Abstract
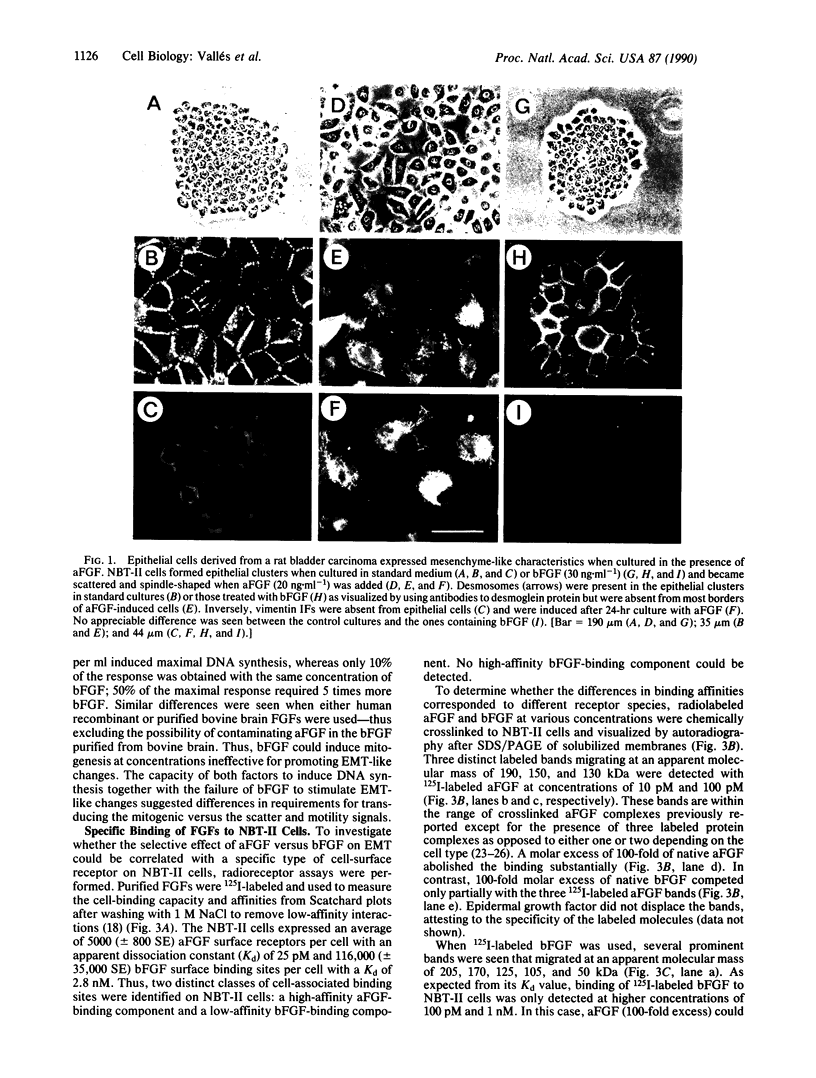
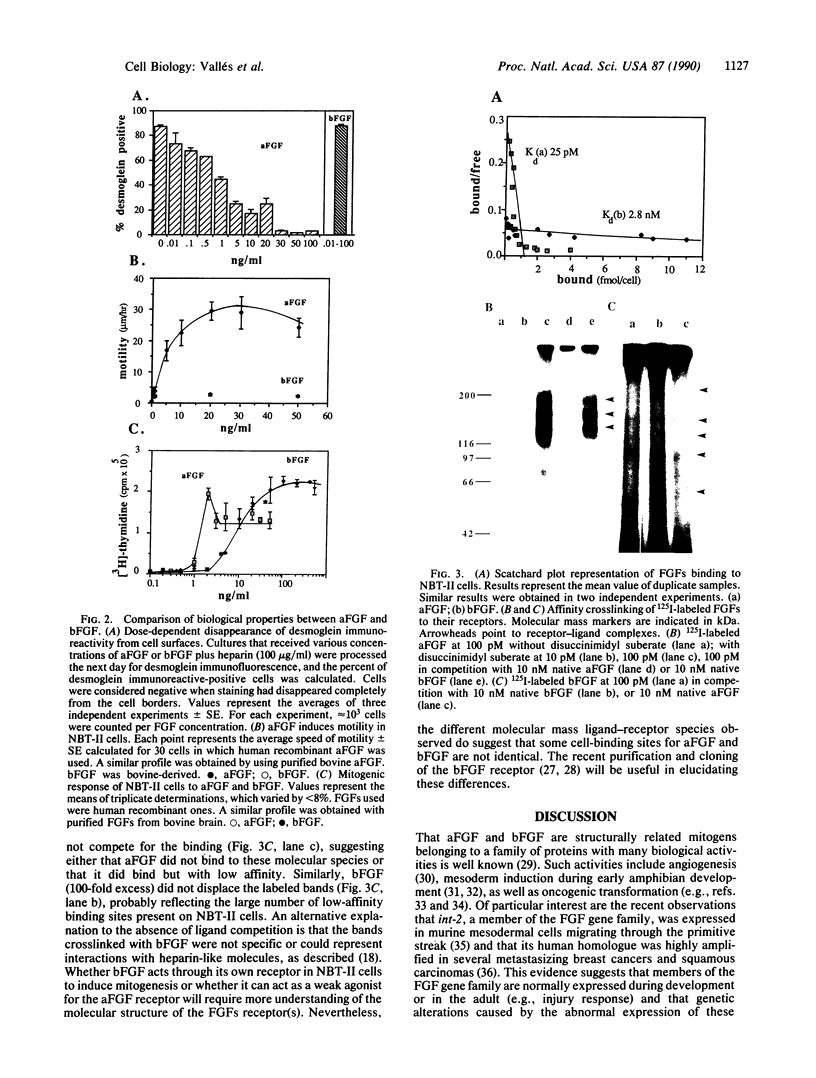
During normal embryogenesis and neoplastic transformation epithelia change their state of differentiation and degree of cohesiveness. It is thus essential to identify the signals modulating these transitions. We report here that acidic fibroblast growth factor (FGF) induces cells derived from a rat bladder carcinoma to lose their epithelial character and to acquire some properties typical of mesenchymal cells. The structurally related basic FGF did not have such an effect; both factors, however, had a mitogenic activity for these cells. Two distinct populations of receptors for acidic FGF and basic FGF were distinguished by their ligand-binding characteristics. The observations that both acidic and basic FGFs had a mitogenic effect on NBT-II cells and that only acidic FGF caused cell dissociation and dispersion strongly suggest that these two biological activities could be medicated through distinct signaling pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battifora H. Spindle cell carcinoma: ultrastructural evidence of squamous origin and collagen production by the tumor cells. Cancer. 1976 May;37(5):2275–2282. doi: 10.1002/1097-0142(197605)37:5<2275::aid-cncr2820370518>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Boyer B., Tucker G. C., Vallés A. M., Franke W. W., Thiery J. P. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. 1989 Oct;109(4 Pt 1):1495–1509. doi: 10.1083/jcb.109.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodak G. W., Hospelhorn V., Judge S. M., Mayforth R., Koeppen H., Sasse J. Increased levels of fibroblast growth factor-like activity in urine from patients with bladder or kidney cancer. Cancer Res. 1988 Apr 15;48(8):2083–2088. [PubMed] [Google Scholar]
- Courty J., Loret C., Chevallier B., Moenner M., Barritault D. Biochemical comparative studies between eye- and brain-derived growth factors. Biochimie. 1987 May;69(5):511–516. doi: 10.1016/0300-9084(87)90088-5. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Henahan M., Bowman M., Okada S., Battifora H., Unger M. Generation of fibroblast-like cells from cloned epithelial mammary cells in vitro: a possible new cell type. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2345–2349. doi: 10.1073/pnas.78.4.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Jackson B. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. IV. Ultrastructure of primary mesenchymal cells and their cell-cell interactions. Differentiation. 1983;25(2):121–141. [PubMed] [Google Scholar]
- Gherardi E., Gray J., Stoker M., Perryman M., Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Halaban R., Ghosh S., Baird A. bFGF is the putative natural growth factor for human melanocytes. In Vitro Cell Dev Biol. 1987 Jan;23(1):47–52. doi: 10.1007/BF02623492. [DOI] [PubMed] [Google Scholar]
- Jaye M., Burgess W. H., Shaw A. B., Drohan W. N. Biological equivalence of natural bovine and recombinant human alpha-endothelial cell growth factors. J Biol Chem. 1987 Dec 5;262(34):16612–16617. [PubMed] [Google Scholar]
- Jaye M., Lyall R. M., Mudd R., Schlessinger J., Sarver N. Expression of acidic fibroblast growth factor cDNA confers growth advantage and tumorigenesis to Swiss 3T3 cells. EMBO J. 1988 Apr;7(4):963–969. doi: 10.1002/j.1460-2075.1988.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan M., DiSorbo D., Hou J. Z., Hoshi H., Mansson P. E., McKeehan W. L. High and low affinity binding of heparin-binding growth factor to a 130-kDa receptor correlates with stimulation and inhibition of growth of a differentiated human hepatoma cell. J Biol Chem. 1988 Aug 15;263(23):11306–11313. [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Wagner J. A., Madison R. D., D'Amore P. A. Acidic fibroblast growth factor enhances regeneration of processes by postnatal mammalian retinal ganglion cells in culture. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2388–2392. doi: 10.1073/pnas.85.7.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenner M., Chevallier B., Badet J., Barritault D. Evidence and characterization of the receptor to eye-derived growth factor I, the retinal form of basic fibroblast growth factor, on bovine epithelial lens cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5024–5028. doi: 10.1073/pnas.83.14.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Odland G., Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968 Oct;39(1):135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. Changing patterns of cytokeratins and vimentin in the early chick embryo. Development. 1989 Jan;105(1):97–107. doi: 10.1242/dev.105.1.97. [DOI] [PubMed] [Google Scholar]
- Ristow H. J., Messmer T. O. Basic fibroblast growth factor and insulin-like growth factor I are strong mitogens for cultured mouse keratinocytes. J Cell Physiol. 1988 Nov;137(2):277–284. doi: 10.1002/jcp.1041370210. [DOI] [PubMed] [Google Scholar]
- Schmelz M., Duden R., Cowin P., Franke W. W. A constitutive transmembrane glycoprotein of Mr 165,000 (desmoglein) in epidermal and non-epidermal desmosomes. II. Immunolocalization and microinjection studies. Eur J Cell Biol. 1986 Dec;42(2):184–199. [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Tchao R. Novel forms of epithelial cell motility on collagen and on glass surfaces. Cell Motil. 1982;2(4):333–341. doi: 10.1002/cm.970020403. [DOI] [PubMed] [Google Scholar]
- Thomas K. A. Fibroblast growth factors. FASEB J. 1987 Dec;1(6):434–440. doi: 10.1096/fasebj.1.6.3315806. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Ito N., Hiasa Y., Kamamoto Y., Makiura S. Tissue culture of urinary bladder tumor induced in a rat by N-butyl-N-(-4-hydroxybutyl)nitrosamine: establishment of cell line, Nara Bladder Tumor II. J Natl Cancer Inst. 1971 Nov;47(5):979–985. [PubMed] [Google Scholar]
- Walicke P. A., Feige J. J., Baird A. Characterization of the neuronal receptor for basic fibroblast growth factor and comparison to receptors on mesenchymal cells. J Biol Chem. 1989 Mar 5;264(7):4120–4126. [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. J., Casey G., Cline M. J. Amplification of human int-2 in breast cancers and squamous carcinomas. Oncogene. 1988 Mar;2(3):279–282. [PubMed] [Google Scholar]





