific features of this cancer, to investigate the promising markers emerging. It can be expected that combinations of different molecular and histopathological biomarkers will be introduced into the clinical setting in the near future [45].
MATERIALS AND METHODS
Study population
The study population included 255 newly diagnosed, histologically confirmed cases of BC recruited at Città della Salute e della Scienza Hospital, Turin (Italy), between 2006 and 2012. All subjects were men aged 40–75 years, living in the Turin metropolitan area that signed an informed consent form. Clinical and socio-demographic information were collected from all patients. Staging and grading were performed by expert uropathologists according to the tumor-node-metastases (TNM) 2002 classification and both the 1973 and 2004 World Health Organization (WHO) classifications. Patients were classified according to current European Organization for Research and Treatment of Cancer (EORTC) risk tables [46]. Recurrence was defined as the occurrence of any CIS and/or papillary Ta-T1 tumor during follow-up, whereas the finding of T>1 was considered as progression. Progression for MIBC patients was defined as the presence of a local, distal or local-distal extravesical disease. Causes of death were retrieved from local demographic offices and death certificates. All treatments were recorded. At 3-months follow-up, urine cytology and cystoscopy with TUR of all visible lesions and a biopsy mapping when appropriate were undertaken. Successively, follow-up was scheduled as per European Association of Urology (EAU) guidelines [5]. Subjects were defined as former smokers if they had quitted smoking ≥1 year before the enrollment. This study was performed according to the principles of the Declaration of Helsinki and in agreement with ethical requirements. An internal ethical review board at Human Genetics Foundation of Turin (HuGeF committee/17-11-2011) approved the study.
Urine sample collection and DNA isolation from exfoliated cells
All subjects recruited in the study provided a urine sample at the time of diagnosis before any treatment. A subgroup of patients (n=81) under surveillance after diagnosis provided additional urine samples, approximately every six months from the recruitment, for a total of four collections. Freshly voided urine (10-100 mL) was collected before cystoscopy and stored at 4°C until their processing. Cells were pelleted by centrifugation for 10 minutes at 1,500 rpm and washed twice with 10 mL phosphate buffered saline (PBS). Finally, the cell pellet was stored at −80°C until DNA isolation. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer's protocol. DNA concentration was measured with Quant-iT High-Sensitivity DNA Assay Kit (Life Technologies), and all samples were diluted to a fixed concentration of 5 ng/μl.
Mutation analyses
The mutation assays were performed as described by van Oers and colleagues [47]. Briefly, we performed three multiplex polymerase chain reactions (PCR) of the exons containing the most common mutations in the genes of interest. Each multiplex PCR reaction was performed in a total volume of 10 μl containing KAPA2G Robust HotStart ReadyMix 2X (KAPA Biosystems), 0.5 μl of the appropriate primer combination, and 5 ng of genomic DNA as template. Thermal cycling conditions consisted of initial denaturation at 95°C for 3 minutes, followed by 40 cycles each consisting of 95°C for 15 seconds, 55°C for 15 seconds, and 72°C for 20 seconds. The final elongation step was 72°C for 10 minutes. Unincorporated primers and deoxynucleotide triphosphates were removed from PCR products by addition of 2 units of Exonuclease I (ExoI) and 1.5 units of Shrimp Alkaline Phosphatase (SAP, USB Corporation) at 37°C for 60 minutes and 72°C for 15 minutes.
PCR products were subsequently analyzed for mutations using probes for each of the possible mutation sites and SNaPshot Multiplex Kit (Life Technologies). The mutation detection reactions were performed in a total volume of 10 μl containing 2.5 μl of SNaPshot Multiplex Ready Reaction Mix, 2 μl of BigDye Sequencing buffer, 1 μl of probe mix, and 1 μl of SAP/ExoI treated PCR product. Extension reactions consisting of 35 cycles of denaturation at 96°C for 10 seconds and annealing/extension at 58.5°C for 40 seconds were performed in a thermal cycler. After extension, the excess of labelled dideoxynucleotide triphosphates was removed by treatment with 1 unit of SAP at 37°C for 60 minutes and 72°C for 15 minutes. Extended primers were denatured at 95°C for 4 minutes and separated by capillary electrophoresis on an automatic sequencer ABI PRISM 3130 XL Genetic Analyzer (Life Technologies). The presence or absence of a mutation was indicated by the fluorescent label on the incorporated nucleotide. Data were analyzed using GeneScan Analysis Software version 3.7 (Life Technologies) and GeneMarker Software version 2.6 (SoftGenetics LLC).
The first multiplex assay identifies simultaneously seven PIK3CA hotspot mutations (E542K, E545A, E545G, E545K, E545Q, H1047R and H1047L) and four TERT mutations (−124C>T (G>A), -124C>A (G>T), -138_139CC>TT (GG>AA) and -146C>T (G>A)). A second multiplex PCR detects the most frequent FGFR3 mutations in three regions that comprise the following codon mutations: R248C and S249C (exon 7); G372C, S373C, Y375C, G382R, A393E (exon 10); and K652M, K652T, K652E, K652Q (exon 15). Somatic mutations in the HRAS, KRAS and NRAS genes in BC affect codons 12, 13 and 61. To facilitate detection of RAS mutations, we used a multiplex RAS mutation assay that screens for 19 mutations simultaneously, representing 96% of all possibly known mutations in the three RAS genes [12].
Statistical analysis
Descriptive statistics of the baseline characteristics of the patients were reported using means and percentages, for NMIBC and MIBC separately.
The relationships between mutational status and clinical-demographic characteristics of patients were analyzed by T-test, Chi-square test, and Fisher exact test.
Follow-up time was considered time from diagnosis to death or loss of follow-up, depending on which came first. In the OS analysis all deaths were considered as events; in the cancer survival analysis, the events were only deaths of cancer. Kaplan-Meier plots were used to estimate the probability of overall and cancer survival according to the mutational status at diagnosis (log-rank test was performed to compare the curves).
Univariate logistic regression models were used considering the different clinical and demographic factors as predictors. The models were estimated for each outcome of interest, to identify the confounders to be included in the subsequent multivariate analysis.
A logistic regression model was fitted to estimate the odds ratio (OR) of recurrence comparing the several categories of mutational status for each gene and the genes combined. An ordered logistic regression model was also applied to estimate the impact of mutational status on the number of recurrences (0, 1, ≥2). In both analyses two different models were used: one unadjusted, and another adjusted for age, smoking status, stage, grade and therapy.
Finally, different prediction models for recurrence were built: 1) a model including age, smoking status, and risk of recurrence as predictors (Model A); 2) Model A with the additional predictor “mutational status at diagnosis” (Model B); and 3) Model A with the additional predictor “mutational status at diagnosis and follow-up” (Model C). ROC curves were plotted and AUC were calculated for each model to evaluate their performance prediction (range between 0.5 -random discrimination- and 1.0 -perfect discrimination-).
All analyses were performed using STATA version 13 (StataCorp, LP).
SUPPLEMENTARY MATERIAL FIGURES AND TABLES
Acknowledgments
The authors are very thankful to all volunteers that participated in the present study. The authors are very thankful to B. O'Brien and E. Van Emburgh for their technical support.
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest were disclosed.
FUNDING
The study was funded by Compagnia di San Paolo, Turin, Italy and by Grant 2016 MIUR ex-60%, Italy.
Authors contribution
Conception and design: R Critelli, G Matullo, A Naccarati, S Polidoro, P Vineis, E C Zwarthoff.
Development of methodology: R Critelli, I Lurkin, S Polidoro, E C Zwarthoff.
Acquisition of data: R Critelli, M B Assumma, C Viberti, G Cucchiarale, P Gontero, M Oderda, M Preto, C Sarcedote.
Analysis and interpretation of data: F Fasanelli, R Critelli, A Naccarati.
Writing, review and/or revision of the manuscript: R Critelli, A Naccarati, F Fasanelli, G Matullo, M Oderda, P Vineis.
Study supervision: G Matullo, P Vineis.
REFERENCES
- 1.Guillaume L, Guy L. [Epidemiology of and risk factors for bladder cancer and for urothelial tumors]. [Article in French] Rev Prat. 2014;64:1372–1374. 1378-1380. [PubMed] [Google Scholar]
- 2.Eble J.N. SG, Epstein J.I., Sesterhenn I.A., editors. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press; 2004. World Health Organization Classification of Tumours. [Google Scholar]
- 3.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. discussion 475-467. [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M, van Rhijn BW, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Dancik GM, Owens CR, Iczkowski KA, Theodorescu D. A cell of origin gene signature indicates human bladder cancer has distinct cellular progenitors. Stem Cells. 2014;32:974–982. doi: 10.1002/stem.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anastasiadis A, Cordeiro E, Bus MT, Alivizatos G, de la Rosette JJ, de Reijke TM. Follow-up procedures for non-muscle-invasive bladder cancer: an update. Expert Rev Anticancer Ther. 2012;12:1229–1241. doi: 10.1586/era.12.98. [DOI] [PubMed] [Google Scholar]
- 8.Wang QY, Zhao Y, Zhang R. The role of mutations and overexpression of the fibroblast growth factor receptor-3 in bladder cancer. Minerva Med. 2015;106:333–337. [PubMed] [Google Scholar]
- 9.Cappellen D, De Oliveira C, Ricol D, de Medina SGD, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 10.Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305–316. doi: 10.1007/s10555-009-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kompier LC, Lurkin I, van der Aa MN, van Rhijn BW, van der Kwast TH, Zwarthoff EC. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 14.Cheng L, Zhang S, MacLennan GT, Williamson SR, Lopez-Beltran A, Montironi R. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42:455–481. doi: 10.1016/j.humpath.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Netto GJ. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nat Rev Urol. 2012;9:41–51. doi: 10.1038/nrurol.2011.193. [DOI] [PubMed] [Google Scholar]
- 16.Hosen I, Rachakonda PS, Heidenreich B, de Verdier PJ, Ryk C, Steineck G, Hemminki K, Kumar R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int J Cancer. 2015;137:1621–1629. doi: 10.1002/ijc.29526. [DOI] [PubMed] [Google Scholar]
- 17.Bell RJ, Rube HT, Xavier-Magalhaes A, Costa BM, Mancini A, Song JS, Costello JF. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol Cancer Res. 2016;14:315–323. doi: 10.1158/1541-7786.MCR-16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 19.Borah S, Xi L, Zaug AJ, Powell NM, Dancik GM, Cohen SB, Costello JC, Theodorescu D, Cech TR. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347:1006–1010. doi: 10.1126/science.1260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachakonda PS, Hosen I, de Verdier PJ, Fallah M, Heidenreich B, Ryk C, Wiklund NP, Steineck G, Schadendorf D, Hemminki K, Kumar R. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci U S A. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralla B, Stephan C, Meller S, Dietrich D, Kristiansen G, Jung K. Nucleic acid-based biomarkers in body fluids of patients with urologic malignancies. Crit Rev Clin Lab Sci. 2014;51:200–231. doi: 10.3109/10408363.2014.914888. [DOI] [PubMed] [Google Scholar]
- 22.Allory Y, Beukers W, Sagrera A, Flandez M, Marques M, Marquez M, van der Keur KA, Dyrskjot L, Lurkin I, Vermeij M, Carrato A, Lloreta J, Lorente JA, Carrillo-de Santa Pau E, Masius RG, Kogevinas M, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–366. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 23.Sanguedolce F, Bufo P, Carrieri G, Cormio L. Predictive markers in bladder cancer: do we have molecular markers ready for clinical use? Crit Rev Clin Lab Sci. 2014;51:291–304. doi: 10.3109/10408363.2014.930412. [DOI] [PubMed] [Google Scholar]
- 24.Hurst CD, Platt FM, Knowles MA. Comprehensive Mutation Analysis of the TERT Promoter in Bladder Cancer and Detection of Mutations in Voided Urine. Eur Urol. 2014;65:367–369. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 25.van Rhijn BW, Vis AN, van der Kwast TH, Kirkels WJ, Radvanyi F, Ooms EC, Chopin DK, Boeve ER, Jobsis AC, Zwarthoff EC. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol. 2003;21:1912–1921. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 26.Ward DG, Baxter L, Gordon NS, Ott S, Savage RS, Beggs AD, James JD, Lickiss J, Green S, Wallis Y, Wei W, James ND, Zeegers MP, Cheng KK, Mathews GM, Patel P, et al. Multiplex PCR and Next Generation Sequencing for the Non-Invasive Detection of Bladder Cancer. PLoS One. 2016;11:e0149756. doi: 10.1371/journal.pone.0149756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosen I, Rachakonda PS, Heidenreich B, de Verdier PJ, Ryk C, Steineck G, Hemminki K, Kumar R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. International journal of cancer. 2015;137:1621–1629. doi: 10.1002/ijc.29526. [DOI] [PubMed] [Google Scholar]
- 28.Nordentoft I, Lamy P, Birkenkamp-Demtroder K, Shumansky K, Vang S, Hornshoj H, Juul M, Villesen P, Hedegaard J, Roth A, Thorsen K, Hoyer S, Borre M, Reinert T, Fristrup N, Dyrskjot L, et al. Mutational context and diverse clonal development in early and late bladder cancer. Cell Rep. 2014;7:1649–1663. doi: 10.1016/j.celrep.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Wu G, Shan Y, Hartmann C, von Deimling A, Xing M. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle. 2013;12:1637–1638. doi: 10.4161/cc.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuiverloon TC, van der Aa MN, van der Kwast TH, Steyerberg EW, Lingsma HF, Bangma CH, Zwarthoff EC. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16:3011–3018. doi: 10.1158/1078-0432.CCR-09-3013. [DOI] [PubMed] [Google Scholar]
- 31.Kinde I, Munari E, Faraj SF, Hruban RH, Schoenberg M, Bivalacqua T, Allaf M, Springer S, Wang Y, Diaz LA, Jr, Kinzler KW, Vogelstein B, Papadopoulos N, Netto GJ. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013;73:7162–7167. doi: 10.1158/0008-5472.CAN-13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65:367–369. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Liu T, Liu C, Meng Y, Yuan X, Liu L, Ge N, Liu J, Wang C, Ren H, Yan K, Hu S, Xu Z, Fan Y, Xu D. TERT promoter mutations and TERT mRNA but not FGFR3 mutations are urinary biomarkers in Han Chinese patients with urothelial bladder cancer. Oncologist. 2015;20:263–269. doi: 10.1634/theoncologist.2014-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zieger K, Dyrskjot L, Wiuf C, Jensen JL, Andersen CL, Jensen KM, Orntoft TF. Role of activating fibroblast growth factor receptor 3 mutations in the development of bladder tumors. Clin Cancer Res. 2005;11:7709–7719. doi: 10.1158/1078-0432.CCR-05-1130. [DOI] [PubMed] [Google Scholar]
- 35.van Rhijn BW, van der Kwast TH, Liu L, Fleshner NE, Bostrom PJ, Vis AN, Alkhateeb SS, Bangma CH, Jewett MA, Zwarthoff EC, Zlotta AR, Bapat B. The FGFR3 mutation is related to favorable pT1 bladder cancer. J Urol. 2012;187:310–314. doi: 10.1016/j.juro.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Sapre N, Anderson PD, Costello AJ, Hovens CM, Corcoran NM. Gene-based urinary biomarkers for bladder cancer: an unfulfilled promise? Urol Oncol. 2014;32:48. doi: 10.1016/j.urolonc.2013.07.002. e49-17. [DOI] [PubMed] [Google Scholar]
- 37.Tracey E, Watt H, Currow D, Young J, Armstrong B. Investigation of poorer bladder cancer survival in women in NSW, Australia: a data linkage study. BJU Int. 2014;113:437–448. doi: 10.1111/bju.12496. [DOI] [PubMed] [Google Scholar]
- 38.Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69:300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Carbayo M. Urine epigenomics: a promising path for bladder cancer diagnostics. Expert Rev Mol Diagn. 2012;12:429–432. doi: 10.1586/erm.12.42. [DOI] [PubMed] [Google Scholar]
- 40.Kompier LC, van der Aa MN, Lurkin I, Vermeij M, Kirkels WJ, Bangma CH, van der Kwast TH, Zwarthoff EC. The development of multiple bladder tumour recurrences in relation to the FGFR3 mutation status of the primary tumour. J Pathol. 2009;218:104–112. doi: 10.1002/path.2507. [DOI] [PubMed] [Google Scholar]
- 41.Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol. 2014;24:487–491. doi: 10.1097/MOU.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 42.Mbeutcha A, Lucca I, Mathieu R, Lotan Y, Shariat SF. Current Status of Urinary Biomarkers for Detection and Surveillance of Bladder Cancer. Urol Clin North Am. 2016;43:47–62. doi: 10.1016/j.ucl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Birkenkamp-Demtroder K, Nordentoft I, Christensen E, Hoyer S, Reinert T, Vang S, Borre M, Agerbaek M, Jensen JB, Orntoft TF, Dyrskjot L. Genomic Alterations in Liquid Biopsies from Patients with Bladder Cancer. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Matullo G, Naccarati A, Pardini B. microRNA expression profiling in bladder cancer: The challenge of Next Generation Sequencing in tissues and biofluids. Int J Cancer. 2015 doi: 10.1002/ijc.29895. [DOI] [PubMed] [Google Scholar]
- 45.Zuiverloon TC, Beukers W, van der Keur KA, Nieuweboer AJ, Reinert T, Dyrskjot L, Orntoft TF, Zwarthoff EC. Combinations of urinary biomarkers for surveillance of patients with incident nonmuscle invasive bladder cancer: the European FP7 UROMOL project. J Urol. 2013;189:1945–1951. doi: 10.1016/j.juro.2012.11.115. [DOI] [PubMed] [Google Scholar]
- 46.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 47.van Oers JM, Lurkin I, van Exsel AJ, Nijsen Y, van Rhijn BW, van der Aa MN, Zwarthoff EC. A simple and fast method for the simultaneous detection of nine fibroblast growth factor receptor 3 mutations in bladder cancer and voided urine. Clin Cancer Res. 2005;11:7743–7748. doi: 10.1158/1078-0432.CCR-05-1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


