Abstract
Human enterovirus 71 (EV71) is the primary causative agent of recent large-scale outbreaks of hand, foot, and mouth disease (HFMD) in Asia. Currently, there are no drugs available for the prevention and treatment of HFMD. In this study, we compared the anti-EV71 activities of three natural compounds, rheum emodin, artemisinin and astragaloside extracted from Chinese herbs Chinese rhubarb, Artemisia carvifolia and Astragalus, respectively, which have been traditionally used for the treatment and prevention of epidemic diseases. Human lung fibroblast cell line MRC5 was mock-infected or infected with EV71, and treated with drugs. The cytotoxicity of the drugs was detected with MTT assay. The cytopathic effects such as cell death and condensed nuclei were morphologically observed. The VP1-coding sequence required for EV71 genome replication was assayed with qRT-PCR. Viral protein expression was analyzed with Western blotting. Viral TCID50 was determined to evaluate EV71 virulence. Flow cytometry analysis of propidium iodide staining was performed to analyze the cell cycle distribution of MRC5 cells. Rheum emodin (29.6 μmol/L) effectively protected MRC5 cells from EV71-induced cytopathic effects, which resulted from the inhibiting viral replication: rheum emodin treatment decreased viral genomic levels by 5.34-fold, viral protein expression by less than 30-fold and EV71 virulence by 0.33107-fold. The fact that inhibition of rheum emodin on viral virulence was much stronger than its effects on genomic levels and viral protein expression suggested that rheum emodin inhibited viral maturation. Furthermore, rheum emodin treatment markedly diminished cell cycle arrest at S phase in MRC5 cells, which was induced by EV71 infection and favored the viral replication. In contrast, neither astragaloside (50 μmol/L) nor artemisinin (50 μmol/L) showed similar anti-EV71 activities. Among the three natural compounds tested, rheum emodin effectively suppressed EV71 viral replication, thus is a candidate anti-HFMD drug.
Keywords: enterovirus 71; hand, foot, and mouth disease (HFMD); MRC5 cells; rheum emodin; artemisinin; astragaloside; viral replication; cell cycle arrest
Introduction
Hand, Foot, and Mouth Disease (HFMD) is a febrile exanthematous disease that occurs in children <5 years of age. The clinical signs include vesicles on the palmar and plantar surfaces of the hands and feet, buccal mucosa, tongue, and buttocks, and in severe cases, acute flaccid paralysis, pulmonary edema, myocarditis, encephalitis and even death1,2. Recently, several large outbreaks of HFMD have been reported in Asia. These include outbreaks in the Fuyang3, Henan4, Nanchang5, Hubei6, Jilin1 and Taiwan of China, as well as in Japan, and Malaysia7,8. Currently, there are no drugs available for the prevention and treatment of HFMD.
Human enterovirus 71 (EV71) is the causative agent for HFMD outbreaks in Asia1,2,5,9. In a previous study, we demonstrated that EV71 virus induces cell cycle arrest at S phase to promote viral replication, while G0/G1 or G2/M synchronization inhibits viral replication10. This indicates a possibility for developing cell cycle-modifying agents (especially those that induce G0/G1 or G2/M arrest) as a novel strategy to treat HFMD.
Natural products have played pivotal roles in the drug discovery and development process. It has been noted that natural products embody more ''privileged structures''11 than purely synthetic chemical libraries and possess the characteristics and therapeutic effects of Chinese herbs12. Natural product-based drug discovery research programs represent important avenues for drug development13, including anti-viral drugs14,15. For example, emodin (1,3,8-tri-hydroxy-6-methylanthraquinone) is an active ingredient sourced from herbs belonging to genera Rheum, Polygonum, Rhamnus and Senna. Extracts from these herbs have been used for the treatment of epidemic-prone diseases16. Emodin has been shown to inhibit the replication of severe acute respiratory syndrome (SARS) virus17, hepatitis B (HBV)18, and herpes simplex viruses (HSV)19.
Artemisinin, a drug obtained from the plant Artemisia annua, is recommended by the WHO for the treatment of infections, such as drug-resistant Plasmodium falciparum strains, cerebral malaria and malaria in children20. A major saponin of this herb, astragaloside, has been shown to possess anti-cancer, anti-fatigue, anti-coxsackie B virus, and anti-inflammatory effects21. However, to date, its anti-EV71 activity has not been well investigated.
In the present study, we aimed to elucidate the anti-EV71 activity of the natural active components from Rheum emodin, Astragaloside and Artemisinin. We observed that Rheum emodin protects host cells against EV71 infection, decreases viral genomic levels and viral protein expressions, and decreases EV71 virulence by intervening in the cell cycle environment that favors viral replication; however, Astragaloside and Artemisinin did not show similar abilities. These results reinforce our model of the EV71 viral pathogenic mechanisms that require regulation of the cell cycle environment and suggest that Rheum emodin is a potential candidate for the treatment and prevention of HMDF disease.
Materials and methods
Viruses and cells
The Changchun077 strain of EV71 has been reported previously1. This virus strain was propagated in African green monkey kidney cells (Vero). Vero (No CCL-81) and MRC5 (No CCL-171) were purchased from the ATCC (Manassas, VA, USA) and were maintained in Dulbecco's modified Eagle's medium (DMEM) (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (GIBCO BRL, Grand Island, NY, USA).
Viral titer determination
Viral titers were determined by measuring the TCID50 in a microtitration assay using Vero cells, as described previously22. Briefly, Vero cells were seeded onto 96-well plates and incubated at 37 °C for 24 h. Virus-containing supernatant was serially diluted 10-fold and 100 μL was added per well in octuplicate. The cytopathic effects were observed once per day until the experimental endpoint was reached. The TCID50 was determined by the Reed-Muench method23, which is based on the assumption that 1×105 TCID50/mL will produce 0.7×105 plaque forming units/mL (www.protocol-online.org/biology-forums/posts/1664.html).
Infection
Cells were mock-infected or infected with EV71 at an MOI of 1 for 2 h. Aafter 2 h of virus adsorption, cells were washed with PBS once and then fresh culture medium was added.
Cell growth inhibition test
Inhibition of cell growth was determined by MTT (Sigma, St Louis, MO, USA) assay. MRC5 cells (1.0×104 cells/well) were seeded onto 96-well culture plates (Nunc, Roskilde, Denmark). After a 24 h incubation, different concentrations of Rheum emodin (Rheu), Artemisinin (Arte) and Astragaloside (Astra), which were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), were added individually to the plates. Following incubation, cell growth was measured at 48 h by the addition of 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/mL) at 37 °C for 2 h; DMSO (150 μL) was added to dissolve the formazan crystals. Absorbance was measured at 492 nm with an enzyme-linked immunosorbent assay plate (ELISA) reader (Bio-Rad, Hercules, CA, USA). The percentage of inhibition was calculated as follows:
Inhibitory ratio (%)=[A492(control)−A492(sample)]/[A492(control)–A492(blank)]×100%.
Cytopathic effect
For observing the cytopathic effects, cells were grown on a culture dish and infected by EV71 for 24 h. The morphological changes were observed and microscopic photographs were taken (Olympus, Tokyo, Japan).
Immunofluorescence assay and Hoechst 33258 staining
The nuclear stain Hoechst 33258 (Sigma, St Louis, MO, USA) was used to visualize nuclear changes by fluorescence microscopy. Briefly, MRC5 cells were plated onto 6-well plastic culture dishes (4×105 cells/well) and infected with EV71 for 2 h; treated with drugs for 22 h; washed with PBS and fixed in 3.7% formaldehyde for 1 h; washed with PBS and then stained with 5 mg/L Hoechst 33258 for 30 min. Nuclear changes were observed under a fluorescence microscope at an excitation wavelength of 350 nm with an emission filter at 460 nm (Leica, Nussloch, Germany).
Cell cycle analysis by flow cytometry
Nuclear DNA content was measured using propidium iodide (PI) staining and fluorescence-activated cell sorting (FACS). Adherent cells were collected by treatment with trypsin and then washed with phosphate-buffered saline (PBS). Cells were fixed in 1 mL of cold 70% ethanol overnight at 4 °C and resuspended in staining buffer (50 μg/mL PI [Sigma] and 20 μg/mL RNase in PBS) for 2 h at 4 °C. The PI-stained cells were then analyzed using FACS (FACScan; BD), and at least 10 000 cells were counted for each sample. Data analysis was performed using ModFit LT, version 2.0 (Verity Software House).
Western blot analysis
To analyze the virus present in the supernatant, intracellular virus and total virus were made up to 5 mL with DMEM medium and subjected to repeated freezing and thawing for three cycles. Subsequently, these samples were centrifuged at 3000 rounds per minute for 20 min and then the supernatant containing the virus was collected. Intracellular virus and total virus were obtained by centrifugation at 30 000 rounds per minute for three hours with 30% sucrose using a type 70.1 Ti Rotor in a Beckman ultracentrifuge (Optimal L-100XP ultracentrifuge Beckman Coulter). The samples were then collected for Western blot analysis.
For determining cyclin A2 and CDK 2 expression, cells were collected at 36 h after EV71 infection and lysed directly in sodium dodecyl sulfate (SDS) sample buffer [60 mmol/L Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, and 0.01% bromophenol blue], followed by boiling for 10 min. Whole-cell lysates were further subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Bio-Rad) and detected with the corresponding primary and alkaline phosphatase-conjugated secondary antibodies. The membranes were then reacted with 5-bromo-4-chloro-39-indolylphosphate (BCIP) and nitro-blue tetrazolium (NBT) substrate (Sigma). The anti-CDK 2 (Boster) and anti-cyclin A2 (Proteintech) mouse or rabbit antibodies were used in the Western blot analyses. Anti-VP1 polyclonal antibodies from rabbit were prepared in our laboratory. Rabbit or mouse secondary antibodies were obtained from Proteintech. The intensity of the bands was calculated by ImageJ (NIH ImageJ), and the value of the Con/Drug refers to the intensity of the VP1 protein band from the control group/the intensity of the VP1 protein band of the drug treatment group in the supernatant, intracellular and total virus.
Quantitative real-time PCR
RNA was extracted from infected cells treated control medium and drugs using the Trizol reagent (Gibco-BRL, Rockville, MD, USA) and isolated as specified by the manufacturer. The RNA was treated with DNAse (DNase I-RNase-Free, Ambion) to remove any contaminating DNA. Then, 200 ng of total RNA was reverse-transcribed with oligo dT primers using the High Capacity cDNA RT Kit (Applied Biosystems) in a 20 μL cDNA reaction as specified by the manufacturer. For quantitative PCR, the template cDNA was added to a 20 μL reaction with SYBR GREEN PCR Master Mix (Applied Biosystems) and 0.2 μmol/L of primers for VP1 and GAPDH.
The forward and reverse primer sequences for VP1 are 5′-AGCACCCACAGGCCAGAACACAC-3′ and 5′-ATCCCGCCCTACTGAAGAAACTA-3′ and those for GAPDH are 5′-GCAAATTCCATGGCACCGT-3′ and 5′-TCGCCCCACTTGATTTTGG-3′.
Amplification was carried out using an ABI Prism 7000 for 40 cycles with the following conditions: an initial denaturation at 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 1 min; then 1 cycle of 95 °C for 1 min, 55 °C for 30 s and 95 °C for 30 s. The fold changes were calculated relative to GAPDH using the ΔΔCt method for VP1-coding sequence analysis.
Statistical analysis
Data are presented as the mean±standard deviation (SD). Between-group differences were assessed using Student's t-test. P values of <0.05 were considered statistically significant.
Results
Cytotoxicity of Rheum emodin, Artemisinin, and Astragaloside
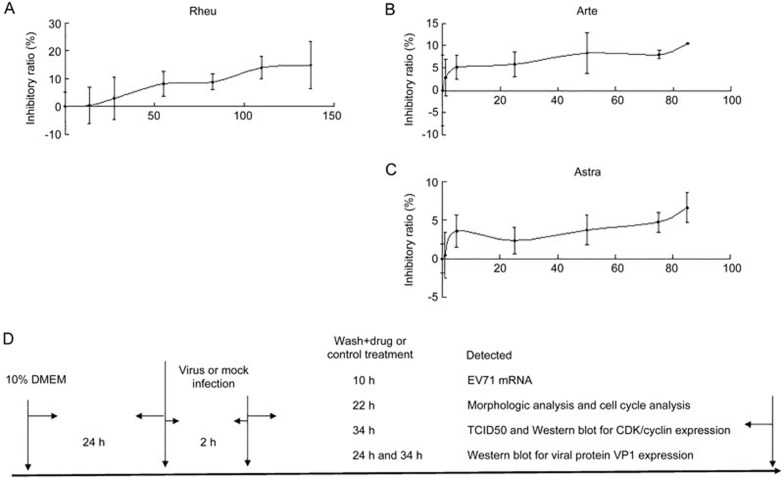
To prove that the anti-virus effects of Rheum emodin, Artemisinin and Astragaloside in MRC5 cells are not due to their cytotoxicity, MRC5 cells were treated with Rheum emodin, Artemisinin or Astragaloside at various doses to confirming their ability to inhibit cell growth. MRC5 are human lung fibroblast cells. Although EV71 infection mainly leads to central nervous system symptoms, the lungs can also be infected by the EV71 virus in vivo24, meaning that MRC5 cells can be used as a cell model. The cell inhibitory ratio was detected via MTT analysis at 48 h post-treatment. After 48 h of treatment with 0, 13.69, 27.38, 54.77, 82.15, 109.53, and 136.92 μmol/L of Rheum emodin, the concentrations with inhibitory ratios below 10% ranged from 0 to 82.15 μmol/L (Figure 1A). After 0, 1, 5, 25, 50, 75, and 85 μmol/L of Artemisinin or Astragaloside treatment for 48 h, the concentrations of Artemisinin with inhibitory ratios below 10% ranged from 0 to 75 μmol/L (Figure 1B) and the concentration of Astragaloside with inhibitory ratios below 10% ranged from 0 to 85 μmol/L (Figure 1C). Therefore, the range of concentrations for Rheum emodin (0 to 82.15 μmol/L), Artemisinin (0 to 75 μmol/L) and Astragaloside (0 to 85 μmol/L) did not affect cell survival. Based on the results of both a published study25,26,27 and our MTT assay, we chose the following concentrations for further study: 29.60 μmol/L of Rheum emodin, 50 μmol/L of Artemisinin or 50 μmol/L of Astragaloside for anti-EV71 activity. Figure 1D illustrates the flow diagram of how to treat MRC5 cells in the designed experiment.
Figure 1.
Cytotoxicity of Rheum emodin, Artemisinin and Astragaloside via MTT assay. (A) Inhibitory effects of Rheum emodin (Rheu) on MRC5 cell growth. Cells (1×104 cells/well) were incubated with 0, 13.69, 27.38, 54.77, 82.15, 109.53, or 136.92 μmol/L of Rheum emodin for 48 h. (B) The inhibitory effects of Artemisinin (Arte) on MRC5 cell growth. The cells (1×104 cells/well) were incubated with 0, 1, 5, 25, 50, 75, or 85 μmol/L of Artemisinin for 48 h. (C) The inhibitory effects of Astragaloside (Astra) on MRC5 cell growth. The cells (1×104 cells/well) were incubated with 0, 1, 5, 25, 50, 75, or 85 μmol/L of Astragaloside for 48 h. The results indicate the mean±SD of one experiment and were representative of three independent experiments. (D) Flow diagram showing the experimental treatment protocol for MRC5 cells.
Rheum emodin protects host cells from damage induced by EV71 viral infection
To further exclude the possibility that the anti-viral effects of Rheum emodin, Artemisinin or Astragaloside was due to their cytotoxic effects, as well as to confirm their protective effect on host cells, 29.6 μmol/L of Rheum emodin, 50 μmol/L of Artemisinin and 50 μmol/L of Astragaloside were chosen based on the previous results to treat mock-infected and infected cells for 22 h. There were no effects observed for either cell viability or morphology in the infected cells (Figure 2A2, 2A3, and 2A4) when compared with mock-infected cells (Figure 2A1). Meanwhile, it was observed that numerous cells were rounded up and detached from the bottom of the dish in the cell morphological analysis (Figure 2B1) compared to the mock-infected cells at 24 h post-infection of EV71 infection at a MOI of 1 (Figure 2A1). While Rheum emodin treatment (Figure 2B2) inhibited cell morphologic changes including being rounded up and detached from the bottom of the dish due to EV71 infection, Artemisinin (Figure 2B3) and Astragaloside (Figure 2B4) did not. Nuclear staining tests were also completed to confirm the effects of Rheum emodin, Artemisinin and Astragaloside on cell death. After the EV71-infected cell nuclei became condensed and bright (Figure 2C1), which indicated cell death, Rheum emodin treatment inhibited the appearance of condensed and bright nuclei induced by EV71 infection (Figure 2C2), while neither Artemisinin (Figure 2C3) nor Astragaloside inhibited cell death induced by EV71 infection (Figure 2C4). Therefore, Rheum emodin possessed the ability to protect host cells from EV71 viral damage, while Artemisinin or Astragaloside were unable to achieve the same results.
Figure 2.
Effects of Rheum emodin, Artemisinin and Astragaloside on the cytopathic effects induced by EV71 infection. (A) Morphological analysis of the effects by Rheum emodin, Artemisinin or Astragaloside on cell growth. Cell growth was visualized by light microscopy at 22 h after treatment with 29.60 μmol/L of Rheum emodin, 50 μmol/L of Astragaloside or 50 μmol/L of Artemisinin. Rheu, Rheum emodin; Arte, Artemisinin; Astra, Astragaloside; Mock, mock infection; Con, 0.1% DMSO in 10% DMEM. Bar=45 μm. (B) Morphological analysis of the effect of Rheum emodin, Artemisinin or Astragaloside on cell growth after EV71 infection. Mock-infected (mock) or MRC5 cells infected with EV71 (infected) at an MOI of 1. Twenty-two hours after treatment with 29.60 μmol/L of Rheum emodin, 50 μmol/L of Astragaloside or 50 μmol/L of Artemisinin, cell growth was visualized by light microscopy. Rheu, Rheum emodin; Arte, Artemisinin; Astra, Astragaloside; Con, 0.1% DMSO in 10% DMEM. Bar=45 μm. (C) MRC5 cells were mock-infected (mock) or infected with EV71 (infected) at an MOI of 1. Twenty-two hours after treatment with 29.60 μmol/L of Rheum emodin, 50 μmol/L of Astragaloside or 50 μmol/L of Artemisinin, cells were stained with Hoechst 33258. Condensed nuclei were observed by fluorescence microscopy. Rheu, Rheum emodin; Arte, Artemisinin; Astra, Astragaloside; Con, 0.1% DMSO in 10% DMEM. Bar=15 μm. The results were from one experiment and were representative of three independent experiments.
Effect of Rheum emodin, Artemisinin and Astragaloside on viral genomic RNA replication
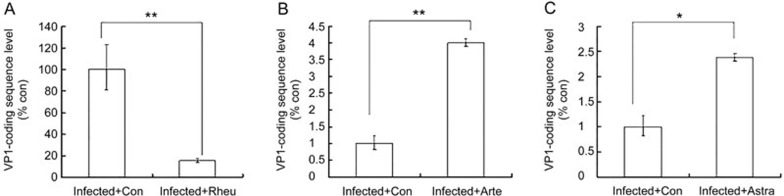
To examine whether viral RNA replication and/or transcription was influenced by treatment with these drugs, we assessed EV71 VP1-coding sequence levels by qRT PCR at 12 h post-infection (EV71 has a single-stranded, positive-sense RNA genome, so detecting the VP1-coding sequence level is equivalent to detecting the level of the EV71 genome). EV71 RNA levels in Rheum emodin-treated cells were significantly lower than those in the control cells (15.75%±1.81% of control cells, Figure 3A, P<0.01). Treatment with Artemisinin (Figure 3B) or Astragaloside (Figure 3C) increased EV71 genomic RNA levels compared to the control cells. Thus, only Rheum emodin decreased viral genomic RNA levels.
Figure 3.
Effect of Rheum emodin, Artemisinin and Astragaloside on EV71 viral genomic levels. At 12 h post-infection, intracellular EV71 RNA levels were detected in control medium (Con) or drug-containing medium-treated MRC5 cells by real-time quantitative PCR. (A) 29.60 μmol/L of Rheum emodin (Rheu) treatment for 10 h. The results are standardized using GAPDH RNA as a control and normalized to 100 in control cells. (B) 50 μmol/L of Artemisinin (Arte) for 10 h. The results are standardized using GAPDH RNA as a control and normalized to 1.0 in control cells. (C) 50 μmol/L of Astragaloside (Astra) treatment for 10 h. The results are standardized using GAPDH RNA as a control and normalized to 1.0 in control cells (C). The results indicate the mean±SD of one experiment and were representative of three independent experiments. *P<0.05, **P<0.01.
Effect of Rheum emodin, Artemisinin and Astragaloside on viral protein expression
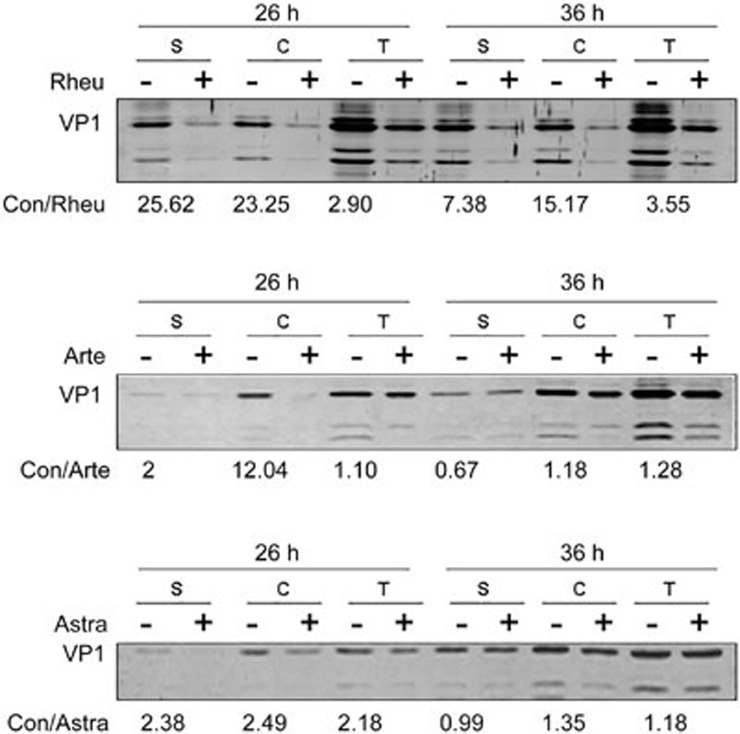
To further confirm the effects of the drugs on viral protein expression, we assessed EV71 viral protein expression using Western blot at 26 h and 36 h post-infection. Intracellular viral proteins were collected by cell lysis, the supernatant viral proteins by ultracentrifugation of viruses present in the culture medium, and the total viral proteins by ultracentrifugation of the cellular and medium viruses. Rheum emodin treatment was found to have reduced the supernatant, intracellular and total viral VP1 protein expression at both 26 h and 36 h. Artemisinin and Astragaloside both decreased the supernatant, intracellular and total viral protein expression at 26 h; however, the inhibitory effect at 36 h was similar to the mock-treatment (Figure 4). Thus, only Rheum emodin persistently inhibited the expression of viral proteins, while Artemisinin or Astragaloside did not show a sustained inhibitory effect on viral protein expression over an extended period of time.
Figure 4.
Western blotting shows the effects of Rheum emodin, Artemisinin and Astragaloside on EV71 protein expression. VP1 expression was determined after growth in control medium (–) or drug medium (+) at 26 h and 36 h post-infection. S, supernatant protein; C, intracellular protein; T, supernatant and intracellular protein. Rheu, Rheum emodin; Arte, Artemisinin; Astra, Astragaloside; Con, 0.1% DMSO in 10% DMEM. The numerical value is the ratio of VP1 protein intensity in the control group to that in the drug treatment group. Data were representative of three independent experiments.
Effects of Rheum emodin, Artemisinin and Astragaloside on EV71 virulence
To understand the inhibitory effect of the drugs on EV71 production, Viral TCID50 was examined at 36 h post-infection to evaluate the viral virulence. The TCID50/mL was found to be 3.16×109 in the mock-treated cells and 2.37×102 in the Rheum emodin-treated cells. The TCID50/mL remained at 3.16×109 in both the Artemisinin- and Astragaloside-treated cells (Table 1). Furthermore, we also observed a 1.33×107-fold increase in the TCID50/mL of mock-treated cells over the Rheum emodin-treated cells (Table 2). Thus, Artemisinin and Astragaloside did not inhibit viral production, while Rheum emodin exhibited a significant inhibitory effect on viral reproduction.
Table 1.
| Treatment | Infected+con | Infected+Rheu | Infected+Arte | Infected+Astra |
|---|---|---|---|---|
| TCID50/mL | 3.16×109 | 2.37×102 | 3.16×109 | 3.16×109 |
Infected, infected EV71; con, control; Rheu, Rheum emodin; Arte, Artemisinin; Astra, Astragaloside
Table 2.
| Treatment | (Infected+con)/(Infected+Rheu) | (Infected+con)/(Infected+Arte) | (Infected+con)/(Infected+Arte) |
|---|---|---|---|
| The fold of TCID50/mL | 1.33×107 | 1 | 1 |
Infected, infected EV71; con, control; Rheu, Rheum emodin; Arte, Artemisinin; Astra, Astragaloside
Effects of Rheum emodin on the host cell
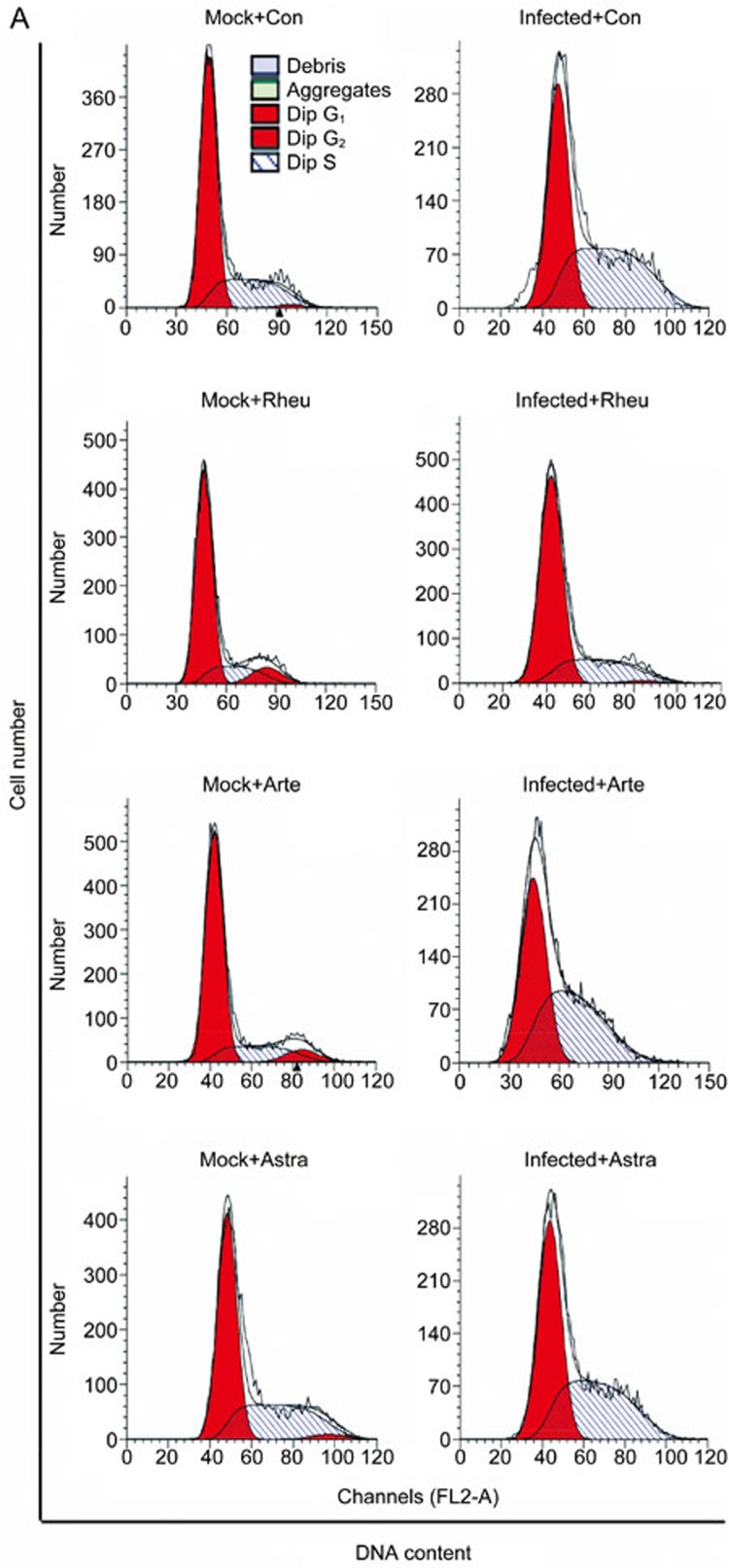
In our previous study, we found that the EV71 virus induced cell cycle arrest at S phase to promote viral replication and that G0/G1 or G2/M synchronization inhibited viral replication. Therefore, we examined whether the effects of emodin were mediated by interfering with the cell cycle environment favored by the EV71 virus in this study. Cell cycle profiles were determined via flow cytometry after drug treatment or EV71 infection (Figure 5A). At 24-h post-infection, an obvious accumulation in S phase was observed by the ModFit analysis in EV71-infected cells, with an increase from 31.15%±1.25% to 50.74%±1.13% (increase of 62.89%, P<0.01) compared to the mock-treated cells. After Rheum emodin treatment, there was a decrease in S phase from 50.74%±1.13% to 28.31%±1.16% compared to the mock-treated cells (P<0.01) after EV71 infection (Figure 5B). Artemisinin treatment decreased S phase from 54.08%±1.24% to 47.65%±0.23% (P<0.01) after EV71 infection (Figure 5D); Astragaloside treatment also induced a decrease from 50.74%±1.13% to 46.01%±1.16% (P<0.01) post-EV71 infection (Figure 5F). To further emphasize the effects of Rheum emodin, Artemisinin and Astragaloside treatment on S phase in EV71-infected cells, we analyzed the percentage of S phase cells after EV71 infection. The percentage of S phase cells after Rheum emodin treatment was 55.78% of that observed in the mock-treated cells (Figure 5C). The corresponding S phase percentages in the Astragaloside- and Artemisinin-treated cells were 90.68% (Figure 5E) and 88.11% of mock-treated cells (Figure 5G), respectively. Thus, our findings suggest that Rheum emodin inhibits the S-phase accumulation induced by EV71 infection.
Figure 5A.
Effect of Rheum emodin, Artemisinin and Astragaloside on the cell cycle manipulation of EV71 infection. Cell cycle profiles were determined by flow cytometry after drug treatment or EV71 infection (A).
Figure 5B–5G.
Effect of Rheum emodin, Artemisinin and Astragaloside on the cell cycle manipulation of EV71 infection. Histograms were analyzed by the ModFit LT program to determine the percentage of cells in each phase of the cell cycle at 24 h post-infection in the mock-infected (Mock) and EV71-infected (infected) MRC5 cells in (B, D, and F). Control medium (Con) and 29.60 μmol/L of Rheum emodin (Rheu) treatment for 22 h (B). Control medium (Con) and 50 μmol/L of Artemisinin (Arte) treatment for 22 h (D). Control medium (Con) and 50 μmol/L of Astragaloside (Astra) treatment for 22 h (F). The S phase percentage was compared between the control medium (Con) and the drug treatment groups in the EV71-infected (Infected) MRC5 cells in (C, E, and G). Control medium (Con) and 29.60 μmol/L of Rheum emodin treatment (Rheu) for 10 h (C). Control medium (Con) and 50 μmol/L of Artemisinin (Arte) treatment for 10 h (E). Control medium (Con) and 50 μmol/L of Astragaloside (Astra) treatment for 10 h (G). The results indicate the mean±SD of one experiment and are representative of three independent experiments. **P<0.01.
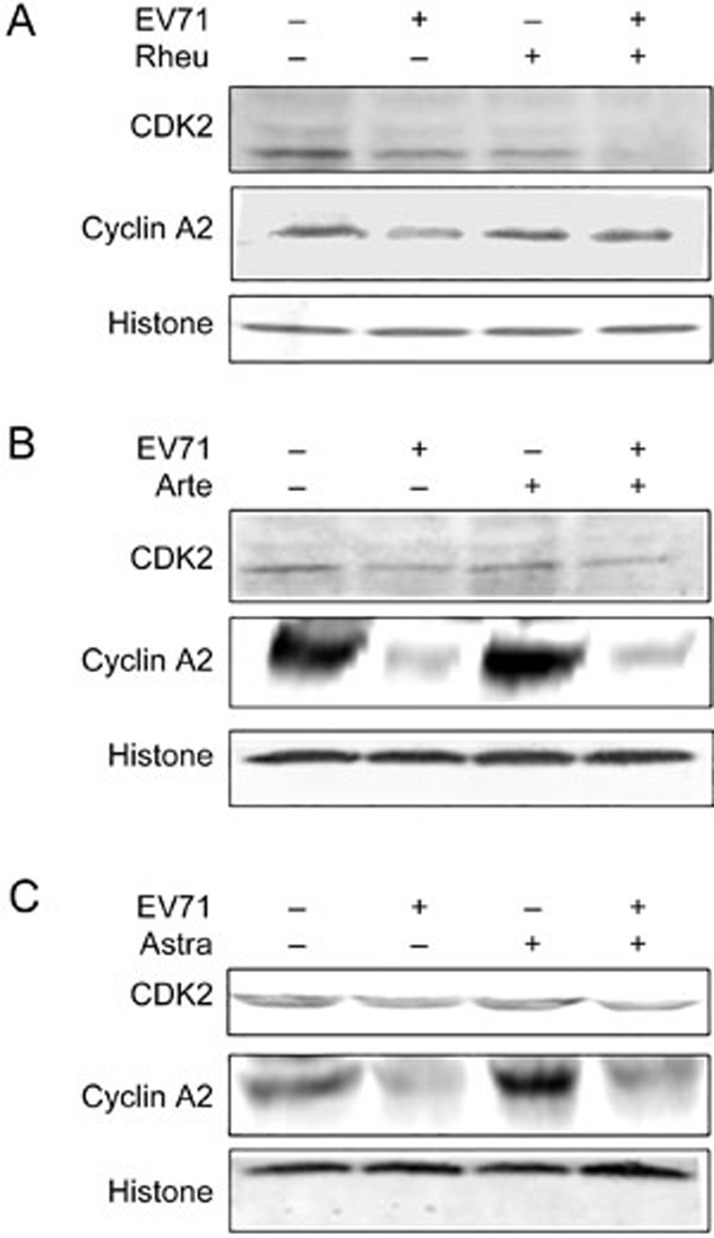
Effects of Rheum emodin on key cell cycle regulators
Western blotting was performed to observe the expression of CDK 2 and cyclin A2 at 36 h post-infection; this time point was chosen according to our previous study28. A significant decrease in CDK 2 and cyclin A2 was observed in the viral-infected cells compared to the mock-infected cells. Rheum emodin further decreased the expression of CDK 2 and increased the expression of cyclin A2 in EV71-infected cells (Figure 6A). This effect was not observed with either Artemisinin (Figure 6B) or Astragaloside treatment (Figure 6C). Therefore, Rheum emodin appeared to inhibit the manipulation of the host cell cycle by EV71 virus by regulating CDK 2 and cyclin A2 expression.
Figure 6.
Western blotting shows the effects of Rheum emodin, Artemisinin and Astragaloside on expression of host cell cycle regulatory proteins after EV71 infection. MRC5 cells were mock-infected or infected with EV71 at a MOI of 1 for 2 h. Two hours later, the cells were treated with drugs that lasted for 34 h, and then the cells were collected for lysis. Cyclin A2 and CDK2 expression was detected by Western blot analysis. Control medium and 29.60 μmol/L of Rheum emodin (Rheu) treatment for 34 h (A). Control medium and 50 μmol/L of Artemisinin (Arte) treatment for 34 h (B). Control medium and 50 μmol/L of Astragaloside (Astra) treatment for 34 h (C). Histone or Tubulin was shown as a loading control. The results are representative of three independent experiments.
Discussion
Human enterovirus 71 (EV71) is the primary causative agent for HFMD outbreaks in Asian countries1,2,5,9. Development of EV71 viral vaccines29,30 and inhibitors that target various phases of the EV71 lifecycle is currently at a preclinical stage31. Currently available antiviral drugs have limited efficacy against EV71 infection. Chinese herbs have been used for the prevention and treatment of epidemic diseases since ancient times. In this study, we investigated the effect of three natural ingredients, Rheum emodin, Astragaloside and Artemisinin, which are extracted from Chinese herbs on EV71 viral replication.
Cell death led to the loss of function in damaged cells; for example, neural cell death will lead to amnesia, mental disorder and other conditions32 and muscle cell death will lead to outcomes such as paralysis or myasthenia33. Cell death also leads to the release of cellular contents and eventually to inflammation. Many studies have been completed in vivo and in vitro to identify the inflammatory factor released after cell death34,35,36. In this study, we found that Rheum emodin has the ability to protect host cells from the damage associated with EV71 viral infection. Therefore, Rheum emodin inhibits cell death caused by EV71 virus infection, which ultimately inhibits the loss of host function and inflammatory occurrence.
Cell death from viral infection is usually due to viral replication. Hence, we investigated whether Rheum emodin inhibited viral replication. Our results clearly indicate that Rheum emodin decreased the levels of viral genomic level, viral protein expression, and EV71 virulence compared to the control-treated group. Therefore, Rheum emodin inhibits cell death by inhibiting viral production. The fold difference in the TCID50/mL, mRNA levels, and protein expression between the control-treated and Rheum emodin-treated cells is 1.33×107, 6.34, and less than 30, respectively, indicating that while Rheum emodin inhibits viral gene replication and viral expression, it mainly inhibits maturation, which is the final step for viral assembly. Rheum emodin also suppressed viral replication for 36 h. Similar anti-EV71 effects were not observed for either Artemisinin or Astragaloside. It should be noted from practitioners of traditional Chinese medicine that the three natural products Rheum emodin, Artemisinin, and Astragaloside were extracted from Chinese rhubarb (also from Polygonum cuspidatum Sieb.et Zucc; Rumex patientia Linn; Fagopyrum dibotrys (D Don) Hara; Rumex japonicus Houtt; Fallopia multiflora (Thunb) Harald), Artemisia carvifolia and Astragalus, respectively. This study suggests that Chinese herb decoction for treating HFMD should include Chinese rhubarb (also Polygonum cuspidatum Sieb.et Zucc; Rumex patientiaLinn; Fagopyrum dibotrys (D Don) Hara; Rumex japonicus Houtt; or Fallopia multiflora (Thunb) Harald)37, but not Artemisia carvifolia or Astragalus.
In the previous study, we showed that EV71 induced cell cycle arrest in S phase to facilitate viral production10. However, Rheum emodin decreased the amount of S phase favored by EV71 to increase the ratio of G0/G1 phase compared with the control treatment in this study. Additionally, the expression of CDK 2 and cyclin A2 further demonstrated the role of Rheum emodin in cell cycle regulation. It was therefore concluded that Rheum emodin inhibited EV71 viral replication by intervening in the cell cycle environment favored by the virus. Although Artemisinin and Astragaloside also displayed the ability to decrease the amount of S phase favored by the EV71 virus, the effect was not as potent as Rheum emodin. Indeed, EV71 appeared to overcome the inhibitory effect of Artemisinin and Astragaloside over 26 h.
In a previous study, exogenous expression of 3D, an RNA-dependent RNA polymerase, was shown to mediate cell cycle arrest. Therefore, it is speculated that Rheum emodin might affect the function of 3D in viral replication. To our knowledge, members of the Flaviviridae family, which include hepatitis C (HCV)38, dengue virus2,39, and classic swine fever40, also induce S-phase arrest. Notably, Picornaviridae and Flaviviridae share the common characteristic of being single-stranded, positive-sense RNA viruses. Therefore, Rheum emodin may be effective against single-stranded, positive-sense RNA viruses that favor S phase.
These results further prove our understanding of the EV71 viral pathogenic mechanisms and indicate that Rheum emodin is a potential candidate for the treatment and prevention of HMDF disease.
Author contribution
Jing-hua YU designed the experiments and wrote the paper; Ting ZHONG conducted the experiments; Li-ying ZHANG, Zeng-yan WANG, Yue WANG, Feng-mei SONG, and Ya-hong ZHANG analyzed the data and prepared the viruses, cells and reagents.
Acknowledgments
This work was supported by funding from the National Natural Science Foundation of China (81301416), the Postdoctoral Science Foundation of China (2014M561302 and 2015T80299), the Norman Bethune Program of Jilin University (2015202), the Jilin Provincial Science and Technology Department (20140204004YY and 20160414025GH), and the Department of Human Resources and Social Security of Jilin Province (2016014), the National Natural Science Foundation of Henan Province (132300410228).
References
- Wang X, Zhu C, Bao W, Zhao K, Niu J, Yu XF, et al. Characterization of full-length enterovirus 71 strains from severe and mild disease patients in northeastern China. PLoS One 2012; 7: e32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J 2010; 7: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Jiang J, Liu Y, Huang X, Wang P, Liu L, et al. Detection of human enterovirus 71 and Coxsackievirus A16 in an outbreak of hand, foot, and mouth disease in Henan Province, China in 2009. Virus Genes 2012; 46: 1–9. [DOI] [PubMed] [Google Scholar]
- Liu MY, Liu W, Luo J, Liu Y, Zhu Y, Berman H, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6: e25287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huo X, Dai Y, Yang Z, Lei Y, Jiang Y, et al. Evidences for intertypic and intratypic recombinant events in EV71 of hand, foot and mouth disease during an epidemic in Hubei Province, China, 2011. Virus Res 2012; 169: 195–202. [DOI] [PubMed] [Google Scholar]
- Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis 2000; 31: 678–83. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Utama A, Yoshii K, Yoshida H, Yoneyama T, Sinniah M, et al. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997-1998. Jpn J Infect Dis 1999; 52: 12–5. [PubMed] [Google Scholar]
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10: 778–90. [DOI] [PubMed] [Google Scholar]
- Yu JH, Zhang LY, Ren PY, Zhong T, Li ZL, Wang ZY, et al. Enterovirus 71 mediates cell cycle arrest in S phase through non-structural protein 3D. Cell Cycle 2015; 14: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Privileged structures: applications in drug discovery. Comb Chem High Throughput Screen 2004; 7: 473–94. [DOI] [PubMed] [Google Scholar]
- Breinbauer R, Vetter IR, Waldmann H. From protein domains to drug candidates-natural products as guiding principles in the design and synthesis of compound libraries. Angew Chem Int Ed Engl 2002; 41: 2879–90. [DOI] [PubMed] [Google Scholar]
- Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2002; 2: 143–8. [DOI] [PubMed] [Google Scholar]
- Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, et al. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol 2005; 79: 8014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Wang Y, Wong J, Zhang J, McManus BM, Luo H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J Virol 2007; 81: 3142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev 2007; 27: 609–30. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Wang K, Yu W, Sun B, Schwarz W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antiviral Res 2011; 90: 64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuangsuo D, Zhengguo Z, Yunru C, Xin Z, Baofeng W, Lichao Y, et al. Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit 2006; 12: BR302–6. [PubMed] [Google Scholar]
- Xiong HR, Luo J, Hou W, Xiao H, Yang ZQ. The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J Ethnopharmacol 2010; 133: 718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg 2004; 71: 179–86. [PubMed] [Google Scholar]
- Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang CB, et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-beta1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol 2011; 658: 168–74. [DOI] [PubMed] [Google Scholar]
- Gay RT, Belisle S, Beck MA, Meydani SN. An aged host promotes the evolution of avirulent coxsackievirus into a virulent strain. Proc Natl Acad Sci U S A 2006; 103: 13825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ. A simple method of estimating fifty percent endpoints. Am J Hyg 1983; 27: 493–7. [Google Scholar]
- Shen FH, Shen TJ, Chang TM, Su IJ, Chen SH. Early dexamethasone treatment exacerbates enterovirus 71 infection in mice. Virology 2014; 464-465: 218–27. [DOI] [PubMed] [Google Scholar]
- Flobinus A, Taudon N, Desbordes M, Labrosse B, Simon F, Mazeron MC, et al. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J Antimicrob Chemother 2013; 69: 34–40. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Lin HJ, Chen TH, Hsu YA, Liu CS, Hwang GY, et al. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS One 2015; 10: e0117602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu H, Huang C, Cui X, Gao Y, Huang Y, et al. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-gamma. J Cardiovasc Pharmacol 2006; 47: 190–5. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Ren P, Zhong T, Li Z, Wang Z, et al. Enterovirus 71 mediates cell cycle arrest in S phase through non-structural protein 3D. Cell Cycle 2015; 14: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Li J, Zhang X, Meng F, Zhou Y, Yao X, et al. Validation and evaluation of serological correlates of protection for inactivated enterovirus 71 vaccine in children aged 6–35 months. Hum Vaccin Immunother 2016; 12: 916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Liang ZL, Li XL, Ge HM, Meng FY, Mao QY, et al. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2013; 381: 1037–45. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang J, Ma S, Liu Y. Anti-enterovirus 71 agents of natural products. Molecules 2015; 20: 16320–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Chen W, Sinha B, Tu Y, Manning S, Thomas N, et al. Neuroprotective agents for neonatal hypoxic-ischemic brain injury. Drug Discov Today 2015; 20: 1372–81. [DOI] [PubMed] [Google Scholar]
- Burr AR, Molkentin JD. Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ 2015; 22: 1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SC, Lin JY, Lo LY, Li ML, Shih SR. Diverse apoptotic pathways in enterovirus 71-infected cells. J Neurovirol 2004; 10: 338–49. [DOI] [PubMed] [Google Scholar]
- Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ, et al. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol 2004; 78: 7916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Zheng X, Wei R, Zhang N, Zhu K, Xu B, et al. The cytokine and chemokine profiles in patients with hand, foot and mouth disease of different severities in Shanghai, China, 2010. PLoS Negl Trop Dis 2013; 7: e2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Liu J, Li Y, Zhao J, Zhang X. Emodin inhibits homocysteine-induced C-reactive protein generation in vascular smooth muscle cells by regulating PPARgamma expression and ROS-ERK1/2/p38 signal pathway. PLoS One 2015; 10: e0131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Liu J, Ye L, Liao QJ, Wu JG, Gao JR, et al. HCV NS2 protein inhibits cell proliferation and induces cell cycle arrest in the S-phase in mammalian cells through down-regulation of cyclin A expression. Virus Res 2006; 121: 134–43. [DOI] [PubMed] [Google Scholar]
- Helt AM, Harris E. S-phase-dependent enhancement of dengue virus 2 replication in mosquito cells, but not in human cells. J Virol 2005; 79: 13218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QH, Zhang YM, Fan L, Tong G, He L, Dai C. Classic swine fever virus NS2 protein leads to the induction of cell cycle arrest at S-phase and endoplasmic reticulum stress. Virol J 2010; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]