Abstract
Background
Various factors may affect the clinical prognosis of lymph node-negative gastric cancer (GC) patients. This study aimed to provide evaluable prognostic information of combination of tumor size (Ts), lymph nodes count (LNs) and lymphovascular invasion (LVI) in lymph node-negative GC patients.
Methods
A total of 1,019 node-negative GC patients were enrolled in this retrospective study from 2000 to 2010. The cutoff points of Ts and LNs were determined using X-tile and patients were randomly categorized into training and validation sets by the sample size ratio 1:1. The clinicopathologic characteristics were analyzed and survival prognostic factors were identified, whereas the survival prediction accuracy was also compared by C-index during the different independent prognostic factors.
Results
The cutoff points for Ts were 3cm and 5cm, while 14 was the cutoff point for LNs. Age, T stage, Ts, LNs and LVI were identified as independent prognostic factors in node-negative GC patients, and a new prognostic predictive model, TsNL staging system which was composed of Ts, LNs and LVI, was proposed in this study. Compared with T staging system, significant improvement of predictive accuracy for TsNL system was found. Furthermore, nomogram based on TsNL was more accurate in prognostic prediction than that based on Ts, LNs and LVI, separately.
Conclusions
Age, T stage, Ts, LNs and LVI were independent prognostic factors in lymph node-negative GC patients. The TsNL staging system, composed of Ts, LNs and LVI, which was closely associated with clinicopathologic features, may improve the prognostic prediction accuracy in node-negative GC patients.
Keywords: predictive model, gastric cancer, tumor size, lymphovascular invasion, lymph nodes count
INTRODUCTION
Despite declining global incidence, gastric cancer (GC) remains one of the most common malignances nowadays, with the secondary leading cause of cancer-related mortality in China [1]. Being widely regarded to be the most important prognostic indicators for GC, depth of tumor invasion (T stage) and status of lymph nodes (N stage), have been enrolled in tumor-node-metastasis (TNM) staging system not only in the American Joint Committee on Cancer (AJCC) [2] but in the Japanese Gastric Cancer Association (JGCA) [3], which is due to the consideration that, this staging system is able to provide accurate prognostic estimation and guidance of choosing appropriate therapeutic protocols for GC patients, and to distinguish the prognostic differences among several subgroups of patients. Lymph node-negative GC patients have been demonstrated in previous studies [4, 5] to present better survival than those with positive lymph nodes involvement, nevertheless, even among the node-negative patients, the survival rate for certain subgroups were worse than others, and some of them still were at the risk of recurrence or cancer-related death. Although several investigators reported that, apart from the most important prognostic factor, T stage, various clinicopathologic factors such as lymphovascular invasion (LVI) [6–9], tumor size (Ts) [4, 10, 11], lymph node count (LNs) [12–15] and perineural invasion [16], were additionally confirmed as independent prognostic factors which were significantly associated with survival for node-negative GC patients followed curative resection, unfortunately, no consensus on this issue by far has been yet reached and few studies focused on prognostic role of the combination of these prognostic factors [6].
In light of these consideration mentioned above, it is highly necessary to analyze independent prognostic factors among a series of clinicopathologic features for node-negative GC patients underwent curative gastrectomy. Therefore, we conducted this study to identify the independent prognostic factors and to dig out some valuable prognostic information about the combination of these factors, trying to explore a more appropriate staging system based on these identified factors than the well-known prognostic factor, T stage, for precise and accurate prediction of the prognosis on overall survival in node-negative GC patients after curative surgery.
RESULTS
Optimal cutoff points for tumor size and lymph nodes count
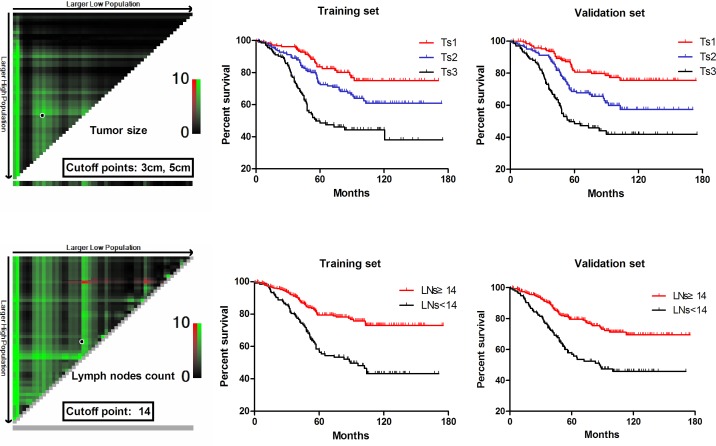
X-tile plots, constructed in Figure 1, indicated that the optimal cutoff points for tumor size (Ts) were 3.0cm and 5.0cm by minimum P value from log-rank χ2 test, based on which patients were divided into three groups, Ts1: ≤ 3cm, Ts2: 3-5cm, Ts3: ≥5cm, with the strongest discriminatory capacity. The count of lymph nodes retrieved in our study ranged from 8 to 59, with a median of 26 and a mean of 25.02 ± 8.80, and according to the optimal cutoff point for the lymph nodes count (LNs), 14, which was produced by X-tile shown in Figure 1, we defined LNs≥14 and LNs < 14 as N0 and N1, respectively. Consequently, a total of 1019 patients enrolled in our study were randomly separated into the training set (n = 510) and the validation set (n = 509), and there were no significant difference existing between these two sets in terms of different clinicopathologic factors (all of the P* value >0.05, illustrated in Table 1), which meant baseline for the two sets was balanced.
Figure 1. Division of patients by the cutoff points produced by X-tile plot.
Table 1. Correlation between TsNL stage and clinicopathologic factors in the training set and validation set.
| Factors | Training set | Validation set | P* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I (n=139) |
II (n=129) |
III (n=107) |
IV (n=99) |
V (n=36) |
Total (n=510) |
P | I (n=131) |
II (n=130) |
III (n=105) |
IV (n=109) |
V (n=34) |
Total (n=509) |
P | ||
| Gender | <0.001 | 0.003 | 0.058 | ||||||||||||
| Male | 94 | 113 | 33 | 88 | 29 | 357 | 101 | 99 | 93 | 71 | 19 | 383 | |||
| Female | 45 | 16 | 74 | 11 | 7 | 153 | 30 | 31 | 12 | 38 | 15 | 126 | |||
| Age (years) | 0.002 | 0.001 | 0.095 | ||||||||||||
| <65 | 71 | 52 | 39 | 24 | 9 | 185 | 79 | 61 | 32 | 28 | 11 | 211 | |||
| ≥65 | 68 | 77 | 68 | 75 | 27 | 325 | 52 | 69 | 73 | 81 | 23 | 298 | |||
| Tumor location | <0.001 | <0.001 | 0.057 | ||||||||||||
| Upper third | 21 | 23 | 24 | 15 | 8 | 91 | 22 | 14 | 17 | 11 | 10 | 74 | |||
| Middle third | 40 | 33 | 22 | 41 | 17 | 153 | 27 | 41 | 39 | 79 | 7 | 193 | |||
| Lower third | 78 | 71 | 57 | 36 | 3 | 245 | 81 | 74 | 45 | 13 | 9 | 222 | |||
| ≥2/3 stomach | 0 | 2 | 4 | 7 | 8 | 21 | 1 | 1 | 4 | 6 | 8 | 20 | |||
| Macroscopic type | <0.001 | <0.001 | 0.080 | ||||||||||||
| Type 0-II | 119 | 108 | 81 | 70 | 16 | 394 | 108 | 101 | 73 | 72 | 15 | 369 | |||
| Type III-V | 20 | 21 | 26 | 29 | 20 | 116 | 23 | 29 | 32 | 37 | 19 | 140 | |||
| Tumor differentiation | <0.001 | <0.001 | 0.054 | ||||||||||||
| Well/Moerately | 56 | 33 | 16 | 11 | 8 | 124 | 61 | 31 | 25 | 26 | 8 | 151 | |||
| Poorly | 83 | 96 | 91 | 88 | 28 | 386 | 70 | 99 | 80 | 83 | 26 | 358 | |||
| Perineural invasion | 0.002 | 0.011 | 0.234 | ||||||||||||
| Negative | 120 | 113 | 88 | 73 | 13 | 407 | 112 | 118 | 91 | 89 | 11 | 421 | |||
| Positive | 19 | 16 | 19 | 26 | 23 | 103 | 19 | 12 | 14 | 20 | 23 | 88 | |||
| T Stage | <0.001 | <0.001 | 0.084 | ||||||||||||
| T1-T2 | 116 | 88 | 33 | 48 | 5 | 290 | 108 | 82 | 29 | 39 | 4 | 262 | |||
| T3-T4b | 23 | 41 | 74 | 51 | 31 | 220 | 23 | 48 | 76 | 70 | 30 | 247 | |||
| Ts | <0.001 | <0.001 | 0.654 | ||||||||||||
| ≤3cm (Ts1) | 139 | 33 | 33 | 0 | 0 | 205 | 131 | 32 | 37 | 0 | 0 | 200 | |||
| 3-5cm (Ts2) | 0 | 96 | 45 | 38 | 0 | 179 | 0 | 98 | 50 | 43 | 0 | 191 | |||
| ≥5cm (Ts3) | 0 | 0 | 29 | 61 | 36 | 126 | 0 | 0 | 18 | 66 | 34 | 118 | |||
| LNs | <0.001 | <0.001 | 0.127 | ||||||||||||
| ≥14 (N0) | 139 | 102 | 72 | 46 | 0 | 359 | 131 | 107 | 76 | 66 | 0 | 380 | |||
| <14 (N1) | 0 | 27 | 35 | 53 | 36 | 151 | 0 | 23 | 29 | 43 | 34 | 129 | |||
| LVI | <0.001 | <0.001 | 0.259 | ||||||||||||
| Negative(L0) | 139 | 117 | 91 | 72 | 0 | 419 | 131 | 112 | 84 | 77 | 0 | 404 | |||
| Positive (L1) | 0 | 12 | 16 | 27 | 36 | 91 | 0 | 18 | 21 | 32 | 34 | 105 | |||
P*: the difference between the training set and the validation set; Ts: tumor size; LVI: lymphovascular invasion; LNs: lymph nodes count; N0: LNs≥14; N1: LNs<14.
Multivariate analyses for patients' prognosis and the proposal of TsNL staging system
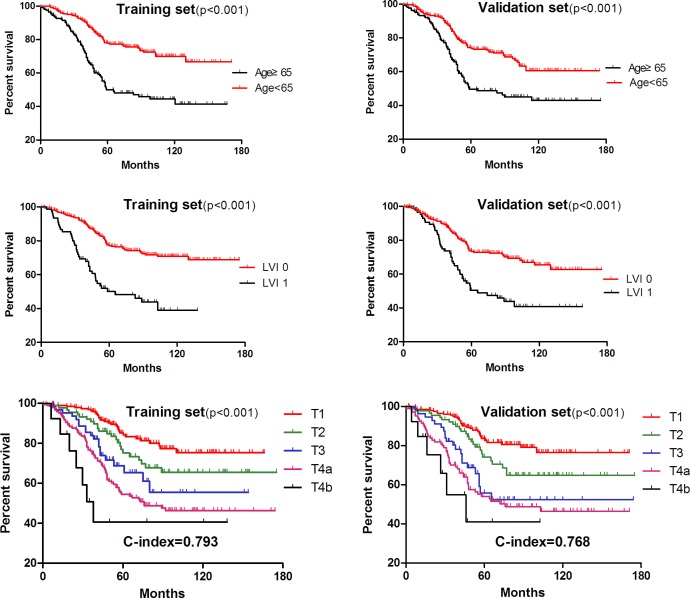
As demonstrated in Table 2, multivariate analysis by Cox regression model showed that age, tumor size (Ts), lymph nodes count (LNs), lymphovascular invasion (LVI) and T stage were independent prognostic factors of overall survival for lymph node-negative gastric cancer patients both in the training set and validation set. Moreover, survival curves related to these factors were illustrated in Figures 1 & 2, and significant difference was found in terms of all of these independent factors(p < 0.001).
Table 2. Multivariate analyses of clinicopathologic factors associated with OS by Cox regression model.
| Factors | Training set (n = 510) | Validation set (n = 509) | ||
|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | |
| Gender | 0.996(0.866-1.105) | 0.087 | 0.896(0.762-1.054) | 0.101 |
| Age | 1.325(1.101-1.980) | 0.039 | 1.425(1.003-2.027) | 0.048 |
| Tumor location | 0.894(0.788-1.002) | 0.091 | 0.956(0.897-1.153) | 0.132 |
| Macroscopic type | 1.003(0.823-1.145) | 0.202 | 1.001(0.891-1.106) | 0.156 |
| Tumor differentiation | 0.978(0.879-1.084) | 0.124 | 0.961(0.858-1.078) | 0.119 |
| Tumor size(Ts) | 1.554(1.232-1.967) | 0.001 | 1.442(1.127-1.844) | 0.004 |
| Perineural invasion | 1.254(0.732-1.629) | 0.233 | 1.132(0.892-1.567) | 0.341 |
| Lymph nodes count (LNs) | 1.401(1.012-2.275) | 0.024 | 1.698(1.126-2.562) | 0.012 |
| Lymphovascular invasion (LVI) | 0.612(0.390-0.873) | <0.001 | 0.536(0.376-0.765) | 0.002 |
| T stage | 1.439(1.069-1.702) | 0.001 | 1.348(1.178-1.544) | <0.001 |
OS: overall survival; HR: Hazard Ratio; CI: Confidence Interval.
Figure 2. Kaplan-Meier survival analysis in terms of age, LVI and T stage.
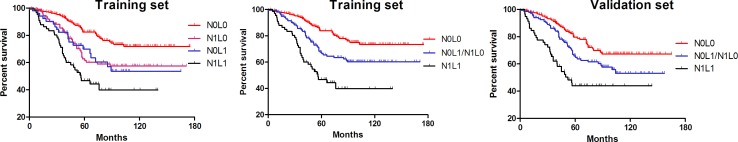
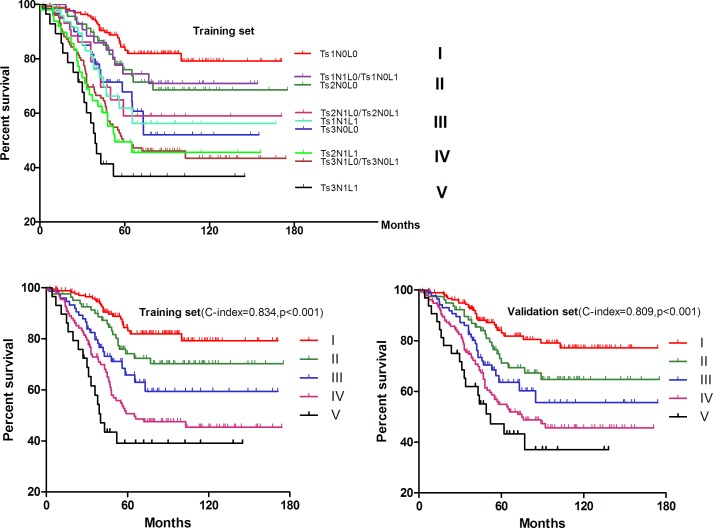
In order to dig out detailed prognostic information of these independent factors, we firstly combined LNs and LVI to make Kaplan-Meier survival analysis and found that there was a cross line between the N0L1 and N1L0 (p = 0.498) in Figure 3. In addition, the survival curves suggested a largely improved discriminatory ability after the integration of N0L1 and N1L0 both in the training set (p < 0.001) and validation set (p < 0.001). Furtherly, Ts and LNs as well as LVI were combined together to make survival analyses in Figure 4, illustrating that overlapping survival curves presented and no significant difference was found between Ts1N1L0/Ts1N0L1 and Ts2N0L0 by log rank test (p = 0.732). Interestingly, similarity was also found among Ts1N1L1, Ts3N0L0 and Ts2N1L0/Ts2N0L1 (p = 0.429), and between Ts2N1L1 and Ts3N1L0/Ts3N0L1 (p = 0.791). Therefore, we tried to integrate them respectively into stage II, III, IV, whereas Ts1N0L0 was regarded as stage I with Ts3N1L1 defined as stage V. Given that this new stage-integrating strategy just mentioned before was surprisingly able to utilize both in the training set and validation set (Figure 4), we proposed a new staging system, TsNL which was composed of Ts, LNs and LVI, illustrated in Table 3.
Figure 3. Kaplan-Meier survival analysis in terms of combination of LVI and LNs.
Figure 4. Kaplan-Meier survival analysis of the TsNL staging system.
Table 3. TsNL staging system.
| N0L0 | N0L1/N1L0 | N1L1 | |
|---|---|---|---|
| Ts1 | I | II | III |
| Ts2 | II | III | IV |
| Ts3 | III | IV | V |
Ts: tumor size; N: lymph nodes count (LNs); L: lymphovascular invasion (LVI);
Ts1:≤3cm; Ts2: 3-5cm; Ts3:≥5cm; N0: LNs≥14; N1: LNs<14; L0: LVI (−); L1: LVI (+).
Clinicopathologic factors and correlation analysis
Clinicopathologic factors were compared among the five stages, as shown in Table 1. Both in the training set and validation set, TsNL stage was significantly related to gender, age, tumor location, macroscopic type, tumor differentiation and perineural invasion as well as T stage. Compared with the TsNL stage IV and V, patients with stage II and III were found more frequently in male and in the age of ≥65 years, having a higher proportion in macroscopic type 0-II, in well/moderate differentiation and in early T stage as well as negative perineural invasion.
As demonstrated in Table 4, logistic regression analyses were performed respectively to determine the risk factors for those four independent prognostic factors identified by Cox regression analysis. As a result, T stage and Ts were mutually evaluated as the risk factor for each other (p < 0.05), indicating that T stage was closely correlated to Ts and that multicollinearity between them was found. That was one of the reason why T stage was not taken into account for our TsNL staging system. However, no correlation was found during other factors, such as Ts, LNs and LVI.
Table 4. Logistic regression analysis of the risk factors for the independent prognostic factors.
| Factors | T stage | Ts | LNs | LVI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | p value | OR(95%CI) | p value | OR(95%CI) | P value | OR(95%CI) | p value | ||||
| T stage | - | - | 2.552(2.314-2.785) | 0.009 | 0.904(0.693-1.421) | 0.492 | 1.105(0.898-1.374) | 0.124 | |||
| Ts | 2.478(2.032-2.931) | 0.021 | - | - | 0.929(0.798-1.221) | 0.146 | 0.921(0.793-1.101) | 0.145 | |||
| LNs | 0.932(0.643-1.403) | 0.402 | 0.891(0.776-1.164) | 0.132 | - | - | 1.108(0.989-1.385) | 0.533 | |||
| LVI | 1.057(0.933-1.439) | 0.105 | 0.903(0.728-1.098) | 0.123 | 1.019(0.913-1.234) | 0.365 | - | - | |||
| Age | 1.187(0.982-1.415) | 0.063 | 1.123(0.829-1.428) | 0.605 | 1.172(0.875-1.654) | 0.413 | 1.057(0.933-1.439) | 0.105 | |||
| Gender | 1.102(0.994-1.508) | 0.201 | 1.320(0.794-1.508) | 0.541 | 1.026(0.774-1.508) | 0.532 | 1.002(0.849-1.307) | 0.243 | |||
| Tumor location | 1.131(0.953-1.457) | 0.182 | 1.036(0.979-1.346) | 0.071 | 1.215(0.789-1.751) | 0.323 | 1.102(0.994-1.508) | 0.101 | |||
| Macroscopic type | 1.147(1.089-1.378) | 0.041 | 1.063(0.902-1.278) | 0.062 | 1.009(0.889-1.428) | 0.132 | 1.163(0.872-1.327) | 0.198 | |||
| Perineural invasion | 1.016(0.781-1.369) | 0.069 | 1.146(0.891-1.428) | 0.091 | 1.105(0.767-1.323) | 0.292 | 1.263(0.991-1.713) | 0.198 | |||
| Tumor differentiation | 1.208(0.665-1.537) | 0.219 | 1.106(0.804-1.425) | 0.197 | 1.326(0.701-1.843) | 0.197 | 1.106(0.934-1.425) | 0.067 | |||
Ts: tumor size; LNs: lymph nodes count; LVI: lymphovascular invasion; OR: Odds Ratio; CI: Confidence Interval.
Survival analysis and prognostic accuracy of TsNL staging system
The 1-year, 3-year and 5-year overall survival (OS) rates of each TsNL stage for the training and validation set were shown in Table 5. The 5-year OS of stage I, II, III, IV, V were 86.1%, 75.2%, 66.4%, 48.1%, 40.3% for the training set and 87.8%,72.4%,61.6%,54.1%,43.9% for the validation set, respectively.
Table 5. Survival analysis of patients in the training set and validation set in terms of TsNL staging system.
| Training set (n= 510) | Validation set (n= 509) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage | 1-yr OS | 3-yr OS | 5-yr OS | MS (month) | 1-yr OS | 3-yr OS | 5-yr OS | MS(month) | ||
| I | 94.6% | 91.8% | 86.1% | 107.0(1.8-170.0) | 95.1% | 90.3% | 87.8% | 94.3(1.5-173.3) | ||
| II | 93.4% | 88.9% | 75.2% | 98.2(0.9-172.1) | 94.1% | 86.6% | 72.4% | 87.0(1.9-173.1) | ||
| III | 93.3% | 80.5% | 66.4% | 72.9(2.5-170.8) | 93.9% | 82.1% | 61.6% | 68.0(3.2-171.8) | ||
| IV | 92.5% | 72.2% | 48.1% | 72.8(1.2-172.0) | 93.1% | 74.6% | 54.1% | 65.4(1.4-169.0) | ||
| V | 88.6% | 60.4% | 40.3% | 40.4(0.7-146.0) | 89.9% | 63.0% | 43.9% | 45.2(0.9-134.2) | ||
OS: overall survival; MS: median survival time.
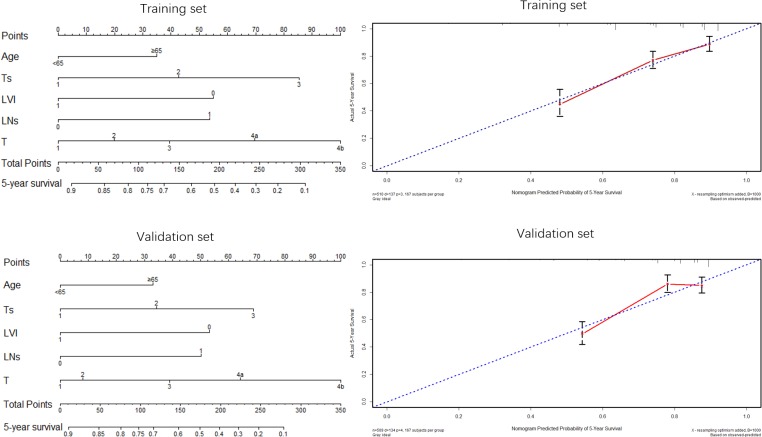
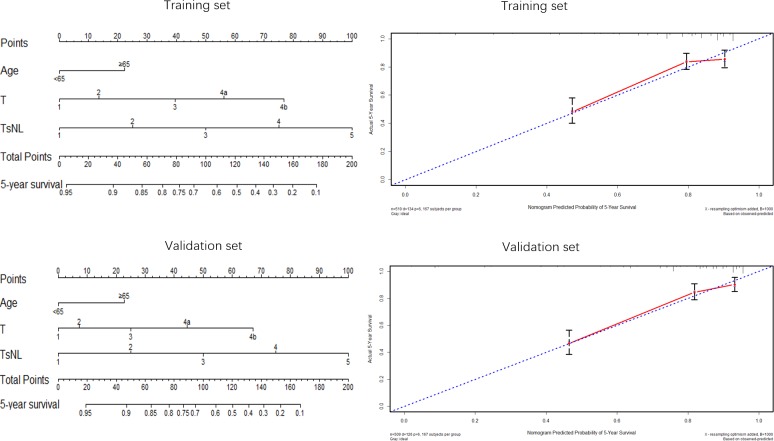
Nomogram was applied to predict 5-year OS of patients (Figures 5 & 6). Both in the training set and validation set, factors such as age, Ts, LVI, LNs and T stage, were enrolled in the nomogram plots (Figure 5), demonstrating that these five factors were independent factors and that age ≥65, larger tumor size, positive LVI and LNs < 14 as well as advanced T stage were adverse prognostic factors, which was consistent with the aforementioned results displayed by Cox regression analyses in this study. Nomograms based on TsNL staging system for the training set and validation set were illustrated in Figure 6, and the corresponding calibration curves in the two sets suggested that the predictive probability of 5-year survival were much more closely to the actual 5-year survival than that of calibration curves produced in Figure 5.
Figure 5. Nomogram plots and calibration curves based on age, Ts, LVI, LNs and T stage.
Figure 6. Nomogram plots and calibration curves based on the TsNL staging system.
Moreover, the concordance index (C-index) in R was used to compare the prognostic accuracy between TsNL stage and T stage system. To be specific, TsNL staging system (c-index = 0.834, 95%CI: 0.790-0.881, Figure 4) was found to be significantly superior to T stage in the training set (c-index = 0.793, 95%CI: 0.723-0.827, Figure 2) in survival prediction accuracy (p < 0.05), and similar result also appeared in the validation set as shown in Figure 2 &4.
DISCUSSION
For lymph node-negative GC patients who underwent curative gastrectomy, T stage has been considered as the most important prognostic predictor according to the TNM staging system [2, 3]. In this study, in addition to T stage, clinicopathologic features such as age, tumor size (Ts), lymphovascular invasion (LVI) and lymph nodes count (LNs), were identified as independent prognostic factors by multivariate Cox regression analysis.
The optimal cutoff points for Ts were 3cm and 5cm in this study, which could produce minimum p value by log-rank and maximum discrimination ability on prognostic prediction both in the training set and validation set. As an important prognostic factor, Ts has already been integrated into the TNM staging system for liver cancer, lung cancer and breast cancer, but not for gastric cancer. In our previous study, Ts was found no superiorities than T stage for node-negative GC patients, but it was more accurate in combination with N stage than TNM staging system in survival prediction [11]. Moreover, the status of lymphovascular invasion (LVI) has been previously observed as an important factor, influencing the clinical outcome of gastric cancer patients who underwent radical gastrectomy, and the presence of LVI has been identified to be of significantly relevance to a poor overall survival for advancer GC patients in several studies [17–19], while some researchers proposed that LVI was just associated with the survival prognosis for early GC patients or node-negative GC patients [6–9]. Our findings revealed a significant difference between node-negative GC patients with LVI and those without LVI on overall survival, which was in accordance with the latter point of view.
The removal of no less than 15 regional lymph nodes count (LNs) at the time of lymphadenectomy for gastric cancer during surgical treatment has been largely demonstrated to improve survival outcomes [12, 14, 20–22]. Given that GC patients might be staged incorrectly because of an insufficient number of LNs, which could lead to miss an inappropriate adjuvant therapy [23], a minimum of 15 LNs is recommended to be retrieved in lymphadenectomy for the sake of nodal metastatic status determination for GC patients in the NCCN guidelines and JGCA [2, 3, 24]. For node-negative GC patients, the number of LNs was also found to be significantly associated with the prognosis, but there have long been controversies over how many LNs should be removed in radical gastrectomy, with the cutoff numbers ranging from 15 to 25 in several studies [12, 21, 25, 26]. Theoretically, an increasing number of LNs indicates a comparatively accurate N stage, especially for lymph node-negative GC patients, due to that these patients have a great risk of being misclassified when few nodes are harvested and their clinical survival outcomes are likely to be changed if they are given timely adjuvant therapy because of the stage migration from negative to positive lymph nodes. In our study, the LNs was demonstrated to be an independent prognostic factor as well, but the optimal cutoff point was 14, which was inconsistent with previous studies. This might be explained by that for the total number of LNs retrieved in lymph-node negative GC patients were less than that in node-positive patients. Studies on population registries have reported that only18-31% of cases were harvested 15 or more LNs [26, 27]. That could be also the reason why node-negative (N0) stage is defined as any gastric cancer with all examined LNs negative, regardless of the total number of LNs in the 7th edition of the TNM classification [2].
Furthermore, T stage and Ts were both demonstrated to be independent prognostic factors in our study and showed similar prognostic power independently. However, logistic regression analyses were performed in this study to identify the risk factors for T stage, Ts, LNs and LVI as well, indicating that T stage was closely correlated to Ts and that multicollinearity between them was found, which reminded us that T and Ts could not be integrated into one staging model. That was one of the reason why T stage was not taken into account for our TsNL staging system. In order to make the utmost use of these independent factors to offer detailed prognostic information, we integrated the independent factors, Ts, LNs and LVI, together to propose a new staging system, TsNL, which could provide powerful survival discrimination ability and enhance the prognostic accuracy for node-negative GC patients. The patients in this study were divided into five stages according to the TsNL staging system both in the training set and validation set, and TsNL stage was significantly associated with clinicopathologic features, such as gender, age, tumor location, macroscopic type, tumor differentiation and perineural invasion as well as T stage. Patients with late TsNL stage were likely to be diagnosed with worse biological behavior and more aggressive features than those with early TsNL stage.
Nomogram, as an effective method to evaluate survival prognosis for patients, was used in this study to show visually the prognostic significance of some important factors on the GC patients. As independent prognostic factors, age, Ts, LVI, LNs and T stage, were enrolled in the nomogram plots. Nomograms and calibration curves based on TsNL staging system revealed a much closer predictive probability of 5-year survival to the actual 5-year survival, according to which we could believe that nomogram based on TsNL staging system showed an improved predictive capability of 5-year overall survival. Additionally, the prognostic accuracy between TsNL stage and T stage system was compared using C-index, as the T stage was the most important prognostic predictor for node-negative GC patients according to the TNM staging system. C-indexes for TsNL stage were observed significantly larger than that for T stage both in the training set and validation set in our study, which illustrated that TsNL stage was more accurate in prognostic prediction than T stage. Given that selection of an appropriate therapy strategy for GC patients in accordance with tumor stage is extremely important and essential to optimize patient prognosis, perhaps node-negative GC patients could benefit a lot from this new staging system, not only because of its powerful discrimination ability in survival estimation but also due to its improved accuracy in prognostic prediction.
There were also limitations in our study. First of all, our findings we got were just on the basis of a retrospective single-center study, which could have been observed by chance in spite of the large sample. In addition, we were lack of another separated validation set to evaluate the predictive power of TsNL staging system. Therefore, large scale and prospective multicenter studies are needed to evaluate the TsNL staging system can whether or not be an important prognostic index for the node-negative GC patients before stronger statement can be done.
In conclusion, age, T stage, Ts, LNs and LVI in our study were independent prognostic factors for lymph node-negative GC patients. Moreover, composed of Ts, LNs and LVI, the TsNL staging system, which was closely associated with clinicopathologic features, could improve the prognostic prediction accuracy in node-negative GC patients.
PATIENTS AND METHODS
Patients
The West China Hospital Research Ethics Committee approved the retrospective analysis of anonymous data involved in this study. The data retrieval of this study was based on the Surgical Gastric Cancer Patient Registry in West China Hospital [28]. Patient records were anonymized and de-identified prior to analysis, and signed patient informed consent was waived per the committee approval because of the retrospective nature of the analysis.
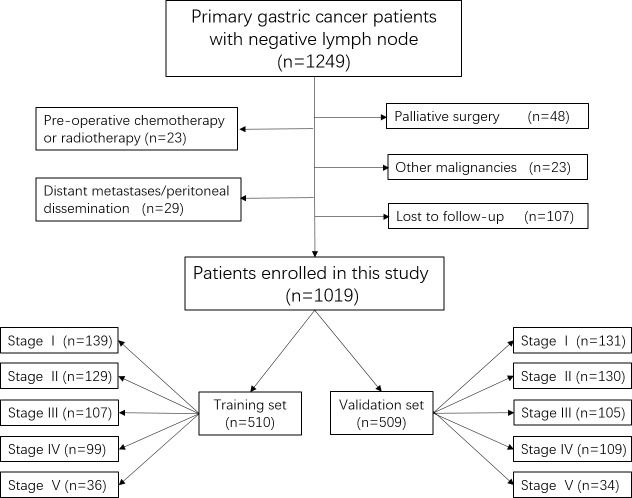
From 2000 January to 2010 December, a total of 1249 consecutive lymph node-negative GC patients who received gastrectomy at the Department of Gastrointestinal Surgery, West China Hospital, were retrospectively evaluated in this study. The diagnosis of primary gastric cancer for all patients was confirmed by upper gastrointestinal endoscopy and biopsy. Patients were excluded on the condition that: (1) patients who underwent palliative surgery with positive residual margins; (2) patients with any pre-operative chemotherapy or radiotherapy; (3) patients with another malignancy or any other life-threatening diseases diagnosed during three years prior to the operation; (4) patients with surgical findings of distant metastasis or peritoneal dissemination. (5) patients who were lost to follow-up. Finally, 109 patients were lost to follow-up and the follow-up rate was 91.43% in this study. A total of 1019 patients were enrolled in this study as shown in Figure 7. The clinicopathological characteristics including of gender, age, tumor location, macroscopic type, tumor differentiation, perineural invasion, T stage, defined as the depth of tumor invasion according to the Japanese gastric cancer treatment guidelines 2010 (version 3) [3], and follow-up information were collected.
Figure 7. The flow chart of patients in this study.
Definition of TsNL staging system
Tumor size (Ts), was divided into three groups (Ts1: ≤3cm, Ts2: 3-5cm, Ts3: ≥5cm) by the cutoff points of 3.0cm and 5.0cm using X-tile, and the lymph nodes count (LNs) was categorized into N0 (LNs≥14) and N1 (LNs < 14) by the cutoff point of 14. Lymphovascular invasion (LVI) was defined as status of tumor invasion of lymphatics or small veins, and L0 was regarded as negative LVI whereas L1 symbolized positive LVI. Consequently, TsNL staging system shown in Table 3, was designed as combination of Ts, LNs and LVI, based on which patients were randomly categorized into the training set and the validation set by the sample size ratio 1:1 using X-tile.
Statistical analysis
Optimal cutoff points for survival were determined by minimum P value from log-rank χ2 statistics using the X-tile program (Version 3.1.2, Yale University) [29]. Chi-square test in the SPSS version 19.0 was applied to analyze unordered categorical variables, whereas Mann-Whitney U test was performed to evaluate ranked variables. Logistic regression analysis was used to analyze the multicollinearity or multivariate correlation. Univariate and multivariate survival analyses were performed by Cox's proportional hazard regression model with conditional backward stepwise. The cumulative survival rates were calculated using the Kaplan-Meier method and life-table in the SPSS, with subgroups compared by the log-rank test through GraphPad Prism 5. Nomogram and calibration curve were displayed with the package of Regression Modeling Strategies (URL http://CRAN.R-project.org/package = rms) in R (version3.1.2.URL http://www.R-project.org/.) Comparisons between the different staging systems for the prognostic prediction were conducted with the package of Harrell Miscellanceous (URL http://CRAN.R-project.org/package = Hmisc.) and were evaluated by the concordance index (C-index). The larger the C-index, the more accurate was the prognostic prediction [30]. A p value of < 0.05 (two side) was defined to be statistically significant.
Acknowledgments
The authors thank the Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China, for the substantial work in data collection and follow-up of the database.
Footnotes
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest
FUNDING SOURCES
This study was supported by grants from National Natural Science Foundation of China (No. 81372344) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
REFERENCES
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016 doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 3.Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa Y, Ohtaka M, Maekawa S, Fukasawa M, Nakayama Y, Yamaguchi T, Inoue T, Uetake T, Sakamoto M, Sato T, Kawaguchi Y, Fujii H, Mochizuki K, Hada M, Oyama T, Yasumura T, et al. Stratifying the risk of lymph node metastasis in undifferentiated-type early gastric cancer. World J Gastroenterol. 2015;21:2683–2692. doi: 10.3748/wjg.v21.i9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, He Q, Cui J, Bian S, Chen L. Lymph node metastasis in early gastric cancer. Chin Med J (Engl) 2014;127:560–567. [PubMed] [Google Scholar]
- 6.Lee JH, Kim MG, Jung MS, Kwon SJ. Prognostic significance of lymphovascular invasion in node-negative gastric cancer. World J Surg. 2015;39:732–739. doi: 10.1007/s00268-014-2846-y. [DOI] [PubMed] [Google Scholar]
- 7.Jin LX, Moses LE, Squires MH, 3rd, Poultsides GA, Votanopoulos K, Weber SM, Bloomston M, Pawlik TM, Hawkins WG, Linehan DC, Strasberg SM, Schmidt C, Worhunsky DJ, Acher AW, Cardona K, Cho CS, et al. Factors Associated With Recurrence and Survival in Lymph Node-negative Gastric Adenocarcinoma: A 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg. 2015;262:999–1005. doi: 10.1097/SLA.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 8.Kunisaki C, Makino H, Kimura J, Takagawa R, Kosaka T, Ono HA, Akiyama H, Fukushima T, Nagahori Y, Takahashi M. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147:204–211. doi: 10.1016/j.surg.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, Shi YQ. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol. 2012;18:3610–3616. doi: 10.3748/wjg.v18.i27.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Cai H, Shi Y, Wang Y. Prognostic factors in patients with node-negative gastric cancer: a single center experience from China. J Gastrointest Surg. 2012;16:1123–1127. doi: 10.1007/s11605-012-1881-y. [DOI] [PubMed] [Google Scholar]
- 11.Zhao LY, Zhang WH, Chen XZ, Yang K, Chen XL, Liu K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Prognostic Significance of Tumor Size in 2405 Patients With Gastric Cancer: A Retrospective Cohort Study. Medicine (Baltimore) 2015;94:e2288. doi: 10.1097/MD.0000000000002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan JW, Ji L, Friedman G, Senthil M, Dyke C, Lum SS. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg. 2015;150:37–43. doi: 10.1001/jamasurg.2014.678. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Zhang R, Zhang L, Liu Y, Hao X, Liang H. Negative node count improvement prognostic prediction of the seventh edition of the TNM classification for gastric cancer. PLoS One. 2013;8:e80082. doi: 10.1371/journal.pone.0080082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu JT, Lin CJ, Sung CM, Yeh HC, Chen TH, Chen TC, Chiang KC, Yeh TS, Hwang TL, Jan YY. Prognostic significance of the number of examined lymph nodes in node-negative gastric adenocarcinoma. Eur J Surg Oncol. 2013;39:1287–1293. doi: 10.1016/j.ejso.2013.07.183. [DOI] [PubMed] [Google Scholar]
- 15.Biffi R, Botteri E, Cenciarelli S, Luca F, Pozzi S, Valvo M, Sonzogni A, Chiappa A, Leal Ghezzi T, Rotmensz N, Bagnardi V, Andreoni B. Impact on survival of the number of lymph nodes removed in patients with node-negative gastric cancer submitted to extended lymph node dissection. Eur J Surg Oncol. 2011;37:305–311. doi: 10.1016/j.ejso.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Scartozzi M, Galizia E, Verdecchia L, Berardi R, Graziano F, Catalano V, Giordani P, Mari D, Silva RR, Marmorale C, Zingaretti C, Cascinu S. Lymphatic, blood vessel and perineural invasion identifies early-stage high-risk radically resected gastric cancer patients. Br J Cancer. 2006;95:445–449. doi: 10.1038/sj.bjc.6603286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee IS, Park YS, Ryu MH, Song MJ, Yook JH, Oh ST, Kim BS. Impact of extranodal extension on prognosis in lymph node-positive gastric cancer. Br J Surg. 2014;101:1576–1584. doi: 10.1002/bjs.9640. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JE, Hong JY, Kim JE, Shim HJ, Bae WK, Hwang EC, Jeong O, Park YK, Lee KH, Lee JH, Cho SH, Chung IJ. Prognostic significance of the concomitant existence of lymphovascular and perineural invasion in locally advanced gastric cancer patients who underwent curative gastrectomy and adjuvant chemotherapy. Jpn J Clin Oncol. 2015;45:541–546. doi: 10.1093/jjco/hyv031. [DOI] [PubMed] [Google Scholar]
- 19.Li P, He HQ, Zhu CM, Ling YH, Hu WM, Zhang XK, Luo RZ, Yun JP, Xie D, Li YF, Cai MY. The prognostic significance of lymphovascular invasion in patients with resectable gastric cancer: a large retrospective study from Southern China. BMC Cancer. 2015;15:370. doi: 10.1186/s12885-015-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Ramos D, Calero A, Escrig-Sos J, Mingol F, Daroca-Jose JM, Sauri M, Arroyo A, Salvador-Sanchis JL, de Juan M, Calpena R, Lacueva FJ. Prognosis for gastric carcinomas with an insufficient number of examined negative lymph nodes. Eur J Surg Oncol. 2014;40:358–365. doi: 10.1016/j.ejso.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Jiao XG, Deng JY, Zhang RP, Wu LL, Wang L, Liu HG, Hao XS, Liang H. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J Gastroenterol. 2014;20:3640–3648. doi: 10.3748/wjg.v20.i13.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14:317–328. doi: 10.1245/s10434-006-9218-2. [DOI] [PubMed] [Google Scholar]
- 23.Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143–2151. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 24.Aurello P, D'Angelo F, Rossi S, Bellagamba R, Cicchini C, Nigri G, Ercolani G, De Angelis R, Ramacciato G. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am Surg. 2007;73:359–366. [PubMed] [Google Scholar]
- 25.Xu D, Huang Y, Geng Q, Guan Y, Li Y, Wang W, Yuan S, Sun X, Chen Y, Li W, Zhou Z, Zhan Y. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PLoS One. 2012;7:e38681. doi: 10.1371/journal.pone.0038681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, Rosa F, Marchet A, Coniglio A, Saragoni L, Veltri M, Pacelli F, Roviello F, Nitti D, Giulini SM, De Manzoni G. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70–73. doi: 10.1097/SLA.0b013e3181e4585e. [DOI] [PubMed] [Google Scholar]
- 27.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 28.Zhang WH, Chen XZ, Liu K, Chen XL, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Outcomes of surgical treatment for gastric cancer patients: 11-year experience of a Chinese high-volume hospital. Med Oncol. 2014;31:150. doi: 10.1007/s12032-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 29.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 30.Huitzil-Melendez FD, Capanu M, O'Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889–2895. doi: 10.1200/JCO.2009.25.9895. 2810.1200/JCO.2009.2825.9895. Epub 2010 May 2810. [DOI] [PMC free article] [PubMed] [Google Scholar]