Abstract
Two independent regions within HNF1B are consistently identified in prostate and ovarian cancer genome-wide association studies (GWAS); their functional roles are unclear. We link prostate cancer (PC) risk SNPs rs11649743 and rs3760511 with elevated HNF1B gene expression and allele-specific epigenetic silencing, and outline a mechanism by which common risk variants could effect functional changes that increase disease risk: functional assays suggest that HNF1B is a pro-differentiation factor that suppresses epithelial-to-mesenchymal transition (EMT) in unmethylated, healthy tissues. This tumor-suppressor activity is lost when HNF1B is silenced by promoter methylation in the progression to PC. Epigenetic inactivation of HNF1B in ovarian cancer also associates with known risk SNPs, with a similar impact on EMT. This represents one of the first comprehensive studies into the pleiotropic role of a GWAS-associated transcription factor across distinct cancer types, and is the first to describe a conserved role for a multi-cancer genetic risk factor.
Keywords: HNF1B, eQTL, prostate, ovarian, cancer
INTRODUCTION
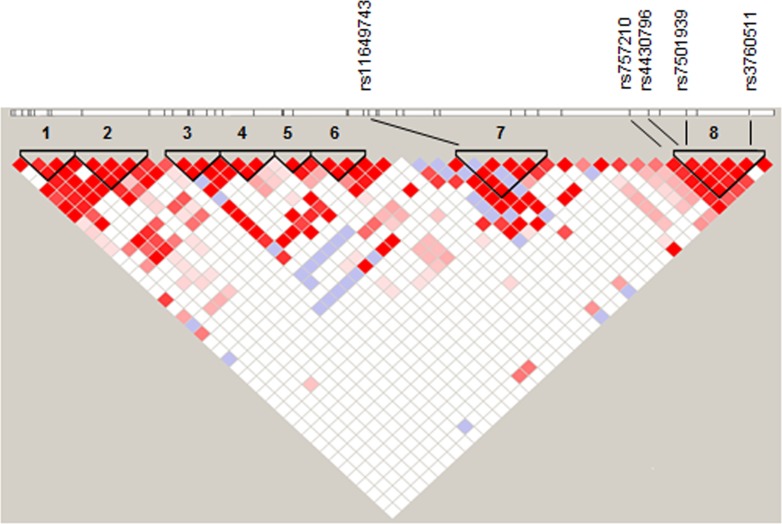
Genome-wide association studies (GWAS) and fine-mapping have identified several distinct variants within the hepatocyte nuclear factor 1b (HNF1B) gene associated with increased risk of prostate cancer [1–8] and high-grade serous and clear cell epithelial ovarian cancer (OC) [9, 10]. It has also been associated with both type I and II endometrial cancer risk in a large meta-analysis [11]. HNF1B has three main regions of risk association (Figure 1) in which the four most significant risk variants for these two cancer types lie: rs11649743 in linkage block 7, rs4430796 and rs7501939 (all prostate) are in strong linkage disequilibrium in region 8 (r2 = 0.764) (Table 1), with the most significant SNP for serous invasive ovarian cancer risk rs757210 immediately adjacent. Prostate cancer (PC) risk SNP rs3760511 is within 1kb of the transcription start site (TSS) and was identified in a GWAS of four European populations; although not the most statistically significant marker, the rs-3760511-C allele is associated with an odds ratio of 1.16 [2].
Figure 1. Overall LD within HNF1B highlighting eight regions (black triangles), with rs11649743 in region 7 and rs4430796, rs7501939 and rs3760511 in region 8.
Ovarian cancer risk SNP rs757210 lies immediately adjacent to region 8. D′ values were derived from 1000 genomes project CEU data (http://www.1000genomes.org/), and linkage plots generated in Haploview using 95 % confidence intervals (Gabriel 2002).
Table 1. Pair-wise estimates of LD between each of the five markers studied (r2; CEU population), confirming three distinct groupings of the SNPs studied.
| rs11649743 | rs757210 | rs4430796 | rs7501939 | rs3760511 | |
|---|---|---|---|---|---|
| rs11649743 | 1 | 0.005 | 0.014 | 0.025 | 0.116 |
| rs757210 | 1 | 0.543 | 0.739 | 0.163 | |
| rs4430796 | 1 | 0.764 | 0.337 | ||
| rs7501939 | 1 | 0.257 | |||
| rs3760511 | 1 |
HNF1B encodes a transcription factor with a central role in vertebrate development and embryonic survival [12]. Rare exonic mutations result in uro-genital defects, pancreatic atrophy and maturity onset diabetes of the young 5 (MODY5) [13], but its role in tumor development is yet to be described. Based on the identification of several cancer-associated SNPs within HNF1B, we aimed to define the function of HNF1B in this context. In vitro functional assays together with gene expression data from matched cancer and non-cancer patient tissue suggest that HNF1B exerts a tumor-suppressive effect when over-expressed in cancer cells. This protective effect is lost following DNA silencing by promoter methylation in the development of prostate cancer. To determine whether HNF1B expression can act as a brake on cancer development, we assessed the phenotypic effects of ectopic HNF1B over-expression in cancer cells, and observed a reversal of epithelial-to-mesenchymal transition (EMT). This represents one of the first comprehensive studies to demonstrate a functional role for a GWAS-identified target from prostate cancer, and to show that a similar mechanism operates in other cancer types. Our data support an emerging hypothesis that common functional mechanisms underlie the biology of neoplastic development at pleotropic risk loci identified by GWAS [14]. This work highlights the potential relevance of GWAS-findings across different but related complex disease types, providing a starting point for other functional follow-up studies and further collaborations.
RESULTS
HNF1B expression correlates with risk SNP genotype in prostate tissues
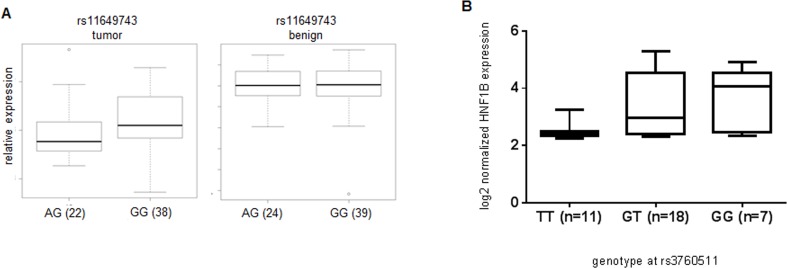
To determine whether any of the known, significant PC risk SNPs correlated with HNF1B expression levels, we genotyped each SNP in 65 British patients, and compared these to mRNA levels of HNF1B in tumor tissue and, where possible, matched non-tumor tissue from the same individuals. We found a significant correlation between rs11649743-G and elevated HNF1B levels in tumor tissue (n = 66) (p = 0.038) (Figure 2A), corresponding to the identification of the G-allele as the risk allele for PC [4]. In a smaller cohort of laser-captured micro-dissected prostate tumor tissue from 36 Danish patients, we found a significant correlation between risk SNP rs3760511-G [2] and elevated HNF1B levels (p = 0.018) (Figure 2B). These two SNPs are only in weak LD (Figure 1), and so these could represent distinct expression quantitative trait loci (eQTL) signals. Alternatively, they may interact at the chromatin level when HNF1B executes its functions as a transcription factor, and so together they could effectively constitute one signal. We found no association between rs4430796 (in LD with rs7501939; r2 = 0.764) and HNF1B expression levels, which may be due to our relatively small sample size compared to Grisanzio et al. (2012)[15], who identified a correlation between rs4430796 and HNF1B expression levels in benign tissues in three ethnic cohorts – European, Japanese and African Americans (total n = 407).
Figure 2. Association between genotype and increased gene expression at rs11649743 with HNF1B levels in prostate tumor tissue, but not benign (Kruskal-Wallis p = 0.038) in British samples (A), between rs3760511-G and HNF1B levels in laser-capture micro-dissected Danish prostate tumor samples (Kruskal-Wallis p = 0.018) (B).
HNF1B promoter methylation correlates with prostate cancer risk SNP genotype
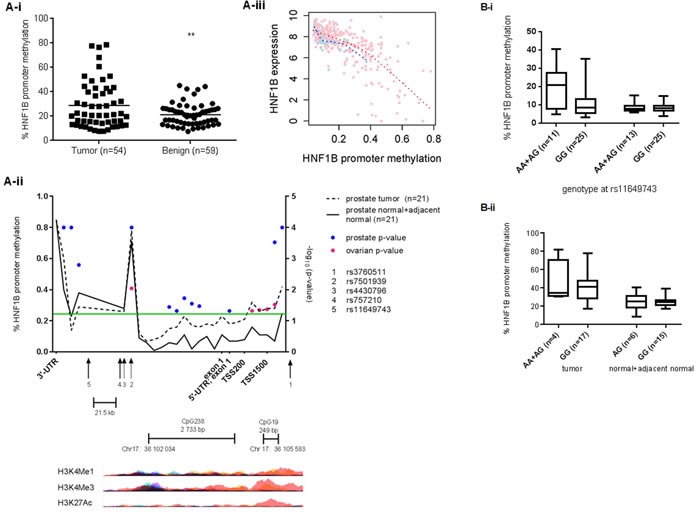
Promoter methylation plays a key role in transcriptional regulation, with the presence of methyl groups on cytosine bases at gene promoters essentially silencing gene expression. The HNF1B promoter harbors a CpG island that is a known target of epigenetic inactivation in high-grade serous (HGS) OC, as well as ovarian, colorectal, gastric and pancreatic cell lines [16, 17]. This CpG island has not previously been reported as a methylation target in PC (http://pubmeth.org). To determine whether epigenetic inactivation could play a role in regulating HNF1B expression levels in prostate cancer, we compared methylation levels at the HNF1B promoter between cancer and matched non-cancer samples in two cohorts (see Methods). Tumor tissues show significant hyper-methylation at this promoter compared to matched non-tumor tissues in both British (n = 59, p = 0.0076; Figure 3Ai) and Danish prostate cancer patients (n = 21, p = 0.0003; Figure 3Aii, blue dots). TCGA prostate cancer methylation data further confirm elevated HNF1B promoter methylation in tumor compared to normal tissue (Supplementary Figure 1). Hypermethylation at this promoter is also associated with reduced HNF1B expression in these same samples (Figure 3A-iii).
Figure 3. Methylation at the HNF1B promoter is significantly higher in prostate tumor tissues compared to matched benign in British (t-test p = 0.0076) (A) and Danish samples (B) (ranksum t-test; blue spots indicate statistical significance at the corresponding CpG probe).
The significance threshold p < 0.05 (log10(p) > 1.301) is indicated by the green line. Ovarian cancer risk SNP rs757210 is also associated with HNF1B promoter methylation (pink spots) in 231 HGS cases (see also Supplementary Table 7). Histone modifications typical of active promoters – H3K4Me1, H3K4Me3 and H3K27Ac – overlap with the transcription start site, both small and large CpG islands and risk SNP rs3760511 (UCSC Genome browser, hg19). Figure 3Aiii. Increased methylation at HNF1B promoter correlates strongly with reduced levels of HNF1B expression prostate tissue (TCGA data). Pink spots = tumor; blue spots = matched normal tissue. Figure 3-Bi. Prostate cancer risk allele rs11649743-G is associated with reduced levels of HNF1B promoter methylation in tumor tissue, but not benign tissue in British samples (p = 0.0296), while risk allele rs3760511-G is associated with reduced levels of HNF1B methylation (p = 0.0283) in 21 pairs of Danish prostate cancer samples (3B-ii).
In addition, PC risk SNP rs11649743-G was associated with significantly lower levels of HNF1B promoter methylation in tumor tissues in British samples (p = 0.0296; Figure 3B-i), while promoter region risk allele rs3760511-G associated with reduced HNF1B promoter methylation in the Danish cohort of 21 pairs of laser-capture micro-dissected (LCM) tumor and normal and adjacent-normal samples (p = 0.0283; Figure 3B-ii).
Essentially, two established, independent PC risk alleles variants – rs11649743-G and rs3760511-G – are both associated with reduced promoter methylation at HNF1B, and simultaneously increased HNF1B expression in two different clinical prostate cohorts, suggesting a plausible mechanism for this observation. We found no association between any other PC risk SNP tested and promoter methylation in either cohort (Supplementary Figure 2A and 2B.).
HNF1B has functional roles significant in prostate cancer
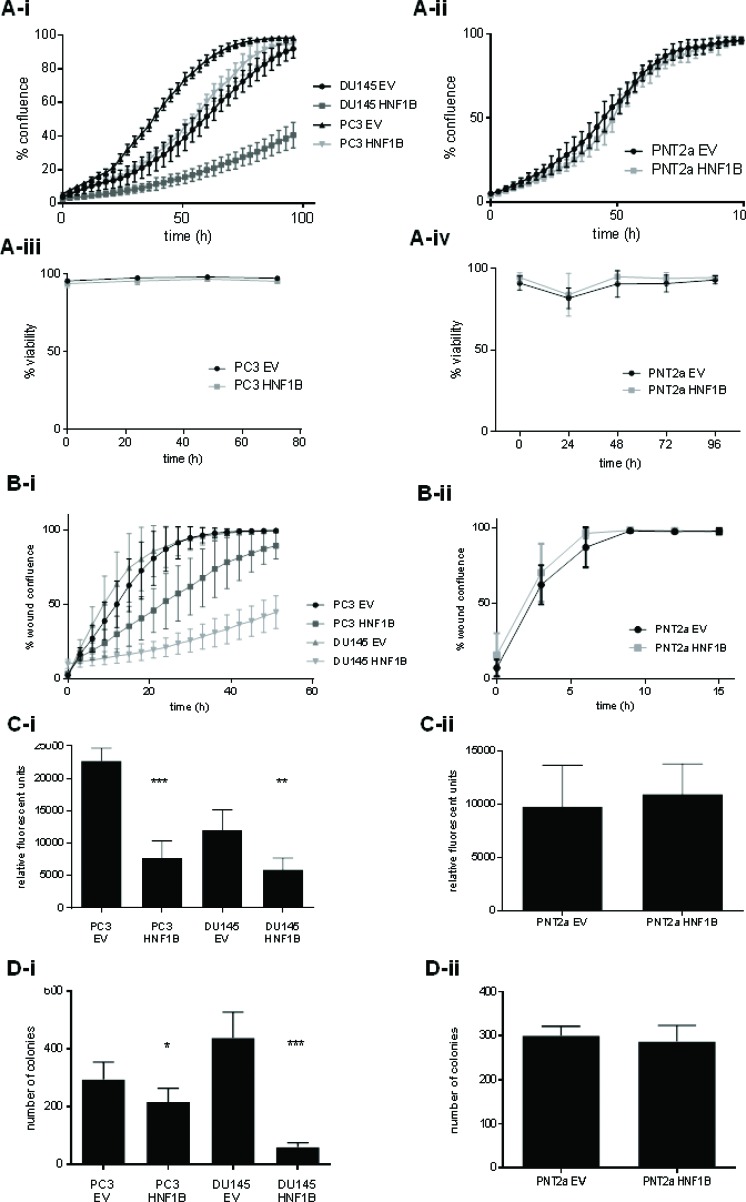
To identify biological pathways regulated by HNF1B in the context of cancer, we generated stable cell lines over-expressing HNF1B (see Methods). PC3 and DU145 are prostate cancer cells derived from bone and dura mater metastases (aggressive tumours), while PNT2a cells originate from normal prostate epithelial cells. Total RNA from PC3 cell line expressing empty vector (PC3-EV) and derived PC3-HNF1B cells was assayed on Illumina HT12 gene expression arrays, since this was the cell line with the highest over-expression of HNF1B (Supplementary Figure 3A-C). In total, 60 down- and 150 up-regulated genes were identified from two biological replicates, and used in subsequent analyses (Methods, Supplementary methods and Supplementary Table 1). Gene ontology (GO) enrichment analysis of the DEG following over-expression of HNF1B in this prostate cancer model suggested that biological pathways in cellular movement, growth and proliferation were over-represented (Supplementary Figure 4).
Over-expression of HNF1B corresponded with a marked reduction in proliferation in PC3 and DU145 prostate cancer cells compared to empty vector (EV) controls (p < 0.0001, Figure 4A-i); no effect was observed in normal prostate PNT2a model (p = 0.6629; Figure 4A-ii), while viability was unaffected in all cell lines (Figures 4A-iii and 4A-iv) HNF1B over-expression also led to a decrease in the rate of cell migration in prostate cancer models (p < 0.0001) (Figure 4B-i), but HNF1B levels had no effect on cell migration in PNT2a cells (p = 0.7771; Figure 4B-ii). Invasiveness of normally highly metastatic PC3 and DU145 cells was significantly reduced on over-expression of HNF1B (p < 0.0001 and p < 0.0003, respectively) (Figure 4C-i), but HNF1B expression had no effect on the invasive potential of normal prostate cells (p = 0.5358; Figure 4-Cii). In addition, clonogenic potential was significantly reduced in PC3-HNF1B and DU145-HNF1B cells compared to EV (p = 0.01 and p < 0.0001 respectively) (Figure 4D-i), but was unaffected in PNT2a cells (p = 0.2784, Figure 4D-ii).
Figure 4. The differential effects of HNF1B in cancer and normal prostate cellular contexts.
Over-expression of HNF1B was associated with a reduction in rate of proliferation in prostate cancer lines PC3 and DU145 compared to empty vector (4A-i), but had no effect in normal PNT2a cells (4A-ii). No effect on viability on any cell line was observed (4A-iii, iv). HNF1B over-expression also correlated with a reduction in cell migration in PC3 and DU145 cells (p < 0.0001)(4B-i), but had no effect on cell migration in normal PNT2a prostate cells (p = 0.7771; 4B-ii). The invasiveness of PC3 and DU145 cancer cells was also significantly reduced with over-expression of HNF1B (p < 0.0001 and p < 0.0003, respectively; Figure 4C-i). No difference was seen in normal PNT2a cells (p = 0.5358; Figure 4Cii). Clonogenic potential was significantly reduced in cancer models PC3 and DU145 over-expressing HNF1B (p = 0.01 and p < 0.0001 respectively) (Figure 4D-i), but showed no change in the PNT2a model (p = 0.2784, Figure 4D-ii).
Over-expression of HNF1B also effected an obvious change in morphology. PC3-EV cells normally have an elongated mesenchymal morphology, but PC3-HNF1B cells acquired a flattened, epithelial-like morphology (Supplementary Figure 5A-C), an effect also observed in endometrial cells [17]. Paxillin is a major component of focal adhesion complexes and a ligand of integrin, and participates in cell adhesion-mediated signal transduction. As such, it has an important role in the regulation of cell shape and movement, and the morphological changes observed were associated with concomitant changes in the localization of the adaptor protein paxillin in these cells (Supplementary Figure 6A-F), as well as in DU145-HNF1B cells compared to DU145-EV controls (Supplementary Figure 6G-L). Neither cell line normally exhibits large peripheral adhesions; however, both PC3 and DU145 cells over-expressing HNF1B displayed large, prominent peripheral paxillin-associated adhesions. These changes were not evident in normal prostate PNT2a-EV or PNT2a-HNF1B cells (Supplementary Figure 6M-R), which both displayed very few paxillin-associated adhesions. A reduction in proliferation can affect some trans-well migration assays, but DU145 cells with larger paxillin-associated adhesions have been shown to migrate more slowly [18], so this is more likely to be the reason HNF1B over-expressing cells migrate less than their EV counterparts. The redistribution of paxillin into large peripheral adhesions was independent of total paxillin levels, which were comparable between PC3-EV and PC3-HNF1B, and DU145-EV and DU145-HNF1B cells (Supplementary Figure 7A and B).
The changes in localization of paxillin within the cell together with alterations in migration potential and prominent clustering at the cell periphery when HNF1B is over-expressed in prostate cancer models strongly suggest that loss of HNF1B expression has a fundamental role in EMT.
HNF1B-related gene networks are enriched in clinical cancer studies
Using gene set enrichment analysis (GSEA)[19] we compared the 210-gene signature (Supplementary Table 1) associated with HNF1B over-expression in vitro, with gene expression data from five clinical prostate cancer studies, [20–24] to identify key genes related to functional phenotypes observed. The HNF1B gene signature was significantly enriched in four of five prostate cohorts (Supplementary Table 2 & Supplementary Figure 8A-D). Leading edge analysis in each cohort identified a subset of 129 key genes driving this enrichment (Supplementary Table 3). Pathway analysis suggested that chemotaxis and integrin- and cadherin-mediated cell adhesion were the most functionally relevant processes (GeneGo Metacore; Supplementary Figure 9), with two genes present in every sample type comparison (e.g. benign vs. tumor) across all studies - FLRT3 and SLC14A1.
Both targets are co-expressed with HNF1B in clinical prostate samples, and in each case we found elevated levels associated with relapse-free survival (p = 0.00025 and p = 0.00026, respectively) (Supplementary Figure 10 A-B) [21]. This mirrors the association reported by Glinsky et al., (2004), where increased levels of HNF1B were significantly associated with improved prognosis (p = 0.0093) in a primary prostate tumor expression data set (Supplementary Figure 10 C) [24]. mRNA levels of FLRT3 and SLC14A1 also showed a stepwise reduction from benign to primary prostate tumor and metastatic samples in other clinical prostate datasets (Supplementary Figure 11)[20–22, 25].
Mechanistic role of HNF1B
Using quantitative RT-PCR, we subsequently confirmed over-expression of novel targets FLRT3 and SLC14A1 and two known HNF1B target genes [26] in PC3-HNF1B and DU145-HNF1B cells, but not in PNT2a cells despite significant over-expression of HNF1B in this model (Supplementary Figure 12 A-E). Finally, chromatin immunoprecipitation (ChIP) for HNF1B in PC3-HNF1B cells followed by qPCR also showed enrichment of FLRT3 and SLC14A1, indicating a direct interaction between HNF1B transcription factor at these target genes at the chromatin level (Supplementary Figure 13). This suggests that epigenetic inactivation of HNF1B could bring about the phenotypic changes observed in the cell-based functional assays via these genes.
FLRT3 is a member of the fibronectin leucine-rich transmembrane protein family, is expressed at the cell surface and has an established role in the regulation of FGF receptor signaling [27] and cadherin-mediated cell adhesion and morphogenesis [28, 29]. Our functional and bioinformatic analyses support this, and suggest that candidates associated with these processes in particular may be relevant to the effects of HNF1B in a cancer context. SNP variants within SLC14A1 have been associated with urinary bladder cancer risk [30], and identified as a potential biomarker in prostate cancer [31]. SLC14A1 encodes urea transporter B (UT-B), which facilitates the clearance of urea (metabolized from L-arginine) from cells. The loss of this transporter in bladder results in the accumulation of urea and reduced expression of arginase I [32], with a concomitant reduction in levels of ornithine and polyamines, and an increase in nitric oxide (NO) production. This makes cells vulnerable to DNA damage, with DNA-damaging reactive NO up-regulated [32] and DNA-stabilising polyamines down-regulated [33]. Interestingly, an eQTL at prostate cancer risk locus SLC22A3 (encoding polyamine transporter OCT-3) was recently reported [15], with the PC risk allele associated with lower levels of SLC22A3 in prostate tissues, and reduced viability in vitro [15].
HNF1B has similar functions and mechanism in ovarian cancer
HNF1B is part of a five-gene expression signature predictive of relapse in PC patients [24], and is also a biomarker that can distinguish clear cell OC from other subtypes[34]. Different SNPs in HNF1B are associated with each disease [9, 17, 35] and different subtypes of OC [9, 17, 36]. Since the rs757210 OC risk-associated SNP is in LD with key prostate cancer risk SNPs (r2 = 0.739 with rs7501939; r2 = 0.543 with rs4430796) (Table 1), we tested whether we could re-capitulate our key findings in an ovarian context.
Firstly, we found similar pathways relating to extra-cellular matrix re-modelling and cellular adhesion enriched in an OC model, following shRNA knock-down of HNF1B in RMG2 ovarian cancer cells (GEO, GSE37290) (Supplementary Figure 14 & Methods). In addition, the 210-gene signature associated with HNF1B over-expression in prostate was enriched in two clinical OC cohorts [37] [38] (Supplementary Figure 15), where FLRT3 was identified in the leading edge gene set in each case (Supplementary Tables 4 & 5). SLC14A1 was only marginally significant.
We compared the prostate (129) and ovarian (45) leading edge gene, and identified 37 genes common to both diseases (Supplementary Figure 16A; Supplementary Table 6). Chemotaxis and cadherin-mediated adhesion to ECM are key biological processes related to this gene set (Supplementary Figure 16B), which is altered (amplified/deleted/mutated) in 72% of 246 PC cases and 82% 316 OC cases, based on The Cancer Genome Atlas (TCGA) data. None is a known cancer gene (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/). FLRT3 is most strongly co-expressed with tumor suppressor gene TP63 and angiogenesis regulator gene NTN4 (Pearson correlation r = 0.83) (CBioportal), which suggests possible mechanisms by which HNF1B could exert the effects observed here – a control switch preventing EMT in non-tumor tissue.
We have shown that HNF1B promoter methylation in PC is associated with known PC risk SNPs (Figure 2). Using 450K methylation array data from the Mayo Clinic [39, 40], we found a significant association between a linked OC risk SNP in HNF1B rs757210, and tumor methylation in n = 231 high-grade serous OC (Figure 3B pink dots, and Supplementary Table 7). Although this specific variant was not associated with HNF1B expression levels in 182 HGS ovarian cancer samples (p = 0.2032) (Supplementary Table 8A), it was borderline significant in a much smaller set of 21 clear cell ovarian cancer patients (p = 0.0732) (Supplementary Table 8B) based on gene expression array data from samples genotyped as part of the Collaborative Oncological Gene-environment Study (COGS)[9]. However, we did identify a significant association between this SNP rs757210 and tumor methylation in HGS OC (Figure 3A and Supplementary Table 7), consistent with the report of Pharoah et al. (2013). TCGA data and fine-mapping of the region has previously identified two loci 6,8 kb apart, associated with increased promoter methylation in high-grade serous cases (rs7405776) and increased HNF1B expression in clear cell OC cases (rs11651755)[17], confirming our results: both of these SNPs - independent of each other - correlate with rs757210 (r2 = 0.6 and r2 = 0.97, respectively), which lies half-way between them. Furthermore, rs11651755 is also in strong LD with PC risk SNP rs4430796 (r2 = 0.97).
There is an obvious overlap between regions of methylation within HNF1B in prostate and ovarian cancer tissues, with a methylation signal at the 3'-UTR in both. However, the most significant methylation occurs upstream of the TSS at the small CpG: 19 island and coincides with poised and active enhancers, identified by the presence of H3K27Ac, H3K4Me1 and H3K3Me3 histone marks [41](Figure 3Aii). Poised enhancers can be activated during differentiation or in response to external stimuli, and HNF1B is known to be important in embryonic urogenital development, where its role in EMT– as suggested by the sum of our functional data here – would be appropriate. However, if this role were disrupted by stressors typical of tumor cells, such as aberrant metabolism (a hallmark of cancer), HNF1B would then be tumor promoting. Indeed, the deregulation of pathways that maintain quiescence of ovarian surface epithelial cells has been shown to be instrumental in the progression to serous ovarian cancer [38], and would present this sort of insult. A similar effect has also been observed in renal cell carcinoma, where HNF1B expression correlated with malignant transformation and progression, with elevated levels of HNF1B expression in primary tumor associated with better prognosis [42]. Our findings highlight the multiple, small blocks of linkage disequilibrium within HNF1B, and may explain the complex associations observed at this locus between different risk SNP alleles, HNF1B expression and promoter methylation depending on cancer or histological subtype.
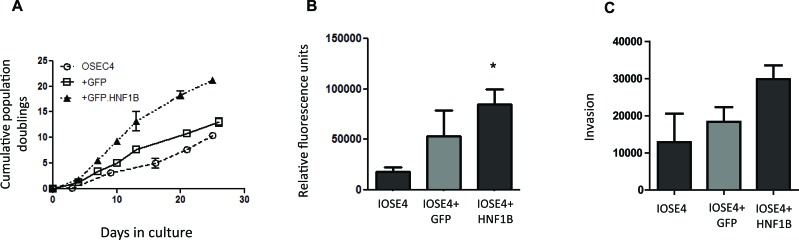
Finally, to assess the phenotypic effects of HNF1B in the context of ovarian cancer, we over-expressed HNF1B in IOSE4 ovarian epithelial cells and tested typical tumor phenotypes: we observed significant effects on proliferation (Figure 5A), migration (5B) and invasiveness (Figure 5C) in IOSE4-HNF1B cells compared to IOSE4-EV control cells. There is considerable evidence to indicate that HNF1B is over-expressed and behaves like an oncogene in clear cell OC [43], but is lost and acts as a tumor suppressor in HGS OC [44, 45]. Our data further support this. Similarly, our data suggests that that HNF1B may act as a tumor suppressor in benign prostate tissue, where it works normally to suppress classic features of tumorigenesis, by stimulating transcription of genes with clear roles in controlling cellular proliferation, adhesion and movement. On inactivation by DNA methylation in the progression to more aggressive tumors, these protective effects are lost.
Figure 5. Over-expression of HNF1B in ovarian IOSE4 cells is associated with increased proliferation (5A), migration (5B) and invasion (5C) compared to IOSE4 cells expressing control GFP.
DISCUSSION
HNF1B appears to play a significant role in the etiology and early stage neoplastic development of both prostate and ovarian cancers. We link intronic SNPs with HNF1B expression levels and epigenetic silencing in multiple populations in both prostate and ovarian cancers, thereby suggesting a plausible mechanism of action. In addition, our experimental data show that similar mechanisms contribute to prostate and serous ovarian cancer development and progression, and demonstrate the value of cross-site cancer analyses to functionally validate consistent GWAS findings across different disease types. In a clinical setting, the implication is that individuals carrying the high-risk genetic variants (rs11649743-G and rs3760511-G in prostate cancer; rs757210-G in serous ovarian cancer) are primed for EMT, which could be driven by additional stressors typical of tumor cells, such as aberrant metabolism – a hallmark of cancer. Most importantly, both increased transcript expression levels and reduced degree of promoter methylation are tagged by the same easily-screened genetic markers. The independent expression-methylation quantitative trait locus (eQTL-mQTL) associations identified here support the idea of pleiotropy as a common functional mechanism underlying the biology of neoplastic development at risk loci identified by GWAS. More comprehensive whole-genome eQTL-mQTL investigations may be informative for HNF1B specifically, as well as more generally for (epi)genome-wide association studies (EWAS/GWAS) This finding further emphasizes the usefulness of considering disease mechanisms across distinct cancer types to describe a conserved and significant general mechanism of action.
MATERIALS AND METHODS
Patient samples
British prostate cancer and matched benign samples were taken from patients recruited under the Prostate Mechanisms for Progression and Treatment (ProMPT) study MREC 01/4/061, and have been previously described [46]. Ovarian cancer samples were taken from patients recruited into the OCAC consortium[9] at the Mayo Clinic, under approval of the Mayo Clinic Institutional Review, and have been previously described [9] [39, 40]. Danish prostate cancer and matched normal and adjacent normal samples were from patients recruited at Aarhus university hospital, under approval of the Aarhus University Regional Ethical committee and the Danish Data Protection Agency.
All participants at each of the three study sites (Cambridge, Aarhus, Mayo Clinic) provided written informed consent; all experimental protocols at each of the three sites were approved by named local research ethics committees. All testing was undertaken in accordance with local, approved guidelines and regulations at each site.
Cell culture
PC3, DU145, and PNT2a cell lines were obtained from ATCC, and grown in RPMI supplemented with 10% FBS (Invitrogen) at 37C, 5% CO2. Stable PC3-HNF1B and DU145-HNF1B cell lines were grown under selection of 100 μg/ml geneticin (Gibco); PNT2a cells were grown under selection of 2 μg/ml puromycin (Invitrogen). IOSE4 cells were grown under selection of 400 ng/ml puromycin.
Stable cell lines over-expressing HNF1B
PC3 and DU145 cells were transfected with a modified rc/CMV vector to over-express HNF1B, to generate stable cell lines PC3-HNF1B and DU145-HNF1B. PNT2a-HNF1B and OSE4-HNF1B cells were generated as described [17]. Parental cells were transfected with the corresponding empty vector (EV) or GFP as a control.
siRNA
Cells were transfected with 50 nM siRNA with RNAi Max (Invitrogen) by reverse transfection, according to manufacturer's instructions. HNF1B was silenced using 50 mM siRNAs SASI_Hs02_00302585 and SASI_Hs02_00302586 in combination (Sigma). Universal negative control #1 (SIC001, Sigma) was used as a negative control.
Western blots
Whole cell lysates were run on 4-16% SDS-PAGE gels (ThermoFisher) and transferred to PVDF membranes (Invitrogen). Antibodies used were: anti-HNF1B (H-85) (SC-22840, Santa Cruz), anti-B-actin (AC-15) (ab6276, Abcam), anti-paxillin [Y-113] (ab32084, Abcam), anti-integrin α2 (611016, BB Biosciences), anti-integrin β1 (610467, BD Biosciences).
Genotyping
British prostate samples were genotyped as part of the PRACTICAL consortium on custom SNP Illumina arrays (iCOGS)[47]. Danish prostate samples were genotyped using commercial TaqMan assays C___2559918_10 (rs11649743), C___2559889_10 (rs4430796), C___2960803_10 (rs7501939) and C__26657407_10 (rs3760511). Ovarian samples were genotyped as part of the OCAC consortium using Illumina infinium iSelect BeadChips and iCOGS arrays[9].
Gene expression microarrays
Cell lines: Total RNA was harvested from 2 biological replicates (4 technical replicates each) of PC3-EV and PC3-HNF1B cells (RiboPure, Ambion). UK clinical material: Total RNA was extracted from prostate tissue sections from fresh-frozen radical prostatectomy biopsies (AllPrep, Qiagen). Tumor or non-tumor was selected by pathologist A.Y.W. Danish clinical material: normal and cancer tissue sections were laser capture micro-dissected, RNA extracted and assayed on Affymetrix U133A gene expression arrays. See also Supplementary Methods.
Promoter methylation analysis
British prostate samples: gDNA was extracted from fresh, frozen radical prostatectomy tissue (AllPrep, Qiagen). Unmethylated cytosine bases were sodium bisulfite converted with EpiTect Bisulfite kits (Qiagen). Converted gDNA was amplified at HNF1B promoter CpG islands using assay PM00178808 and PyroMark® kits (Qiagen). Methylation levels at each amplicon were determined using Pyrosequencing – see Supplementary Methods. Danish prostate samples: gDNA from 21 Danish patient prostate cancer tumor samples (T), 12 adjacent non-malignant prostate tissue (AN) and 9 normal prostate tissue (N) from patients with bladder cancer but no prostate cancer were assayed on Illumina 450K methylation arrays. Mayo Clinic ovarian cancer sample data were from 450K methylation arrays, generated as previously described [39, 40].
Chromatin immunoprecipitation
ChIP reactions were performed as described previously [48], using anti-HNF1B antibody (H-85) (SC-22840, Santa Cruz.
Data access
in vitro HNF1B over-expression gene expression assay data are entered at GEO under GSE63134.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
We are grateful to study volunteers for their participation and to staff at the Wellcome Trust Clinical Research Facility, Addenbrooke's Clinical Research Centre, Cambridge for their help in conducting the study.
Footnotes
CONFLICTS OF INTERESTS
None.
FINANCIAL SUPPORT
We acknowledge the support of the National Cancer Research Prostate Cancer: Mechanisms of Progression and Treatment (ProMPT) collaborative (grant code G0500966/75466), which has funded tissue and urine collections in Cambridge, as well as the Human Research Tissue bank, which is supported by the NIHR Cambridge Biomedical Research Centre. We acknowledge the support of The University of Cambridge, Cancer Research UK and Hutchison Whampoa Limited, and the support of Cancer Research UK Cambridge Institute Genomics and Bioinformatics core facilities. We thank Dr Thorunn Rafnar for helpful discussions and critical review of the manuscript, and Dr Gerhart Ryffel for his kind gift of the HNF1B plasmid. This work was funded by a CRUK program grant awarded to DEN. HR-A and SB were supported by EU Framework Programme 7 grant 202059 (ProMark;http://www.promark-fp7.eu/). The Aarhus Prostate Cancer study is supported by the Danish Strategic Research Council and The Danish Cancer Society. This work was in part supported by the NIH grants R01 CA122443 and P30-CA13083 to ELG and P50-CA136393 to Mayo Clinic SPORE Ovarian Cancer, to ELG. I.G.M. is an Associate Professor at Queen's University Belfast and funded through the PCUK/Movember Centre of Excellence for Prostate Cancer Research. He is a visiting scientist and honorary senior visiting research fellow at Cambridge University.
Editorial note
This paper has been accepted based in part on peer-review conducted by another journal and the authors' response and revisions as well as expedited peer-review in Oncotarget.
REFERENCES
- 1.Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, Agnarsson BA, Benediktsdottir KR, Magnusdottir DN, Orlygsdottir G, Jakobsdottir M, Stacey SN, Sigurdsson A, Wahlfors T, et al. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nature genetics. 2009;41:1122–6. doi: 10.1038/ng.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nature genetics. 2007;39:977–83. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 3.Takata R, Akamatsu S, Kubo M, Takahashi A, Hosono N, Kawaguchi T, Tsunoda T, Inazawa J, Kamatani N, Ogawa O, Fujioka T, Nakamura Y, Nakagawa H. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nature genetics. 2010;42:751–4. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Zheng SL, Wiklund F, Isaacs SD, Purcell LD, Gao Z, Hsu FC, Kim ST, Liu W, Zhu Y, Stattin P, Adami HO, Wiley KE, et al. Evidence for two independent prostate cancer risk-associated loci in the HNF1B gene at 17q12. Nature genetics. 2008;40:1153–5. doi: 10.1038/ng.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nature genetics. 2008;40:310–5. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher FR, Berndt SI, Siddiq A, Jacobs KB, Wang Z, Lindstrom S, Stevens VL, Chen C, Mondul AM, Travis RC, Stram DO, Eeles RA, Easton DF, et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Human molecular genetics. 2011;20:3867–75. doi: 10.1093/hmg/ddr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, Muir K, Hopper JL, Henderson BE, Haiman CA, Schleutker J, Hamdy FC, Neal DE, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nature genetics. 2009;41:1116–21. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, Field HI, Southey MC, Severi G, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nature genetics. 2008;40:316–21. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 9.Pharoah PDP, Tsai Y-Y, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, Buckley M, Fridley BL, Tyrer JP, Shen H, Weber R, Karevan R, Larson MC, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nature genetics. 2013;45:362–70. doi: 10.1038/ng.2564. doi http://www.naturecom/ng/journal/v45/n4/abs/ng2564.html supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, Cicek MS, Tyrer J, Stram D, Larson MC, Kobel M, Consortium P, Ziogas A, et al. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nature communications. 2013;4:1628. doi: 10.1038/ncomms2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setiawan VW, Haessler J, Schumacher F, Cote ML, Deelman E, Fesinmeyer MD, Henderson BE, Jackson RD, Vockler JS, Wilkens LR, Yasmeen S, Haiman CA, Peters U, et al. HNF1B and endometrial cancer risk: results from the PAGE study. PLoS One. 2012;7:e30390. doi: 10.1371/journal.pone.0030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P. Role of the Hepatocyte Nuclear Factor-1β (HNF-1β) C-terminal Domain in Pkhd1 (ARPKD) Gene Transcription and Renal Cystogenesis. Journal of Biological Chemistry. 2005;280:10578–86. doi: 10.1074/jbc.M414121200. [DOI] [PubMed] [Google Scholar]
- 13.Bingham C, Hattersley AT. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrology, dialysis, transplantation. 2004;19:2703–8. doi: 10.1093/ndt/gfh348. [DOI] [PubMed] [Google Scholar]
- 14.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nature reviews Genetics. 2013;14:483–95. doi: 10.1038/nrg3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grisanzio C, Werner L, Takeda D, Awoyemi BC, Pomerantz MM, Yamada H, Sooriakumaran P, Robinson BD, Leung R, Schinzel AC, Mills I, Ross-Adams H, Neal DE, et al. Genetic and functional analyses implicate the NUDT11, HNF1B, and SLC22A3 genes in prostate cancer pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11252–7. doi: 10.1073/pnas.1200853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terasawa K, Toyota M, Sagae S, Ogi K, Suzuki H, Sonoda T, Akino K, Maruyama R, Nishikawa N, Imai K, Shinomura Y, Saito T, Tokino T. Epigenetic inactivation of TCF2 in ovarian cancer and various cancer cell lines. British journal of cancer. 2006;94:914–21. doi: 10.1038/sj.bjc.6602984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, Cicek MS, Tyrer J, Stram D, Larson MC, Kobel M, Ziogas A, Zheng W, et al. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nature communications. 2013;4:1628. doi: 10.1038/ncomms2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells CM, Whale AD, Parsons M, Masters JRW, Jones GE. PAK4: a pluripotent kinase that regulates prostate cancer cell adhesion. Journal of cell science. 2010;123:1663–73. doi: 10.1242/jcs.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, Pienta KJ, Ghosh D, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Integrative molecular concept modeling of prostate cancer progression. Nature genetics. 2007;39:41–51. doi: 10.1038/ng1935. doi http://www.naturecom/ng/journal/v39/n1/suppinfo/ng1935_S1html. [DOI] [PubMed] [Google Scholar]
- 24.Glinsky GV, Glinskii AB, Stephenson AJ, Hoffman RM, Gerald WL. Gene expression profiling predicts clinical outcome of prostate cancer. Journal of Clinical Investigation. 2004;113:913–23. doi: 10.1172/jci200420032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 26.Ryffel G. The Transcription Factor Encyclopedia. Genome biology. 2012;13:R24. doi: 10.1186/gb-2012-13-3-r24. HNF1B. In Yusuf, D et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacy SEBC, Buzney EA, Kunkel LM. Identification of FLRT1, FLRT2, and FLRT3: a novel family of transmembrane leucine-rich repeat proteins. Genomics. 1999;62:417–26. doi: 10.1006/geno.1999.6033. doi. [DOI] [PubMed] [Google Scholar]
- 28.Karaulanov EE, Böttcher RT, Niehrs C. A role for fibronectin-leucine-rich transmembrane cell-surface proteins in homotypic cell adhesion. EMBO reports. 2006;7:283–90. doi: 10.1038/sj.embor.7400614. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Koh E, Yoder M, Gumbiner BM. A Protocadherin-Cadherin-FLRT3 Complex Controls Cell Adhesion and Morphogenesis. PloS one. 2009;4:e8411. doi: 10.1371/journal.pone.0008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafnar T, Vermeulen SH, Sulem P, Thorleifsson G, Aben KK, Witjes JA, Grotenhuis AJ, Verhaegh GW, Hulsbergen-van de Kaa CA, Besenbacher S, Gudbjartsson D, Stacey SN, Gudmundsson J, et al. European genome-wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Human molecular genetics. 2011;20:4268–81. doi: 10.1093/hmg/ddr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guyon I. Biomarkers downregulated in prostate cancer. US 20090305257 A1. US patent. 2009 doi.
- 32.Dong Z, Ran J, Zhou H, Chen J, Lei T, Wang W, Sun Y, Lin G, Bankir L, Yang B. Urea Transporter UT-B Deletion Induces DNA Damage and Apoptosis in Mouse Bladder Urothelium. PloS one. 2013;8:e76952. doi: 10.1371/journal.pone.0076952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rider JE, Hacker A, Mackintosh CA, Pegg AE, Woster PM, Casero RA., Jr Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids. 2007;33:231–40. doi: 10.1007/s00726-007-0513-4. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, Ohki M, Yasugi T, Taketani Y, Hirohashi S. Expression Profiling in Ovarian Clear Cell Carcinoma: Identification of Hepatocyte Nuclear Factor-1β as a Molecular Marker and a Possible Molecular Target for Therapy of Ovarian Clear Cell Carcinoma. The American journal of pathology. 2003;163:2503–12. doi: 10.1016/s0002-9440(10)63605-x. doi http://dxdoiorg/101016/S0002-9440(10)63605-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott KS, Zeggini E, McCarthy MI, Gudmundsson J, Sulem P, Stacey SN, Thorlacius S, Amundadottir L, Gronberg H, Xu J, Gaborieau V, Eeles RA, Neal DE, et al. Evaluation of association of HNF1B variants with diverse cancers: collaborative analysis of data from 19 genome-wide association studies. PloS one. 2010;5:e10858. doi: 10.1371/journal.pone.0010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadiyeh N, Pomerantz MM, Grisanzio C, Herman P, Jia L, Almendro V, He HH, Brown M, Liu XS, Davis M, Caswell JL, Beckwith CA, Hills A, et al. 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proceedings of the National Academy of Sciences. 2010;107:9742–6. doi: 10.1073/pnas.0910668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partheen K, Levan K, Österberg L, Horvath G. Expression analysis of stage III serous ovarian adenocarcinoma distinguishes a sub-group of survivors. European journal of cancer. 2006;42:2846–54. doi: 10.1016/j.ejca.2006.06.026. doi http://dxdoiorg/101016/jejca200606026. [DOI] [PubMed] [Google Scholar]
- 38.Bowen NJ, Walker LD, Matyunina LV, Logani S, Totten KA, Benigno BB, McDonald JF. Gene expression profiling supports the hypothesis that human ovarian surface epithelia are multipotent and capable of serving as ovarian cancer initiating cells. BMC medical genomics. 2009;2:71. doi: 10.1186/1755-8794-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Cicek MS, Charbonneau B, Kalli KR, Armasu SM, Larson MC, Konecny GE, Winterhoff B, Fan J-B, Bibikova M, Chien J, Shridhar V, Block MS, et al. Tumor Hypomethylation at 6p21.3 Associates with Longer Time to Recurrence of High-Grade Serous Epithelial Ovarian Cancer. Cancer research. 2014;74:3084–91. doi: 10.1158/0008-5472.can-13-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cicek MS, Koestler DC, Fridley BL, Kalli KR, Armasu SM, Larson MC, Wang C, Winham SJ, Vierkant RA, Rider DN, Block MS, Klotzle B, Konecny G, et al. Epigenome-wide ovarian cancer analysis identifies a methylation profile differentiating clear-cell histology with epigenetic silencing of the HERG K+ channel. Human molecular genetics. 2013;22:3038–47. doi: 10.1093/hmg/ddt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21931–6. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchner A, Castro M, Hennig A, Popp T, Assmann G, Stief CG, Zimmermann W. Downregulation of HNF-1B in Renal Cell Carcinoma Is Associated With Tumor Progression and Poor Prognosis. Urology. 2010;76:507. doi: 10.1016/j.urology.2010.03.042. E6-.e11 doi: http://dx.doi.org/10.1016/j.urology.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 43.Kato NMT. Hepatocyte nuclear factor-1β (HNF-1β) in human urogenital organs: its expression and role in embryogenesis and tumorigenesis Histology and Histopathology. 2009;24:8. doi: 10.14670/HH-24.1479. doi. [DOI] [PubMed] [Google Scholar]
- 44.Kato N, Sasou S-i, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2005;19:83–9. doi: 10.1038/modpathol.3800492. doi. [DOI] [PubMed] [Google Scholar]
- 45.Kato N, Tamura G, Motoyama T. Hypomethylation of hepatocyte nuclear factor-1beta (HNF-1beta) CpG island in clear cell carcinoma of the ovary. Virchows Archiv. 2008;452:175–80. doi: 10.1007/s00428-007-0543-z. [DOI] [PubMed] [Google Scholar]
- 46.Ross-Adams H, Lamb AD, Dunning MJ, Halim S, Lindberg J, Massie CM, Egevad LA, Russell R, Ramos-Montoya A, Vowler SL, Sharma NL, Kay J, Whitaker H, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EBioMedicine. 2015;2:1133–44. doi: 10.1016/j.ebiom.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nature genetics. 2013;45:385–91. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods. 2009;48:240–8. doi: 10.1016/j.ymeth.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.