Abstract
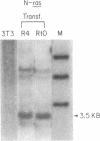
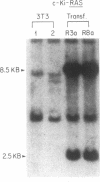
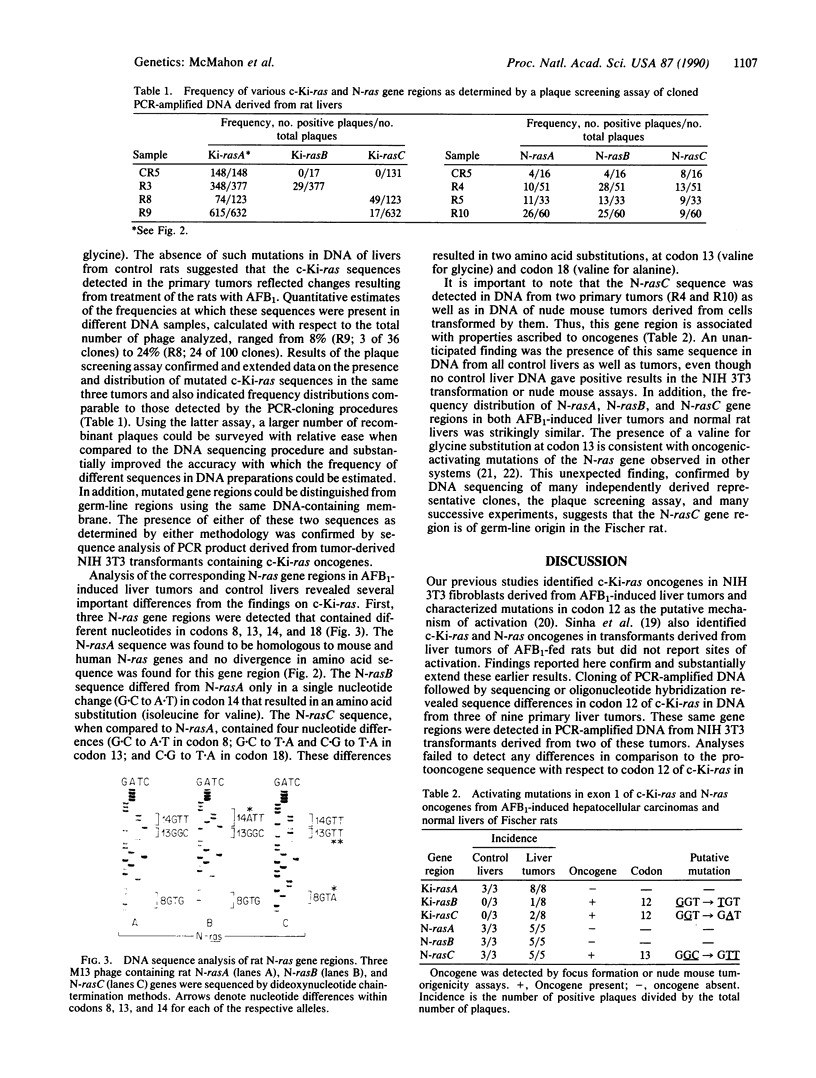
c-Ki-ras and N-ras oncogenes have been characterized in aflatoxin B1-induced hepatocellular carcinomas. Detection of different protooncogene and oncogene sequences and estimation of their frequency distribution were accomplished by polymerase chain reaction, cloning, and plaque screening methods. Two c-Ki-ras oncogene sequences were identified in DNA from liver tumors that contained nucleotide changes absent in DNA from livers of untreated control rats. Sequence changes involving G.C to T.A or G.C to A.T nucleotide substitutions in codon 12 were scored in three of eight tumor-bearing animals. Distributions of c-Ki-ras sequences in tumors and normal liver DNA indicated that the observed nucleotide changes were consistent with those expected to result from direct mutagenesis of the germ-line protooncogene by aflatoxin B1. N-ras oncogene sequences were identified in DNA from two of eight tumors. Three N-ras gene regions were identified, one of which was shown to be associated with an oncogene containing a putative activating amino acid residing at codon 13. All three N-ras sequences, including the region detected in N-ras oncogenes, were present at similar frequencies in DNA samples from control livers as well as liver tumors. The presence of a potential germ-line oncogene may be related to the sensitivity of the Fischer rat strain to liver carcinogenesis by aflatoxin B1 and other chemical carcinogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Toksoz D., Marshall C. J., Verlaan-de Vries M., Veeneman G. H., van der Eb A. J., van Boom J. H., Janssen J. W., Steenvoorden A. C. Amino-acid substitutions at codon 13 of the N-ras oncogene in human acute myeloid leukaemia. 1985 Jun 27-Jul 3Nature. 315(6022):726–730. doi: 10.1038/315726a0. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Essigmann J. M., Reinhold V. N., Wogan G. N. Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1745–1749. doi: 10.1073/pnas.75.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy R. G., Wogan G. N. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res. 1981 Jan;41(1):197–203. [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Miller J. H. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci U S A. 1983 May;80(9):2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Loss of the normal N-ras allele in a mouse thymic lymphoma induced by a chemical carcinogen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7810–7814. doi: 10.1073/pnas.82.23.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W., Matheson D. W. GC-rich regions in genomes as targets for DNA alkylation. Carcinogenesis. 1988 Nov;9(11):2065–2072. doi: 10.1093/carcin/9.11.2065. [DOI] [PubMed] [Google Scholar]
- McMahon G., Davis E., Wogan G. N. Characterization of c-Ki-ras oncogene alleles by direct sequencing of enzymatically amplified DNA from carcinogen-induced tumors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4974–4978. doi: 10.1073/pnas.84.14.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Hanson L., Lee J. J., Wogan G. N. Identification of an activated c-Ki-ras oncogene in rat liver tumors induced by aflatoxin B1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9418–9422. doi: 10.1073/pnas.83.24.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench K. F., Misra R. P., Humayun M. Z. Sequence specificity in aflatoxin B1--DNA interactions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):6–10. doi: 10.1073/pnas.80.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. J., Cunningham J. M., Parada L. F., Dautry F., Lebowitz P., Weinberg R. A. The HL-60 transforming sequence: a ras oncogene coexisting with altered myc genes in hematopoietic tumors. Cell. 1983 Jul;33(3):749–757. doi: 10.1016/0092-8674(83)90017-x. [DOI] [PubMed] [Google Scholar]
- Newberne P. M., Wogan G. N. Sequential morphologic changes in aflatoxin B carcinogenesis in the rat. Cancer Res. 1968 Apr;28(4):770–781. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sinha S., Webber C., Marshall C. J., Knowles M. A., Proctor A., Barrass N. C., Neal G. E. Activation of ras oncogene in aflatoxin-induced rat liver carcinogenesis. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3673–3677. doi: 10.1073/pnas.85.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson D. H., Miller E. C., Miller J. A. Aflatoxin B1-2,3-oxide: evidence for its formation in rat liver in vivo and by human liver microsomes in vitro. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1036–1043. doi: 10.1016/0006-291x(74)90417-3. [DOI] [PubMed] [Google Scholar]
- Tahira T., Hayashi K., Ochiai M., Tsuchida N., Nagao M., Sugimura T. Structure of the c-Ki-ras gene in a rat fibrosarcoma induced by 1,8-dinitropyrene. Mol Cell Biol. 1986 Apr;6(4):1349–1351. doi: 10.1128/mcb.6.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]