Abstract
Toll-like receptor 4 (TLR4)-mediated signaling plays a critical role in sepsis-induced acute lung injury (ALI). LYRM03 (3-amino-2-hydroxy-4-phenyl-valyl-isoleucine) is a novel derivative of ubenimex, a widely used antineoplastic medicine. We previously found that LYRM03 has anti-inflammatory effects in cecal ligation puncture mouse model. In this study we determined whether LYRM03 attenuated LPS-induced ALI in mice. LPS-induced ALI mouse model was established by challenging the mice with intratracheal injection of LPS (5 mg/kg), which was subsequently treated with LYRM03 (10 mg/kg, ip). LYRM03 administration significantly alleviated LPS-induced lung edema, inflammatory cell (neutrophils and macrophages) infiltration and myeloperoxidase (MPO) activity, decreased pro-inflammatory and chemotactic cytokine (TNF-α, IL-6, IL-1β, MIP-2) generation and reduced iNOS and COX-2 expression in the lung tissues. In cultured mouse alveolar macrophages in vitro, pretreatment with LYRM03 (100 μmol/L) suppressed LPS-induced macrophage activation by reducing Myd88 expression, increasing IκB stability and inhibiting p38 phosphorylation. These results suggest that LYRM03 effectively attenuates LPS-induced ALI by inhibiting the expression of pro-inflammatory mediators and Myd88-dependent TLR4 signaling pathways in alveolar macrophages. LYRM03 may serve as a potential treatment for sepsis-mediated lung injuries.
Keywords: LYRM03, ubenimex, LPS, acute lung injury, alveolar macrophage, pro-inflammatory cytokines, toll-like receptor 4, Myd88, IκB, p38
Introduction
Acute lung injury (ALI) is a serious clinical condition that is often caused by devastating diseases, including sepsis, acid aspiration and trauma. ALI is characterized by severe and acute inflammatory responses within the lungs1,2,3,4, and its most severe form is defined as acute respiratory distress syndrome (ARDS). ALI and ARDS are the major causes of morbidity and mortality in the clinic.
The pathogenesis of ALI is not entirely clear. Generally, the recruitment of inflammatory cells (ie, neutrophils and macrophages) into the lung is considered a primary event in the initiation and development of ALI5,6. The over-activated inflammatory cells lead to the excessive release of pro-inflammatory cytokines [ie, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), etc], chemokines [ie, macrophage inflammatory protein 2 (MIP-2)], proteolytic enzymes (iNOS, COX-2, etc), oxidants and reactive nitrogen species; this results in direct tissue injury and lung inflammation3,4.
The intratracheal administration of LPS, an endotoxin, has been commonly used to simulate and study the pathogenesis of human ALI, along with potential intervention strategies7. LPS is a specific ligand of TLR4 and the primary stimulus in macrophage activation8,9. LPS binding to TLR4 triggers downstream signaling events that mainly occur through two adaptors —Myd88 (myeloid differentiation factor 88) and TRIF (TIR-domain-containing adapter-inducing interferon-β)10,11. Myd88 is the major activator of three mitogen-activated protein kinase (MAPK) pathways, including the c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 pathways12,13. Activation of Myd88-dependent TLR4 signaling also induces degradation of IκB and promotes the nuclear translocation and activation of nuclear factor-kappa B (NF-κB); this results in the overexpression of inflammatory mediators and subsequent pulmonary damage that precedes ALI14.
There are no proved drugs for treating ARDS in the world because no specific ALI therapies exist15. Recent scientific interests have become focused on new applications for old drugs and/or their derivatives as potential therapeutic agents in combating human diseases. Ubenimex (Figure 1A), more commonly known as Bestatin®, is an inhibitor of several proteases, including aminopeptidase B, aminopeptidase N (APN), leukotriene A4 hydrolase and leukotriene D4 hydrolase16,17,18. It is derived from Streptomyces olivoreticuli19 and has been widely used to treat acute myelocytic leukemia20. Ubenimex treatment reportedly inhibits the production of pro-inflammatory mediators, such as IL-6 and IL-8, by LPS-stimulated monocytes and alveolar macrophages from patients with sarcoidosis21. This study suggests that ubenimex and its derivatives may provide a basis for developing novel anti-inflammatory agents to treat inflammatory diseases. LYRM03 (3-amino-2-hydroxy-4-phenyl-valyl-isoleucine) is a novel derivative of ubenimex (Figure 1B). We observed that LYRM03 protected mice from bacterial infection-induced death in the cecal ligation puncture (CLP) model (data not published). However, the role of LYRM03 in inflammation and ALI remains undefined.
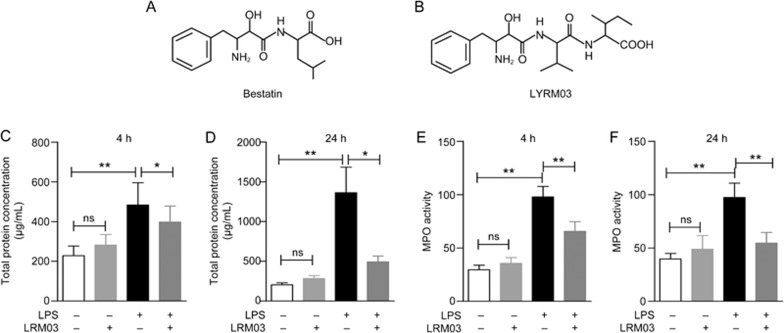
Figure 1.
LYRM03 attenuated inflammatory cell accumulation in the lung tissues of ALI mice. Mice were challenged with 5 mg/kg of LPS or 10 mg/kg of LYRM03 by an intratracheal injection. Subsequent intraperitoneal (ip) injections with 10 mg/kg of LYRM03 were administered once (4 h groups) or twice (24 h groups) before the collections. Control mice received PBS alone by injection. (A, B) The chemical structures of Bestatin (ubenimex, A) and LYRM03 (B). (C, D) The total BALF protein concentrations from mice after the LPS challenge for 4 h (C) and 24 h (D) were determined. (E, F) LYRM03 reduced MPO activity in LPS-induced ALI mice. Values represent the mean±SD. n>=5 mice in each group. *P<0.05, **P<0.01.
In the present study, the protective effect of LYRM03 against LPS-induced ALI was characterized. The LYRM03 treatment remarkably attenuated lung inflammation and decreased the expression of pro-inflammatory cytokines, chemokines and inflammation-associated enzymes in the lungs of ALI mice. The inhibitory effects of LYRM03 on alveolar macrophage activation and TLR4-mediated signaling were also determined in vitro.
Materials and methods
Animals
Male C57BL/6 mice (6–12 weeks old, 22±3 g, Specific Pathogen-Free) were purchased from Slac Laboratory Animal Corporation (Shanghai, China). The study adhered to the Principles of Laboratory Animal Care (NIH publication No 85–23, revised 1996). All experimental protocols for this study were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University.
Compound and reagents
LYRM03 (3-amino-2-hydroxy-4-phenyl-valyl-isoleucine) (Figure 1B) was synthesized at the Shanghai Laiyi Center for Biopharmaceutical R&D. Its purity was >98% by HPLC. The original LYRM03 stock was stored as a solid powder at -20 °C. The working solution was freshly prepared before use by dissolving 1 mg/mL (2.5 mmol/L) into PBS; it was stored at 4 °C for no more than 12 h.
The primers for this study were synthesized by HuaGene Biotech Co, Ltd (Shanghai, China). The primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA); they included antibodies against iNOS, COX-2, Myd88, IκB, phosphor-p38 (T334), total p38, and β-actin. ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). Other chemical reagents without special indications were obtained from Sigma.
ALI mouse model
Mice were randomly divided into the following 7 groups: control, LYRM03 (4 and 24 h) groups, LPS (4 and 24 h) groups, and LPS+LYRM03 (4 and 24 h) groups. Each group contained five mice. LYRM03 and LPS were separately dissolved in PBS. Mice were administered an intratracheal injection of LPS (5 mg/kg) or LYRM03 (10 mg/kg) and a subsequent intraperitoneal (ip) injection of LYRM03 (10 mg/kg). For the 24-h LPS treatment group, another ip injection with LYRM03 was administered 12 h after the LPS challenge. Mice in the control group received a PBS injection without the LPS challenge. At the indicated time points (4 and 24 h), mice were euthanized by CO2 inhalation, and samples were collected.
BALF acquisition and analysis
The lungs were lavaged three times with 1 mL PBS, and the BALF was centrifuged at 4 °C. The cell-free supernatant was harvested for a total protein analysis, which was performed using the bicinchoninic acid (BCA) protein assay kit (Beyotime, Shanghai, China).
MPO activity assay
The largest (right) lobes of the lungs were collected and subjected to three freeze-thaw cycles. The supernatants were collected at 4 °C, and their protein concentrations were determined as described above. Absorbance changes at 655 nm were measured with a microplate reader (FlexStation 3, Molecular Devices, California, CA, USA). The MPO activity value was defined as the absorbance change per min per gram protein22.
RNA isolation, reverse transcription and quantitative PCR
Frozen lungs (each from the right upper lobe) were homogenized, and the total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was prepared with the ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan) and amplified by real-time PCR on a StepOne Plus (Thermo Fisher Scientific, Waltham, MA, USA) with primer sets for TNF-α (forward, 5′-TTCTCATTCCTGCTTGTGG-3'; reverse, 5'-ACTTGGTGGTTTGCTACG-3'), IL-1β (forward, 5'-CCAGCTTCAAATCTCACAGCAG-3'; reverse, 5'-CTTCTTTGGGTATTGCTTGGGATC-3'), IL-6 (forward, 5'-CTTCTTGGGACTGATG-3'; reverse, 5'-CTGGCTTTGTCTTTCT-3'), MIP-2 (forward, 5'-CCAAGGGTTGACTTCAAGAAC-3'; reverse, 5'-AGCGAGGCACATCAGGTACG-3') and GAPDH (forward, 5'-TGCGACTTCAACAGCAACTC-3'; reverse, 5'-CTTGCTCAGTGTCCTTGCTG-3').
Histopathology
Lung tissues (left lobe) were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 5-μm thick sections in a microtome (RM2235, Leica Biosystems, Wetzlar, Germany). The sections were stained with hematoxylin and eosin, and images were captured by microscopy (RX51, Olympus Optical Co Ltd, Tokyo, Japan).
Isolation of alveolar macrophages and cell culture
Alveolar macrophages were prepared by flushing the cells from the lung tissue with 10 mL PBS per mouse. Cells were collected and cultured in DMEM (HyClone Logan, UT, USA) plus 10% fetal bovine serum (FBS) (Biological Industries, Kibbutz Beit-Haemek, Israel). Cells were treated with the indicated concentrations of LYRM03 and/or LPS according to the experimental requirements.
ELISA
The IL-6 and TNF-α concentrations in the BALF were quantified using an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. All experiments were performed in triplicate.
Western blot analysis
After the indicated treatments, cells were collected and lysed in RIPA buffer (WEIAO BioTech Co Ltd, Shanghai, China) plus 1 mmol/L PMSF. Equal protein concentrations were loaded onto 10% SDS/PAGE gels, transferred to nitrocellulose filter membranes and incubated with indicated primary antibodies overnight at 4 °C. Membranes were incubated with secondary antibodies (KPL, Gaithersburg, MD, USA) for 1 h at room temperature. Western blot quantifications were analyzed with ImageJ software (National Institute of Mental Health, Bethesda, MD, USA).
Quantitative determination of nitrite levels
NO2- levels were determined using Griess reagent. Briefly, 5×105 macrophages were seeded into each well of a 24-well plate. After incubation overnight, cells were treated with LPS (100 ng/mL) and different concentrations of LYRM03 for 24 h. Supernatants were collected, and nitric oxide (NO) levels were measured using the Nitric Oxide Assay Kit (Beyotime, Shanghai, China).
Statistical analysis
The data are presented as the mean±SD and were obtained from at least three independent tests. Student's t-test (paired comparison) was performed using Prism 5 (GraphPad, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
LYRM03 inhibited inflammatory cell infiltration into the lungs of ALI mice
To determine whether LYRM03 alleviated sepsis-induced ALI, we challenged mice with intratracheal injections of LPS (5 mg/kg) and treated mice with LYRM03 (10 mg/kg) by intraperitoneal administration. The total protein concentrations in the BALF were elevated after the LPS challenge at both 4 h (Figure 1C, P<0.01) and 24 h (Figure 1D, P<0.01). The LPS-induced increase in the BALF protein concentration was relieved by the LYRM03 treatment for 4 h (Figure 1C, P<0.05), with an approximate decrease of 32.7%. During the 24-h LPS challenge, LYRM03 was additionally administered 12 h after the LPS injection. The BALF protein concentration in the LYRM03/LPS group (24 h) was remarkably reduced compared to that of the LPS group (Figure 1D, P<0.05).
MPO activity in lung tissue is a specific marker for neutrophil infiltration and ALI severity. The MPO activity assay indicated that the intratracheal LPS injection caused evident neutrophil accumulation in the lung tissue (Figure 1E, P<0.01; Figure 1F, P<0.01). Mice that were treated with LPS and LYRM03 for 4 and 24 h displayed significant reductions in MPO activity (Figure 1E and 1F, P<0.05). The LYRM03 treatment without the LPS challenge did not induce a change in BALF protein concentration (Figure 1C and 1D) nor did it affect MPO activity (Figure 1E and 1F).
LYRM03 ameliorated the LPS-induced pulmonary injury
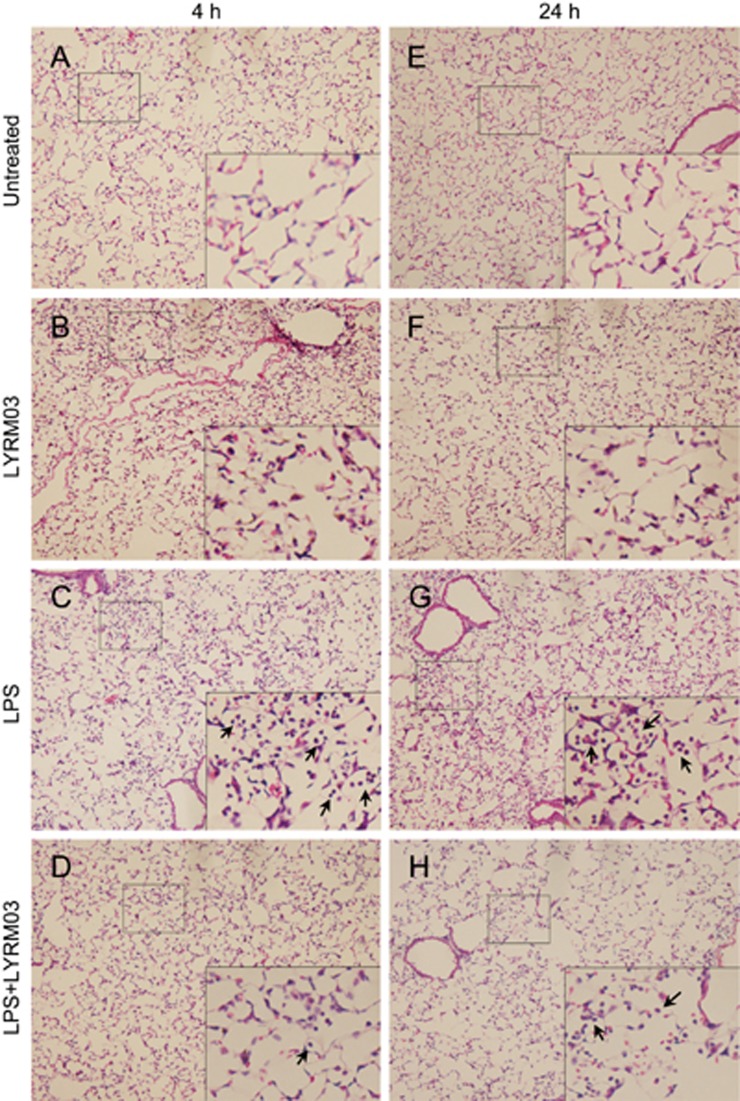
The largest lobe of the lungs was collected and subjected to a histologic assessment. Compared to the control group (Figure 2A and 2D), mice that were challenged with LPS (5 mg/kg) for 4 h (Figure 2B) and 24 h (Figure 2E) showed evident inflammation in their lung tissues. Inflammatory cell infiltration and inter-alveolar septal thickening were also observed. These LPS-induced pathologic changes were attenuated in the lungs of ALI mice that were treated with LYRM03 (10 mg/kg) for 4 h (Figure 2C) and 24 h (Figure 2F). The histological assays were consistent with the protein concentration and MPO activity assay data, which suggested that LYRM03 inhibited inflammatory cell infiltration during ALI development. In the analysis above, the LYRM03 injection without the LPS challenge did not induce pathologic changes in the lung tissue.
Figure 2.
LYRM03 attenuated pathological changes in ALI mice. The lung lobes were collected, fixed and stained with H&E to assess ALI inflammation. Panels in the left lane show histopathological images that represent the lungs of naïve mice that were treated for 4 h with PBS alone (A, untreated), LYRM03/PBS (B, LYRM03), LPS/PBS (C, LPS) or LPS/LYRM03 (D, LPS+LYRM03). Panels in the right lane show histopathological images for naïve mice that were treated for 24 h with PBS alone (E, untreated), LYRM03/PBS (F, LYRM03), LPS/PBS (G, LPS) or LPS/ LYRM03 (H, LPS+LYRM03). Representative images at the original magnification (×100) are shown with partially enlarged details. The arrows indicate the infiltrated inflammatory cells.
LYRM03 decreased inflammatory cytokines and factors in ALI mice
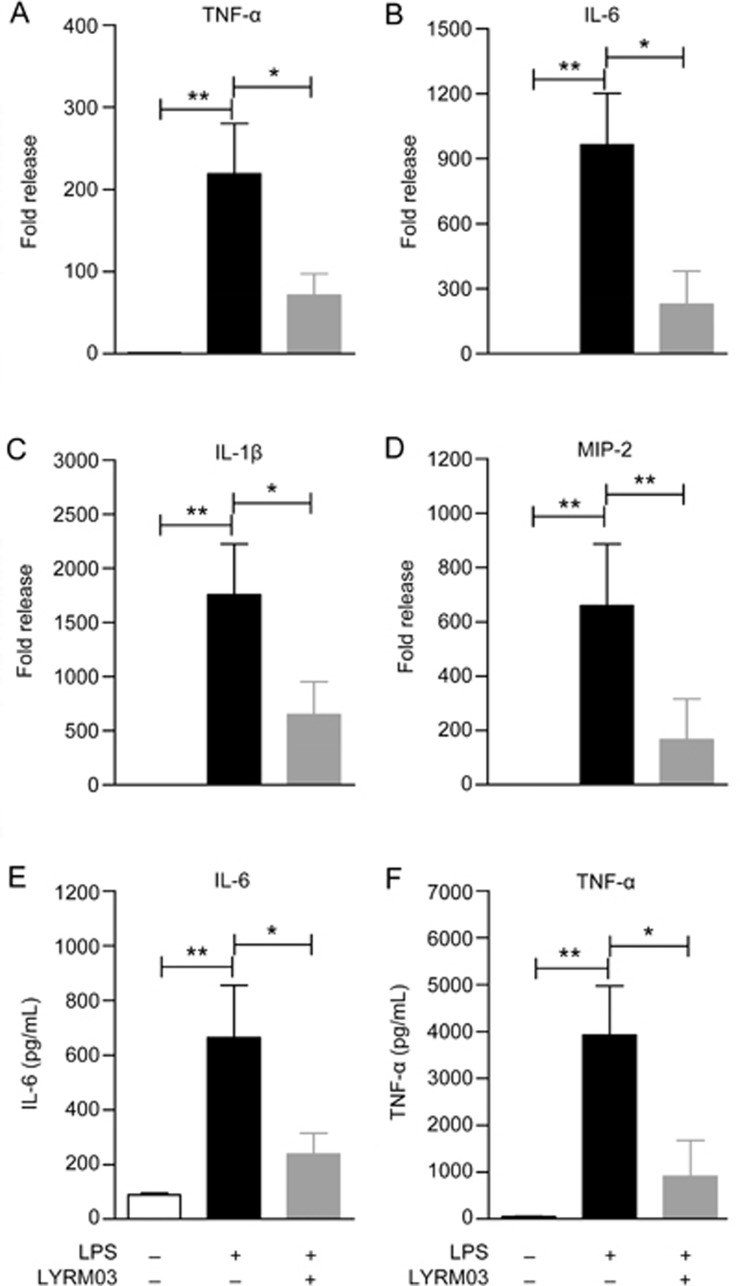
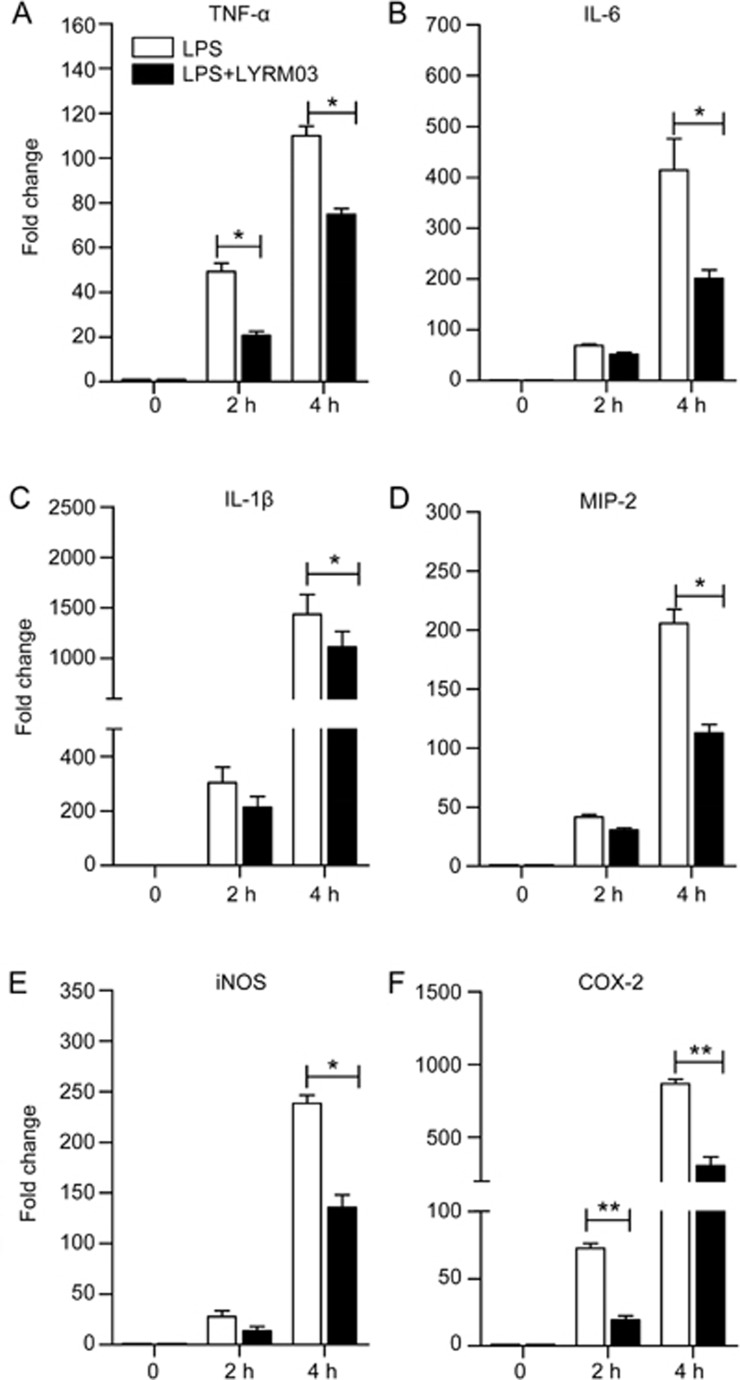
To further determine the anti-inflammatory role of LYRM03 in ALI, we measured the expression of pro-inflammatory and chemotactic cytokines. The quantitative PCR analysis indicated that the mRNA levels of TNF-α, IL-1β, IL-6, and MIP-2 significantly increased (Figure 3A–3D, P<0.01) in the lungs of mice that were treated with LPS for 4 h compared with the control group, which only received PBS. The LYRM03 treatment remarkably decreased the LPS-induced mRNA expression of these pro-inflammatory cytokines (Figure 3A–3D, P<0.05). The mRNA levels of TNF-α, IL-1β, IL-6, and MIP-2 were reduced to approximately 30% in response to LYRM03 (Figure 3A–3D). The enzyme linked immunosorbent assays (ELISA) results for the two critical inflammatory cytokines (IL-6 and TNF-α) confirmed that LYRM03 attenuated LPS-induced IL-6 and TNF-α production in the ALI mice (Figure 3E and 3F, P<0.05).
Figure 3.
LYRM03 decreased pro-inflammatory cytokine expression and release in ALI mice. The lung lobes and BALF from mice that were treated with PBS, LPS/PBS and LPS/LYRM03 for 4 h were individually collected. (A–D) Total RNA was isolated from each lung homogenate, and mRNA expression levels of TNF-α (A), IL-6 (B), IL-1β (C), and MIP-2 (D) were quantified by real-time PCR. The values represent the mean±SD. n=5. *P<0.05, **P<0.01. (E, F) The IL-6 (E) and TNF-α (F) concentrations in the BALF were quantified using commercial ELISA kits. The values represent the mean±SD. n=4. *P<0.05, **P<0.01.
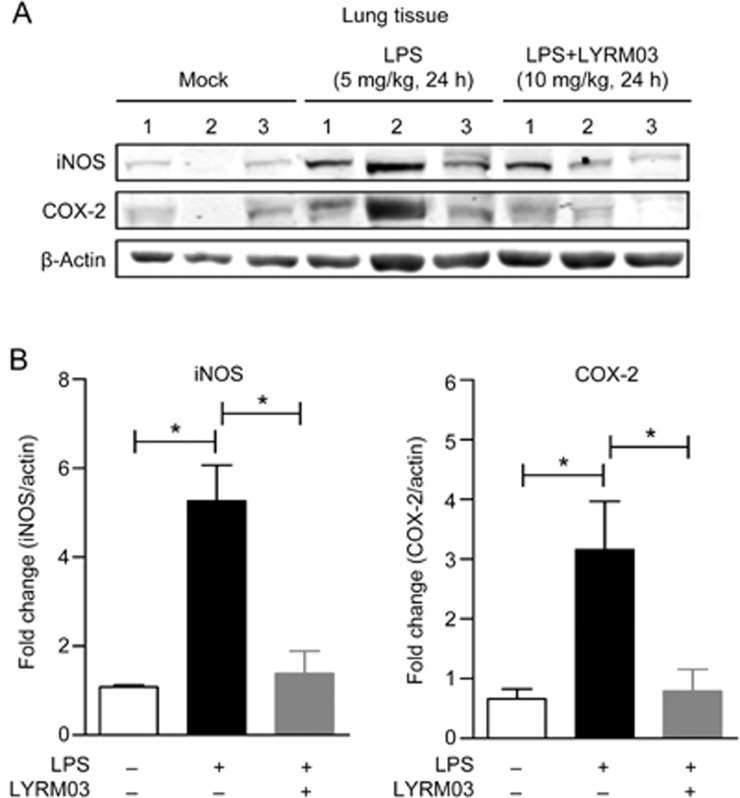
Both iNOS and COX-2 are important inflammatory factors that contribute to ALI. The minor lobes of the lungs were collected and prepared to determine iNOS and COX-2 expression by Western blot. The LPS challenge for 24 h clearly induced iNOS and COX-2 expression (Figure 4A) in the lungs of ALI mice. LYRM03 injections after the LPS challenge led to observable decreases in iNOS and COX-2 expression (Figure 4A). The statistical analysis of the Western blot result indicated that LYRM03 significantly suppressed LPS-induced iNOS and COX-2 expression (Figure 4B, P<0.05).
Figure 4.
LYRM03 inhibited iNOS and COX-2 expression in the lungs of ALI mice. The lung lobes of mice that were treated with PBS, LPS/PBS and LPS/LYRM03 for 24 h were collected. (A) Western blot analysis of iNOS and COX-2 protein expression in the lungs of control mice, ALI mice and ALI mice that were treated with LYRM03. (B) The ratios of iNOS or COX-2 to β-actin are shown in the densitometry. Statistical analyses were performed with the Student's t-test. *P<0.05. n= 3.
LYRM03 inhibited LPS-induced alveolar macrophage activation
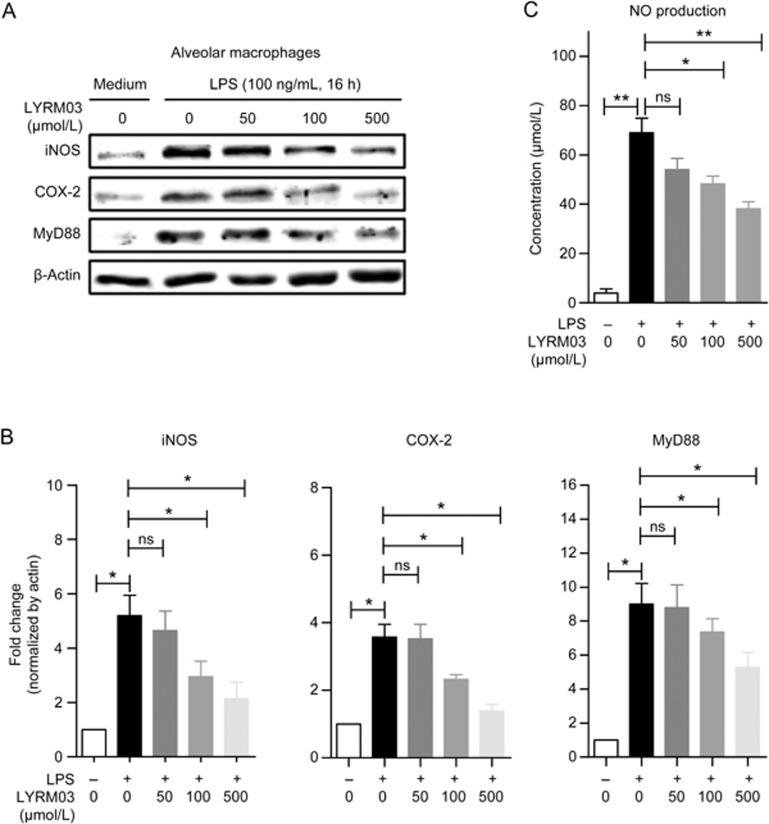
Alveolar macrophages serve as the first line of defense against pathogen invasion and infection. To investigate the underlying mechanism of the anti-inflammatory effects of LYRM03 in ALI, we isolated alveolar macrophages (AM) from murine lung tissues. Alveolar macrophages were incubated with LYRM03 for 1 h before the LPS (100 ng/mL) treatment. After subsequent 2- or 4-h incubations, alveolar macrophages were collected, and quantitative PCR assays were performed to determine the mRNA levels of TNF-α, IL-1β, IL-6, and MIP-2. After 2- and 4-h LPS treatments, the inflammatory cytokines and mediators that were expressed by the AMs were dramatically induced (Figure 5). The LYRM03 (100 μmol/L) pretreatment inhibited the expression of these inflammatory factors (Figure 5, P<0.05). The Western blot analysis confirmed the dose-dependent suppression of iNOS and COX-2 expression by LYRM03 after the 16-h LPS treatment (Figure 6A and 6B). Increased iNOS expression is responsible for the excessive production of NO during the inflammatory process. Accordingly, our result showed that LYRM03 also significantly inhibited NO that was produced by AMs after the 24-h LPS stimulation (Figure 6C).
Figure 5.
LYRM03 inhibited the LPS-induced expression of inflammatory factors in alveolar macrophages. Alveolar macrophages were isolated from murine lung tissues. Cells were collected and incubated with or without LYRM03 (100 μmol/L) for 1 h, followed by treatments with LPS (100 ng/mL) for 0, 2, and 4 h. The expression levels of TNF-α (A), IL-6 (B), IL-1β (C), MIP-2 (D), iNOS (E), and COX-2 (F) were measured by quantitative PCR. The values represent the mean±SD. n= 4. *P<0.05, **P<0.01.
Figure 6.
Dose-dependent reduction of iNOS, COX-2, and Myd88 expression by LYRM03 in LPS-activated alveolar macrophages. Alveolar macrophages were collected and incubated with or without LYRM03 (0, 50, 100, and 500 μmol/L) for 1 h. Cells were treated with LPS (100 ng/mL) for 16 h. Control cells were incubated with the cell culture medium. (A) Western blot analysis of the iNOS, COX-2, and Myd88 proteins in alveolar macrophages. β-Actin was used as the loading control. (B) The ratios of iNOS, COX-2, and Myd88 to β-actin are shown in the densitometry. (C) The culture supernatant was collected, and the NO levels were measured using Griess reagent at 24 h. Statistical analyses were performed with Student's t-test. *P<0.05, **P<0.01. ns (not significant). n= 3.
LYRM03 suppressed the Myd88-dependent TLR4 signaling pathway
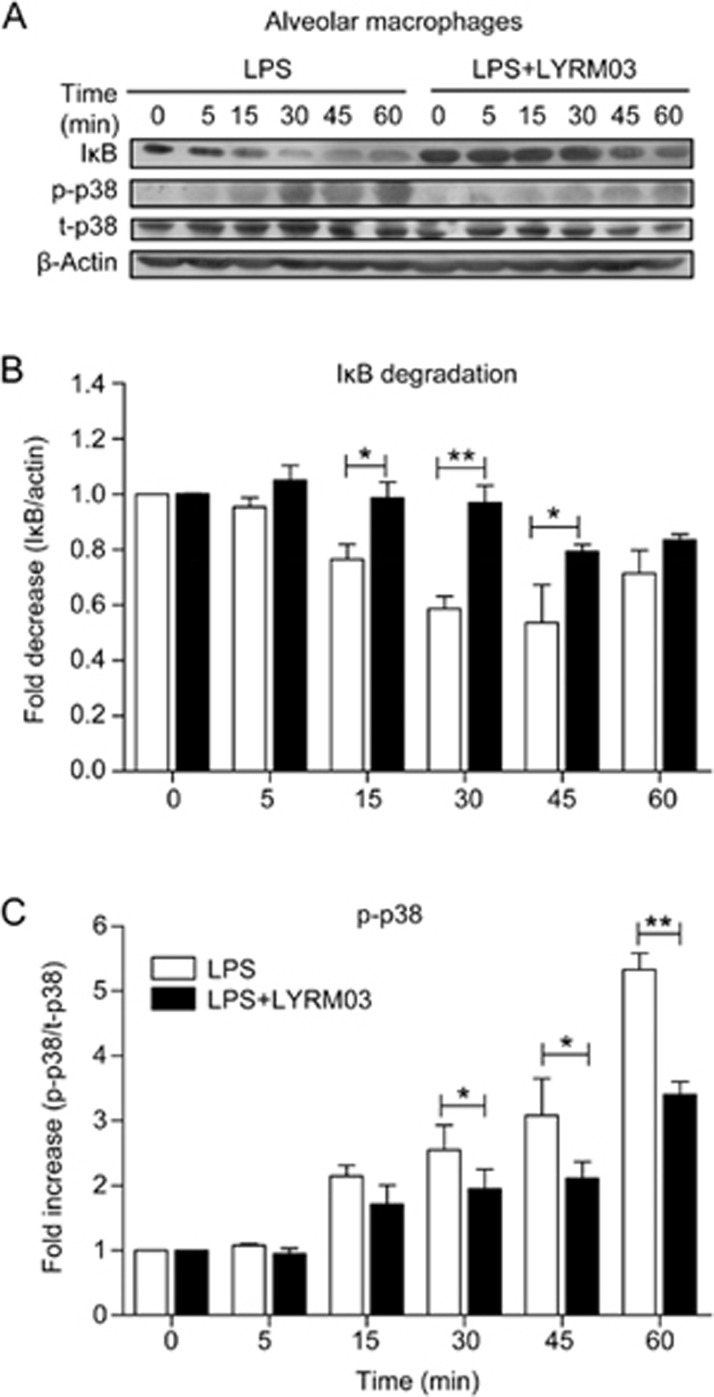
As the direct receptor of LPS, TLR4 plays a critical role in LPS-induced macrophage activation and inflammatory factor generation. We investigated whether LYRM03 attenuated LPS-induced ALI by modulating the TLR4 signaling pathway. The Western blot result suggested a dose-dependent inhibition by LYRM03 of the LPS-induced expression of Myd88 (Figure 6A and 6B), a crucial adaptor protein for the activated TLR4 response. Two traditional downstream pathways (IκB/NF-κB and MAPK) were also evaluated. Alveolar macrophages were pretreated with LYRM03 (100 μmol/L) for 1 h and stimulated with LPS (100 ng/mL) for 5, 15, 30, 45, and 60 min. IκB degradation and p38 MAPK activation were assessed by Western blot. After LPS treatments for 15, 30, and 45 min, IκB degradation was significantly reduced in LYRM03-pretreated macrophages (Figure 7A and 7B, P<0.05), which indicated that LPS-induced IκB degradation was inhibited by LYRM03. The phosphorylated p38 MAPK was also reduced in the AMs that were treated with LYRM03/LPS compared to the AMs that were treated with LPS (Figure 7A and 7B), while the total p38 levels remained similar between the two groups (Figure 7A). Our result demonstrated that LYRM03 attenuated TLR4/Myd88-dependent signaling pathways, where both IκB degradation and p38 MAPK activation were inhibited.
Figure 7.
LYRM03 suppressed IκB degradation and p38 activation in alveolar macrophages. Alveolar macrophages were pre-incubated with or without LYRM03 (100 μmol/L) for 1 h. Cells were subsequently stimulated with LPS (100 ng/mL) for 0, 5, 15, 30, 45, and 60 min. Control cells were incubated with the cell culture medium. (A) Western blotting for IκB, phosphor-p38 (p-p38) and total p38 (t-p38) in alveolar macrophages. β-actin was used as the loading control. (B) The ratios of IκB to β-actin and phosphor-p38 (p-p38) to total-p38 are shown in the densitometry. Statistical analyses were performed with Student's t-test. *P<0.05, **P<0.01. n=4.
Discussion
Acute lung injury (ALI) is a life-threatening disorder that is often complicated by shock, sepsis, ischemia reperfusion, hyperoxia, and other ailments1,2. Despite advances in the understanding of ALI pathophysiology, effective therapies and specific drugs for ALI treatments are lacking. Thus, ALI and its severe form, ARDS, remain problematic in the clinic, with 30% to 40% mortality rates23.
LYRM03 (3-amino-2-hydroxy-4-phenyl-valyl-isoleucine) is a novel derivative of ubenimex (a.k.a.Bestatin®), which is a widely used anti-tumor drug that is administered in combination with chemotherapy and radiotherapy in the clinic. Ubenimex is a known inhibitor of various proteases, including aminopeptidase N (APN/CD13). APN/CD13 expression is dysregulated in inflammatory diseases and cancers19. It is likely that novel anti-cancer and anti-inflammatory drugs will be developed by designing new APN inhibitors or by synthesizing derivatives of existing inhibitors, such as ubenimex19. However, there is no evidence that addresses whether ubenimex or its derivatives play roles in sepsis-mediated inflammation. The present study focused on the effect of LYRM03, an ubenimex derivative, in an LPS-induced murine ALI model that was broadly used to recapitulate the characteristics of human ALI24.
During the ALI process, common pathological changes are observed, such as injuries to the capillary endothelium and pulmonary epithelium and the increased permeability of the alveolar-capillary barrier1,2; this leads to flooding of the airspaces by protein-rich edema fluid and severe gas-exchange abnormalities. In our study, the LYRM03 treatment improved the lung pathologic changes and reduced the BALF protein concentrations in mice that were challenged with LPS for 4 and 24 h. The anti-inflammatory effects of LYRM03 were more prominent than those of its parent molecule, ubenimex (data not shown). Therefore, our subsequent studies focused on how LYRM03 alleviated endotoxin-induced ALI.
Inflammatory cell infiltration into the lung tissue is widely accepted as a typical characteristic of ALI. Two major inflammatory cell types, neutrophils and macrophages, significantly accumulate in the lung and contribute to ALI25,26. MPO activity is proportional to the neutrophil number and is a key marker of lung injury27. The results of the MPO activity assay were consistent with the pulmonary edema and histological analyses, which suggested that LYRM03 could alleviate LPS-induced lung inflammation during the entire ALI process.
Based on the lung microenvironment, on lung disease progression and in response to various stimulations, alveolar macrophages (AMs) switch to the classically activated phenotype or the alternatively activated phenotype28. Activation of AMs into the classically activated phenotype is typical of LPS-induced ALI. In response to LPS stimulation, classically activated AMs excessively release pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which elicit the inflammatory cascade and cause further damage to epithelial and endothelial cells in the lung29. MIP-2, a chemotactic cytokine, is primarily produced by LPS-activated AMs and further induces the synthesis and release of TNF-α, IL-1β, and IL-6 from alveolar epithelial cells and macrophages; MIP-2 also triggers neutrophil accumulation into the lung tissue. iNOS and COX-2 are two major products of classically activated AMs, where they are highly expressed. iNOS and COX-2 are responsible for NO and prostaglandin E2 (PGE2) production, respectively. Excessive amounts of NO react with superoxide to form peroxynitrite and directly damage the capillary endothelium and pulmonary epithelium, which are key events in sepsis-induced ALI pathogenesis30,31. COX-2 overexpression and high PGE2 levels in response to inflammatory stimuli also contribute to various inflammatory diseases, including ALI32. The LYRM03 treatment in ALI mice significantly decreased the production of the inflammatory cytokines and factors in the lung and BALF. Moreover, LYRM03 also significantly reduced the expression of these pro-inflammatory mediators in LPS-activated AMs in vitro. Therefore, LYRM03 exerted protective effects against LPS-induced ALI by inhibiting the inflammatory mediators.
LPS-TLR4 signaling is important for AM activation and plays a pivotal role in the initiation, amplification and perpetuation of the inflammatory response during ALI. Previous studies have shown that TLR4 mutant (C3H/HeJ) mice are hypo-responsive to LPS33 and do not develop ALI after an aerosolized LPS treatment34. TLR4 gene knockdown with small interference RNA significantly reduces LPS-induced production of pro-inflammatory cytokines in vitro and in vivo, which results in the alleviation of LPS-induced acute lung injury35. These findings suggest that TLR4 signaling serves as a critical pathway in LPS-induced ALI. The results of our in vitro analysis indicated that LYRM03 significantly decreased the expression of Myd88, which is one of two crucial adapter proteins in the activated TLR4 response. The subsequent analysis confirmed that LYRM03 suppressed the Myd88-dependent LPS-TLR4 signaling pathways, including the p38 MAPK and IκB/NF-κB pathways. The pretreatment with LYRM03 inhibited p38 MAPK phosphorylation and delayed IκB protein degradation, which masked the NF-κB nuclear localization signals to maintain NF-κB in an inactive state. The p38 MAPK suppression and prolonged NF-κB inactivation inhibited downstream transcription and expression of pro-inflammatory mediators, including cytokines (TNF-α, IL-1β, and IL-6), chemokines (MIP-2), and inflammation-related proteins (iNOS and COX-2)23. Our study suggested that LYRM03 inhibited inflammatory mediator production by suppressing the Myd88-dependent TLR4 pathway. Additionally, although LYRM03 is a derivative of ubenimex, its substrate is unlikely to be APN/CD13. A recent study revealed that myeloid APN/CD13 negatively regulated TLR4 signaling by governing TLR4 internalization and subsequent innate signaling cascades36. It is likely that an APN/CD13 inhibitor will enhance TLR4-mediated inflammatory responsiveness. Consequently, the molecular target of LYRM03 in macrophage activation and TLR4-mediated signaling will need to be defined further.
Conclusion
The present study showed that LYRM03, a synthetic derivative of ubenimex, exerted a protective effect against LPS-induced ALI. The LYRM03 treatment in ALI mice reduced inflammatory cell infiltration, decreased pro-inflammatory cytokine expression and alleviated pathologic changes in the lung tissue. In vitro analyses indicated that LYRM03 inhibited the LPS-stimulated expression of inflammatory cytokines (TNF-α, IL-6, IL-1β, and MIP-2) and proteins (iNOS and COX-2) in alveolar macrophages that were isolated from the murine lung. The analyses further revealed that LYRM03 suppressed Myd88 expression and significantly repressed the MAPK and IκB/NF-κB pathways in a time-dependent manner by inhibiting p38 MAPK phosphorylation and IκB degradation. Our results suggest that LYRM03 primarily attenuated LPS-induced inflammation and lung injury by suppressing Myd88-dependent TLR4 signaling in alveolar macrophages. As a novel derivative of the old drug, LYRM03 may be a potential therapeutic agent for future ALI treatments.
Author contribution
Feng QIAN and Hui-qiong HE designed the study and drafted the manuscript; Hui-qiong HE performed the experiments; Ya-xian WU and Yun-juan NIE assisted with the animal experiments; Mei GE assisted with the data analyses; Jun WANG and Mei GE were involved in experimental discussions. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 81373424, 81573438, and 81401302), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No 20130073120108).
References
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–49. [DOI] [PubMed] [Google Scholar]
- Gordon DR, Ellen C, Eve P, Jim W, Diane PM, Margaret N, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–93. [DOI] [PubMed] [Google Scholar]
- Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003; 31: S195–9. [DOI] [PubMed] [Google Scholar]
- Lee WL, Gregory PD. Neutrophil activation and acute lung injury. Curr Opin Crit Care 2001; 7: 1–7. [DOI] [PubMed] [Google Scholar]
- Corteling R, Wyss D, Trifilieff A. In vivo models of lung neutrophil activation. Comparison of mice and hamsters. BMC Pharmacol 2002; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes TJ, Joanna HZ, Gregory PD. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol 2006; 13: 21–7. [DOI] [PubMed] [Google Scholar]
- George CL, Jin H, Christine LW. Marsha EO', John CP, Patrick O'S, et al. Endotoxin responsiveness and subchronic grain dust-induced airway disease. Am J Physiol Lung Cell Mol Physiol 2001; 280: L203–13. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003; 21: 335–76. [DOI] [PubMed] [Google Scholar]
- Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, et al. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest 2009; 119: 1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001; 413 (6851): 78–83. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott, Eva M, O'Neill, Luke AJ. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004; 113: 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlaar E, Brown Z. P38 MAPK signaling cascades in inflammatory disease. Mol Med Today 1999; 5: 439–46. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu BE, Karadikar M, Berman K, Cobb ME. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrinol Rev 2001; 22: 153–83. [DOI] [PubMed] [Google Scholar]
- Arancibia SA, Beltrán CJ, Aguirre IM, Silva P, Peralta AL, Malinarich F, Hermoso MA. Toll-like receptors are key participants in innate immune responses. Biol Res 2007; 40: 97–112. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Peter AW. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res 2016; 167: 183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot 1976; 29: 97–9. [DOI] [PubMed] [Google Scholar]
- Muskardin DT, Voelkel NF, Fitzpatrick FA. Modulation of pulmonary leukotriene formation and perfusion pressure by Bestatin, an inhibitor of leukotriene A4 hydrolase. Biochem Pharmacol 1994; 48: 131–7. [DOI] [PubMed] [Google Scholar]
- Sekine K, Fujii H, Abe F. Induction of apoptosis by Bestatin (ubenimex) in human leukemic cell lines. Leukemia 1999; 13: 729–34. [DOI] [PubMed] [Google Scholar]
- Bauvois B, Dauzonne D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: chemistry, biological evaluations, and therapeutic prospects. Med Res Rev 2006; 26: 88–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama Y, Sakamaki S, Takayanagi N, Tsuji Y, Sagawa T, Chiba H, et al. Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients-effects, complications and long-term survival. Cancer Chemother 2003; 30: 1113–8. [PubMed] [Google Scholar]
- Lkhagvaa B, Tani K, Sato K, Toyoda Y, Suzuka C, Sone S. Bestatin, an inhibitor for aminopeptidases, modulates the production of cytokines and chemokines by activated monocytes and macrophages. Cytokine 2008; 44: 386–91. [DOI] [PubMed] [Google Scholar]
- Qian F, Deng J, Gantner BN, Flavell RA, Dong C, Christman JW, et al. MAP kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2012; 302: L866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med 2003; 167: 1027–35. [DOI] [PubMed] [Google Scholar]
- Marshall HE, Potts EN, Kelleher ZT, Stamler JS, Foster WM, Auten RL. Protection from lipopolysaccharide-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med 2009; 180: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Faurschou M, Niels B. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 2003; 5: 1317–27. [DOI] [PubMed] [Google Scholar]
- Corteling R, Wyss D, Trifilieff A. In vivo models of lung neutrophil activation. Comparison of mice and hamsters. BMC Pharmacol 2002; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes TJ, Joanna HZ, Gregory PD. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol 2006; 13: 21–7. [DOI] [PubMed] [Google Scholar]
- Qian F, Deng J, Lee YG, Zhu J, Karpurapu M, Chung S, et al. The transcription factor PU.1 promotes alternative macrophage polarization and asthmatic airway inflammation. J Mol Cell Biol 2015; 7: 557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagisawa R, et al. Rosmarinic acid inhibits lung injury induced by diesel exhaust particles. Free Radical Bio Med 2003; 34: 1060–9. [DOI] [PubMed] [Google Scholar]
- Hinder F, Stubbe HD, Van Aken H, Waurick R, Booke M, Meyer J. Role of nitric oxide in sepsis-associated pulmonary edema. Am J Respir Crit Care Med 1999; 159: 252–7. [DOI] [PubMed] [Google Scholar]
- Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med 1998; 158: 1883–9. [DOI] [PubMed] [Google Scholar]
- Gust R, Kozlowski JK, Stephenson AH, Schuster DP. Schuster role of cyclooxygenase-2 in oleic acid-induced acute lung injury. Am J Respir Crit Care Med 1999; 160: 1165–70. [DOI] [PubMed] [Google Scholar]
- Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol 1979; 28: 293–450. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun 2005; 73: 1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Liu Y, Lv X, Miao X, Sun Y, Yu W. Small interference RNA targeting TLR4 gene effectively attenuates pulmonary inflammation in a rat model. J Biomed Biotechnol 2012; 2012: 406435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Subramani J, Rahman MM, Shapiro LH. CD13 restricts TLR4 endocytic signal transduction in inflammation. J Immunol 2015; 194: 4466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]