Abstract
In 2014, NM-2201 (CBL-2201), a novel synthetic cannabinoid (SC), was detected by Russian and United States laboratories. It was already added to the scheduled drugs list in Japan, Sweden and Germany. Unfortunately, no human metabolism data are currently available, making it challenging to confirm its intake because all previous investigated SCs were extensively metabolized. The present study aims to recommend appropriate marker metabolites by investigating NM-2201 metabolism in human hepatocytes and confirm the results in authentic human urine specimens. For the metabolic stability assay, 1 μM NM-2201 was incubated in human liver microsomes (HLMs) for up to 1 h; for metabolite profiling, 10 μM of NM-2201 was incubated in human hepatocytes for 3 h. Two authentic urine specimens from NM-2201 positive cases were analyzed after β-glucuronidase hydrolysis. Metabolite identification in hepatocyte samples and urine specimens was achieved with high-resolution mass spectrometry via information-dependent acquisition. NM-2201 was quickly metabolized in HLMs with an 8.0 min half-life. In human hepatocyte incubation samples, a total of thirteen NM-2201 metabolites were identified, generated mainly from ester hydrolysis and further hydroxylation, oxidative defluorination and subsequent glucuronidation. M13 (5-fluoro PB-22 3-carboxyindole) was the major metabolite. In the urine specimens, the parent drug NM-2201 was not detected; M13 was the predominant metabolite after β-glucuronidase hydrolysis. Therefore, based on our study, we recommend the M13 as a suitable urinary marker metabolite for confirming NM-2201 and/or 5F-PB-22 intake.
Keywords: NM-2201, CBL-2201, Synthetic cannabinoid, In vitro human hepatocyte metabolism, High-resolution mass spectrometry, Authentic human urine specimen
Introduction
Since 2008, synthetic cannabinoids (SCs) are commonly abused as recreational drugs by the smoked or inhaled routes for their psychoactive effects [1, 2]. SCs were initially developed as pharmacological probes for investigating the endocannabinoid system and developing potential therapeutic compounds [3, 4]. Many SCs and their metabolites possess higher binding affinity to cannabinoid receptor 1 (CB1) and CB2 than conventional cannabinoids such as Δ9-tetrahydrocannabinol, producing greater cannabinoid-receptor-mediated effects in the central and peripheral nervous systems [5]. Novel psychoactive substances (NPSs) are manufactured by clandestine chemical laboratories and generally emerge in Europe or Japan before identification in the United States [6]. Abuse of these SCs can result in severe toxic effects such as psychotic episodes, kidney failure, acute cerebral ischemia, myocardial infarction and even death [7-10]. Dependence to these compounds can develop, especially with daily SC intake [11]. For these reasons, many SCs were scheduled in the European Union countries, United States, China, Japan, Russia, Australia, and other countries [6, 12].
To bypass scheduling laws, more structurally diverse analogs are continuously released to the illegal drug market. A total of 858 SCs were scheduled in Japan as narcotics or designated substances as of April 2015 [13]. Besides structural analogs, a new trend is forming, namely positional isomers are now appearing on the illegal drug market. For instance, THJ-2201 was scheduled in the United States in February 2015, and in Japan in August 2014; its positional isomer, FUBIMINA (BIM-2201) quickly emerged and became popular among drug users. FUBIMINA and THJ-2201 shared the same metabolic pathway and their major metabolites were also pairs of isomers, making it challenging to distinguish their intake [14]. This new phenomenon makes it more challenging to confirm which SC was consumed. In most cases, the detailed pharmacological activities of these SCs are unknown and unpredictable, making the easy access and uncontrolled dissemination a serious threat to public health and safety. Potential drug-drug interactions may increase adverse effects when metabolism pathways are altered with simultaneous intake of multiple synthetic drugs [15]. In some SC cases, human metabolism leads to differential pharmacodynamic and pharmacokinetic properties and may produce clinical toxicity such as occurred with JWH-018 and AM-2201 [16].
NM-2201 [naphthalen-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate], also known as CBL-2201, is a recently emerging SC (Fig. 1a). NM-2201 is structurally similar to 5F-PB-22 (Fig. 1b), with the only difference being the naphthalene or quinoline ring, respectively, connected to the N-fluoropentyl indole core via an ester bond. Compared to AM-2201 (Fig. 1c), the linkage between naphthalene and the indole core changed from the carbonyl to the ester in NM-2201. The United States Army Criminal Investigation Laboratory (USACIL) first reported positive NM-2201 cases in October 2014 (unpublished data). NM-2201 also was detected by Russian researchers in 2014 and 2015 [17, 18]. Proliferation of NPSs is a global challenge; identifying NPS intake and associating specific adverse effects with the causative agent requires rapid elucidation of the NPS and their major urinary metabolites, because SCs are usually extensively metabolized and the parent drug is rarely present in urine [17, 19, 20]. To the best of our knowledge, there are no clinical studies investigating pharmacological and toxicological effects and the pharmacokinetics of NM-2201. The limited available data about their effects are found on Internet drug user forums, for instance, www.drug-forum.com, www.bluelight.org. Drug users reported that NM-2201 was potent, strong, and does not last as long as JWH-018 [21]. Caution is warranted when inferring pharmacologic effects, since drug users are frequently unaware of what compounds they are actually taking.
Fig. 1.
Structures of synthetic cannabinoids NM-2201 (a), 5F-PB-22 (b), AM-2201 (c), THJ-2201 (d)
To date, all investigated SCs are extensively metabolized in humans and are predominantly excreted as metabolites in urine [6, 16, 22, 23], complicating detection, as metabolites are initially unknown. Knowledge of human metabolism is essential for verifying NM-2201 consumption since urine testing is routine for workplace, clinical and forensic testing. Unfortunately, controlled human pharmacokinetic studies are not yet possible due to the lack of pharmacology, toxicity and safety data for SCs. Although there are no published data for NM-2201, in vitro metabolism studies of their analogs 5F-PB-22 and AM-2201 are available [12, 22]. 5F-PB-22 underwent extensive ester hydrolysis, followed by oxidative defluorination [12]. However, no human urine samples were studied to confirm the hepatocyte results. For AM-2201, oxidative defluorination with subsequent carboxylation and glucuronidation is the primary metabolic pathway [22]. In is unknown whether the minor structural change from 5F-PB-22 or AM-2201 to NM-2201 alters the metabolic profile.
In the present study, we evaluated the metabolic stability of NM-2201 in human liver microsomes (HLMs); then incubated NM-2201 in human hepatocytes for metabolite profiling. Hepatocytes are superior to HLMs for metabolite profiling because they better mimic the physiological environment [24, 25]. Our previous human hepatocyte metabolism studies proved the success of the hepatocyte incubation model for identifying appropriate marker metabolites, such as AB-FUBINACA [26], AB-PINACA [20], FDU-PB-22 and FUB-PB-22 [27]. Two urine samples collected from individuals suspected of driving under the influence of drugs were also investigated. Metabolite elucidation was achieved with high-performance liquid chromatography (HPLC) coupled to the 5600+ TripleTOF quadrupole/time-of-flight high-resolution mass spectrometer (HR-MS). Human hepatocyte metabolites were compared to urinary metabolites and the optimal metabolites for identifying NM-2201 intake identified.
Our goal was to investigate in vitro human metabolism of NM-2201 and confirm marker metabolites in authentic urine specimens. The identified optimal urinary markers can be incorporated into screening methods for documenting SC intake, associating adverse events with the causative substances, and providing reference standard manufacturers with the most critical metabolites for their synthesis efforts.
Materials and methods
Chemicals and reagents
NM-2201 (95.92% pure) was kindly provided by the Special Testing and Research Laboratory (Dulles, VA, USA), US Drug Enforcement Administration, Department of Justice, USA. 5-Fluoro PB-22 3-carboxyindole metabolite (5F-PI-COOH, ≥98%), PB-22 N-(5-hydroxypentyl)-3-carboxyindole metabolite (5′-OH-PI-COOH, ≥98%), and PB-22 N-pentanoic acid-3-carboxyindole metabolite (PI-COOH pentanoic acid, ≥98%) were purchased from Cayman Chemical (Ann Arbor, MI, USA); HLMs (50-donor pool), NADPH, 10-donor pooled cryopreserved human hepatocytes, Gro CP and Gro KHB buffer from BioreclamationIVT (Baltimore, MD, USA); Red Abalone BG100® β-glucuronidase from Kura Biotec (100,000 U/mL, Puerto Varas, Chile); liquid chromatography-mass spectrometry (LC-MS) grade acetonitrile and ethyl acetate from Sigma-Aldrich (St. Louis, MO, USA); LC-MS grade water, acetic acid, and formic acid from Fisher Scientific (Waltham, MA, USA). Isolute (1 mL) supported liquid extraction (SLE+) cartridges (Biotage, Charlotte, NC, USA) were used in urine sample extraction.
Metabolic stability assay in HLMs
Metabolic stability of NM-2201 was evaluated in HLM suspensions with slight modification with previous descriptions [28, 29]. In brief, the HLM incubation mixture contained 50 mM potassium phosphate buffer (pH 7.4), NADPH regenerating system (glucose-6-phosphate, MgCl2, and glucose-6-phosphate dehydrogenase), and 1 μM NM-2201. Incubation was conducted at 37°C for 1 h in a shaking water bath. Final percentage of organic solvent was <1%. One hundred microliter samples were collected at 0, 3, 8, 13, 20, 30, 45, and 60 min and added to 100 μL ice-cold acetonitrile. Samples were centrifuged (15,000 g, 4°C, 10 min), and then the supernatant was removed and stored at −80°C. After thawing and vortexing, samples were centrifuged (15,000 g, 4°C, 5 min); 10 μL supernatant was diluted with 990 μL mobile phase A/B (90: 10, v/v) and 10 μL injected into the liquid chromatography-tandem mass spectrometry (LC-MS/MS) instrument.
The HPLC system consisted of two LC-20ADxr pumps, a DGU-20A3R degasser, a SIL-20ACxr auto-sampler, and a CTO-20A column oven (Shimadzu, Columbia, MD, USA). Chromatography was performed on a Kinetex™ C18 column (100 mm×2.1 mm ID, 2.6 μm) with a KrudKatcher Ultra HPLC in-line filter (0.5 μm×0.1 mm ID) (Phenomenex, Torrance, CA, USA). Mobile phases were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B); the gradient remained at 10% B for 0.5 min, ramped to 95% B at 10 min, then held until 12.5 min before re-equilibrating at 10% B for another 2.5 min. Total run time was 15 min at 0.3 mL/min. Column and autosampler temperatures were 40 and 4°C, respectively.
NM-2201 concentrations in HLM incubation samples were determined on a 3200 QTRAP mass spectrometer (SCIEX, Redwood City, CA, USA) via positive electrospray ionization (+ESI). +ESI source parameters were: source temperature, 500°C; ion spray voltage, 4000 V; curtain gas, 30 psi; gas 1, 50 psi; gas 2, 50 psi. A pair of multiple reaction monitoring transitions were monitored for NM-2201 (m/z 376.2→232.2; m/z 376.2→144.2). Declustering potential was 36 V (target ion, T) and 50 V (qualifier ion, Q); collision energies were 25 eV (T) and 51 eV (Q).
Peak areas of remaining NM-2201 were plotted versus time points, and microsomal half-life (T1/2) and intrinsic clearance (CLint, micr) were calculated [30]. Intrinsic clearance (CLint) was scaled from microsomal intrinsic clearance in whole-liver dimensions. Human hepatic clearance (CLH) and extraction ratio (ER) were estimated without considering plasma-protein binding.
Metabolic identification in human hepatocytes
Hepatocyte incubation was performed as previously described [29, 31]. Chemical stability of NM-2201 in incubation buffer also was performed (37°C, 3 h) to determine whether metabolite formation is dependent on hepatocyte enzymes. Samples were kept at −80 °C until analysis.
Samples were thawed and vortexed thoroughly. A 100-μL volume of acetonitrile was added to 100 μL sample, and the mixture was vortexed and centrifuged at 15,000 g (4°C, 5 min), and the supernatant transferred to another new tube. After evaporation to dryness under nitrogen at 40°C, and reconstitution in 150 μL mobile phase A/B (80:20, v/v), a 15-μL aliquot was injected for analysis.
The HPLC system consisted of two LC-20ADxr pumps, a DGU-20A5R degasser, a SIL-20ACxr autosampler, and a CTO-20 AC column oven (Shimadzu). Chromatographic separation was achieved on an Ultra Biphenyl column (100 mm×2.1 mm ID, 3 μm; Restek, Bellefonte, PA, USA) equipped with a guard column containing identical packing material. Gradient elution was performed with 0.1 % formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at 0.5 mL/min. Starting gradient was 20% B, held for 0.5 min; then increased to 95% B over 10.5 min, held until 13.0 min; and returned to 20% B at 13.1 min and held until 15.0 min. HPLC eluent was diverted to MS between 2.0 and 13.0 min. The column oven and auto-sampler were set at 30 and 4°C, respectively.
A 5600+ TripleTOF mass spectrometer (SCIEX) was used for data acquisition in +ESI mode. MS data were acquired by information-dependent acquisition (IDA) in combination with multiple mass defect filters (MDF) and dynamic background subtraction. +ESI source parameters were: source temperature, 650°C; ion spray voltage, 4000 V; gas 1, 60 psi; gas 2, 75 psi; curtain gas, 45 psi; declustering potential, 80 V; collision entrance potential, 10 V. For IDA, spectra exceeding 100 cps were selected for the dependent MS/MS scan, isotopes within 1.5 Da were excluded, and mass tolerance was 50 mDa. Spectra were acquired by scanning a mass range of m/z 100–1000 followed by product ion scanning from m/z 60 to 1000. Collision energy spread was 35 ±15 eV. The mass spectrometer was automatically calibrated every three injections.
Acquired MS data were processed by MetabolitePilot (version 1.5, SCIEX) incorporating different peak-finding algorithms (product ion and neutral loss, MDF, predicted biotransformation, and generic LC peak-finding) for mining possible metabolites. LC peak, MS, and MS/MS intensity thresholds were set at 500, 100 and 50 cps, respectively.
Analysis of authentic human urine specimens
Two urine samples collected from individuals suspected of driving under the influence of drugs were provided by the National Board of Forensic Medicine in Linköping, Sweden. These two specimens were not from human experimental investigations. Specimens were anonymized and de-identified before shipping to our laboratory for analysis. These specimens are exempt from the institutional review board approval as they are anonymized and do not fall under human experimental investigations. The corresponding blood samples of the two urine samples were positive for NM-2201.
Urine samples were prepared with and without enzymatic hydrolysis. β-Glucuronidase hydrolysis was performed as described previously with slight modifications [19, 32]. Briefly, 250 μL urine was diluted with 600 μL ammonium acetate buffer (0.4 M, pH 4.0). β-Glucuronidase (40 μL, 15625 IU/mL) was added and mixed; then the mixture was incubated at 55°C for 1 h before being quenched by 200 μL acetonitrile. For non-enzymatic hydrolysis samples, 250 μL urine was diluted with 640 μL ammonium acetate buffer (0.4 M, pH 4.0) and 200 μL acetonitrile. After being centrifuged (15,000 g, 4°C, 5 min), all samples were loaded onto the Isolute SLE+ cartridges and eluted twice with 3 mL ethyl acetate. Extracts were dried at 45°C under nitrogen, and reconstituted in 250 μL mobile phase A/B (80:20, v/v). A 25-μL volume of reconstituted solution was injected for analysis; data acquisition and processing were the same as for hepatocyte samples.
Results
Metabolic stability of NM-2201 in HLMs
In HLM, the in vitro T1/2 of NM-2201 was calculated as 8.0 ± 1.5 min; in vitro CLint, micr was 0.088 mL/min/mg, corresponding to an intrinsic clearance (CLint) of 81.6 mL/min/kg after scaling to whole-liver-dimensions [33]. Without considering plasma-protein binding and using a simplified Rowland’s equation [30, 34], we calculated the human CLH as 16.1 mL/min/kg and ER to be 0.80.
Analysis of NM-2201 and reference standards of three possible metabolites
NM-2201 and three possible metabolites’ chromatographic and MS fragmentation were studied first with reference standards, i.e., 5F-PI-COOH, 5′-OH-PI-COOH, and PI-COOH pentanoic acid. The fragmentation patterns of major product ions were characterized based on accurate mass measurement, and used to facilitate elucidation of potential metabolites. The three metabolites are supposed to be shared by NM-2201 and 5F-PB-22 (Fig. 1a, b) after ester hydrolysis, as shown previously [12].
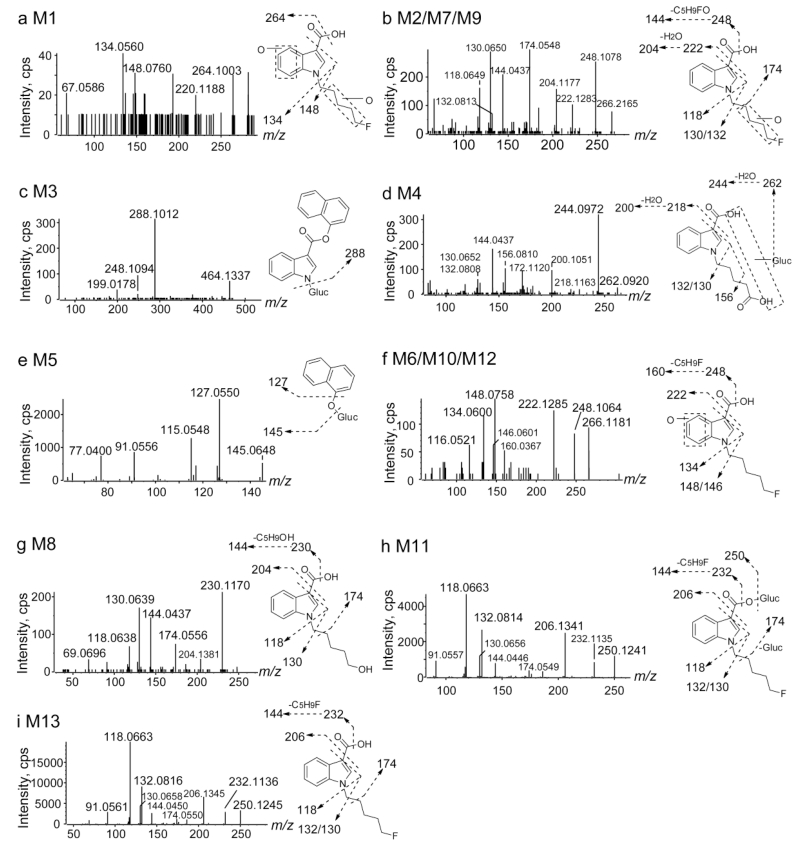
NM-2201 with [M + H]+ at m/z 376.1717 eluted at 9.60 min and showed fragments at m/z 144.0446, 171.0445, 206.1343, 232.1140 and 358.1611 (Fig. 2a, b). The base peak ion at m/z 232.1140 was generated by cleavage of the ester bond; further neutral loss of CO or the C5H9F chain led to m/z 206.1343 and 144.0446, respectively; m/z 171.0445 derived from a bond cleavage between the ester and the indole core.
Fig. 2.
Extracted ion chromatograms, corresponding product ion spectra and proposed fragmentation modes of reference standards NM-2201 (m/z 376.1703, a & b), 5-fluoro PB-22 3-carboxyindole metabolite (5F-PI-COOH; m/z 250.1250, c & d), PB-22 N-(5-hydroxypentyl)-3-carboxyindole metabolite (5′-OH-PI-COOH; m/z 248.1289, e & f), and PB-22 N-pentanoic acid-3-carboxyindole metabolite (PI-COOH pentanoic acid; m/z 262.1079, g & h)
5F-PI-COOH eluted at 6.06 min and displayed a protonated molecular ion at m/z 250.1250. It produced the characteristic fragments at m/z 118.0662, 130.0659/132.0816, 144.0450, 174.0552, 206.1344 and 232.1139 (Fig. 2c, d). Fragments at m/z 232.1139 and 206.1344 were generated via neutral loss of H2O or CO2 from its precursor m/z 250.1246. The most intense ions at m/z 132.0816 and 118.0662 represent the indole core with or without α-methylene. Ion m/z 174.0552 was formed by cleavage of the α and β carbon-carbon bond; m/z 144.0450 represents the indole ring with the attached carbonyl group.
5′-OH-PI-COOH possessed the protonated ion at m/z 248.1289 with a retention time of 4.18 min. It yielded characteristic fragments at m/z 118.0663, 130.0658, 144.0449, 174.0556, 186.1281, 204.1399 and 230.1186 (Fig. 3e, f). 5′-OH-PI-COOH is the oxidative defluorination product of 5F-PI-COOH; this pathway was frequently reported for ω-fluoropentyl chain containing SCs [14, 35]. 5′-OH-PI-COOH and 5F-PI-COOH behaved similarly in fragmentation patterns. Fragments at m/z 230.1186 and 204.1399 were generated via neutral loss of H2O or CO2 from its precursor m/z 248.1289. Other fragments were the same as those of 5F-PI-COOH.
Fig. 3.
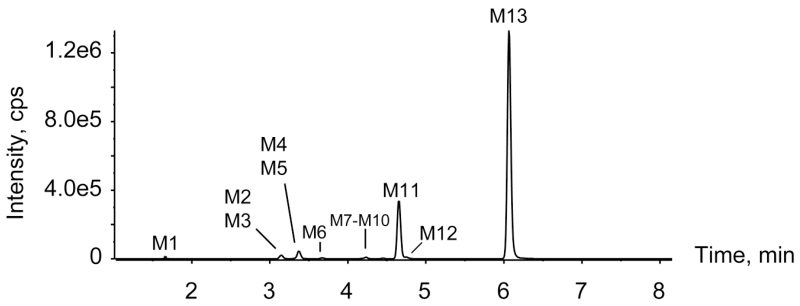
High-performance liquid chromatogram after being processed by MetabolitePilot showing metabolic profile of NM-2201 after three hour incubation in human hepatocytes
PI-COOH pentanoic acid with [M + H]+ at m/z 262.1079 eluted at 4.21 min and yielded characteristic fragments at m/z 118.0651, 144.0443, 156.0810, 172.1120, 200.1073, 218.1187 and 244.0973 (Fig. 3g, h). PI-COOH pentanoic acid is the further oxidation product of 5′-OH-PI-COOH. Peak fragment m/z 244.0973 was from neutral loss of H2O, and further loss of CO2 took place, leading to m/z 200.1073. Fragment m/z 144.0443 was the same as that from NM-2201 and 5F-PI-COOH; m/z 156.0810 and 172.1120 were generated from neutral loss of HCOOH and further loss of HCOOH (or CH3COOH) from its precursor m/z 262.1079.
All of the above fragments were used as diagnostic ions for metabolite elucidation as similar cleavage patterns could be expected in metabolites.
NM-2201 metabolites in human hepatocytes
In the three hour hepatocyte incubation sample, nine phase I and four phase II metabolites were detected for NM-2201; the parent compound was absent (Fig. 3, Table 1). None of these metabolites were observed after incubating NM-2201 in the buffer only for three hours, indicating metabolites formation was enzyme-dependent. Table 1 lists all the metabolites with their metabolic pathways, retention times, detected m/z, mass errors, elemental composition, fragment ions and peak areas in the three hour sample. Metabolites were labeled “M” in the order of retention time. An overview of the metabolic pathway is shown in Fig. 4. Detailed metabolite elucidation is explained as follows.
Table 1.
Identification of NM-2201 metabolites after three hour incubation with human hepatocytes
| ID | Biotransformation | Retention time (min) |
[M + H]+ (m/z) |
Mass error (ppm) |
Formula | Fragment ions | Peak area | Rank |
|---|---|---|---|---|---|---|---|---|
| M1 | 5F-PI-COOH di-oxidation | 1.67 | 282.1131 | −1.9 | C14H16NO4F | 134.0560, 148.0760, 264.1003 | 1.11E+04 | 11 |
| M2 | 5F-PI-COOH oxidation (fluoropentyl) | 3.15 | 266.1186 | −0.2 | C14H16NO3F | 118.0649, 130.0650, 144.0437, 174.0548, 204.1177, 222.1283, 248.1078 |
4.38E+04 | 4 |
| M3 | N-dealkylation + glucuronidation | 3.15 | 464.1321 | −4.0 | C25H21NO8 | 288.1012 | 2.88E+04 | 5 |
| M4 | 5F-PI-COOH oxidative defluorination to carboxylic acid + glucuronidation |
3.33 | 438.1410 | 0.7 | C20H23NO10 | 130.0652, 132.0808, 144.0437, 172.1120, 200.1051, 244.0972, 262.1076 |
9.35E+03a | 13 |
| M5 | Naphthol + glucuronidation | 3.37 | 321.0975 | 2.0 | C16H16O7 | 115.0548, 127.0550, 145.0648 | 1.35E+05a | 3 |
| M6 | 5F-PI-COOH oxidation (indole) | 3.67 | 266.1187 | −0.1 | C14H16NO3F | 116.0521, 134.0600, 146.0601, 148.0758, 160.0367, 222.1285, 248.1064 |
1.86E+04 | 9 |
| M7 | 5F-PI-COOH oxidation (fluoropentyl) | 4.10 | 266.1191 | 1.5 | C14H16NO3F | 118.0640, 130.0669, 144.0466, 174.0550, 184.1146, 222.1292, 248.1078 |
2.15E+04 | 8 |
| M8 | 5F-PI-COOH oxidative defluorination | 4.18 | 248.1297 | 6.2 | C14H17NO3 | 118.0638, 130.0639, 144.0437, 174.0556, 204.1381, 230.1170 |
1.05E+04 | 12 |
| M9 | 5F-PI-COOH oxidation (fluoropentyl) | 4.23 | 266.1191 | 1.5 | C14H16NO3F | 130.0646, 132.0815, 144.0412, 174.0552, 204.1135, 222.1277, 248.1052 |
2.76E+04 | 6 |
| M10 | 5F-PI-COOH oxidation (indole) | 4.45 | 266.1181 | −2.2 | C14H16NO3F | 116.0499, 134.0597, 148.0768, 160.0404, 222.1288, 248.1126 |
1.34E+04 | 10 |
| M11 | 5F-PI-COOH glucuronidation | 4.64 | 426.1558 | −0.3 | C20H24NO8F | 118.0663, 130.0656, 132.0814, 144.0446, 174.0549, 206.1341, 232.1135, 250.1241 |
1.05E+06a | 2 |
| M12 | 5F-PI-COOH oxidation (indole) | 4.75 | 266.1188 | 0.2 | C14H16NO3F | 116.0523, 134.0594, 148.0763, 160.0391, 222.1273, 248.1096 |
2.20E+04 | 7 |
| M13 | Ester hydrolysis to 5F-PI-COOH | 6.06 | 250.1242 | 1.9 | C14H16NO2F | 118.0663, 130.0658, 132.0816, 144.0450, 174.0550, 206.1345, 232.1136 |
4.36E+06 | 1 |
ID, identification; 5F-PI-COOH, 5-fluoro PB-22 3-carboxyindole
area of corresponding aglycone due to in-source fragmentation
Fig. 4.
Metabolic pathway of NM-2201 in human hepatocytes. Gluc, glucuronide. Bold arrows implicate major pathways
Ester hydrolysis
NM-2201 was extensively hydrolyzed via ester hydrolysis, generating M13, and 1-naphthol, which was identified in the form of naphthol glucuronide (M5, Fig. 4).
M13 displayed a protonated molecular ion [M + H]+ at m/z 250.1242 and eluted at 6.06 min. Its product ion spectrum revealed fragments at m/z 118.0663, 130.0658/132.0816, 144.0450, 174.0550, 206.1345 and 232.1136 (Fig. 5i). M13 chromatographic and MS behaviors (i.e., retention time, product ions and relative ion ratios) were identical to 5F-PI-COOH reference standard (Fig. 2d). Therefore, M13 was assigned as 5F-PI-COOH.
Fig. 5.
Product ion spectra, proposed structures and fragmentation of NM-2201 metabolites. Gluc, glucuronide
M5 (naphthol glucuronide) eluted early at 3.37 min and produced fragments at m/z 115.0548, 127.0550 and 145.0648 (Fig. 5e). Product ion m/z 145.0648 was formed by neutral loss of 176.0327 (glucuronide); further loss of water led to m/z 127.0550. M5 (naphthol glucuronide) was also observed as a minor metabolite of FDU-PB-22 after ester hydrolysis [27].
M13 further hydroxylation or glucuronidation
The predominant metabolite M13 underwent further hydroxylation and/or glucuronidation, yielding ten metabolites (M1, M2, M4, and M6 – M12).
Direct glucuronidation of M13 led to M11. M11 eluted at 4.64 min and revealed product ions at m/z 118.0663, 130.0656/132.0814, 144.0446, 174.0549, 206.1341, 232.1135, and 250.1241 (Fig. 5h). The product ion m/z 250.1241 was formed by neutral loss of glucuronide (−176.0317 Da) from precursor ion m/z 426.1558; other product ions were the same as those of M13, suggesting M11 was a glucuronide of M13.
Further metabolism of M13 generated six mono-hydroxylated and one di-hydroxylated metabolite. Among these, three were hydroxylated at the fluoropentyl chain (i.e., M2, M7, M9), three were hydroxylated on the indole moiety (i.e., M6, M10, M12), and the di-hydroxylated metabolite M1 was hydroxylated at both the fluoropentyl chain and indole moiety.
M2, M7 and M9 showed precursor ions at m/z 266.1186 and shared similar fragments at m/z 118.0649, 130.0650/132.0813, 144.0437, 174.0548, 204.1177, 222.1283, and 248.1078 (Fig. 5b). Precursor ion m/z 266.1186 and product ions m/z 222.1283 and 248.1078 were 15.9944 Da (+ O) larger than corresponding ions of M13 (5F-PI-COOH). Other fragments m/z 118.0649, 130.0650/132.0813, 144.0437, 174.0548 were the same as those of M13, indicating the indole moiety was unmodified. Therefore, we propose the hydroxylation to be on the fluoropentyl chain.
M6, M10 and M12 displayed precursor ions at m/z 266.1187 and shared similar fragments at m/z 116.0521, 134.0600, 148.0758, 160.0367, 222.1285 and 248.1064 (Fig. 5F). Precursor ion m/z 266.1187, and product ions m/z 134.0600, 148.0758, 160.0367, 222.1285, and 248.1064 were 15.9945 Da (+ O) larger than corresponding ions of M13 (5F-PI-COOH), indicating the indole moiety was hydroxylated. Another ion m/z 116.0521 was generated from neutral loss of H2O from m/z 134.0600.
The earliest eluting metabolite, M1 is di-hydroxylated metabolite of M13. M1 demonstrated a precursor ion at m/z 282.1131 and fragments at m/z 134.0560, 148.0760, 220.1188, and 264.1003 (Fig. 5A). Precursor ion m/z 282.1131 and product ion m/z 264.1003 were 31.9889 Da (+ 2O) larger than those of M13 (i.e., m/z 250.1242 and 232.1136), and product ions m/z 134.0560 and 148.0760 were 15.9957 Da (+ O) larger than corresponding ions of M13 (5F-PI-COOH), indicating the indole moiety was hydroxylated and the other hydroxylation took place at the fluoropentyl chain.
M13 oxidative defluorination and sequential metabolism
Oxidative defluorination of M13 led to the formation of M8 ([M + H]+ at m/z 248.1297). M8 eluted at 4.18 min and produced fragments at m/z 118.0638, 130.0639, 144.0437, 174.0556, 204.1381 and 230.1170 (Fig. 5g). Product ions m/z 204.1381 and 230.1170 were 1.9964 Da (+ OH − F) less than corresponding ions of M13, indicating oxidative defluorination. Furthermore, M8 chromatographic and MS behaviors (i.e., product ions and retention time) were identical to the reference standard of 5′-OH-PI-COOH (Fig. 2f). Therefore, M8 is assigned as 5′-OH-PI-COOH.
Further oxidation and glucuronidation of M8 led to the formation of M4 ([M + H]+ at m/z 438.1410). M4 eluted at 3.33 min and revealed fragments at m/z 130.0652/132.0808, 144.0437, 156.0810, 172.1120, 200.1051, 244.0972, and 262.0920 (Fig. 5d). Product ion m/z 262.0920 was produced by neutral loss of glucuronide from the precursor ion m/z 438.1410. All other fragments and ion abundance ratios were similar to PI-COOH pentanoic acid (Fig. 2h). Therefore, M4 was assigned as the glucuronide of PI-COOH pentanoic acid. PI-COOH pentanoic acid itself was not detected in the hepatocyte incubation samples.
N-Dealkylation and glucuronidation
All the above metabolites are generated from extensive ester hydrolysis of NM-2201. We also observed a minor metabolite M3 without hydrolysis in the three hour hepatocyte incubation sample. M3 eluted at 3.15 min with the protonated molecular ion being m/z 464.1321. The product ion spectrum showed an intensive fragment at 288.1012 (Fig. 5c). Product ion m/z 288.1012 was 176.0325 Da (glucuronide) less than the precursor ion m/z 464.1337, and is also 88.0705 Da less than the protonated molecular ion of NM-2201 (m/z 376.1717), corresponding to loss of the C5H9F chain. Therefore, M3 was assigned as the glucuronide of N-dealkylated NM-2201.
Metabolite profiling in authentic urine specimens
In authentic human urine specimens, several metabolites were observed whereas the parent drug was not detected, suggesting that NM-2201 was highly metabolized in humans. As shown in Fig. 6a, c, three metabolites were identified in authentic urine 1 and 2 before hydrolysis, namely, M7, M11 and M13. Glucuronide conjugate M11, and its aglycone M13 (5F-PI-COOH) were the predominant metabolites.
Fig. 6.
High-performance liquid chromatograms after being processed by MetabolitePilot showing metabolic profiles of NM-2201 in case urine 1 (a, unhydrolyzed; b, hydrolyzed) and case urine 2 (c, unhydrolyzed; d, hydrolyzed)
After hydrolysis with β-glucuronidase, M11 disappeared, and the abundance of M13 increased significantly (Fig. 6b, d). This phenomena further confirmed M11 being the glucuronide of M13 (Fig. 4). Besides M13, three mono-hydroxylated metabolites M7, M9 and M12 were detected, at much lower abundance than M13. M9 and M12 were not detected before hydrolysis, probably because the glucuronides’ MS signals were low due to in-source fragmentation.
Discussion
HLM metabolic stability
In the pharmaceutical industry, in vitro T1/2 and CLint estimate a drug’s susceptibility to metabolism, and assist in predicting in vivo hepatic clearance, in vivo half-life, and bioavailability [30]. Short T1/2, high CLint and estimated ER indicate that NM-2201 is a rapidly metabolized drug [33, 36]. HLMs are rich in carboxylesterases and cytochrome P450 oxidases [37]; probably the liver carboxylesterases catalyzed the hydrolysis of NM-2201.
Metabolism of NM-2201 as compared to 5F-PB-22 and AM-2201
As shown in Fig. 1, NM-2201 and 5F-PB-22 possess quite similar substructures, i.e., an indole connects with naphthalene or quinoline via an ester bond. NM-2201 was metabolized significantly after 3 h incubation in hepatocytes. Ideally, intense metabolites that are specific for SCs should be targeted in forensic cases. In our study, however, no specific marker for NM-2201 could be identified because NM-2201 and 5F-PB-22 are hydrolyzed quickly to the same primary metabolite, 5F-PI-COOH (M13), after losing essential components of the original structures. Though not perfect, 5F-PI-COOH is considered the best target metabolite for NM-2201 based on our results. However, it is challenging to distinguish the consumption of NM-2201 from 5F-PB-22 based on the detection of 5F-PI-COOH in human urine. NM-2201 generated a specific alcohol conjugated metabolite M5 (naphthol glucuronide), which is not detected in 5F-PB-22. Unfortunately, the structures of M5 is too common and simple to be a representative marker of NM-2201 as it can also derive from other drugs of abuse (i.e., FDU-PB-22), medicine, or food supplements [38].
As described in previous studies, oxidative defluorination and subsequent carboxylation were the major metabolic pathways for ω-fluoropentyl chain containing drugs, such as AM-2201 [22], 5F-AKB-48 [35], THJ-2201 [39], and FUBIMINA [14]. However, in this study, although oxidative defluorination and carboxylation metabolites of 5F-PI-COOH (M13) were observed i.e., M8 and M4, they were only minor metabolites as compared to their abundance as metabolites of AM-2201, THJ-2201 and FUBIMINA. This was also observed in the metabolic profile of 5F-PB-22, where the hydrolysis metabolite 5F-PI-COOH was the predominant metabolite [12]. We conclude that SCs with a N-fluoropentyl indole (or indazole) connected to a naphthalene (or quinoline) via a carbonyl linkage do not generally undergo ester hydrolysis, but oxidative defluorination and subsequent carboxylation dominate metabolism. For SCs with N-fluoropentyl indole (or indazole) connected to naphthalene (or quinoline) via an ester bond linkage, there is extensive ester hydrolysis, while oxidative defluorination and subsequent carboxylation occur only to a minor extent after significant ester hydrolysis.
Urinary metabolites for documenting NM-2201 intake
Urine is a common matrix for drug detection and screening due to its non-invasive collection, adequate specimen volume, and wider detection windows and higher drug concentrations than blood; however, SC metabolites predominate in urine [19, 22, 27], hence showing the importance of identifying urinary metabolites. When extrapolating the hepatocyte metabolite profile to human urine, some factors such as extrahepatic metabolism [40], kidney uptake or efflux transporters [41], metabolites’ enrichment in urine [42-44], and time intervals after drug consumption may affect the abundance ratios of urinary metabolites. Therefore, it is important to evaluate the metabolic profiles in real urine specimens if available, to further document NM-2201 urinary markers.
In our study, we analyzed two urine specimens collected from individuals suspected of driving under the influence of drugs. The corresponding blood samples for urine samples 1 and 2 were positive for NM-2201. For both urine samples, four metabolites were detected after β-glucuronidase hydrolysis, namely, M13 (5F-PI-COOH), and three hydroxylated metabolites of M13 (M7, M9, and M12). Similar to the metabolic profile in the three hour hepatocyte incubation sample, M13 also was observed to be the predominant metabolite in both urine specimens. The three hydroxylated metabolites were too minor to be considered marker metabolites for NM-2201.
In some jurisdictions, unequivocal identification of the substance consumed is required for forensic testing, especially when identical metabolites may arise from a scheduled and non-scheduled compound. Therefore, metabolite M13 (5F-PI-COOH) is a good urinary marker to confirm consumption of NM-2201 and/or 5F-PB-22. However, it is not easy to distinguish NM-2201 from 5F-PB-22 intake based on urine results only, because the same primary metabolite M13 is produced by extensive ester hydrolysis. Other metabolites derived from M13, were similar but present only as minor metabolites. As discussed above, M5 (naphthol) and quinolinol can derive from many origins and their structures are too simple to be specific for NM-2201 or 5F-PB-22. Therefore, we cannot determine which SC was consumed based on the urine results only. Definitive differentiation of NM-2201 from 5F-PB-22 intake would require identification of the parent compound in blood or oral fluid; however, the windows of detection of the potent parent compounds in these matrices are expected to be short due to their metabolic instability and low dosage.
Success of hepatocyte model in proposing urine marker metabolites
Human hepatocytes offer many advantages over HLMs for identifying human metabolites of NPSs despite higher cost. Hepatocytes are more representative of the physiological liver environment containing all phase I and II drug-metabolizing enzymes, cofactors (such as NADPH), and drug transporters [25, 45]. In addition, compounds must penetrate across cell membranes before being metabolized, which is not the case in HLMs. The hepatocyte model proved to be successful in confirming consumption of AB-FUBINACA [26], AB-PINACA [20], FDU-PB-22 and FUB-PB-22 [27]. In the current study, the primary metabolite in hepatocytes, M13 (5F-PI-COOH), was also the dominant metabolite in clinical urine specimens after β-glucuronidase hydrolysis.
Our data help clinical laboratories to target urinary markers of NM-2201 intake. These data also enable linkage of adverse events to specific SCs or in this case to NM-2201. Our hepatocyte incubation and HR-MS analysis workflow is applicable for studies of newly emerging SCs.
Conclusions
We characterized, for the first time, in vitro and in vivo human metabolism of NM-2201. The hepatocyte model proved successful in recommending major urinary metabolites. In authentic urine specimens, the parent drug was not detected; M13 (5F-PI-COOH) and M11 (the glucuronide of M13) were the primary metabolites. M11 was completely converted to M13 after β-glucuronidase hydrolysis. Thus, we propose M13 (5F-PI-COOH) as the best urinary marker for confirming NM-2201 consumption. However, it should be noted that 5F-PI-COOH is also the major metabolite of 5F-PB-22, which is a structural analog of NM-2201. Definitive distinguishing NM-2201 from 5F-PB-22 intake requires identification of the parent compound in blood or oral fluid. Our data lay the foundation for future forensic and clinical scientists to develop analytical screening methods for identifying NM-2201 intake.
Acknowledgments
This research is supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. NM-2201 was donated generously by the US Drug Enforcement Administration. We also appreciate Dr. Ariane Wohlfarth’s help in performing the HLMs metabolic stability assays.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 2.Namera A, Kawamura M, Nakamoto A, Saito T, Nagao M. Comprehensive review of the detection methods for synthetic cannabinoids and cathinones. Forensic Toxicol. 2015;33:175–194. doi: 10.1007/s11419-015-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(Suppl 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huffman JW, Dai D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–566. [Google Scholar]
- 5.Cooper ZD. Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr Psychiatry Rep. 2016;18:52. doi: 10.1007/s11920-016-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheidweiler KB, Jarvis MJ, Huestis MA. Nontargeted SWATH acquisition for identifying 47 synthetic cannabinoid metabolites in human urine by liquid chromatography-high-resolution tandem mass spectrometry. Anal Bioanal Chem. 2015;407:883–897. doi: 10.1007/s00216-014-8118-8. [DOI] [PubMed] [Google Scholar]
- 7.Hermanns-Clausen M, Kneisel S, Szabo B, Auwarter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 8.Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrester MB, Kleinschmidt K, Schwarz E, Young A. Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol. 2012;31:1006–1011. doi: 10.1177/0960327111421945. [DOI] [PubMed] [Google Scholar]
- 10.Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med. 2012;30:1320, e1325–e1327. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Ustundag MF, Ozhan Ibis E, Yucel A, Ozcan H. Synthetic cannabis-induced mania. Case Rep Psychiatry. 2015:310930. doi: 10.1155/2015/310930. 2015. doi: 10.1155/2015/310930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlfarth A, Gandhi AS, Pang S, Zhu M, Scheidweiler KB, Huestis MA. Metabolism of synthetic cannabinoids PB-22 and its 5-fluoro analog, 5F-PB-22, by human hepatocyte incubation and high-resolution mass spectrometry. Anal Bioanal Chem. 2014;406:1763–1780. doi: 10.1007/s00216-014-7668-0. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama N, Asakawa K, Kikura-Hanajiri R, Tsutsumi T, Hakamatsuka T. A new pyrazole-carboxamide type synthetic cannabinoid AB-CHFUPYCA [N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1H-pyrazole-5-carboxamide] identified in illegal products. Forensic Toxicol. 2015;33:367–373. doi: 10.1007/s11419-015-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao X, Scheidweiler KB, Wohlfarth A, Zhu M, Pang S, Huestis MA. Strategies to distinguish new synthetic cannabinoid FUBIMINA (BIM-2201) intake from its isomer THJ-2201: metabolism of FUBIMINA in human hepatocytes. Forensic Toxicol. 2016 doi: 10.1007/s11419-016-0312-2. doi: 10.1007/s11419-016-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm NB, Nielsen LM, Linnet K. CYP3A4 mediates oxidative metabolism of the synthetic cannabinoid AKB-48. AAPS J. 2015;17:1237–1245. doi: 10.1208/s12248-015-9788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Baranovsky A, Shafran Y. Synthetic cannabinoids as designer drugs: new representatives of indol-3-carboxylates series and indazole-3-carboxylates as novel group of cannabinoids. Identification and analytical data. Forensic Sci Int. 2014;244:263–275. doi: 10.1016/j.forsciint.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Kondrasenko AA, Goncharov EV, Dugaev KP, Rubaylo AI. CBL-2201. Report on a new designer drug: napht-1-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate. Forensic Sci Int. 2015;257:209–213. doi: 10.1016/j.forsciint.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Castaneto MS, Wohlfarth A, Pang S, Zhu M, Scheidweiler KB, Kronstrand R, Huestis MA. Identification of AB-FUBINACA metabolites in human hepatocytes and urine using high-resolution mass spectrometry. Forensic Toxicol. 2015;33:295–310. [Google Scholar]
- 20.Wohlfarth A, Castaneto MS, Zhu M, Pang S, Scheidweiler KB, Kronstrand R, Huestis MA. Pentylindole/pentylindazole synthetic cannabinoids and their 5-fluoro analogs produce different primary metabolites: metabolite profiling for AB-PINACA and 5F-AB-PINACA. AAPS J. 2015;17:660–677. doi: 10.1208/s12248-015-9721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [Accessed 28 April, 2016];2016 www.bluelight.org. http://www.bluelight.org/vb/threads/721477-Any-Information-about-NM-2201.
- 22.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal. 2012;4:745–753. doi: 10.1002/dta.1418. [DOI] [PubMed] [Google Scholar]
- 23.Andersson M, Diao X, Wohlfarth A, Scheidweiler KB, Huestis MA. Metabolic profiling of new synthetic cannabinoids AMB and 5F-AMB by human hepatocyte and liver microsome incubations and high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2016;30:1067–1078. doi: 10.1002/rcm.7538. [DOI] [PubMed] [Google Scholar]
- 24.Diao X, Pang X, Xie C, Guo Z, Zhong D, Chen X. Bioactivation of 3-n-butylphthalide via sulfation of its major metabolite 3-hydroxy-NBP: mediated mainly by sulfotransferase 1A1. Drug Metab Dispos. 2014;42:774–781. doi: 10.1124/dmd.113.056218. [DOI] [PubMed] [Google Scholar]
- 25.Soars MG, McGinnity DF, Grime K, Riley RJ. The pivotal role of hepatocytes in drug discovery. Chem-Biol Interact. 2007;168:2–15. doi: 10.1016/j.cbi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Castaneto MS, Wohlfarth A, Pang SK, Zhu MS, Scheidweiler KB, Kronstrand R, Huestis MA. Identification of AB-FUBINACA metabolites in human hepatocytes and urine using high-resolution mass spectrometry. Forensic Toxicol. 2015;33:295–310. [Google Scholar]
- 27.Diao X, Scheidweiler KB, Wohlfarth A, Pang S, Kronstrand R, Huestis MA. In vitro and in vivo human metabolism of synthetic cannabinoids FDU-PB-22 and FUB-PB-22. AAPS J. 2016;18:455–464. doi: 10.1208/s12248-016-9867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P, Zhao Y, Zhu Y, Sun J, Yerke A, Sang S, Yu Z. Metabolism of dictamnine in liver microsomes from mouse, rat, dog, monkey, and human. J Pharm Biomed Anal. 2016;119:166–174. doi: 10.1016/j.jpba.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Ellefsen KN, Wohlfarth A, Swortwood MJ, Diao X, Concheiro M, Huestis MA. 4-Methoxy-α-PVP: in silico prediction, metabolic stability, and metabolite identification by human hepatocyte incubation and high-resolution mass spectrometry. Forensic Toxicol. 2016;34:61–75. doi: 10.1007/s11419-015-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, Garberg P, Postlind H. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58:453–472. [PubMed] [Google Scholar]
- 31.Swortwood MJ, Carlier J, Ellefsen KN, Wohlfarth A, Diao X, Concheiro-Guisan M, Kronstrand R, Huestis MA. In vitro, in vivo and in silico metabolic profiling of alpha-pyrrolidinopentiothiophenone, a novel thiophene stimulant. Bioanalysis. 2016;8:65–82. doi: 10.4155/bio.15.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Chen H, Sang S. Trapping methylglyoxal by genistein and its metabolites in mice. Chem Res Toxicol. 2016;29:406–414. doi: 10.1021/acs.chemrestox.5b00516. [DOI] [PubMed] [Google Scholar]
- 33.McNaney CA, Drexler DM, Hnatyshyn SY, Zvyaga TA, Knipe JO, Belcastro JV, Sanders M. An automated liquid chromatography-mass spectrometry process to determine metabolic stability half-life and intrinsic clearance of drug candidates by substrate depletion. Assay Drug Dev Technol. 2008;6:121–129. doi: 10.1089/adt.2007.103. [DOI] [PubMed] [Google Scholar]
- 34.Diao XX, Zhong K, Li XL, Zhong DF, Chen XY. Isomer-selective distribution of 3-n-butylphthalide (NBP) hydroxylated metabolites, 3-hydroxy-NBP and 10-hydroxy-NBP, across the rat blood-brain barrier. Acta Pharmacol Sin. 2015;36:1520–1527. doi: 10.1038/aps.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vikingsson S, Josefsson M, Green H. Identification of AKB-48 and 5F-AKB-48 metabolites in authentic human urine samples using human liver microsomes and time of flight mass spectrometry. J Anal Toxicol. 2015;39:426–435. doi: 10.1093/jat/bkv045. [DOI] [PubMed] [Google Scholar]
- 36.Lave T, Dupin S, Schmitt C, Valles B, Ubeaud G, Chou RC, Jaeck D, Coassolo P. The use of human hepatocytes to select compounds based on their expected hepatic extraction ratios in humans. Pharmaceut Res. 1997;14:152–155. doi: 10.1023/a:1012036324237. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen R, Nielsen LM, Holm NB, Rasmussen HB, Linnet K. Synthetic cannabimimetic agents metabolized by carboxylesterases. Drug Test Anal. 2015;7:565–576. doi: 10.1002/dta.1731. [DOI] [PubMed] [Google Scholar]
- 38.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1999;16:697–709. doi: 10.1039/a809408j. [DOI] [PubMed] [Google Scholar]
- 39.Diao X, Wohlfarth A, Pang S, Scheidweiler KB, Huestis MA. High-resolution mass spectrometry for characterizing the metabolism of synthetic cannabinoid THJ-018 and its 5-fluoro analog THJ-2201 after incubation in human hepatocytes. Clin Chem. 2016;62:157–169. doi: 10.1373/clinchem.2015.243535. [DOI] [PubMed] [Google Scholar]
- 40.Li XD, Xia SQ, Lv Y, He P, Han J, Wu MC. Conjugation metabolism of acetaminophen and bilirubin in extrahepatic tissues of rats. Life Sci. 2004;74:1307–1315. doi: 10.1016/j.lfs.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Gao C, Zhang H, Guo Z, You T, Chen X, Zhong D. Mechanistic studies on the absorption and disposition of scutellarin in humans: selective OATP2B1-mediated hepatic uptake is a likely key determinant for its unique pharmacokinetic characteristics. Drug Metab Dispos. 2012;40:2009–2020. doi: 10.1124/dmd.112.047183. [DOI] [PubMed] [Google Scholar]
- 42.Xie C, Zhou J, Guo Z, Diao X, Gao Z, Zhong D, Jiang H, Zhang L, Chen X. Metabolism and bioactivation of famitinib, a novel inhibitor of receptor tyrosine kinase, in cancer patients. Br J Pharmacol. 2013;168:1687–1706. doi: 10.1111/bph.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao R, Li L, Xie C, Diao X, Zhong D, Chen X. Metabolism and pharmacokinetics of morinidazole in humans: identification of diastereoisomeric morpholine N+-glucuronides catalyzed by UDP glucuronosyltransferase 1A9. Drug Metab Dispos. 2012;40:556–567. doi: 10.1124/dmd.111.042689. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Wang K, Jiang YZ, Chang XW, Dai CF, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits human ovarian cancer cell proliferation. Cell Oncol. 2014;37:429–437. doi: 10.1007/s13402-014-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li AP, Gorycki PD, Hengstler JG, Kedderis GL, Koebe HG, Rahmani R, de Sousas G, Silva JM, Skett P. Present status of the application of cryopreserved hepatocytes in the evaluation of xenobiotics: consensus of an international expert panel. Chem-Biol Interact. 1999;121:117–123. doi: 10.1016/s0009-2797(99)00081-2. [DOI] [PubMed] [Google Scholar]