Abstract
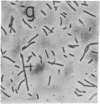
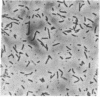
In Escherichia coli, selection of the proper division site at midcell requires the specific inhibition of septation at two other potential division sites, located at each of the cell poles. This site-specific inhibition of septation is mediated by the gene products of the minicell locus (the minB operon) that includes three genes, minC, minD, and minE. In this paper we show that one of the components of this division-inhibition system, the minC gene product, is also an essential component of another division-inhibition system, which is induced by derepression of the dicB gene and leads to inhibition of septation at all potential division sites. The two minC-dependent division-inhibition systems could be functionally distinguished by their different responses to the minE gene product. The results suggest a model in which a common mechanism, mediated by MinC, is responsible for the division block in a class of division-inhibition systems that can be independently activated by different proteins that determine the specific properties of these systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjar S., Bouché F., Bouché J. P. Cell division inhibition gene dicB is regulated by a locus similar to lambdoid bacteriophage immunity loci. Mol Gen Genet. 1988 Apr;212(1):11–19. doi: 10.1007/BF00322439. [DOI] [PubMed] [Google Scholar]
- Cam K., Béjar S., Gil D., Bouché J. P. Identification and sequence of gene dicB: translation of the division inhibitor from an in-phase internal start. Nucleic Acids Res. 1988 Jul 25;16(14A):6327–6338. doi: 10.1093/nar/16.14.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G., Belin D., Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984 Nov;31(1-3):165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Huisman O. Novel mechanism of cell division inhibition associated with the SOS response in Escherichia coli. J Bacteriol. 1983 Oct;156(1):243–250. doi: 10.1128/jb.156.1.243-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., Gottesman S. Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Holland I. B. Role of the SulB (FtsZ) protein in division inhibition during the SOS response in Escherichia coli: FtsZ stabilizes the inhibitor SulA in maxicells. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6045–6049. doi: 10.1073/pnas.82.18.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labie C., Bouché F., Bouché J. P. Isolation and mapping of Escherichia coli mutations conferring resistance to division inhibition protein DicB. J Bacteriol. 1989 Aug;171(8):4315–4319. doi: 10.1128/jb.171.8.4315-4319.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983 Jun;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J., Sanjanwala B., Lowe M. Overproduction of FtsZ suppresses sensitivity of lon mutants to division inhibition. J Bacteriol. 1986 Jun;166(3):756–762. doi: 10.1128/jb.166.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguin E., Lutkenhaus J., D'Ari R. Reversibility of SOS-associated division inhibition in Escherichia coli. J Bacteriol. 1986 Jun;166(3):733–738. doi: 10.1128/jb.166.3.733-738.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Collins J. F., Donachie W. D. Quantal behavior of a diffusible factor which initiates septum formation at potential division sites in Escherichia coli. J Bacteriol. 1974 May;118(2):407–413. doi: 10.1128/jb.118.2.407-413.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto S., Ohtsubo E. Effect of the pem system on stable maintenance of plasmid R100 in various Escherichia coli hosts. Mol Gen Genet. 1989 Feb;215(3):463–468. doi: 10.1007/BF00427044. [DOI] [PubMed] [Google Scholar]
- Ward J. E., Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985 Oct;42(3):941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- de Boer P. A., Crossley R. E., Rothfield L. I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989 Feb 24;56(4):641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- de Boer P. A., Crossley R. E., Rothfield L. I. Isolation and properties of minB, a complex genetic locus involved in correct placement of the division site in Escherichia coli. J Bacteriol. 1988 May;170(5):2106–2112. doi: 10.1128/jb.170.5.2106-2112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]