Abstract
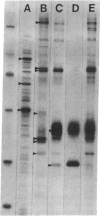
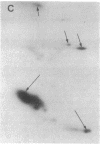
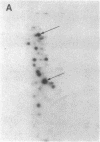
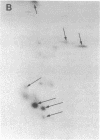
Transection of a peripheral nerve results in Wallerian degeneration of the nerve segment distal to the lesion site and the initiation of axonal regeneration just proximal to it (neuroma site). Nonneuronal cells resident in peripheral nerve are suggested to play an important role in neural repair mechanisms through diffusable molecules that they synthesize and secrete. We examined the array of proteins synthesized and secreted by nonneuronal cells resident in the frog peripheral nerve, which is known for its high regenerative capacity. Nerve segments were incubated in medium containing [35S]methionine, and the secreted radioactively labeled proteins were analyzed by gel electrophoresis. Nerve injury resulted in the complete down-regulation of a group of proteins synthesized and secreted by nonneuronal cells in intact nerve. At the same time, the synthesis and secretion of several proteins were up-regulated in the neuroma and degenerating nerve segments, proximal and distal to the axotomy site, respectively. Proteins secreted by the proximal segment were of apparent kDa/pI (mass/isoelectric point) values of 215/5.6, 76/6.7, 73/7.0, 44/5.2, 36.5/5.6, 35.5/6.0, and 32/6.0. Similar proteins were secreted by the degenerating distal segment but with the exception of variable reductions in the 44- and 32-kDa proteins and increases in proteins of apparent kDa/pI values of 39/5.2 and 29/7.3-7.4. Step gradient ultracentrifugation suggested that the latter two are apolipoproteins. Comparison with plasma apolipoproteins further indicated that nerve and plasma apolipoproteins differ. The up-regulation of the synthesis and secretion of these proteins concurrently with nerve degeneration and regeneration strongly imply that these molecules are involved in neuronal repair mechanisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989 Mar;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Schwab M. E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988 Apr;106(4):1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. A., Schechter N., Williams D. L. Induction of rat E and chicken A-I apolipoproteins and mRNAs during optic nerve degeneration. J Biol Chem. 1986 May 5;261(13):5681–5684. [PubMed] [Google Scholar]
- Eisenberg S., Bilheimer D. W., Levy R. I. The metabolism of very low density lipoprotein proteins. II. Studies on the transfer of apoproteins between plasma lipoproteins. Biochim Biophys Acta. 1972 Sep 7;280(1):94–104. [PubMed] [Google Scholar]
- Hall S. M., Kent A. P. The response of regenerating peripheral neurites to a grafted optic nerve. J Neurocytol. 1987 Jun;16(3):317–331. doi: 10.1007/BF01611344. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Bandtlow C., Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987 Jun;104(6):1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide C., Tohyama K., Yokota R., Nitatori T., Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983 Dec 12;288(1-2):61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Haerter P. J., Pitas R. E., Shooter E. M. Apolipoprotein E in nerve injury and repair. Prog Brain Res. 1987;71:177–184. doi: 10.1016/s0079-6123(08)61822-1. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Gebicke-Härter P. J., Skene J. H., Schilling J. W., Weisgraber K. H., Mahley R. W., Shooter E. M. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. J., Shooter E. M., Pitas R. E., Mahley R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987 May 22;236(4804):959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Ignatius M. J., Hangen D. H., Shooter E. M. Expression of specific sheath cell proteins during peripheral nerve growth and regeneration in mammals. J Cell Biol. 1986 Feb;102(2):393–402. doi: 10.1083/jcb.102.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Politis M. J., Ederle K., Spencer P. S. Tropism in nerve regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res. 1982 Dec 16;253(1-2):1–12. doi: 10.1016/0006-8993(82)90667-9. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Ebendal T. Nerve growth activities in rat peripheral nerve. Brain Res. 1982 Aug 19;246(1):57–64. doi: 10.1016/0006-8993(82)90141-x. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., McGuinness U. M., Aguayo A. J. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980 Mar 20;284(5753):264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985 Sep;5(9):2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Shooter E. M. Denervated sheath cells secrete a new protein after nerve injury. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4169–4173. doi: 10.1073/pnas.80.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1130–1134. doi: 10.1073/pnas.83.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]