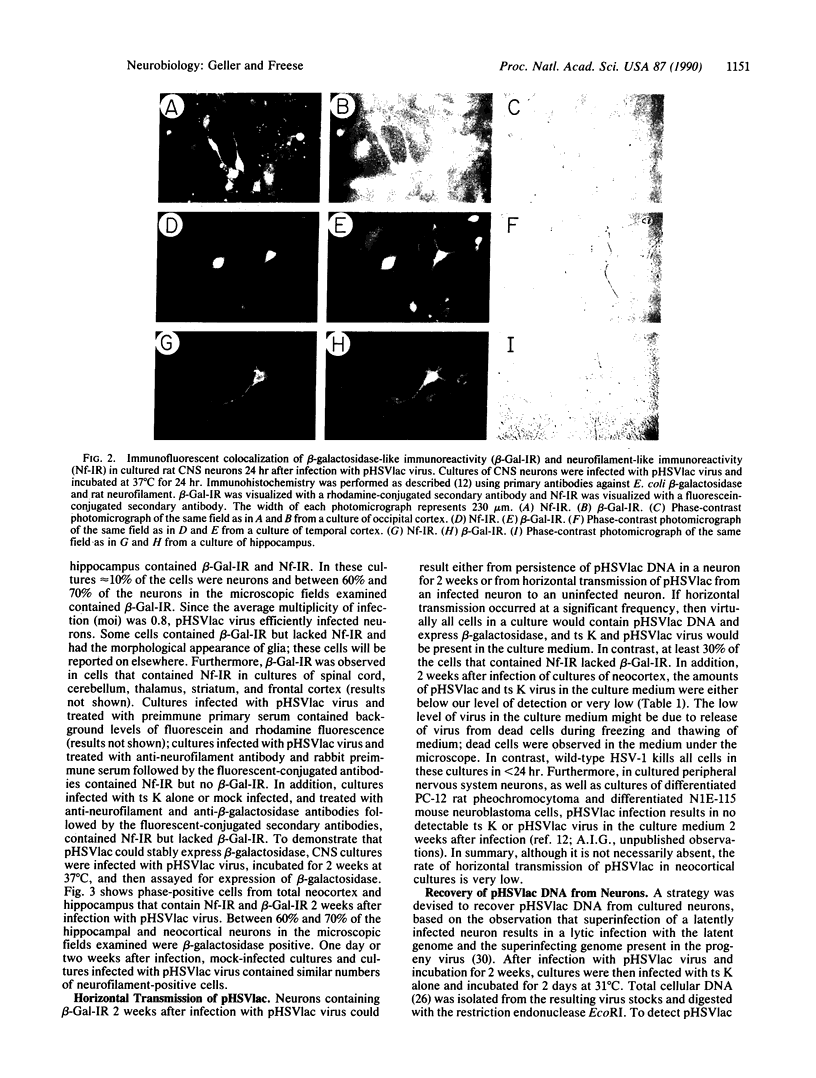
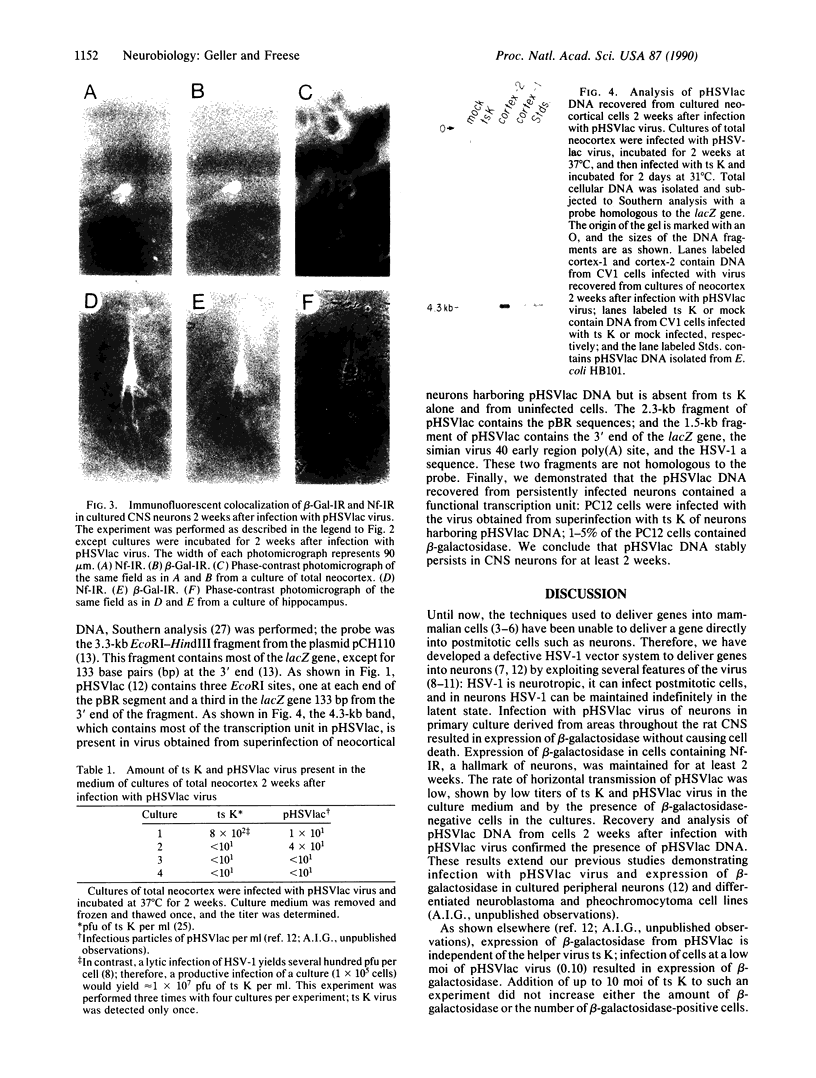
Abstract
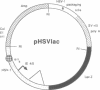
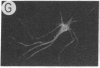
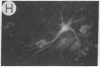
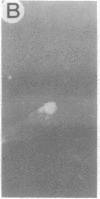
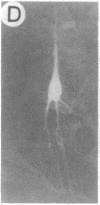
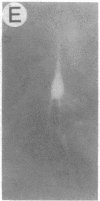
We have developed a defective herpes simplex virus (HSV) vector system that permits the introduction of virtually any gene into mammalian central nervous system neurons. The prototype vector, pHSVlac, contains a transcription unit that places the Escherichia coli lacZ gene under the control of the HSV-1 immediate early 4/5 promoter. pHSVlac was propagated using the HSV-1 temperature-sensitive mutant ts K as helper virus. Infection of rat neurons in primary culture derived from various regions throughout the central nervous system, including spinal cord, cerebellum, thalamus, basal ganglia, hippocampus, occipital cortex, temporal cortex, and frontal cortex, resulted in stable expression of high levels of beta-galactosidase for at least 2 weeks, without cell damage. Since other genes can be expressed from pHSVlac, HSV-1 vectors may prove useful for delivery of genes into central nervous system neurons for studies on nervous system physiology or to perform gene therapy for neurological conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. R., Field H. J. The distribution of herpes simplex type 1 antigen in mouse central nervous system after different routes of inoculation. J Neurol Sci. 1983 Aug;60(2):181–195. doi: 10.1016/0022-510x(83)90061-8. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O., Geller A. I. Gene transfer into the nervous system. Mol Neurobiol. 1987 Winter;1(4):339–371. doi: 10.1007/BF02935741. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Davison M. J., Preston V. G., McGeoch D. J. Determination of the sequence alteration in the DNA of the herpes simplex virus type 1 temperature-sensitive mutant ts K. J Gen Virol. 1984 May;65(Pt 5):859–863. doi: 10.1099/0022-1317-65-5-859. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Kurata T., Yamamoto A., Yamaguchi K. Morphological and physiological studies on cultured nerve cells from guinea pigs infected with herpes simplex virus in vivo. Brain Res. 1983 Feb 28;262(1):79–89. doi: 10.1016/0006-8993(83)90471-7. [DOI] [PubMed] [Google Scholar]
- Geller A. I. A new method to propagate defective HSV-1 vectors. Nucleic Acids Res. 1988 Jun 24;16(12):5690–5690. doi: 10.1093/nar/16.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., Breakefield X. O. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988 Sep 23;241(4873):1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M. T., Courtney R. J., Dunkel E. C. Detection of an immediate early herpes simplex virus type 1 polypeptide in trigeminal ganglia from latently infected animals. Infect Immun. 1981 Dec;34(3):987–992. doi: 10.1128/iai.34.3.987-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hu G. Y., Hvalby O., Walaas S. I., Albert K. A., Skjeflo P., Andersen P., Greengard P. Protein kinase C injection into hippocampal pyramidal cells elicits features of long term potentiation. 1987 Jul 30-Aug 5Nature. 328(6129):426–429. doi: 10.1038/328426a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Lewis M. E., Brown S. M., Warren K. G., Subak-Sharpe J. H. Recovery of herpes simplex virus genetic information from human trigeminal ganglion cells following superinfection with herpes simplex virus type 2 temperature-sensitive mutants. J Gen Virol. 1984 Jan;65(Pt 1):215–219. doi: 10.1099/0022-1317-65-1-215. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Brauer D. H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986 Feb 25;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Hyman R. W. Palindrome and palindrome-like sequences of herpes simplex virus DNA. Virology. 1978 Jun 1;87(1):34–41. doi: 10.1016/0042-6822(78)90155-1. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Reyes G. R., Pitha P. M., Hayward G. S. Differential activation of hybrid genes containing herpes simplex virus immediate-early or delayed-early promoters after superinfection of stable DNA-transfected cell lines. J Virol. 1985 Dec;56(3):867–878. doi: 10.1128/jvi.56.3.867-878.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R. H., Bacchetti S., Smiley J. R. Cells that constitutively express the herpes simplex virus immediate-early protein ICP4 allow efficient activation of viral delayed-early genes in trans. J Virol. 1985 May;54(2):414–421. doi: 10.1128/jvi.54.2.414-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J Virol. 1985 Sep;55(3):849–852. doi: 10.1128/jvi.55.3.849-852.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982 Aug;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]