Abstract
The lymph node ratio (LNR), defined as the relation of tumor-infiltrated to resected lymph nodes, has been identified as an independent prognostic factor for colorectal cancer (CRC) after radical surgery. Recently, new guidelines propose counting tumor deposits (TDs) as positive lymph nodes (pLNs). The aim of this study was to investigate whether a novel LNR (nLNR) that considers TDs as pLNs can be used to accurately predict the long-term outcome of CRC patients. In this multicenter retrospective study, clinicopathological and outcome data from 2,051 stage III CRC patients who underwent R0 resection were collected between January 2004 and December 2011. Disease-free survival (DFS) and overall survival (OS) according to the nLNR category were analyzed using Kaplan-Meier survival curves. Univariate and multivariate analyses were performed to determine significant prognostic factors, and ROC curves were computed to measure the predictive capacity of the nLNR category. The 5-year DFS rates of nLNR1-4 were 68.3%, 48.4%, 33.3% and 16.5%, respectively (P<0.0001), and the 5-year OS rate of nLNR1-4 were 71.8%, 60.1%, 42.7% and 21.8%, respectively (P<0.0001). The area of under curve (AUC) of the nLNR was 0.686 (95% CI 0.663-0.710) and 0.672 (95% CI 0.648-0.697) for predicting DFS and OS. Our results demonstrate that the nLNR predicted long-term outcomes better than the LNR, npN and pN, using the cutoff points 0.250, 0.500 and 0.750.
Keywords: lymph node ratio, tumor deposits, colorectal cancer, prognosis
INTRODUCTION
The presence of tumor deposits (TDs) is a prognostic indicator for colorectal cancer (CRC) [1–4]. The TNM staging system by the American Joint Committee on Cancer (AJCC) issues definitions of what should be considered as positive lymph nodes (pLNs) and TDs [5–7]. The TNM5 classification [5] was the first to evaluate TDs and proposed the 3-mm rule. Later, the TNM6 [6] defined TDs as pLNs when they had the form and smooth contour of LNs while irregular TDs remained in the T category. In 2010, a new pN1c category was defined in TNM7 [7], which considered that T1 and T2 lesions with tumor deposits but lacking regional positive lymph node(s) should be classified as pN1c. This rapidly changing classification criteria impacts the selection of strategies to treat patients, such as postoperative chemoradiotherapy. Recently discussions to simplify the TNM staging system address whether TDs should be considered pLNs [1, 8]. A large-scale study by Li J et al reported that the counting TDs as pLNs in the new pN (npN) category is potentially superior to the classification in the original pN category, in terms of evaluating long-term outcomes for CRC patients [8].
The “lymph node ratio” (LNR) has been used as a predictive factor of the long-term survival status of CRC patients after radical surgery [9, 10]. Here, we conducted a large-scale, multicenter study, analyzing data from 2,051 stage-III CRC patients who received initial radical surgery, in order to investigate whether nLNR (relation of number of pLNs plus TDs to all nodes in resected samples) can accurately predict the long-term outcomes such as 5-year disease-free survival (DFS) and overall survival (OS).
RESULTS
Harvested LNs status and TDs
We harvested a total of 32,505 nodes including 30,707 LNs and 1,798 TDs, in which there were 7,771 pLNs. The number of pLNs plus TDs added to a total of 9,569 positive nodes. The mean number of retrieved LNs was 15.0. The LNR (pLNs to total LNs, 7,771/30,707) was 0.253, while the nLNR (positive nodes to all nodes, 9,569/32,505) was 0.294. We found that 35.0% (717/2,051) of patients had TDs, and there were 39.3% (282/717) of patients with TDs but without pLNs.
Patient characteristics and association of nLNR category with clinicopathologic factors
The median age was 58.0 ± 12.3 years (range 14-84), and the ratio of male to female was 1.26: 1 (1,144/907). In this study, only patients with adenocarcinoma including tubular (91.9%), mucinous (6.1%) or ring cell (2.0%) tumors were included. Patient clinicopathologic features are listed in Table 1. The rates of nLNR categories 1-4 were 53.9%, 26.4%, 11.6%, and 8.1%, respectively. nLNR categories were associated with tumor location (colon or rectum), pT category (7th), pN (7th) or npN category, venous/lymphatic invasion, differentiation grade, pathological category, and histological type (all P<0.05). The distributions of nLNR subgroups were similar with respect to gender, age, tumor size, preoperative carcinoembryonic antigen (CEA) levels, or adjuvant chemoradiotherapy (P>0.05, respectively).
Table 1. Association of nLNR category with clinical and pathological characteristics.
| Variable | All Patients | nLNR Category | X2 | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | nLNR1 | % | nLNR2 | % | nLNR3 | % | nLNR4 | % | |||
| All patients | 2051 | 100.0% | 1105 | 53.9% | 542 | 26.4% | 238 | 11.6% | 166 | 8.1% | ||
| Gender | ||||||||||||
| Male | 1144 | 55.8% | 630 | 57.0% | 301 | 55.5% | 125 | 52.5% | 88 | 53.0% | 2.236 | 0.525 |
| Female | 907 | 44.2% | 475 | 43.0% | 241 | 44.5% | 113 | 47.5% | 78 | 47.0% | ||
| Age, year | ||||||||||||
| ≤60 | 1187 | 57.9% | 630 | 57.0% | 296 | 54.6% | 143 | 60.1% | 118 | 71.1% | 3.622 | 0.057 |
| >60 | 864 | 42.1% | 475 | 43.0% | 246 | 45.4% | 95 | 39.9% | 48 | 28.9% | ||
| Tumor location | ||||||||||||
| Colon | 635 | 31.0% | 393 | 35.6% | 154 | 28.4% | 52 | 21.8% | 36 | 21.7% | 18.865 | <0.0001 |
| Rectum | 1416 | 69.0% | 712 | 64.4% | 388 | 71.6% | 186 | 78.2% | 130 | 78.3% | ||
| Tumor size, diameter | ||||||||||||
| ≤5.0 cm | 1440 | 70.2% | 748 | 67.7% | 418 | 77.1% | 167 | 70.2% | 107 | 64.5% | 5.693 | 0.128 |
| >5.0 cm | 611 | 29.8% | 357 | 32.3% | 124 | 22.9% | 71 | 29.8% | 59 | 35.5% | ||
| Preoperative CEA levels | ||||||||||||
| <5.0 ng/ml | 1102 | 53.7% | 607 | 54.9% | 297 | 54.8% | 118 | 49.6% | 80 | 48.2% | 9.169 | 0.164 |
| ≥5.0 ng/ml | 704 | 34.3% | 378 | 34.2% | 169 | 31.2% | 92 | 38.7% | 65 | 39.2% | ||
| Unknown | 245 | 11.9% | 120 | 10.9% | 76 | 14.0% | 28 | 11.8% | 21 | 12.7% | ||
| pT category (7th) | ||||||||||||
| pT2 | 128 | 6.2% | 81 | 7.3% | 36 | 6.6% | 7 | 2.9% | 4 | 2.4% | 18.107 | 0.006 |
| pT3 | 642 | 31.3% | 350 | 31.7% | 181 | 33.4% | 69 | 29.0% | 42 | 25.3% | ||
| pT4 | 1281 | 62.5% | 674 | 61.0% | 325 | 60.0% | 162 | 68.1% | 120 | 72.3% | ||
| pN category (7th) | ||||||||||||
| pN1a | 533 | 26.0% | 476 | 43.1% | 51 | 9.4% | 4 | 1.7% | 2 | 1.2% | 117.1 | <0.0001 |
| pN1b | 539 | 26.3% | 342 | 31.0% | 156 | 28.8% | 37 | 15.5% | 4 | 2.4% | ||
| pN1c | 282 | 13.7% | 197 | 17.8% | 79 | 14.6% | 4 | 1.7% | 2 | 1.2% | ||
| pN2a | 374 | 18.2% | 80 | 7.2% | 185 | 34.1% | 88 | 37.0% | 21 | 12.7% | ||
| pN2b | 323 | 15.7% | 10 | 0.9% | 71 | 13.1% | 105 | 44.1% | 137 | 82.5% | ||
| npN category | ||||||||||||
| pN1a | 526 | 25.6% | 508 | 46.0% | 18 | 3.3% | 0 | 0.0% | 0 | 0.0% | 136.21 | <0.0001 |
| pN1b | 629 | 30.7% | 453 | 41.0% | 156 | 28.8% | 16 | 6.7% | 4 | 2.4% | ||
| pN2a | 495 | 24.1% | 134 | 12.1% | 258 | 47.6% | 82 | 34.5% | 21 | 12.7% | ||
| pN2b | 401 | 19.6% | 10 | 0.9% | 110 | 20.3% | 140 | 58.8% | 141 | 84.9% | ||
| Venous/lymphatic invasion | ||||||||||||
| Yes | 230 | 11.2% | 75 | 6.8% | 66 | 12.2% | 38 | 16.0% | 51 | 30.7% | 75.987 | <0.0001 |
| No | 1821 | 88.8% | 1030 | 93.2% | 476 | 87.8% | 200 | 84.0% | 115 | 69.3% | ||
| Differentiation grade | ||||||||||||
| Well | 208 | 10.1% | 126 | 11.4% | 62 | 11.4% | 14 | 5.9% | 6 | 3.6% | 215.17 | <0.0001 |
| Moderately | 1507 | 73.5% | 877 | 79.4% | 401 | 74.0% | 80 | 33.6% | 80 | 48.2% | ||
| Poorly | 336 | 16.4% | 102 | 9.2% | 79 | 14.6% | 75 | 31.5% | 80 | 48.2% | ||
| Pathological category | ||||||||||||
| Tubular adenocarcinoma | 1885 | 91.9% | 1042 | 94.3% | 502 | 92.6% | 220 | 92.4% | 121 | 72.9% | 83.956 | <0.0001 |
| Mucinous adenocarcinoma | 125 | 6.1% | 54 | 4.9% | 34 | 6.3% | 6 | 2.5% | 31 | 18.7% | ||
| Ring cell cancer | 41 | 2.0% | 9 | 0.8% | 6 | 1.1% | 12 | 5.0% | 14 | 8.4% | ||
| Histological type | ||||||||||||
| Protrude | 1250 | 60.9% | 684 | 61.9% | 345 | 63.7% | 144 | 60.5% | 77 | 46.4% | 68.773 | <0.0001 |
| Ulcer | 638 | 31.1% | 336 | 30.4% | 180 | 33.2% | 75 | 31.5% | 47 | 28.3% | ||
| Infiltrative | 163 | 7.9% | 85 | 7.7% | 17 | 3.1% | 19 | 8.0% | 42 | 25.3% | ||
| Adjuvant therapy | ||||||||||||
| Yes | 1827 | 89.1% | 991 | 89.7% | 486 | 89.7% | 220 | 92.4% | 130 | 78.3% | 10.261 | 0.097 |
| No | 224 | 10.9% | 114 | 10.3% | 56 | 10.3% | 18 | 7.6% | 36 | 21.7% | ||
LNR versus nLNR as a prognostic for DFS and OS
During a 61-months (median; range 2-136) follow-up visit, a total of 838 patients (43.1%) were detected with failure including 11.5% of patients (235/2,051) with LR, 34.2% (701/2,051) with DM, 4.8% (98/2,051) with both LR and DM. For all 2,051 patients, the rates of 5-year DFS and OS were 55.1% and 62.0%, respectively. Table 2 lists the association of clinical and pathologic factors with long-term outcomes. The 5-year DFS rates of nLNR1-4 were 68.3%, 48.4%, 33.3% and 16.5%, respectively (P<0.0001). By contrast, the 5-year rates of DFS for LNR1-4 were 66.7%, 41.0%, 37.3% and 16.6%, respectively (P<0.0001). The 5-year OS for nLNR1-4 were 71.8%, 60.1%, 42.7% and 21.8%, respectively (P<0.0001). Compared to the nLNR category, the 5-year OS for LNR1-4 were 71.7%, 53.4%, 40.6% and 20.3%, respectively (P<0.0001).
Table 2. Impact of clinical and pathological characteristics on 5-year DFS and OS.
| Variable | All patients | All Failure (LR and/or DM) | 5-Years DFS Rate | X2 | P | 5-Year OS Rate | X2 | P | |
|---|---|---|---|---|---|---|---|---|---|
| No. cases | % | ||||||||
| All patients | 2051 | 883 | 43.1% | 55.1% | 62.0% | ||||
| Gender | |||||||||
| Male | 1144 | 502 | 43.9% | 54.1% | 0.065 | 0.800 | 62.0% | 2.963 | 0.085 |
| Female | 907 | 381 | 42.0% | 56.2% | 60.2% | ||||
| Age, year | |||||||||
| ≤60 | 1187 | 497 | 41.9% | 57.1% | 0.078 | 0.005 | 64.1% | 12.258 | <0.0001 |
| >60 | 864 | 386 | 44.7% | 52.1% | 57.1% | ||||
| Tumor location | |||||||||
| Colon | 635 | 270 | 42.5% | 56.7% | 0.100 | 0.751 | 62.9% | 0.609 | 0.435 |
| Rectum | 1416 | 613 | 43.3% | 54.2% | 60.5% | ||||
| Tumor size, diameter | |||||||||
| ≤5.0 cm | 1440 | 608 | 42.2% | 56.3% | 8.186 | 0.004 | 66.8% | 19.960 | <0.0001 |
| >5.0 cm | 611 | 275 | 45.0% | 52.2% | 56.0% | ||||
| Preoperative CEA levels | |||||||||
| <5.0 ng/ml | 1102 | 404 | 36.7% | 62.4% | 29.562 | <0.0001 | 68.9% | 61.925 | <0.0001 |
| ≥5.0 ng/ml | 704 | 363 | 51.6% | 45.3% | 51.2% | ||||
| Unknown | 245 | 116 | 47.3% | 49.5% | 53.9% | ||||
| pT category (7th) | |||||||||
| pT2 | 128 | 26 | 20.3% | 78.6% | 80.647 | <0.0001 | 82.8% | 61.982 | <0.0001 |
| pT3 | 642 | 226 | 35.2% | 63.5% | 68.5% | ||||
| pT4 | 1281 | 631 | 49.3% | 48.4% | 55.3% | ||||
| pN category (7th) | |||||||||
| pN1a | 533 | 149 | 28.0% | 71.5% | 210.812 | <0.0001 | 74.3% | 241.467 | <0.0001 |
| pN1b | 539 | 215 | 39.9% | 57.8% | 63.7% | ||||
| pN1c | 282 | 87 | 30.9% | 69.9% | 73.9% | ||||
| pN2a | 374 | 207 | 55.3% | 39.8% | 49.9% | ||||
| pN2b | 323 | 225 | 69.7% | 25.7% | 32.9% | ||||
| npN category | |||||||||
| pN1a | 526 | 141 | 26.8% | 72.4% | 243.677 | <0.0001 | 77.2% | 252.334 | <0.0001 |
| pN1b | 629 | 207 | 32.9% | 65.6% | 68.4% | ||||
| pN2a | 495 | 252 | 50.9% | 46.0% | 57.2% | ||||
| pN2b | 401 | 283 | 70.6% | 26.0% | 35.9% | ||||
| Venous/lymphatic invasion | |||||||||
| Yes | 230 | 151 | 65.7% | 28.1% | 82.275 | <0.0001 | 34.7% | 84.399 | <0.0001 |
| No | 1821 | 732 | 40.2% | 58.3% | 64.3% | ||||
| Differentiation grade | |||||||||
| Well | 208 | 56 | 26.9% | 71.8% | 77.730 | <0.0001 | 75.4% | 86.138 | <0.0001 |
| Moderately | 1507 | 627 | 41.6% | 56.6% | 63.1% | ||||
| Poorly | 336 | 200 | 59.5% | 37.4% | 42.6% | ||||
| Pathological category | |||||||||
| Tubular adenocarcinoma | 1885 | 799 | 42.4% | 55.7% | 12.542 | 0.002 | 62.5% | 32.689 | <0.0001 |
| Mucinous adenocarcinoma | 125 | 58 | 46.4% | 55.2% | 50.4% | ||||
| Ring cell cancer | 41 | 26 | 63.4% | 34.7% | 35.6% | ||||
| Histological type | |||||||||
| Protrude | 1250 | 496 | 39.7% | 58.9% | 32.074 | <0.0001 | 63.8% | 20.552 | <0.0001 |
| Ulcer | 638 | 298 | 46.7% | 50.9% | 58.7% | ||||
| Infiltrative | 163 | 89 | 54.6% | 41.0% | 50.3% | ||||
| Adjuvant therapy | |||||||||
| Yes | 1827 | 771 | 42.2% | 55.8% | 11.710 | 0.001 | 63.9% | 30.826 | <0.0001 |
| No | 224 | 112 | 50.0% | 50.4% | 51.7% | ||||
| LNR category | |||||||||
| LNR1 | 1270 | 412 | 32.4% | 66.7% | 222.124 | <0.0001 | 71.7% | 245.392 | <0.0001 |
| LNR2 | 442 | 245 | 55.4% | 41.0% | 53.4% | ||||
| LNR3 | 186 | 107 | 57.5% | 37.3% | 40.6% | ||||
| LNR4 | 153 | 119 | 77.8% | 16.6% | 20.3% | ||||
| nLNR category | |||||||||
| nLNR1 | 1105 | 339 | 30.7% | 68.3% | 300.214 | <0.0001 | 71.8% | 261.948 | <0.0001 |
| nLNR2 | 542 | 266 | 49.1% | 48.4% | 60.1% | ||||
| nLNR3 | 238 | 150 | 63.0% | 33.3% | 42.7% | ||||
| nLNR4 | 166 | 128 | 77.1% | 16.5% | 21.8% | ||||
The results of univariate analysis indicated that these thirteen clinical or pathologic factors including age, tumor size, preoperative CEA levels, pT or pN category, npN category, venous/lymphatic invasion, differentiation grade, pathological category, histological type, adjuvant chemoradiotherapy, LNR and nLNR categories, were all correlated with DFS and OS (all P<0.05). On the other hand, gender and tumor location could not predict the long-term outcomes of CRC patients (all P>0.05). Figure 1 shows the DFS and OS curves for both LNR and nLNR categories.
Figure 1. The DFS and OS curves for nLNR and LNR categories.
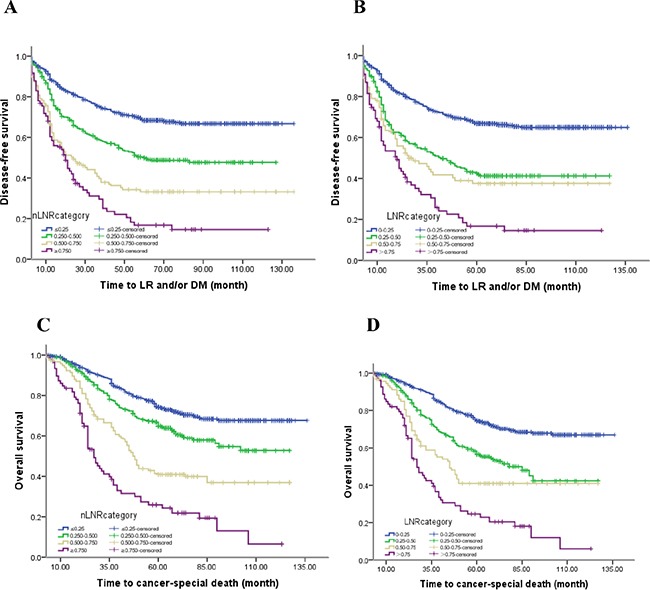
The 5-year DFS rates for A. the nLNR category (68.3%, 48.4%, 33.3% and 16.5%, P<0.0001; all statistically different, P<0.005), and B. the LNR category (66.7%, 41.0%, 37.3% and 16.6%, P<0.0001; all statistically different, P<0.001, except LNR 2 versus LNR 3; X2=1.989, P=0.158), The 5-year OS rates of C. the nLNR category (71.8%, 60.1%, 42.7% and 21.8%, P<0.0001; all statistically different, P<0.0001), and D. the LNR category (71.7%, 53.4%, 40.6% and 20.3%, P<0.0001; all statistically different, P<0.002).
In a previous study, we indicated that the npN category was superior to the pN category as a predictor of prognosis. In addition to the high correlation with each other, only the npN category was considered in multivariate models. Given that nLNR and LNR were highly correlated, multivariate analyses for all patients assessed both variables separately to avoid potential bias (Table 3 and 4). Both the nLNR and LNR categories were determined to be independent prognostic factors for DFS (HR 1.497, 95% CI 1.306 to 1.576, P < 0.0001; HR 1.411, 95% CI 1.294 to 1.537, P=0.001) and OS (HR 1.425, 95% CI 1.291 to 1.583; HR 1.418, 95% CI 1.288 to 1.561, both P< 0.0001). In order to identify which variable was superior in predicting DFS and OS, the area under the curve (AUC) was calculated for nLNR and LNR ROC curves. nLNR was found to be superior to LNR (AUC = 0.686, 95% CI 0.663-0.710 vs. 0.668, 95% CI 0.644-0.691) in predicting DFS. Similarly, nLNR was a more accurate indicator than LNR to assess OS (AUC = 0.672, 95% CI 0.648-0.697 vs. 0.667, 95% CI 0.643-0.692) (Figure 2). For those patients with less than 12 nodes (TDs plus pLNs), nLNR was also superior to LNR in assessing DFS (AUC = 0.670, 95%CI 0.629-0.711 vs. 0.666, 95% CI 0.625-0.708) and OS (AUC = 0.675, 95% CI 0.634-0.717 vs. 0.689, 95% CI 0.647-0.730) (Figure 3).
Table 3. Multivariate analysis for 5-year DFS and OS when nLNR enrolled.
| Variables | 5-Year DFS | 5-Year OS | ||||
|---|---|---|---|---|---|---|
| HR | 95.0% CI | P | HR | 95.0% CI | P | |
| Age | 1.139 | (1.139 to 1.307) | 0.063 | 1.306 | (1.127 to 1.514) | 0.000 |
| Tumor size | 0.975 | (0.975 to 1.135) | 0.747 | 1.042 | (0.887 to 1.225) | 0.615 |
| Preoperative CEA levels | 0.857 | (0.769 to 0.956) | 0.006 | 0.890 | (0.792 to 1.000) | 0.051 |
| pT category (7th) | 1.287 | (1.198 to 1.396) | 0.000 | 1.249 | (1.196 to 1.463) | 0.000 |
| npN category | 1.164 | (1.065 to 1.271) | 0.000 | 1.186 | (0.571 to 0.857) | 0.000 |
| Venous/lymphatic invasion | 0.671 | (0.557 to 0.810) | 0.000 | 0.700 | (0.571 to 0.857) | 0.001 |
| Differentiation grade | 1.194 | (1.037 to 1.374) | 0.014 | 1.217 | (1.047 to 1.414) | 0.010 |
| Pathological category | 0.937 | (0.790 to 1.110) | 0.454 | 1.094 | (0.920 to 1.301) | 0.308 |
| Histological type | 1.099 | (0.991 to 1.218) | 0.072 | 1.058 | (0.946 to 1.184) | 0.323 |
| Adjuvant therapy | 1.324 | (1.136 to 1.544) | 0.000 | 1.369 | (1.122 to 1.560) | 0.000 |
| LNR category | 1.411 | (1.294 to 1.537) | 0.001 | 1.418 | (1.288 to 1.561) | 0.000 |
Table 4. Multivariate analysis for 5-year DFS and OS when LNR enrolled.
| Variables | 5-Year DFS | 5-Year OS | ||||
|---|---|---|---|---|---|---|
| HR | 95.0% CI | P | HR | 95.0% CI | P | |
| Age | 1.133 | (0.988 to 1.300) | 0.074 | 1.296 | (1.118 to 1.501) | 0.001 |
| Tumor size | 0.980 | (0.842 to 1.420) | 0.790 | 1.049 | (0.893 to 1.233) | 0.560 |
| Preoperative CEA levels | 0.853 | (0.853 to 0.952) | 0.004 | 0.880 | (0.783 to 0.990) | 0.033 |
| pT category (7th) | 1.240 | (1.176 to 1.504) | 0.000 | 1.301 | (1.256 to 1.491) | 0.000 |
| npN category | 1.337 | (1.216 to 1.469) | 0.000 | 1.145 | (1.035 to 1.267) | 0.009 |
| Venous/lymphatic invasion | 0.673 | (0.558 to 0.812) | 0.000 | 0.684 | (0.559 to 0.838) | 0.000 |
| Differentiation grade | 1.198 | (1.043 to 1.376) | 0.011 | 1.265 | (1.092 to 1.466) | 0.002 |
| Pathological category | 0.934 | (0.788 to 1.108) | 0.435 | 1.116 | (0.940 to 1.326) | 0.211 |
| Histological type | 1.100 | (0.788 to 1.108) | 0.069 | 1.067 | (0.953 to 1.194) | 0.260 |
| Adjuvant therapy | 1.309 | (1.112 to 1.527) | 0.001 | 1.371 | (1.129 to 1.566) | 0.000 |
| nLNR category | 1.497 | (1.306 to 1.576) | 0.000 | 1.425 | (1.291 to 1.583) | 0.000 |
Figure 2. The ROC curves of nLNR, npLNs (TDs plus pLNs), LNR, and pLNs for predicting DFS and OS in all patients.
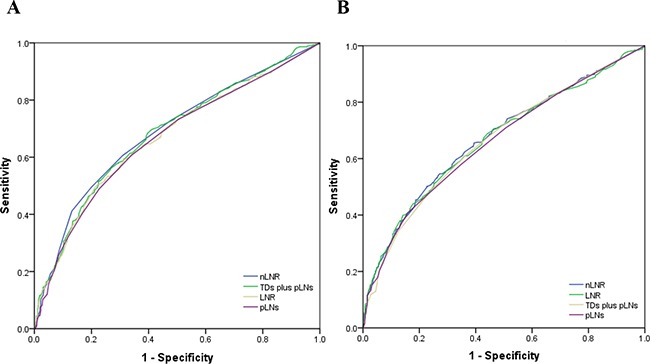
A. The AUC of nLNR, npLNs, LNR, and pLNs for predicting DFS were: 0.686 (95% CI 0.663-0.710), 0.681 (95% CI 0.657-0.704), 0.668 (95% CI 0.644-0.691), and 0.664 (95% CI 0.640-0.688), respectively (all P<0.0001). B. The AUC of nLNR, LNR, npLNs, and pLNs for predicting OS were: 0.672 (95% CI 0.648-0.697), 0.667 (95% CI 0.643-0.692), 0.663 (95% CI 0.638-0.688), and 0.659 (95% CI 0.635-0.684), respectively (all P<0.0001).
Figure 3. The ROC curves of nLNR, npLNs (TDs plus pLNs), LNR, and pLNs for predicting DFS and OS in patients with less than 12 nodes.
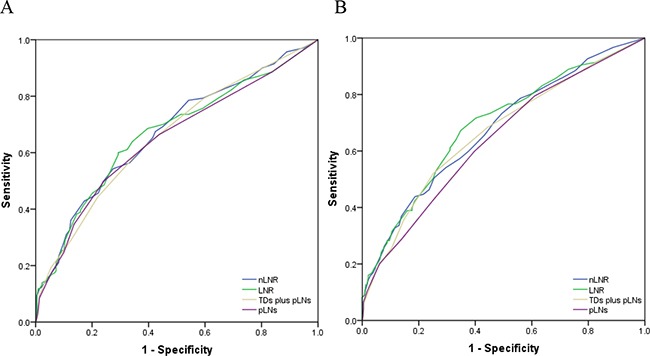
A. The AUC of nLNR, npLNs, LNR, and pLNs for predicting DFS were: 0.670 (95% CI 0.629-0.711), 0.666 (95% CI 0.625-0.708), 0.658 (95% CI 0.616-0.699), and 0.648 (95% CI 0.606-0.690), respectively (all P<0.0001). B. The AUC of nLNR, LNR, npLNs, and pLNs for predicting OS were: 0.675 (95% CI 0.634-0.717), 0.689 (95% CI 0.647-0.730), 0.664 (95% CI 0.621-0.707), and 0.640 (95% CI 0.597-0.683), respectively (all P<0.0001).
DISCUSSION
Lymph node (LN) involvement has been widely identified as one of the most important predictor of poor prognosis, and the pN staging strategy has been established by the AJCC TNM staging system according to the number of positive lymph nodes (pLNs) [5–7]. Recently, however, there has been increasing evidence that the pN staging based on the number of pLNs alone may not predict the long-term outcomes of patients accurately [11, 12]. In addition, although tumor deposits (TDs) are taken into account in TNM7 for colorectal cancer (CRC), which defines a new pN1c category (TDs are considered as pN1c when pT1-2 lesions lack pLNs), it is difficult to discriminate TDs from all nodes that include LNs [8].
Considering the shortcomings of pN, efforts have been made to identify more reliable prognostic markers related to LN status, such as LNR. The LNR is the ratio of pLNs to all resected LNs. Indeed, the LNR might be a good predictor of long-term survival in CRC [9, 10, 13, 14]. Rosenberg et al. [9] analyzed the data from a total of 17,309 CRC patients who underwent resection with a 5.9-year follow-up, finding that the LNR category could be used as an independent prognostic factor. Nonetheless, since guidelines regarding the use of LNR are lacking, it is not used widely in clinical practice.
TDs predict poor prognosis in CRC. However, the pN staging strategy does not consider the impact of the number of TDs on prognosis; therefore, recent studies have investigated whether a TD could be counted as a pLN. Results from such studies prompted the definition of a new pN (npN) category to evaluate long-term outcomes for CRC, which takes into account the number of both pLNs and TDs [1]. We recently investigated the feasibility of using the npN category to predict prognosis, analyzing data from 4,021 CRC patients who received radical surgery [8]. In that study, we proposed that TDs counted as pLNs could simplify the pN category (without pN1c), and found that the npN category is superior to the pN category in predicting DFS and OS [8]. Using the npN category, we did not find a need to differentiate TDs from nodes when the LN structure disappears totally.
In the present study, due to “no need to distinguish TDs and pLNs” [8], we designed the nLNR category calculating the ratio between the number of TDs plus pLNs and the number of TDs plus all harvested LNs. Besides, unlike other studies using variable cutoff values to classify the nLNR, we chose the standard 0.250, 0.500 and 0.750 as cutoff values based on statistical analyses, thereby circumventing the poor reproducibility of the LNR category. We investigated the feasibility of using the nLNR category to predict prognosis in CRC. Our findings from univariate and multivariate analyses indicated that the nLNR category could be used as an independent prognostic factor of both DFS and OS for CRC after radical surgery, similar to the LNR category [9, 10, 13, 14]. However, our ROC curve analyses indicated that the nLNR was superior to the LNR, npN, and pN in assessing DFS and OS in CRC. Thus, it is feasible to use the nLNR category to predict the long-term prognosis of CRC patients with greater accuracy than LNR category.
For patients with preoperative chemotherapy, the total number of harvested nodes can be less than 12 [11, 15–17]. Yet, according to the TNM7 staging criteria, at least 12 nodes are necessary to accurately use the pN category. Therefore, the nLNR category may be helpful to select reasonable treatment strategies for patients with less than 12 nodes. Indeed, our results here showed that nLNR was better than LNR, npN or pN in predicting the long-term survival of patients with less than 12 nodes.
Although our large-scale study included multicenter databases, it suffered from several limitations such as its retrospective design and lack of more long-term follow-up visits by patients. Previous studies have reported that patients can suffer a continuous increase in local recurrence for up to 10 years [18]. A randomized controlled trial, with different therapeutic strategies with or without adjuvant chemotherapy after radical surgery, should be done to validate our results. Besides, Colon and rectal cancer have different staging and treatment algorithms, even though similar outcomes were identified in this series. Nonetheless, our results warrant further prospective studies as well as the use of the nLNR category with the cutoff values indicated to predict the long-term prognosis of CRC patients.
PATIENTS AND METHODS
Inclusion and exclusion criteria
We examined the records of 2,051 patients with stage III colorectal adenocarcinoma who received initial radical surgery at seven study sites in China between January 2004 and December 2011. We excluded patients that: 1) had distant metastasis detected pre- or peri-operatively; 2) suffered from colorectal cancer before; 3) had synchronous tumors; 4) received preoperative chemoradiotherapy; 5) had multiple adenocarcinomas; 6) died of surgical complications in the immediate postoperative period; 7) lacked complete pathological slides; 8) lost follow-up visit within 5 years.
Surgical and adjuvant treatments
All patients received R0 resection without preoperative radiotherapy and/or chemotherapy. The standard total mesorectal excision (TME) was performed for rectal cancer patients. After surgery, patients were treated with radiotherapy and/or chemotherapy or not according to body situation, tumor location, and TNM staging system. Patients with rectal cancer were treated with adjuvant chemoradiotherapy (40-50Gy/2Gy/20-25F and Xeloda), while patients with colon cancer were managed with Xeloda plus 5-Fu regimens. A total of 224 (10.9%, 224/2051) patients who were at high risk (venous/lymphatic invasion, poor differentiation, or advanced stage) of local recurrence (LR) and distant metastasis (DM) due to rejection, poor physical condition or side effects, did not receive adjuvant therapy.
Pathologic examination and staging strategy
Slides from all resected specimens were reexamined by local pathologists who were blinded to the patients' clinical outcomes according to a standardized protocol including determination of the AJCC TNM7 classification, differentiation degree, histological type, numbers of resected and involved lymph nodes, and presence or absence of lymphatic or venous invasion. Negative, microscopic and macroscopic involvement was recorded as R0, R1 and R2, respectively. TDs were assessed using the 3-mm (TNM5) and contour (TNM6) rules [5, 6]. Regular tumor nodules were classified as positive LN. Irregular nodules without any residual tissues of LN were considered as TD if their diameters were > 3mm measured with a ruler. Otherwise, we irregular nodules were considered as pT3 if their diameters were ≤3mm. We counted TDs as pLNs in a new pN category, which included four tiers as follows: npN1a (one tumor node), npN1b (two to three tumor nodes), npN2a (four to six tumor nodes), and npN2b (≥ seven tumor nodes). All patients were classified depending on TNM7 [8]. A new LNR (nLNR) category was defined with four tiers as follows: nLNR1: ≤ 0.25; nLNR2: > 0.25 but ≤ 0.50; nLNR3: > 0.50 but ≤ 0.75; nLNR4: > 0.75.
Follow-up
Follow-up results were collected from all seven hospitals. The last follow-up date for this study was May 2015. The median time of follow-up was 61 months (range: 2-136 months). All time-to-event end points were measured from date of radical surgery. DFS and OS were calculated from radical surgery to finding evidence of local recurrence and/or distant metastasis, and death of any cause, respectively.
Statistical analysis
LR and DM analyses were performed for all eligible patients who received R0 resection. Statistical analysis was performed using SPSS software (version 20.0). Differences were evaluated with the log-rank test. Multivariate models were performed using the Cox proportional hazards model. All significant variables in the univariate analysis were included in multivariate Cox regression models in a forward-step procedure. The variables were entered into the regression models with increasing complexity, in order, according to clinical relevance, and significance was assessed using variance analysis. The predictive power of the individual model was evaluated using a receiver operating characteristic (ROC) curve. A two-sided P value <0.05 was considered to be of statistical significance.
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest to declare.
FUNDING
This study was financially supported by Scientific Research Project of Clinical Medical College of Chengdu University (grant number 201509) and Project of Sichuan Provincial Department of Health (grant number 090112).
Author contributions
JL and JY designed the study. JL, SKY, HL, JJH, FD, JY, SL, CL, SSX, JTY and BL provided study materials or patients. JL, JY, SSX, and SKY performed the analyses. JL, JY, SL and FD prepared all figures and tables. JL and JY wrote the main manuscript. All authors reviewed the manuscript.
REFERENCES
- 1.Song YX, Gao P, Wang ZN, Liang JW, Sun Z, Wang MX, Dong YX, Wang XF, Xu HM. Can the tumor deposits be counted as metastatic lymph nodes in the UICC TNM staging system for colorectal cancer? PLoS One. 2012;7:e34087. doi: 10.1371/journal.pone.0034087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng P, Xu P, Zhu D, Ji M, Xu J. Tumor deposit is a poor prognostic indicator in patients who underwent simultaneous resection for synchronous colorectal liver metastases. Onco Targets Ther. 2015;22:233–40. doi: 10.2147/OTT.S71414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HC, Pahlman L, Brown JM, Guillou PJ, Quirke P. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011;29:2487–92. doi: 10.1200/JCO.2011.34.6429. [DOI] [PubMed] [Google Scholar]
- 4.Nagtegaal ID, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon: a critical review. Histopathology. 2007;51:141–9. doi: 10.1111/j.1365-2559.2007.02720.x. [DOI] [PubMed] [Google Scholar]
- 5.Fleming ID, Cooper JS, Henson DE, editors. AJCC Cancer Staging Manual. ed 5. Philadelphia, PA: Lippincott Raven; 1998. [Google Scholar]
- 6.Greene FL, Page DL, Fleming ID, editors. AJCC Staging Handbook. (ed 6) New York, NY: Springer; 2002. [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, editors. AJCC cancer staging handbook. 7th edition. New York: Springer; 2010. p. 149. [Google Scholar]
- 8.Li J, Yang S, Hu J, Liu H, Du F, Yin J, Liu S, Li C, Xing S, Yuan J, Lv B, Fan J, Leng S, Zhang X, Wang B. Tumor deposits counted as positive lymph nodes in TNM staging for advanced colorectal cancer: a retrospective multicenter study. Oncotarget. 2016;7:18269–79. doi: 10.18632/oncotarget.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, Friess H, Hölzel D. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–8. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, Grebner A, Ulm K, Höfler H, Nekarda H, Siewert JR. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–78. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 11.Mekenkamp LJ, van Krieken JH, Marijnen CA, van de Velde CJ, Nagtegaal ID, Pathology Review Committee and the Co-operative Clinical Investigators Lymph node retrieval in rectal cancer is dependent on many factors–the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547–53. doi: 10.1097/PAS.0b013e3181b2e01f. [DOI] [PubMed] [Google Scholar]
- 12.Beresford M, Glynne-Jones R, Richman P, Makris A, Mawdsley S, Stott D, Harrison M, Osborne M, Ashford R, Grainger J, Al-Jabbour J, Talbot I, Mitchell IC, et al. The reliability of lymph-node staging in rectal cancer after preoperative chemoradiotherapy. Clin Oncol. 2005;17:448–55. doi: 10.1016/j.clon.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, Mino-Kenudson M, Giovannucci EL, Meyerhardt JA, Fuchs CS. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol. 2010;105:420–33. doi: 10.1038/ajg.2009.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moug SJ, McColl G, Lloyd SM, Wilson G, Saldanha JD, Diament RH. Comparison of positive lymph node ratio with an inflammation-based prognostic score in colorectal cancer. Br J Surg. 2011;98:282–6. doi: 10.1002/bjs.7294. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yuan J, Liu H, Yin J, Liu S, Du F, Hu J, Li C, Niu X, Lv B, Xing S. Lymph nodes regression grade is a predictive marker for rectal cancer after neoadjuvant therapy and radical surgery. Oncotarget. 2016;7:16975–84. doi: 10.18632/oncotarget.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SD, Kim TH, Kim DY, Baek JY, Kim SY, Chang HJ, Park SC, Park JW, Oh JH, Jung KH. Lymph node ratio is an independent prognostic factor in patients with rectal cancer treated with preoperative chemoradiotherapy and curative resection. Eur J Surg Oncol. 2012;38:478–83. doi: 10.1016/j.ejso.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Kang J, Hur H, Min BS, Lee KY, Kim NK. Prognostic impact of the lymph node ratio in rectal cancer patients who underwent preoperative chemoradiation. J Surg Oncol. 2011;104:53–8. doi: 10.1002/jso.21913. [DOI] [PubMed] [Google Scholar]
- 18.Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, Raab HR, Sauer R, Wittekind C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554–1562. doi: 10.1200/JCO.2013.54.3769. [DOI] [PubMed] [Google Scholar]


