Abstract
Zika is an emerging virus whose rapid spread is of great public health concern. Knowledge about transmission remains incomplete, especially concerning potential transmission in geographic areas in which it has not yet been introduced. To identify unknown vectors of Zika, we developed a data-driven model linking vector species and the Zika virus via vector-virus trait combinations that confer a propensity toward associations in an ecological network connecting flaviviruses and their mosquito vectors. Our model predicts that thirty-five species may be able to transmit the virus, seven of which are found in the continental United States, including Culex quinquefasciatus and Cx. pipiens. We suggest that empirical studies prioritize these species to confirm predictions of vector competence, enabling the correct identification of populations at risk for transmission within the United States.
DOI: http://dx.doi.org/10.7554/eLife.22053.001
Research Organism: Virus
eLife digest
Mosquitoes carry several diseases that pose an emerging threat to society. Outbreaks of these diseases are often sudden and can spread to previously unaffected areas. For example, the Zika virus was discovered in 1947, but only received international attention when it spread to the Americas in 2014, where it caused over 100,000 cases in Brazil alone. While we now recognize the threat Zika can pose for public health, our knowledge about the ecology of the disease remains poor. Nine species of mosquitoes are known to be able to carry the Zika virus, but it cannot be ruled out that other mosquitoes may also be able to spread the disease.
There are hundreds of species of mosquitoes, and testing all of them is difficult and costly. So far, only a small number of species have been tested to see if they transmit Zika. However, computational tools called decision trees could help by predicting which mosquitoes can transmit a virus based on common traits, such as a mosquito's geographic range, or the symptoms of a virus.
Evans et al. used decision trees to create a model that predicts which species of mosquitoes are potential carriers of Zika virus and should therefore be prioritized for testing. The model took into account all known viruses that belong to the same family as Zika virus and the mosquitoes that carry them. Evans et al. predict that 35 species may be able to carry the Zika virus, seven of which are found in the United States. Two of these mosquito species are known to transmit West Nile Virus and are therefore prime examples of species that should be prioritized for testing. Together, the ranges of the seven American species encompass the whole United States, suggesting Zika virus could affect a much larger area than previously anticipated.
The next step following on from this work will be to carry out experiments to test if the 35 mosquitoes identified by the model are actually able to transmit the Zika virus.
Introduction
In 2014, Zika virus was introduced into Brazil and Haiti, from where it rapidly spread throughout the Americas. By January 2017, over 100,000 cases had been confirmed in 24 different states in Brazil (http://ais.paho.org/phip/viz/ed_zika_cases.asp), with large numbers of reports from many other counties in South and Central America (Faria et al., 2016). Originally isolated in Uganda in 1947, the virus remained poorly understood until it began to spread within the South Pacific, including an outbreak affecting 75% of the residents on the island of Yap in 2007 (49 confirmed cases) and over 32,000 cases in the rest of Oceania in 2013–2014, the largest outbreak prior to the Americas (2016-present) (Cao-Lormeau et al., 2016; Duffy et al., 2009). Guillian-Barré syndrome, a neurological pathology associated with Zika virus infection, was first recognized at this time (Cao-Lormeau et al., 2016). Similarly, an increase in newborn microcephaly was found to be correlated with the increase in Zika cases in Brazil in 2015 and 2016 (Schuler-Faccini et al., 2016). For this reason, in February 2016, the World Health Organization declared the American Zika virus epidemic to be a Public Health Emergency of International Concern.
Despite its public health importance, the ecology of Zika virus transmission has been poorly understood until recently. It has been presumed that Aedes aegypti and Ae. albopictus are the primary vectors due to epidemiologic association with Zika virus (Messina et al., 2016), viral isolation from and transmission experiments with field populations (especially in Ae. aegypti [Haddow et al., 2012; Boorman and Porterfield, 1956; Haddow et al., 1964]), and association with related arboviruses (e.g. dengue fever virus, yellow fever virus). Predictions of the potential geographic range of Zika virus in the United States,and associated estimates for the size of the vulnerable population, are therefore primarily based on the distributions of Ae. aegypti and Ae. albopictus, which jointly extend across the Southwest, Gulf coast, and mid-Atlantic regions of the United States (Centers for Disease Control and Prevention, 2016). We reasoned, however, that if other, presently unidentified Zika-competent mosquitoes exist in the Americas, then these projections may be too restricted and therefore optimistically biased. Additionally, recent experimental studies show that the ability of Ae. aegypti and Ae. albopictus to transmit the virus varies significantly across mosquito populations and geographic regions (Chouin-Carneiro et al., 2016), with some populations exhibiting low dissemination rates even though the initial viral titer after inoculation may be high (Diagne et al., 2015). This suggests that in some locations other species may be involved in transmission. The outbreak on Yap, for example, was driven by a different species, Ae. hensilli (Ledermann et al., 2014). Closely related viruses of the Flaviviridae family are vectored by over nine mosquito species, on average (see Supplementary Data). Thus, because Zika virus may be associated with multiple mosquito species, we considered it necessary to develop a more comprehensive list of potential Zika vectors.
The gold standard for identifying competent disease vectors requires isolating virus from field-collected mosquitoes, followed by experimental inoculation and laboratory investigation of viral dissemination throughout the body and to the salivary glands (Barnett, 1960; Hardy et al., 1983), and, when possible, successful transmission back to the vertebrate host (e.g. Komar et al., 2003). Unfortunately, these methods are costly, often underestimate the risk of transmission (Bustamante and Lord, 2010), and the amount of time required for analyses can delay decision making during an outbreak (Day, 2001). To address the problem of identifying potential vector candidates in an actionable time frame, we therefore pursued a data-driven approach to identifying candidate vectors aided by machine learning algorithms for identifying patterns in high dimensional data. If the propensity of mosquito species to associate with Zika virus is statistically associated with common mosquito traits, it is possible to rank mosquito species by the degree of risk represented by their traits – a comparative approach similar to the analysis of risk factors in epidemiology. For instance, a model could be constructed to estimate the statistical discrepancy between the traits of known vectors (i.e., Ae. aegypti, Ae. albopictus, and Ae. hensilli) and the traits of all possible vectors. Unfortunately, this simplistic approach would inevitably fail due to the small amount of available data (i.e., sample size of 3). Thus, we developed an indirect approach that leverages the information contained in the associations among many virus-mosquito pairs to inform us about specific associations. Specifically, our method identifies covariates associated with the propensity for mosquito species to vector any flavivirus. From this, we constructed a model of the mosquito-flavivirus network and then extracted from this model the life history profile and species list of mosquitoes predicted to associate with Zika virus, which we recommend be experimentally tested for Zika virus competence.
Results
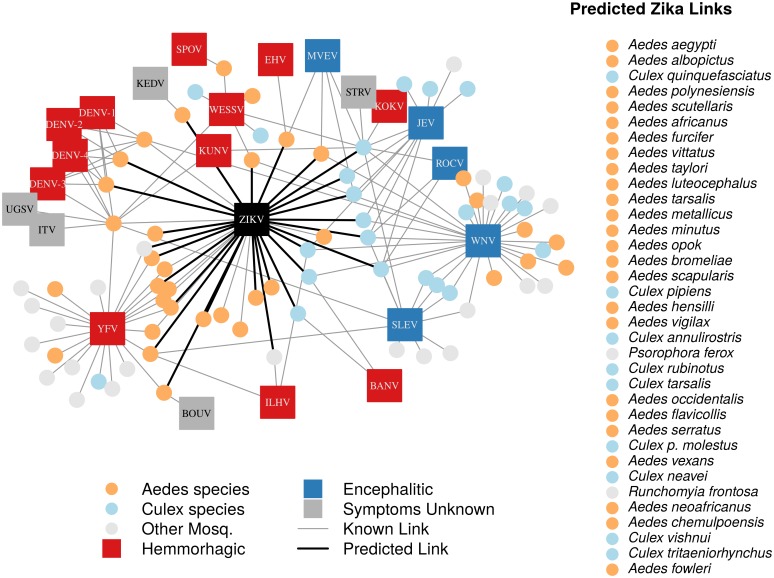
In total, we identified 132 vector-virus pairs, consisting of 77 mosquito species and 37 flaviviruses. The majority of these species were Aedes (32) or Culex (24) species. Our supplementary dataset consisted of an additional 103 mosquito species suspected to transmit flaviviruses, but for which evidence of a full transmission cycle does not exist. This resulted in 180 potential mosquito-Zika pairs on which to predict with our trained model. As expected, closely related viruses, such as the four strains of dengue, shared many of the same vectors and were clustered in our network diagram (Figure 1). The distribution of vectors to viruses was uneven, with a few viruses vectored by many mosquito species, and rarer viruses vectored by only one or two species. The virus with the most known competent vectors was West Nile virus (31 mosquito vectors), followed by yellow fever virus (24 mosquito vectors). In general, encephalitic viruses such as West Nile virus were found to be more commonly vectored by Culex mosquitoes and hemorrhagic viruses were found to be more commonly vectored by Aedes mosquitoes (see Gould and Solomon (2008) for further distinctions within Flaviviridae) (Figure 1).
Figure 1. A network diagram of mosquito vectors (circles) and their flavivirus pairs (rectangles).
The Culex mosquitoes (light blue) and primarily encephalitic viruses (blue) are more clustered than the Aedes (orange) and hemmorhagic viruses (red). Notably, West Nile Virus is vectored by both Aedes and Culex species. Predicted vectors of Zika are shown by bolded links in black. The inset shows predicted vectors of Zika and species names, ordered by the model’s propensity scores. Included flaviviruses are Banzi virus (BANV), Bouboui virus (BOUV), dengue virus strains 1, 2, 3 and 4 (DENV-1,2,3,4), Edge Hill virus (EHV), Ilheus virus (ILHV), Israel turkey meningoencephalomyelitis virus (ITV), Japanese encephalitis virus (JEV), Kedougou virus (KEDV), Kokobera virus (KOKV), Kunjin virus (KUNV), Murray Valley encephalitis virus (MVEV), Rocio virus (ROCV), St. Louis encephalitis virus (SLEV), Spondwendi virus (SPOV), Stratford virus (STRV), Uganda S virus (UGSV), Wesselsbron virus (WESSV), West Nile Virus (WNV), yellow fever virus (YFV), and Zika virus (ZIKV).
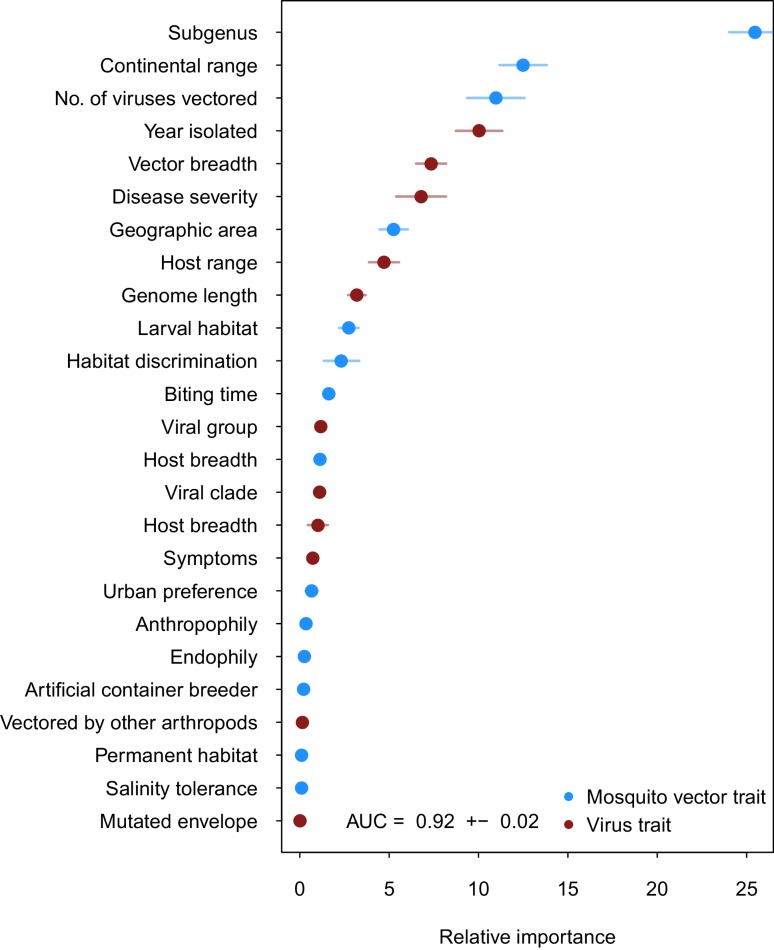
Our ensemble of BRT models trained on common vector and virus traits predicted mosquito vector-virus pairs in the test dataset with high accuracy (AUC = 0.92 ± 0.02; sensitivity = 0.858 ± 0.04; specificity = 0.872 ± 0.04). Due to non-monotonicity and existence of interactions among predictor variables within our model, one cannot make general statements about the directionality of effect. Thus, we focus on the relative importance of different variables to model performance. The most important variable for accurately predicting the presence of vector-virus pair was the subgenus of the mosquito species, followed by continental range (e.g. continents on which species are present). The number of viruses vectored by a mosquito species and number of mosquito vectors of a virus were the third and fifth most important variables, respectively. Unsurprisingly, this suggests that, when controlling for other variables, mosquitoes and viruses with more known vector-virus pairs (i.e., more viruses vectored and more hosts infected, respectively), are more likely to be part of a predicted pair by the model. Mosquito ecological traits such as larval habitat and salinity tolerance were generally less important than a species’ phylogeny or geographic range (Figure 2).
Figure 2. Variable importance by permutation, averaged over 25 models.
Because some categorical variables were treated as binary by our model (i.e. continental range), the relative importance of each binary variable was summed to result in the overall importance of the categorical variable. Mosquito and virus traits are shown in blue and maroon, respectively. Error bars represent the standard error from 25 models.
When applied to the 180 potential mosquito-Zika pairs, the model predicted thirty-five vectors to be ranked above the threshold (set at the value of the lowest-ranked known vector), for a total of nine known vectors and twenty-six novel, predicted mosquito vectors of Zika (Table 1). Of these vectors, there were twenty-four Aedes species, nine Culex species, one Psorophora species, and one Runchomyia species. The GBM model’s top two ranked vectors for Zika are the most highly-suspected vectors of Zika virus, Ae. aegypti and Ae. albopictus.
Table 1.
Predicted vectors of Zika virus, as reported by our model. Mosquito species endemic to the continental United States are bolded. A species is defined as a known vector of Zika virus if a full transmission cycle (see main text) has been observed.
| Species | GBM prediction SD | Known vector? |
|---|---|---|
| Aedes aegypti | 0.81 ± 0.12 | Yes |
| Ae. albopictus | 0.54 ± 0.14 | Yes |
| Culex quinquefasciatus | 0.38 ± 0.14 | No |
| Ae. polynesiensis | 0.36 ± 0.13 | No |
| Ae. scutellaris | 0.33 ± 0.13 | No |
| Ae. africanus | 0.32 ± 0.11 | No |
| Ae. furcifer | 0.31 ± 0.16 | Yes |
| Ae. vittatus | 0.30 ± 0.20 | Yes |
| Ae. taylori | 0.30 ± 0.16 | Yes |
| Ae. luteocephalus | 0.25 ± 0.12 | Yes |
| Ae. tarsalis | 0.18 ± 0.11 | Yes |
| Ae. metallicus | 0.16 ± 0.08 | No |
| Ae. minutus | 0.16 ±0.09 | No |
| Ae. opok | 0.14 ± 0.06 | No |
| Ae. bromeliae | 0.11 ± 0.06 | No |
| Ae. scapularis | 0.10 ± 0.04 | No |
| Cx. pipiens | 0.10 ± 0.04 | No |
| Ae. hensilli | 0.10 ± 0.06 | Yes |
| Ae. vigilax | 0.10 ± 0.05 | No |
| Cx. annulirostrix | 0.08 ± 0.03 | No |
| Psorophora ferox | 0.08 ± 0.05 | No |
| Cx. rubinotus | 0.08 ± 0.07 | No |
| Cx. tarsalis | 0.08 ± 0.03 | No |
| Ae. occidentalis | 0.08 ± 0.05 | No |
| Ae. flavicolis | 0.07 ± 0.04 | No |
| Ae. serratus | 0.07 ± 0.04 | No |
| Cx. p. molestus | 0.07 ± 0.04 | No |
| Ae. vexans | 0.06 ± 0.04 | No |
| Cx. neavei | 0.06 ± 0.02 | No |
| Runchomyia frontosa | 0.06 ± 0.04 | No |
| Ae. neoafricanus | 0.06 ± 0.03 | No |
| Ae. chemulpoensis | 0.06 ± 0.03 | No |
| Cx. vishnui | 0.05 ± 0.01 | No |
| Cx. tritaeniorhynchus | 0.05 ± 0.01 | No |
| Ae. fowleri | 0.04 ± 0.03 | Yes |
Model validation
Our supplementary and primary models generally concur and their ranking of potential Zika virus vectors are highly correlated (ρ = 0.508 and ρ = 0.693 on raw and thresholded predictions, respectively). As one might expect, the supplementary model assigned fewer scores of low propensity (Appendix 1—figure 2), suggesting that incorporating this additional uncertainty in the training dataset eroded the model's ability to distinguish negative links. The supplementary model’s performance on the testing data (AUC = 0.84 ± 0.02), however, indicates that the additional uncertainty did not impede model performance.
When trained on ‘leave-one-out’ datasets, all three models were able to predict the testing data with high accuracy (AUC = 0.91, AUC = 0.91, AUC = 0.92 for West Nile, dengue, and yellow fever viruses, respectively). Performance varied when models were validated against predictions of ‘known outcomes’. A model trained without West Nile virus predicted highly linked vectors reasonably well (AUC = 0.69), however it assigned low scores to rarer ‘known’ vectors, such as Culiseta inornata, which was only associated with West Nile virus. Similarly, the model trained on the dengue-omitted dataset predicted training data and vectors of dengue itself with high accuracy (AUC = 0.92). While the model trained without yellow fever performed well on the testing data, it performed poorly when predicting vectors of yellow fever virus (AUC = 0.47). Unlike West Nile and dengue viruses, the majority of the known vectors of yellow fever are only associated with yellow fever (i.e. a single vector-virus link), and so were excluded completely from the training data when all yellow fever links were omitted. Additionally, several of the vector species are of the Haemagogus genus, which was completely absent from the training data. Given the importance of phylogeny of the vector species in predicting vector-virus links, it follows that a dataset with a novel subgenus would be difficult for the model to predict on, resulting in low model performance. The low performance of this model illustrates that incorporating common traits and additional vector-virus links improves model prediction. When traits were not available in the training dataset, model performance was much lower, suggesting that there exists a statistical association between a vectors’ traits and its ability to transmit a virus.
Discussion
Zika virus is unprecedented among emerging arboviruses in its combination of severe public health hazard, rapid spread, and poor scientific understanding. Particularly crucial to public health preparedness is knowledge about the geographic extent of potentially at risk populations and local environmental conditions for transmission, which are determined by the presence of competent vectors. Until now, identifying additional competent vector species has been a low priority because Zika virus has historically been geographically restricted to a narrow region of equatorial Africa and Asia (Petersen et al., 2016), and the mild symptoms of infection made its range expansion since the 1950’s relatively unremarkable. However, with its relatively recent and rapid expansion into the Americas and its association with severe neurological disorders, the prediction of potential disease vectors in non-endemic areas has become a matter of critical public health importance. We identify these potential vector species by developing a data-driven model that identifies candidate vector species of Zika virus by leveraging data on traits of mosquito vectors and their flaviviruses. We suggest that empirical work should prioritize these species in their evaluation of vector competence of mosquitoes for Zika virus.
Our model predicts that fewer than one third of the potential mosquito vectors of Zika virus have been identified, with over twenty-five additional mosquito species worldwide that may have the capacity to contribute to transmission. The continuing focus in the published literature on two species known to transmit Zika virus (Ae. aegypti and Ae. albopictus) ignores the potential role of other vectors, potentially misrepresenting the spatial extent of risk. In particular, four species predicted by our model to be competent vectors – Ae. vexans, Culex quinquefasciatus, Cx. pipiens, and Cx. tarsalis – are found throughout the continental United States. Further, the three Culex species are primary vectors of West Nile virus (Farajollahi et al., 2011). Cx. quinquefasciatus and Cx. pipiens were ranked 3rd and 17th by our model, respectively, and together these species were the highest-ranking species endemic to the United States after the known vectors (Ae. aegypti and Ae. albopictus). Cx. quinquefasciatus has previously been implicated as an important vector of encephalitic flaviviruses, specifically West Nile virus and St. Louis encephalitis (Turell et al., 2005; Hayes et al., 2005), and a hybridization of the species with Cx. pipiens readily bites humans (Fonseca et al., 2004). The empirical data available on the vector competence of Cx. pipiens and Cx. quinquefasciatus is currently mixed, with some studies finding evidence for virus transmission and others not (Guo et al., 2016; Aliota et al., 2016; Fernandes et al., 2016; Huang et al., 2016). These results suggest, in combination with evidence for significant genotype x genotype effects on the vector competence of Ae. aegypti and Ae. albopictus to transmit Zika (Chouin-Carneiro et al., 2016), that the vector competence of Cx. pipiens and Cx. quinquefasciatus for Zika virus could be highly dependent upon the genetic background of the mosquito-virus pairing, as well as local environmental conditions. Thus, considering their anthropophilic natures and wide geographic ranges, Cx. quinquefasciatus and Cx. pipiens could potentially play a larger role in the transmission of Zika in the continental United States. Further experimental research into the competence of populations of Cx. pipiens to transmit Zika virus across a wider geographic range is therefore highly recommended, and should be prioritized.
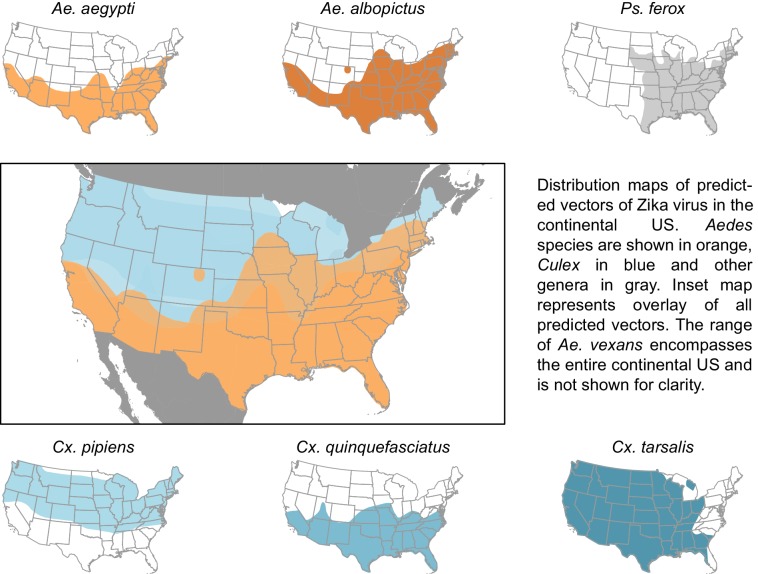
The vectors predicted by our model have a combined geographic range much larger than that of the currently suspected vectors of Zika (Figure 3), suggesting that, were these species to be confirmed as vectors, a larger population may be at risk of Zika infection than depicted by maps focusing solely on Ae. aegypti and Ae. albopictus. The range of Cx. pipiens includes the Pacific Northwest and the upper mid-West, areas that are not within the known range of Ae. aegypti or Ae. albopictus (Darsie and Ward, 2005). Furthermore, Ae. vexans, another predicted vector of Zika virus, is found throughout the continental US and the range of Cx. tarsalis extends along the entire West coast (Darsie and Ward, 2005). On a finer scale, these species use a more diverse set of habitats, with Ae. aegypti and Cx. quinquefasciatus mainly breeding in artificial containers, and Ae. vexans and Ae. albopictus being relatively indiscriminate in their breeding sites, including breeding in natural sites such as tree holes and swamps. Therefore, in addition to the wider geographic region supporting potential vectors, these findings suggest that both rural and urban areas could serve as habitat for potential vectors of Zika. We recommend experimental tests of these species for competency to transmit Zika virus, because a confirmation of these vectors would necessitate expanding public health efforts to these areas not currently considered at risk.
Figure 3. Distribution maps of predicted vectors of Zika virus in the continental US.
Maps of Aedes species are based on Centers for disease control and prevention (2016). All other species’ distributions are georectified maps from Darsie and Ward (2005).
While transmission requires a competent vector, vector competence does not necessarily equal transmission risk or inform vectorial capacity. There are many biological factors that, in conjunction with positive vector competence, determine a vector’s role in disease transmission. For example, although Ae. aegypti mosquitoes are efficient vectors of West Nile virus, they prefer to feed on humans, which are dead-head hosts for the disease, and therefore have low potential to serve as a vector (Turell et al., 2005). Psorophora ferox, although predicted by our model as a potential vector of Zika virus, would likely play a limited role in transmission because it rarely feeds on humans (Molaei et al., 2008). Additionally, vector competence is dynamic, and may be mediated by environmental factors that influence viral development and mosquito immunity (Muturi and Alto, 2011). Therefore, our list of potential vectors of Zika represents a comprehensive starting point, which should be furthered narrowed by empirical work and consideration of biological details that impact transmission dynamics. Given the severe neurological side-effects of Zika virus infection, beginning with the most conservative method of vector prediction ensures that risk is not underestimated, and allows public health agencies to interpret the possibility of Zika transmission given local conditions.
Our model serves as a starting point to streamlining empirical efforts to identify areas and populations at risk for Zika transmission. While our model enables data-driven predictions about the geographic area at potential risk of Zika transmission, subsequent empirical work investigating Zika vector competence and transmission efficiency is required for model validation, and to inform future analyses of transmission dynamics. For example, in spite of its low transmission efficiency in certain geographic regions (Chouin-Carneiro et al., 2016), Ae. aegypti is anthropophilic (Powell and Tabachnick, 2013), and may therefore pose a greater risk of human-to-human Zika virus transmission than mosquitoes that bite a wider variety of animals. On the other hand, mosquito species that prefer certain hosts in rural environments are known to alter their feeding behaviors to bite alternative hosts (e.g., humans and rodents) in urban settings, due to changes in host community composition (Chaves et al., 2010). Environmental factors such as precipitation and temperature directly influence mosquito populations, and determine the density of vectors in a given area (Thomson et al., 2006), an important factor in transmission risk. Additionally, socio-economic factors such as housing type and lifestyle can decrease a populations’ contact with mosquito vectors, and lower the risk of transmission to humans (Moreno-Madriñán and Turell, 2017). Effective risk modeling and forecasting the range expansion of Zika virus in the United States will depend on validating the vector status of these species, as well as resolving behavioral and biological details that impact transmission dynamics.
Although we developed this model with Zika virus in mind, our findings have implications for other emerging flaviviruses and contribute to the recently developed methodology applying machine learning methods to the prediction of unknown agents of infectious diseases. This technique has been used to predict rodent reservoirs of disease (Han et al., 2015) and bat carriers of filoviruses (Han et al., 2016) by training models with host-specific data. Our model, however, incorporates additional data by constructing a vector-virus network that is used to inform predictions of vector-virus associations. The combination of common virus traits with vector-specific traits enabled us to predict potential mosquito vectors of specific flaviviruses, and to train the model on additional information distributed throughout the flavivirus-mosquito network.
Uncertainty in our model arises through uncertainty inherent in our datasets. Vector status is not static (e.g. mutation in the chikungunya virus to increase transmission by Ae. albopictus [Weaver and Forrester, 2015]) and can vary across vector populations (Bennett et al., 2002). When incorporating uncertainty in vector status through our supplementary model, our predictions generally agreed with that of our original model. However, the increased uncertainty did reduce the models’ ability to distinguish negative links, resulting in higher uncertainty in propensity scores (as measured by standard deviation) and a larger number of predicted vectors. Additionally, the model performs poorly when predicting on vector-virus links with trait levels not included in the training data set, as was the case when omitting yellow fever virus. Another source of uncertainty is regarding vector and virus traits. In addition to intraspecific variation in biological traits, many vectors are understudied, and common traits such as biting activity are unknown to the level of species. Additional study into the behavior and biology of less common vector species would increase the accuracy of prediction techniques such as this, and allow for a better of understanding of species’ potential role as vectors.
Interestingly, our constructed flavivirus-mosquito network generally concurs with the proposed dichotomy of Aedes species vectoring hemorrhagic or febrile arboviruses and Culex species vectoring neurological or encephalitic viruses (Grard et al., 2010) (Figure 1). However, there are several exceptions to this trend, notably West Nile virus, which is vectored by several Aedes species. Additionally, our model predicts several Culex species to be possible vectors of Zika virus. While this may initially seem contrary to the common phylogenetic pairing of vectors and viruses noted above, Zika’s symptoms, like West Nile virus, are both febrile and neurological. Thus, its symptoms do not follow the conventional hemorrhagic/encephalitic division. The ability of Zika virus to be vectored by a diversity of mosquito vectors could have important public health consequences, as it may expand both the geographic range and seasonal transmission risk of Zika virus, and warrants further empirical investigation.
Considering our predictions of potential vector species and their combined ranges, species on the candidate vector list need to be validated to inform the response to Zika virus. Vector control efforts that target Aedes species exclusively may ultimately be unsuccessful in controlling transmission of Zika because they do not control other, unknown vectors. For example, the release of genetically modified Ae. aegypti to control vector density through sterile insect technique is species-specific and would not control alternative vectors (Alphey et al., 2010). Additionally, species’ habitat preferences differ, and control efforts based singularly on reducing Aedes larval habitat will not be as successful at controlling Cx. quinquefasciatus populations (Rey et al., 2006). Predicted vectors of Zika virus must be empirically tested and, if confirmed, vector control efforts would need to respond by widening their focus to control the abundance of all predicted vectors of Zika virus. Similarly, if control efforts are to include all areas at potential risk of disease transmission, public health efforts would need to expand to address regions such as the northern Midwest that fall within the range of the additional vector species predicted by our model. An understanding of the capacity of mosquito species to vector Zika virus is necessary to prepare for the potential establishment of Zika virus in the United States, and we recommend that experimental work start with this list of candidate vector species.
Materials and methods
Data collection and feature construction
Our dataset comprised a matrix of vector-virus pairs relating all known flaviviruses and their mosquito vectors. To construct this matrix, we first compiled a list of mosquito-borne flaviviruses to include in our study (Van Regenmortel et al., 2000; Kuno et al., 1998; Cook and Holmes, 2006). Viruses that only infect mosquitoes and are not known to infect humans were not included. Using this list, we constructed a mosquito-virus pair matrix based on the Global Infectious Diseases and Epidemiology Network database (GIDEON, 2016), the International Catalog of Arboviruses Including Certain Other Viruses of Vertebrates (ArboCat) (Karabatsos, 1985), The Encyclopedia of Medical and Veterinary Entomology (Russell et al., 2013)and Mackenzie et al. (2012).
We defined a known vector-virus pair as one for which the full transmission cycle (i.e, infection of mosquito via an infected host (mammal or avian) or bloodmeal that is able to be transmitted via saliva) has been observed. Basing vector competence on isolation or intrathoracic injection bypasses several important barriers to transmission (Hardy et al., 1983), and may not be true evidence of a mosquito’s ability to transmit an arbovirus. We found our definition to be more conservative than that which is commonly used in disease databases (e.g. Global Infectious Diseases and Epidemiology Network database), which often assumes isolation from wild-caught mosquitoes to be evidence of a mosquito’s role as a vector. Therefore, a supplementary analysis investigates the robustness of our findings with regards to uncertainty in vector status by comparing the analysis reported in the main text to a second analysis in which any kind of evidence for association, including merely isolating the virus in wild-caught mosquitoes, is taken as a basis for connection in the virus-vector network (see Appendix 1 for analysis and results).
Fifteen mosquito traits (Appendix 2—table 1) and twelve virus traits (Appendix 2—table 2) were collected from the literature. For the mosquito species, the geographic range was defined as the number of countries in which the species has been collected, based on Walter Reed Biosystematics Unit, (2016). While there are uncertainties in species’ ranges due to false absences, this represents the most comprehensive, standardized dataset available that includes both rare and common mosquito species. A species’ continental extent was recorded as a binary value of its presence by continent. A species’ host range was defined as the number of taxonomic classes the species is known to feed on, with the Mammalia class further split into non-human primates and other mammals, because of the important role primates play in zoonotic spillovers of vector-borne disease (e.g. dengue, chikungunya, yellow fever, and Zika viruses) (Weaver, 2005; Diallo et al., 2005; Weaver et al., 2016). The total number of unique flaviviruses observed per mosquito species was calculated from our mosquito-flavivirus matrix. All other traits were based on consensus in the literature (see Appendix III for sources by species). For three traits – urban preference, endophily (a proclivity to bite indoors), and salinity tolerance – if evidence of that trait for a mosquito was not found in the literature, it was assumed to be negative.
We collected data on the following virus traits: host range (Mahy, 2009; Mackenzie et al., 2012; Chambers and Monath, 2003; Cook and Zumla, 2009b), disease severity (Mackenzie et al., 2012), human illness (Chambers and Monath, 2003; Cook and Zumla, 2009), the presence of a mutated envelope protein, which controls viral entry into cells (Grard et al., 2010), year of isolation (Karabatsos, 1985), and host range (Karabatsos, 1985). Disease severity was based on Mackenzie et al. (2012), ranging from no known symptoms (e.g. Kunjin virus) to severe symptoms and significant human mortality (e.g. yellow fever virus). For each virus, vector range was calculated as the number of mosquito species for which the full transmission cycle has been observed. Genome length was calculated as the mean of all complete genome sequences listed for each flavivirus in the Virus Pathogen Database and Analysis Resource (http://www.viprbrc.org/). For more recently discovered flaviviruses not yet cataloged in the above databases (i.e., New Mapoon Virus, Iquape virus), viral traits were gathered from the primary literature (sources listed in Appendix 3).
Predictive model
Following Han et al. (2015), boosted regression trees (BRT) (Friedman, 2001) were used to fit a logistic-like predictive model relating the status of all possible virus-vector pairs (0: not associated, 1: associated) to a predictor matrix comprising the traits of the mosquito and virus traits in each pair. Boosted regression trees circumvent many issues associated with traditional regression analysis (Elith et al., 2008), allowing for complex variable interactions, collinearity, non-linear relationships between covariates and response variables, and missing data. Additionally, this technique performs well in comparison with other logistic regression approaches (Friedman, 2001). Trained boosted regression tree models are dependent on the split between training and testing data, such that each model might predict slightly different propensity values. To address this, we trained an ensemble of 25 internally cross-validated BRT models on independent partitions of training and testing data. The resulting model demonstrated low variance in relative variable importance and overall model accuracy, suggesting models all converged to a similar result.
Prior to the analysis of each model, we randomly split the data into training (70%) and test (30%) sets while preserving the proportion of positive labels (known associations) in each of the training and test sets. Models were trained using the gbm package in (Ridgeway, 2015), with the maximum number of trees set to 25,000, a learning rate of 0.001, and an interaction depth of 5. To correct for optimistic bias (Smith et al., 2014), we performed 10-fold cross validation and chose a bag fraction of 50% of the training data for each iteration of the model. We estimated the performance of each individual model with three metrics: Area Under the Receiver Operator Curve, specificity, and sensitivity. For specificity and sensitivity, which require a preset threshold, we thresholded predictions on the testing data based on the value which maximized the sum of the sensitivity and specificity, a threshold robust to the ratio of presence to background points in presence-only datasets (Liu et al., 2016). Variable importance was quantified by permutation (Breiman, 2001) to assess the relative contribution of virus and vector traits to the propensity for a virus and vector to form a pair. Because we transformed many categorical variables into binary variables (e.g., continental range as binary presence or absence by continent), the sum of the relative importance for each binary feature was summed to obtain a single value for the entire variable.
Each of our twenty-five trained models was then used to predict novel mosquito vectors of Zika by applying the trained model to a data set consisting of the virus traits of Zika paired with the traits of all mosquitoes for which flaviviruses have been isolated from wild caught individuals, and, depending on the species, may or may not have been tested in full transmission cycle experiments (a total of 180 mosquito species). This expanded dataset allowed us to predict over a large number of mosquito species, while reasonably limiting our dataset to those species suspected of transmitting flaviviruses. The output of this model was a propensity score ranging from 0 to 1. In our case, the final propensity score for each vector was the mean propensity score assigned by the twenty-five models. To label unobserved edges, we thresholded propensity scores at the value of lowest ranked known vector (Liu et al., 2013).
Model validation
In addition to conventional performance metrics, we conducted additional analyses to further validate both this method of prediction, and our model specifically. To account for uncertainty in the vector-virus links in our initial matrix, we repeated our analysis for a vector-virus matrix with a less conservative definition of a positive link (field isolation and above), referred to as our supplementary model. Vector competence is a dynamic trait, and there exists significant intraspecific variation in the ability of a vector to transmit a virus for certain species of mosquitoes (Diallo et al., 2005; Gubler et al., 1979). Our supplementary model is based on a less conservative definition of vector competence and includes species implicated as vectors, but not yet verified through laboratory competence studies, and therefore accounts for additional uncertainty such as intraspecific variation.
While this approach is well-tested in epidemiological applications (Parascandola, 2004), it has only recently been applied to predict ecological associations, and, as such, has limitations unique to this application. To further evaluate this prediction method, we performed a modified ‘leave-one-out’ analysis, whereby we trained a model to a dataset from which a well-studied virus had been omitted, and then predicted vectors for this virus and compared them against a list of known vectors. We repeated this analysis for West Nile, dengue, and yellow fever viruses, following the same method of training as for our original model. While this analysis differs from our original method, it provides a more stringent evaluation of this method of prediction because the model is trained on an incomplete dataset and predicts on unfamiliar data, a more difficult task than that posed to our original model.
Acknowledgements
The authors acknowledge Pasha Feinberg for assistance with data collection.
Appendix 1
Comparison model trained on virus isolation data
The primary model is trained on vector-virus pairs for which the full transmission cycle has been observed. However, many sources, such as the Global Infectious Diseases and Epidemiology Network database (GIDEON), interpret isolation of a virus in wild-caught mosquitoes as evidence of a mosquito’s role as a vector. In order to investigate the robustness of our findings, we conducted a supplementary analysis in which any evidence for association, including isolation of the virus, is used as the basis for a link in the vector-virus network.
Data collection
As in the primary model, the mosquito-virus pair matrix was constructed based on the Global Infectious Diseases and Epidemiology Network database (GIDEON, 2016), the International Catalog of Arboviruses Including Certain Other Viruses of Vertebrates (ArboCat) (Karabatsos, 1985), The Encyclopedia of Medical and Veterinary Entomology (Russell et al., 2013) and (Mackenzie et al., 2012). This resulted in a dataset containing 180 mosquito species and 37 viruses, for a total of 334 vector-virus pairs. The vector and virus trait datasets were identical to those used in the primary model (see Appendix 2 for lists of traits).
Predictive model
We used boosted regression trees (Friedman, 2001) to fit a logistic-like predictive model relating the status of all possible virus-vector pairs (0: not associated, 1:associated) to a predictor matrix comprising the traits of the mosquito and virus traits in each pair. We fit a total of 25 models, applying different training and testing datasets to each, to reduce the dependence dependent on the split between training and testing data. Prior to the analysis of each model, we randomly split the data into training (70%) and test (30%) sets while preserving the proportion of positive labels in each of the training and test sets. Models were trained using the gbm package in (Ridgeway, 2015), with the maximum number of trees set to 25,000 and a learning rate of 0.001. To correct for optimistic bias (Smith et al., 2014), we performed 10-fold cross validation and bagged 50% of the training data for each iteration of the model. These methods are identical to those used to train the primary model. We quantified variable importance by permutation (Breiman, 2001) to assess the relative contribution of virus and vector traits to the propensity for a virus and vector to form a pair.Each of our twenty-five trained models was then used to predict novel mosquito vectors of Zika over the whole virus-vector pair dataset, resulting in twenty-five propensity values assigned to each mosquito species, of which we took the mean. Our prediction dataset, therefore, consisted of the common virus traits of Zika paired with the common traits of all mosquitoes in our flavivirus dataset, for a total of 180 species. The output of this model was a propensity score ranging from 0 to 1. In our case, the final propensity score for each vector was the mean propensity score assigned by the twenty-five models. To label unobserved edges, we thresholded propensity at the value of lowest ranked known vector (Liu et al., 2013).
Results
Boosted regression models trained on the weakest evidence of association accurately predicted mosquito vector-virus associations in the test dataset (AUC= 0.84 ±0.02). When thresholded at the value of the lowest ranked known vector, the model predicted 66 potential vectors of ZIKV, including 42 unknown vectors LABEL:table:predictions. The majority of predicted vectors were Aedes species (39 species), with Culex as the second most predicted genus (15 species). It included all but three of the vectors predicted by the main model (Ae. occidentalis, Ru. frontosa, Cx. rubinotus).
Model comparisons
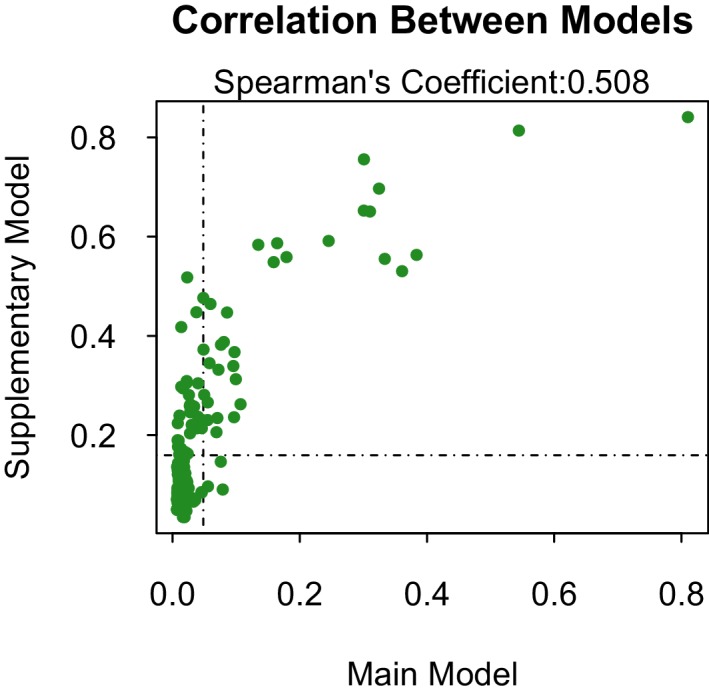
Our supplementary and primary models, trained on virus isolation and above and full transmission cycle, respectively, generally concur. The models are fairly correlated (Spearman’s coefficient, ρ=0.508 when considering the propensities of all 180 species 1. However, when only comparing the correlation of propensities between those vectors above the threshold of lowest ranked known vector, the models become much more correlated (ρ=0.693). This suggests that our model has a higher sensitivity than specificity, and is better able to predict those vectors that are competent for ZIKV than those that are not. The predictive accuracy of our supplementary model was slightly lower than our primary model. However, this may be an indirect effect of a lower positive-negative label ratio in the dataset used in the primary model, which can artificially inflate AUC values (Lobo et al., 2008).
The models differ in their ability to differentiate between vectors and non-vectors. The distribution of propensities for our main model is more skewed towards lower propensity values than is the supplementary model 2. This is logical, as the dataset used to train the main model contains a higher proportion of zeros (e.g. vector-virus pairs with no known association) than the supplementary model. The difference in distributions is accounted for by a similar discrepancy in threshold propensity values based on the lowest ranked known vector. The main model, which has a higher frequency of near-zero propensities, uses a lower threshold value than the supplementary model, however both thresholds qualitatively lie above the majority of the distributions.
Conclusion
In summary, our supplementary model predicts which mosquito species may test positive for ZIKV through isolation in wild-caught individuals. As isolation can be understood as evidence of a vector’s role in transmission of a disease, our supplementary model may also be interpreted as a ranking of potential vectors of ZIKV, similar to our main model. In fact, both models are well correlated in their ranking of species, although the main model, which trains on fewer vector-virus links, predicts fewer vectors than the supplementary model. Those species predicted by both models, such as Cx. quinquefasciatus and Ae. vexans, should be prioritized for further research on their competency to transmit ZIKV. Furthermore, as suggested by the main model, the current geographic range at risk for ZIKV transmission in the United States should be expanded to include the range of these species ranked highly by both our main and supplementary models.
Appendix 1—table 1.
Vector predictions by the supplementary model.
| Vector |
GBM Prediction | SD |
|---|---|---|
| Aedes aegypti | 0.84 | 0.06 |
| Aedes albopictus | 0.81 | 0.07 |
| Aedes vittatus | 0.76 | 0.10 |
| Aedes africanus | 0.70 | 0.11 |
| Aedes taylori | 0.65 | 0.14 |
| Aedes furcifer | 0.65 | 0.14 |
| Aedes luteocephalus | 0.59 | 0.12 |
| Aedes metallicus | 0.59 | 0.13 |
| Aedes opok | 0.58 | 0.13 |
| Culex quinquefasciatus | 0.56 | 0.13 |
| Aedes tarsalis | 0.56 | 0.12 |
| Aedes scutellaris | 0.56 | 0.11 |
| Aedes minutus | 0.55 | 0.12 |
| Aedes polynesiensis | 0.53 | 0.11 |
| Mansonia uniformis | 0.52 | 0.12 |
| Aedes fowleri | 0.48 | 0.14 |
| Aedes vexans | 0.46 | 0.11 |
| Aedes dalzieli | 0.45 | 0.13 |
| Culex annulirostris | 0.45 | 0.08 |
| Mansonia africana | 0.42 | 0.12 |
| Psorophora ferox | 0.39 | 0.14 |
| Culex tarsalis | 0.38 | 0.09 |
| Culex tritaeniorhynchus | 0.37 | 0.08 |
| Culex pipiens | 0.37 | 0.13 |
| Culex neavei | 0.34 | 0.06 |
| Aedes vigilax | 0.34 | 0.07 |
| Aedes flavicollis | 0.33 | 0.14 |
| Aedes scapularis | 0.31 | 0.07 |
| Aedes taeniarostris | 0.31 | 0.13 |
| Aedes jamoti | 0.31 | 0.13 |
| Aedes circumluteolus | 0.30 | 0.13 |
| Eretmapodites inornatus | 0.30 | 0.15 |
| Aedes cumminsii | 0.29 | 0.11 |
| Culex vishnui | 0.28 | 0.05 |
| Aedes lineatopennis | 0.28 | 0.11 |
| Aedes neoafricanus | 0.27 | 0.11 |
| Aedes bromeliae | 0.26 | 0.10 |
| Culex guiarti | 0.26 | 0.06 |
| Culex perfuscus | 0.26 | 0.06 |
| Aedes stokesi | 0.26 | 0.12 |
| Culex telesilla | 0.25 | 0.06 |
| Anopheles gambiae | 0.24 | 0.11 |
| Sabethes chloropterus | 0.24 | 0.11 |
| Aedes hensilli | 0.24 | 0.09 |
| Aedes serratus | 0.23 | 0.06 |
| Aedes chemulpoensis | 0.23 | 0.08 |
| Aedes normanensis | 0.23 | 0.06 |
| Culex bitaeniorhynchus | 0.22 | 0.09 |
| Culex pseudovishnui | 0.22 | 0.05 |
| Aedes argenteopunctatus | 0.21 | 0.06 |
| Wyeomyia vanduzeei | 0.21 | 0.15 |
| Culex p. molestus | 0.21 | 0.06 |
| Culex salinarius | 0.20 | 0.04 |
| Aedes grahami | 0.19 | 0.15 |
| Anopheles coustani | 0.19 | 0.08 |
| Aedes longipalpis | 0.18 | 0.18 |
| Uranotaenia sapphirina | 0.17 | 0.08 |
| Aedes domesticus | 0.17 | 0.06 |
| Aedes abnormalis | 0.17 | 0.06 |
| Aedes natronius | 0.17 | 0.06 |
| Eretmapodites chrysogaster | 0.17 | 0.08 |
| Aedes mcintoshi | 0.17 | 0.06 |
| Aedes ochraceus | 0.16 | 0.06 |
| Culex fatigans | 0.16 | 0.07 |
| Anopheles amictus | 0.16 | 0.06 |
| Eretmapodites quinquevittatus | 0.16 | 0.08 |
Appendix 1—figure 1. Propensity values of the main and supplementary models.
Dashed lines represent corresponding threshold values for each model based on lowest ranked known vector propensities.
Appendix 1—figure 2. Distribution of propensity values for the main and supplementary models.
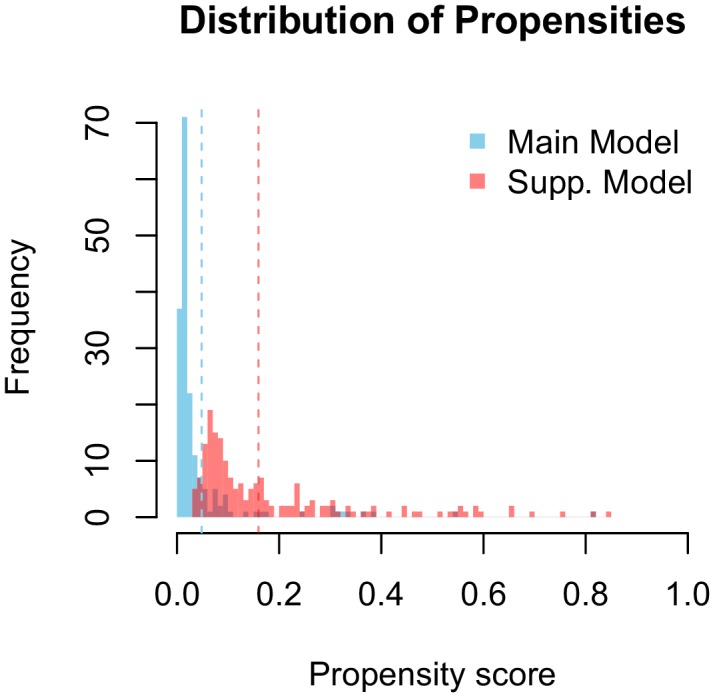
Dashed lines represent corresponding threshold values for each model based on lowest ranked known vector propensities.
Appendix 2
Tables of vector and virus traits
Appendix 2—table 1.
Table of mosquito traits used in model.
| Trait | Type | Subcategories |
|---|---|---|
| Anthropophily | binary | NA |
| Subgenus | factor | NA |
| Host breadth | numeric | NA |
| Host range | binary (x4) | Primate, Non-Primate Mammal, Bird, Cold-Blooded Vertebrate |
| Geographic area | numeric | NA |
| Continental range | binary (x8) | Africa, Middle East, Australia, Pacific, Asia, Europe, North America, South America |
| Biting time | binary (x4) | Dawn, Day, Dusk, Night |
| Artificia container breeder | binary | NA |
| Oviposition site | binary (x8) | Treehole, Natural Container, Permanent Fresh Water, Rockhole, Marsh, Swamp, Temporary Ground Pools, Rice Paddy |
| Habitat discrimination | numeric | NA |
| Salinity tolerance | binary | NA |
| Habitat permanence | binary | NA |
| Urban preference | binary | NA |
| Endophily | binary | NA |
| No. of flaviviruses vectored | numeric | NA |
Appendix 2—table 2.
Table of virus traits used in model.
| Trait | Type | Subcategories |
|---|---|---|
| Group | factor | Japanese Encephalitis, Ntaya, Yellow Fever, Aroa, Dengue, Kokobera, Spondweni |
| Continental range | binary(x8) | Africa, Middle East, Australia, Pacific, Asia, Europe, North America, South America |
| Clade | factor | VI, VII, IX, X, XI, XII, XIV, |
| Year isolated | numeric | NA |
| Mutated envelope | binary | NA |
| Host breadth | numeric | NA |
| Host Range | binary(x6) | Human, Non-Human Primate, Rodent, Other Mammal, Bird, Marsupial |
| Mosquito vector breadth | numeric | NA |
| Vectored by other arthropods | binary | NA |
| Disease symptoms | binary (x2) | Encephalitis, Fever |
| Disease severity | numeric | NA |
| Genome length | numeric | NA |
Appendix 3
Primary sources used for vector and virus traits
Appendix 3—table 1.
Primary sources for mosquito traits.
Appendix 3—table 2.
Primary sources for virus traits.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
National Science Foundation DEB-1640780 to Courtney C Murdock.
University of Georgia Presidential Fellowship to Michelle V Evans.
National Institutes of Health U01GM110744 to John M Drake.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
MVE, Data curation, Formal analysis, Visualization, Writing—original draft, Writing—review and editing.
TAD, Formal analysis, Methodology, Writing—original draft, Writing—review and editing.
BAH, Resources, Formal analysis, Methodology, Writing—original draft, Writing—review and editing.
CCM, Writing—original draft, Writing—review and editing.
JMD, Conceptualization, Methodology, Writing—original draft, Writing—review and editing.
Additional files
Major datasets
The following dataset was generated:
Michelle V Evans,Tad A Dallas,2017,Data and Code to reproduce Evans et al 2017: "Data-driven identification of potential Zika virus vectors",https://doi.org/10.6084/m9.figshare.4042488.v1,Publicly accessible at figshare under a CC-BY licence ((https://figshare.com/).
References
- Adebote AD, Oniye JS, Ndams S, I, Nache KM. The breeding of mosquitoes (Diptera: culicidae) in Peridomestic containers and implication in yellow fever transmission in villages around Zaria, northern Nigeria. Journal of Entomology. 2006;3:180–188. doi: 10.3923/je.2006.180.188. [DOI] [Google Scholar]
- Aitken TH, Anderson CR. Virus transmission studies with trinidadian mosquitoes II. further observations. The American Journal of Tropical Medicine and Hygiene. 1959;8:41–45. doi: 10.4269/ajtmh.1959.8.41. [DOI] [PubMed] [Google Scholar]
- Al-Sheik AA. Larval habitat, ecology, seasonal abundance and vectorial role in malaria transmission of anopheles arabiensis in Jazan region of saudi arabia. Journal of the Egyptian Society of Parasitology. 2011;41:615–634. [PubMed] [Google Scholar]
- Aldemir A, Bedir H, Demirci B, Alten B. Biting activity of mosquito species (Diptera: culicidae) in the Turkey-Armenia border area, ararat valley, turkey. Journal of Medical Entomology. 2010;47:22–27. doi: 10.1093/jmedent/47.1.22. [DOI] [PubMed] [Google Scholar]
- Alencar J, Lorosa ES, Dégallier lN, Serra-Freire NM, Pacheco JB, Guimarães AE. Feeding patterns of haemagogus janthinomys (Diptera: culicidae) in different regions of brazil. Journal of Medical Entomology. 2005;42:981–985. doi: 10.1093/jmedent/42.6.981. [DOI] [PubMed] [Google Scholar]
- Alencar J, Marcondes CB, Serra-Freire NM, Lorosa ES, Pacheco JB, Guimarães AE. Feeding patterns of haemagogus capricornii and haemagogus leucocelaenus (Diptera: culicidae) in two brazilian states (Rio de janeiro and goiás) Journal of Medical Entomology. 2008;45:873–876. doi: 10.1093/jmedent/45.5.873. [DOI] [PubMed] [Google Scholar]
- Alfonzo D, Grillet ME, Liria J, Navarro JC, Weaver SC, Barrera R. Ecological characterization of the aquatic habitats of mosquitoes (Diptera: culicidae) in enzootic foci of venezuelan equine encephalitis virus in western Venezuela. Journal of Medical Entomology. 2005;42:278–284. doi: 10.1093/jmedent/42.3.278. [DOI] [PubMed] [Google Scholar]
- Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. Culex pipiens and aedes triseriatus mosquito susceptibility to zika virus. Emerging Infectious Diseases. 2016;22:1857–1859. doi: 10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector-Borne and Zoonotic Diseases. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerasinghe FP, Indrajith NG. Nocturnal biting rhythms of mosquitoes (Diptera Culicidae) in Sri Lanka. Tropical Zoology. 1995;8:43–53. doi: 10.1080/03946975.1995.10539271. [DOI] [Google Scholar]
- Amerasinghe FP, Munasingha NB. Nocturnal biting rhythms of six mosquito species (Diptera: culicidae) in Kandy, Sri Lanka. Journal of the National Science Council of Sri Lanka. 1994;22:279–290. [Google Scholar]
- Ammar SE, Kenawy MA, Abdel-Rahman HA, Gad AM, Hamed AF. Ecology of the mosquito larvae in urban environments of Cairo Governorate, Egypt. Journal of the Egyptian Society of Parasitology. 2012;42:191–202. doi: 10.12816/0006307. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Main AJ, Ferrandino FJ, Andreadis TG. Nocturnal activity of mosquitoes (Diptera: culicidae) in a west nile virus focus in Connecticut. Journal of Medical Entomology. 2007;44:1102–1108. doi: 10.1093/jmedent/44.6.1102. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of west nile virus in Connecticut: a five-year analysis of mosquito data 1999-2003. Vector-Borne and Zoonotic Diseases. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of culex and other mosquitoes (Diptera: culicidae) in the borough of queens in New York City, with characters and techniques for identification of culex mosquitoes. Journal of Medical Entomology. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Arnell JH. Mosquito studies (DIPTERA, Culicidae) XXXII. a revision of the genus haemagogus. Contributions of the American Entomological Institute. 1973;10:1–176. [Google Scholar]
- Bagirov GA, Gadzhibekova EA, Alirzaev GU. [The attack activity of Uranotaenia Unguiculata Edwards, 1913 mosquitoes on man] Meditsinskaia Parazitologiia I Parazitarnye Bolezni. 1994;3:39–40. [PubMed] [Google Scholar]
- Barker CM, Bolling BG, Black WC, Moore CG, Eisen L. Mosquitoes and west nile virus along a river corridor from prairie to montane habitats in eastern Colorado. Journal of Vector Ecology. 2009;34:276–293. doi: 10.1111/j.1948-7134.2009.00036.x. [DOI] [PubMed] [Google Scholar]
- Barnett H. The incrimination of arthropods as vectors of disease. In: Strouhal H, Beier M, editors. Proceedings of the 11th International Congress of Entomolgoy. Vol. 1962. 1960. pp. 341–345. [Google Scholar]
- Bashar K, Tuno N, Ahmed TU, Howlader AJ. Blood-feeding patterns of anopheles mosquitoes in a malaria-endemic area of Bangladesh. Parasites & Vectors. 2012;5:39. doi: 10.1186/1756-3305-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baton LA, Pacidônio EC, Gonçalves DS, Moreira LA. wFlu: characterization and evaluation of a native Wolbachia from the mosquito aedes fluviatilis as a potential vector control agent. PLoS One. 2013;8:e59619. doi: 10.1371/journal.pone.0059619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Neumann D. Mosquito populations diptera Culicidae of wetlands of an urban area. Zeitschrift Fuer Angewandte Zoologie. 1983;70:73–90. [Google Scholar]
- Begum A, Biswas BR, Elias M. The ecology and seasonal fluctuations of mosquito larvae in a lake in Dhaka City bangladesh. Bangladesh Journal of Zoology. 1986;14:41–48. [Google Scholar]
- Belton P. The mosquitoes of Burnaby lake British-Columbia Canada. Journal of the Entomological Society of British Columbia. 1979;75 [Google Scholar]
- Bennett KE, Olson KE, Muñoz ML, Fernandez-Salas I, Farfan-Ale JA, Higgs S, Black WC, Beaty BJ. Variation in vector competence for dengue 2 virus among 24 collections of aedes aegypti from Mexico and the united states. The American Journal of Tropical Medicine and Hygiene. 2002;67:85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- Bennett KL, Linton YM, Shija F, Kaddumukasa M, Djouaka R, Misinzo G, Lutwama J, Huang YM, Mitchell LB, Richards M, Tossou E, Walton C. Molecular differentiation of the african yellow fever vector aedes bromeliae (Diptera: culicidae) from its sympatric Non-vector sister species, aedes lilii. PLOS Neglected Tropical Diseases. 2015;9:e0004250. doi: 10.1371/journal.pntd.0004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran GW. Handbook of Zoonoses. CRC Press; 1994. Second Edition: Bacterial, Rickettsial, Chlamydial, and Mycotic Zoonoses. [Google Scholar]
- Bhattacharyya DR, Handique R, Dutta LP, Dutta P, Doloi P, Goswami BK, Sharma CK, Mahanta J. Host feeding patterns of culex vishnui sub group of mosquitoes in dibrugarh district of assam. The Journal of Communicable Diseases. 1994;26:133–138. [PubMed] [Google Scholar]
- Bohart RM, Ingram RL. Mosquitoes of Okinawa and Islands in the Central Pacific. US Dept of the Navy, Bureau of Medicine and Surgery; 1946. [Google Scholar]
- Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of zika virus. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1956;50:238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- Boorman JPT. Observations on the habits of mosquitos of plateau province, northern Nigeria, with particular reference to aëdes (Stegomyia) vittatus (Bigot) Bulletin of Entomological Research. 1961;52:709–725. doi: 10.1017/S0007485300055723. [DOI] [Google Scholar]
- Boreham PFL, Chandler JA, Highton RB. Studies on the feeding patterns of mosquitoes of the genera Ficalbia, mimomyia and uranotaenia in the Kisumu area of Kenya. Bulletin of Entomological Research. 1975;65:69. doi: 10.1017/S0007485300005770. [DOI] [Google Scholar]
- Bosak PJ, Reed LM, Crans WJ. Habitat preference of host-seeking Coquillettidia perturbans (Walker) in relation to birds and eastern equine encephalomyelitis virus in new jersey. Journal of Vector Ecology : Journal of the Society for Vector Ecology. 2001;26:103–109. [PubMed] [Google Scholar]
- Boussès P, Dehecq JS, Brengues C, Fontenille D. Inventaire actualisé des moustiques (Diptera : culicidae) de l’île de La Réunion, océan Indien. Bulletin De La Société De Pathologie Exotique. 2013;106:113–125. doi: 10.1007/s13149-013-0288-7. [DOI] [PubMed] [Google Scholar]
- Boxmeyer CE, Palchick SM. Distribution of resting female aedes vexans (Meigen) in wooded and nonwooded areas of metropolitan Minneapolis-St. Paul, Minnesota. Journal of the American Mosquito Control Association. 1999;15:128–132. [PubMed] [Google Scholar]
- Breiman L. Random forests. Machine Learning. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- Brugman VA, Hernández-Triana LM, Prosser SW, Weland C, Westcott DG, Fooks AR, Johnson N. Molecular species identification, host preference and detection of myxoma virus in the anopheles maculipennis complex (Diptera: culicidae) in southern England, UK. Parasites & Vectors. 2015;8:421. doi: 10.1186/s13071-015-1034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno-Marà R, Almeida APG, Navarro JC. Emerging Zoonoses: Eco-Epidemiology, Involved Mechanisms and Public Health Implications. Frontiers Media SA; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DS, Leake CJ. Japanese encephalitis. The Arboviruses: Epidemiology and Ecology. 1988;3:62–92. [Google Scholar]
- Burkett-Cadena ND. Mosquitoes of the Southeastern United States. 1st edition. Tuscaloosa: University of Alabama Press; 2013. [Google Scholar]
- Bustamante DM, Lord CC. Sources of error in the estimation of mosquito infection rates used to assess risk of arbovirus transmission. American Journal of Tropical Medicine and Hygiene. 2010;82:1172–1184. doi: 10.4269/ajtmh.2010.09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan JL, Morris CD. Habitat characteristics of Coquillettidia perturbans in central florida. Journal of the American Mosquito Control Association. 1987;3:176–180. [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawché F. Guillain-Barré syndrome outbreak associated with zika virus infection in french polynesia: a case-control study. The Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CA, Lourenço-de-Oliveira R, Codeço CT, Motta MA. Mosquitoes in bromeliads at ground level of the brazilian Atlantic forest: the relationship between mosquito fauna, water volume, and plant type. Annals of the Entomological Society of America. 2015;108:449–458. doi: 10.1093/aesa/sav040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso JC, de Almeida MA, dos Santos E, da Fonseca DF, Sallum MA, Noll CA, Monteiro HA, Cruz AC, Carvalho VL, Pinto EV, Castro FC, Nunes Neto JP, Segura MN, Vasconcelos PF. Yellow fever virus in haemagogus leucocelaenus and aedes serratus mosquitoes, southern brazil, 2008. Emerging Infectious Diseases. 2010;16:1918–1924. doi: 10.3201/eid1612.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico) University of California Press; 1974. [Google Scholar]
- Centers for Disease Control and Prevention Estimated range of Aedes albopictus and Aedes aegypti in the United States, 2016. 2016 http://www.cdc.gov/zika/vector/range.html
- Chadee DD, Hingwan JO, Persad RC, Tikasingh ES. Seasonal abundance, biting cycle, parity and vector potential of the mosquito haemagogus equinus in Trinidad. Medical and Veterinary Entomology. 1993;7:141–146. doi: 10.1111/j.1365-2915.1993.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Persad RC, Andalcio N, Ramdath W. Distribution of haemagogus mosquitoes on small islands off Trinidad, W. I. Mosquito Systematics. 1985;17:147–153. [Google Scholar]
- Chadee DD, Tikasingh ES, Ganesh R. Seasonality, biting cycle and parity of the yellow fever vector mosquito haemagogus janthinomys in Trinidad. Medical and Veterinary Entomology. 1992;6:143–148. doi: 10.1111/j.1365-2915.1992.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Tikasingh ES. Diel biting activity of culex (Melanoconion) caudelli in Trinidad, west indies. Medical and Veterinary Entomology. 1989;3:231–237. doi: 10.1111/j.1365-2915.1989.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Chalvet-Monfray K, Sabatier P, Bicout DJ. Downscaling modeling of the aggressiveness of mosquitoes vectors of diseases. Ecological Modelling. 2007;204:540–546. doi: 10.1016/j.ecolmodel.2007.01.024. [DOI] [Google Scholar]
- Chambers TJ, Monath TP. The Flaviviruses: Detection, Diagnosis and Vaccine Development. Academic Press; 2003. [Google Scholar]
- Chandler JA, Boreham PF, Highton RB, Hill MN. A study of the host selection patterns of the mosquitoes of the Kisumu area of Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1975;69:415–425. doi: 10.1016/0035-9203(75)90200-X. [DOI] [PubMed] [Google Scholar]
- Chapman HC. Observations on aedes Melanimon and A. dorsalis in Nevada. Annals of the Entomological Society of America. 1960;53:706–708. doi: 10.1093/aesa/53.6.706. [DOI] [Google Scholar]
- Chapman HF, Hughes JM, Jennings C, Kay BH, Ritchie SA. Population structure and dispersal of the saltmarsh mosquito aedes vigilax in Queensland, Australia. Medical and Veterinary Entomology. 1999;13:423–430. doi: 10.1046/j.1365-2915.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Chaves LF, Harrington LC, Keogh CL, Nguyen AM, Kitron UD. Blood feeding patterns of mosquitoes: random or structured? Frontiers in Zoology. 2010;7:3–11. doi: 10.1186/1742-9994-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Lee HL, Lau KW, Abdullah AG, Tan SB, Sa’diyah I, Norma-Rashid Y, Oh PF, Chan CK, Sofian-Azirun M. Biting behavior of malaysian mosquitoes, aedes albopictus Skuse, armigeres kesseli ramalingam, culex quinquefasciatus say, and culex vishnui theobald obtained from urban residential areas in kuala lumpur. Asian Biomedicine. 2014;8:315–321. doi: 10.5372/1905-7415.0803.295. [DOI] [Google Scholar]
- Chevalier V, Mondet B, Diaite A, Lancelot R, Fall AG, Ponçon N. Exposure of sheep to mosquito bites: possible consequences for the transmission risk of rift valley fever in Senegal. Medical and Veterinary Entomology. 2004;18:247–255. doi: 10.1111/j.0269-283X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, Lourenço-de-Oliveira R, Failloux AB. Differential susceptibilities of aedes aegypti and aedes albopictus from the americas to zika virus. PLOS Neglected Tropical Diseases. 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall LT. Anopheles gambiae in Brazil, 1930-40. American Journal of Public Health and the Nations Health. 1944;34:75–76. doi: 10.2105/AJPH.34.1.75. [DOI] [Google Scholar]
- Coimbra TL, Nassar ES, Nagamori AH, Ferreira IB, Pereira LE, Rocco IM, Ueda-Ito M, Romano NS. Iguape: a newly recognized Flavivirus from São Paulo state, Brazil. Intervirology. 1993;36:144–152. doi: 10.1159/000150333. [DOI] [PubMed] [Google Scholar]
- Cook GC, Zumla A. Manson’s Tropical Diseases. Elsevier Health Sciences; 2009. [Google Scholar]
- Cook S, Holmes EC. A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: flaviviridae) and the evolution of vector transmission. Archives of Virology. 2006;151:309–325. doi: 10.1007/s00705-005-0626-6. [DOI] [PubMed] [Google Scholar]
- Cooper RD, Waterson DG, Frances SP, Beebe NW, Sweeney AW. The anopheline fauna of papua new guinea. Journal of the American Mosquito Control Association. 2006;22:213–221. doi: 10.2987/8756-971X(2006)22[213:TAFOPN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Corbet PS. A note on the biting behaviour of the mosquito, aedes ochraceus, in a village in Kenya. East African Medical Journal. 1962;39:511–514. [PubMed] [Google Scholar]
- Crans WJ, Sprenger DA, Mahmood F. The blood-feeding habits of aedes sollicitans (Walker) in relation to eastern equine encephalitis virus in coastal areas of new jersey. 2. results of experiments with caged mosquitoes and the effects of temperature and physiological age on host selection. Journal of Vector Ecology. 1996;21:1–5. [Google Scholar]
- Crans WJ, Sprenger DA. The blood-feeding habits of Aedes sollicitans (Walker) in relation to eastern equine encephalitis virus in coastal areas of New Jersey. 3. Habitat preference, vertical distribution, and diel periodicity of host-seeking adults. Journal of Vector Ecology. 1996;21:6–13. [Google Scholar]
- Crans WJ. New Jersey mosquito species: Rutgers center for vector biology. [02 Mar 2016];2016 http://vectorbio.rutgers.edu/outreach/species/sapp.htm
- Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR. Transmission of eastern equine encephalomyelitis virus in central Alabama. The American Journal of Tropical Medicine and Hygiene. 2003;68:495–500. [PMC free article] [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and Geographical Distribution of the Mosquitos of North America, North of Mexico. University Press of Florida; 2005. [Google Scholar]
- Davies JB. Moonlight and the biting activity of culex (Melanoconion) portesi senevet & abonnenc and C. (M.) taeniopus D. & K. (Diptera, Culicidae) in Trinidad forests. Bulletin of Entomological Research. 1975;65:81–96. doi: 10.1017/S0007485300005794. [DOI] [Google Scholar]
- Davies JB. Attraction of culex portesi senevet & abonnenc and culex taeniopus dyar & knab (Diptera: culicidae) to 20 animal species exposed in a Trinidad forest. I. baits ranked by numbers of mosquitoes caught and engorged. Bulletin of Entomological Research. 1978;68:707–719. doi: 10.1017/S0007485300009664. [DOI] [Google Scholar]
- Davis GE, Philip CB. The identification of the blood-meal in west african mosquitoes by means of the precipitin test. a preliminary report*. American Journal of Epidemiology. 1931;14:130–141. doi: 10.1093/oxfordjournals.aje.a117751. [DOI] [Google Scholar]
- Day JF. Predicting St. louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annual Review of Entomology. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- de Cunha Ramos H, Ribeiro H. Research on the mosquitoes of Angola XXI - Description of Eretmapodites angolensis sp. nov. and Eretmapodites dundo sp. nov. of the oedipodeios group. Garcia De Orta. Série De Zoologia. 1990;17:31–35. [Google Scholar]
- de Oliveria RL, da Silva TF, Heyden R. Alguns aspectos da ecologia dos mosquitos (Diptera: culicidae) de Uma area de planicie (Granjas Calabria), em Jacarepagua, rio de janeiro. II. Frequencia Mensal E No Ciclo Lunar. Memoirs Instituto Oswaldo Cruz-Fiocruz. 1985;80:123–133. doi: 10.1590/s0074-02761986000300003. [DOI] [PubMed] [Google Scholar]
- Degallier N, Pajot F, Kramer R, Claustre J, Bellony S. Biting cycle of Culicidae in french guiana. Cahiers O.R.S.T.O.M. (Office De La Recherche Scientifique Et Technique Outre-Mer) Serie Entomologie Medicale Et Parasitologie. 1978;16:73–84. [Google Scholar]
- DeGroote JP, Sugumaran R. National and regional associations between human west nile virus incidence and demographic, landscape, and land use conditions in the coterminous united states. Vector-Borne and Zoonotic Diseases. 2012;12:657–665. doi: 10.1089/vbz.2011.0786. [DOI] [PubMed] [Google Scholar]
- Derraik JG, Ji W, Slaney D. Mosquitoes feeding on brushtail possums (Trichosurus vulpecula) and humans in a native forest fragment in the Auckland region of New Zealand. The New Zealand Medical Journal. 2007;120:U2830. [PubMed] [Google Scholar]
- Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, Dia I, Faye O, Weaver SC, Sall AA, Diallo M. Potential of selected senegalese aedes spp. mosquitoes (Diptera: culicidae) to transmit zika virus. BMC Infectious Diseases. 2015;15:492. doi: 10.1186/s12879-015-1231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagne N, Fontenille D, Konate L, Faye O, Lamizana MT, Legros F, Molez JF, Trape JF. [Anopheles of Senegal. an annotated and illustrated list] Bulletin De La Société De Pathologie Exotique. 1994;87:267–277. [PubMed] [Google Scholar]
- Diallo D, Diagne CT, Hanley KA, Sall AA, Buenemann M, Ba Y, Dia I, Weaver SC, Diallo M. Larval ecology of mosquitoes in sylvatic arbovirus foci in southeastern senegal. Parasites & Vectors. 2012a;5:286–17. doi: 10.1186/1756-3305-5-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D, Sall AA, Buenemann M, Chen R, Faye O, Diagne CT, Faye O, Ba Y, Dia I, Watts D, Weaver SC, Hanley KA, Diallo M. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern senegal. PLoS Neglected Tropical Diseases. 2012b;6:e1649. doi: 10.1371/journal.pntd.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, Hanley KA, Buenemann M, Weaver SC, Diallo M. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo M, Sall AA, Moncayo AC, Ba Y, Fernandez Z, Ortiz D, Coffey LL, Mathiot C, Tesh RB, Weaver SC. Potential role of sylvatic and domestic african mosquito species in dengue emergence. The American Journal of Tropical Medicine and Hygiene. 2005;73:445–449. [PubMed] [Google Scholar]
- Digoutte JP. An arbovirus disease of present interest: yellow fever, its natural history facing an haemorragic fever, rift valley fever. Bulletin De La Société De Pathologie Exotique. 1999;92:343–348. [PubMed] [Google Scholar]
- Doherty RL, Carley JG, Gorman BM, Buchanan P, Welch JS, Whitehead RH. Studies of arthropod-borne virus infections in Queensland. Australian Journal of Experimental Biology and Medical Science. 1964;42:149–164. doi: 10.1038/icb.1964.16. [DOI] [PubMed] [Google Scholar]
- dos Santos Silva J, Alencar J, Costa JM, Seixas-Lorosa E, Guimarães AÉ. Feeding patterns of mosquitoes (Diptera: culicidae) in six brazilian environmental preservation areas. Journal of Vector Ecology. 2012;37:342–350. doi: 10.1111/j.1948-7134.2012.00237.x. [DOI] [PubMed] [Google Scholar]
- Doucet J, Cachan P. Forest mosquitoes of the ivory coast republic. V. observations on the breeding places of mosquitoes of the genus Eretmapodites in the banco forest, Abidjan. Bulletin De La Société De Pathologie Exotique. 1961;54:1253–1265. [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, federated states of Micronesia. New England Journal of Medicine. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Eastwood G, Goodman SJ, Cunningham AA, Kramer LD. Aedes taeniorhynchus vectorial capacity informs a pre-emptive assessment of west nile virus establishment in galápagos. Scientific Reports. 2013;3:1–8. doi: 10.1038/srep01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, Rochlin I, Longacker J, Kramer LD. Culex restuans (Diptera: culicidae) relative abundance and vector competence for west nile virus. Journal of Medical Entomology. 2005;42:838–843. doi: 10.1093/jmedent/42.5.838. [DOI] [PubMed] [Google Scholar]
- Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. Journal of Animal Ecology. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Ellis BR, Wesson DM, Sang RC. Spatiotemporal distribution of diurnal yellow fever vectors (Diptera: culicidae) at two sylvan interfaces in Kenya, east africa. Vector-Borne and Zoonotic Diseases. 2007;7:129–142. doi: 10.1089/vbz.2006.0561. [DOI] [PubMed] [Google Scholar]
- Evans AM. Notes on Freetown mosquitos, with descriptions of new and Little-Known species. Annals of Tropical Medicine & Parasitology. 1926;20:97–108. doi: 10.1080/00034983.1926.11684481. [DOI] [Google Scholar]
- Fakoorziba MR, Vijayan A. Breeding habitats of culex tritaeniorhynchus (Diptera: culicidae), A Japanese encephalitis vector, and associated mosquitoes in Mysore, India. Journal of the Entomological Research Society. 2008;10:1–9. [Google Scholar]
- Fall AG, Diaïté A, Lancelot R, Tran A, Soti V, Etter E, Konaté L, Faye O, Bouyer J. Feeding behaviour of potential vectors of west nile virus in Senegal. Parasites & Vectors. 2011;4:99. doi: 10.1186/1756-3305-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall AG, Diaïté A, Seck MT, Bouyer J, Lefrançois T, Vachiéry N, Aprelon R, Faye O, Konaté L, Lancelot R. West nile virus transmission in sentinel chickens and potential mosquito vectors, Senegal river Delta, 2008-2009. International Journal of Environmental Research and Public Health. 2013;10:4718–4727. doi: 10.3390/ijerph10104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. "Bird biting" mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infection, Genetics and Evolution. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria NR, Azevedo RS, Kraemer MU, Souza R, Cunha MS, Hill SC, Thézé J, Bonsall MB, Bowden TA, Rissanen I, Rocco IM, Nogueira JS, Maeda AY, Vasami FG, Macedo FL, Suzuki A, Rodrigues SG, Cruz AC, Nunes BT, Medeiros DB, Rodrigues DS, Nunes Queiroz AL, da Silva EV, Henriques DF, Travassos da Rosa ES, de Oliveira CS, Martins LC, Vasconcelos HB, Casseb LM, Simith DB, Messina JP, Abade L, Lourenço J, Carlos Junior Alcantara L, de Lima MM, Giovanetti M, Hay SI, de Oliveira RS, Lemos PS, de Oliveira LF, de Lima CP, da Silva SP, de Vasconcelos JM, Franco L, Cardoso JF, Vianez-Júnior JL, Mir D, Bello G, Delatorre E, Khan K, Creatore M, Coelho GE, de Oliveira WK, Tesh R, Pybus OG, Nunes MR, Vasconcelos PF. Zika virus in the americas: early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng LC. The tree-hole species of mosquitoes of peiping, China. Chinese Medical Journal. 1983;2:503–525. [Google Scholar]
- Fernandes RS, Campos SS, Ferreira-de-Brito A, Miranda RM, Barbosa da Silva KA, Castro MG, Raphael LM, Brasil P, Failloux AB, Bonaldo MC, Lourenço-de-Oliveira R. Culex quinquefasciatus from rio de janeiro is not competent to transmit the local zika virus. PLOS Neglected Tropical Diseases. 2016;10:e0004993. doi: 10.1371/journal.pntd.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro C, Boshell J, Moncayo AC, Gonzalez M, Ahumada ML, Kang W, Weaver SC. Natural enzootic vectors of venezuelan equine encephalitis virus, Magdalena Valley, Colombia. Emerging Infectious Diseases. 2003;9:49–54. doi: 10.3201/eid0901.020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemings MB. An altitude biting study of culex tritaeniorhynchus (Giles) and other associated mosquitoes in japan. Journal of Economic Entomology. 1959;52:490–492. doi: 10.1093/jee/52.3.490. [DOI] [Google Scholar]
- Flordia Medical Entomology Laboratory Mosquito information website. [28 Feb 2016];2016 http://mosquito.ifas.ufl.edu/index.htm
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG. New vectors of rift valley fever in west africa. Emerging Infectious Diseases. 1998;4:289–293. doi: 10.3201/eid0402.980218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forattini OP, Gomes AC, de Castro Gomes A. Biting activity of aedes scapularis (Rondani) and Haemagogus mosquitoes in southern Brazil (Diptera: Culicidae) Revista De Saúde Pública. 1988;22:84–93. doi: 10.1590/S0034-89101988000200003. [DOI] [PubMed] [Google Scholar]
- Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors anopheles coustani s.l. and anopheles squamosus in Macha, Zambia. Vector-Borne and Zoonotic Diseases. 2011;11:1173–1179. doi: 10.1089/vbz.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances SP, Van Dung N, Beebe NW, Debboun M. Field evaluation of repellent formulations against daytime and nighttime biting mosquitoes in a tropical rainforest in northern Australia. Journal of Medical Entomology. 2002;39:541–544. doi: 10.1603/0022-2585-39.3.541. [DOI] [PubMed] [Google Scholar]
- Friedman JH. Greedy function approximation: a gradient boosting machine. The Annals of Statistics. 2001;29:1189–1232. doi: 10.1214/aos/1013203451. [DOI] [Google Scholar]
- Frohne WC. Natural History of Culiseta Impatiens (Wlk.), (Diptera, Culicidae), in Alaska. Transactions of American Microscopical Society. 1953;72:103–118. doi: 10.2307/3223507. [DOI] [Google Scholar]
- Fyodorova MV, Savage HM, Lopatina JV, Bulgakova TA, Ivanitsky AV, Platonova OV, Platonov AE. Evaluation of potential west nile virus vectors in Volgograd region, Russia, 2003 (Diptera: culicidae): species composition, bloodmeal host utilization, and virus infection rates of mosquitoes. Journal of Medical Entomology. 2006;43:552–563. doi: 10.1093/jmedent/43.3.552. [DOI] [PubMed] [Google Scholar]
- Gad AM, Riad IB, Farid HA. Host-feeding patterns of culex pipiens and cx. Antennatus (Diptera: culicidae) from a village in Sharqiya Governorate, Egypt. Journal of Medical Entomology. 1995;32:573–577. doi: 10.1093/jmedent/32.5.573. [DOI] [PubMed] [Google Scholar]
- Galindo P, Carpenter SJ, Trapido H. Ecological observations on forest mosquitoes of an endemic yellow fever area in Panama. The American Journal of Tropical Medicine and Hygiene. 1951;31:98–137. doi: 10.4269/ajtmh.1951.s1-31.98. [DOI] [PubMed] [Google Scholar]
- Galindo P, Trapido H, Carpenter SJ. Observations on diurnal forest mosquitoes in relation to sylvan yellow fever in Panama. The American Journal of Tropical Medicine and Hygiene. 1950;30:533–574. doi: 10.4269/ajtmh.1950.s1-30.533. [DOI] [PubMed] [Google Scholar]
- Galindo P. Bionomics of Sabethes chloropterus humboldt, a vector of sylvan yellow fever in middle america. The American Journal of Tropical Medicine and Hygiene. 1958;7:429–440. doi: 10.4269/ajtmh.1958.7.429. [DOI] [PubMed] [Google Scholar]
- Gamino V, Gutiérrez-Guzmán AV, Fernández-de-Mera IG, Ortíz JA, Durán-Martín M, de la Fuente J, Gortázar C, Höfle U. Natural bagaza virus infection in game birds in Southern Spain. Veterinary Research. 2012;43:65. doi: 10.1186/1297-9716-43-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy B. The aedes (Aedimorphus) Domesticus group (Diptera, Culicidae) Mosquito Systematics. 1987;19:100–110. [Google Scholar]
- Germain M, Sureau P, Herve JP, Fabre J, Mouchet J, Robin Y, Geoffrey B. Isolation of the yellow fever virus from the aedes of the Aedes-Africanus group in the Central-African-Republic the importance of the humid and semi humid savanna in the emergence zone of the amaril virus. Cahiers O.R.S.T.O.M. (Office De La Recherche Scientifique Et Technique Outre-Mer) Serie Entomologie Medicale Et Parasitologie. 1976;14:125–140. [Google Scholar]
- Giberson DJ, Dau-Schmidt K, Dobrin M. Mosquito species composition, phenology and distribution (Diptera: culicidae) on prince Edward island. Journal of the Acadian Entomological Society. 2007;3:7–27. [Google Scholar]
- GIDEON Online Global guide to infectious diseases. [5 February, 2016]; www.gideononline.com
- Gillies MT, De Meillon B. The anophelinae of Africa south of the sahara (Ethiopian zoogeographical region) Publications of the South African Institute for Medical Research. 1968;54:1–343. [Google Scholar]
- Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behavior of anopheles gambiae s.s., anopheles arabiensis, and anopheles funestus and their implications for malaria control. Experimental Parasitology. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- Gomes AdC, Torres MAN, Paula MBd, Fernandes A, Marassá AM, Consales CA, Fonseca DF. Ecologia de Haemagogus e Sabethes (Diptera: Culicidae) em áreas epizoóticas do vírus da febre amarela, Rio Grande do Sul, Brasil. Epidemiologia e Serviços de Saúde. 2010;19:101–113. [Google Scholar]
- Gomes B, Sousa CA, Vicente JL, Pinho L, Calderón I, Arez E, Almeida AP, Donnelly MJ, Pinto J. Feeding patterns of molestus and pipiens forms of culex pipiens (Diptera: Culicidae) in a region of high hybridization. Parasites & Vectors. 2013;6:93. doi: 10.1186/1756-3305-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeev M, I, Zvantsov AB, Goriacheva I, I, ShaÄkevich E, V, Ezhov MN. Description of the new species anopheles artemievi sp.n. Diptera, Culicidae). ResearchGate. 2005;2:4–5. [PubMed] [Google Scholar]
- Gould EA, Solomon T. Pathogenic flaviviruses. The Lancet. 2008;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- Grard G, Moureau G, Charrel RN, Holmes EC, Gould EA, de Lamballerie X. Genomics and evolution of Aedes-borne flaviviruses. Journal of General Virology. 2010;91:87–94. doi: 10.1099/vir.0.014506-0. [DOI] [PubMed] [Google Scholar]
- Gresser I, Hardy JL, Hu SM, Scherer WF. Factors influencing transmission of japanese B encephalitis virus by a colonized strain of culex tritaeniorhynchus giles, from infected pigs and chicks to susceptible pigs and birds. The American Journal of Tropical Medicine and Hygiene. 1958;7:365–373. doi: 10.4269/ajtmh.1958.7.365. [DOI] [PubMed] [Google Scholar]
- Grieco JP, Johnson S, Achee NL, Masuoka P, Pope K, Rejmánková E, Vanzie E, Andre R, Roberts D. Distribution of anopheles albimanus, anopheles vestitipennis, and anopheles crucians associated with land use in northern Belize. Journal of Medical Entomology. 2006;43:614–622. doi: 10.1093/jmedent/43.3.614. [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Nalim S, Tan R, Saipan H, Sulianti Saroso J. Variation in susceptibility to oral infection with dengue viruses among geographic strains of aedes aegypti. The American Journal of Tropical Medicine and Hygiene. 1979;28:1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- Guimarães AE, Gentile C, Lopes CM, de Mello RP. Ecology of mosquitoes (Diptera: culicidae) in areas of serra do mar state Park, state of são paulo, Brazil. III. daily biting rhythms and lunar cycle influence. Memórias Do Instituto Oswaldo Cruz. 2000;95:753–760. doi: 10.1590/S0074-02762000000600002. [DOI] [PubMed] [Google Scholar]
- Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, Sun AJ, Dong YD, Cao WC, Qin CF, Zhao TY. Culex pipiens quinquefasciatus: a potential vector to transmit zika virus. Emerging Microbes & Infections. 2016;5:e102. doi: 10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of zika virus strains: geographic expansion of the asian lineage. PLoS Neglected Tropical Diseases. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bulletin of the World Health Organization. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- Haddow AJ. The mosquito fauna and climate of native huts at Kisumu, Kenya. Bulletin of Entomological Research. 1942;33:91–142. doi: 10.1017/S0007485300026389. [DOI] [Google Scholar]
- Haddow AJ. The mosquitoes of bwamba county, Uganda. IV.- Studies on the genus Eretmapodites, theobald. Bulletin of Entomological Research. 1946a;37:57–82. doi: 10.1017/S0007485300021994. [DOI] [Google Scholar]
- Haddow AJ. The mosquitoes of bwamba county, Uganda. Bulletin of Entomological Research. 1946b;37:57. doi: 10.1017/S0007485300021994. [DOI] [PubMed] [Google Scholar]
- Haddow AJ. Studies on the biting habits and medical importance of east african mosquitos in the genus aëdes. II.—Subgenera Mucidus, Diceromyia, Finlaya and Stegomyia. Bulletin of Entomological Research. 1961;52:317–351. doi: 10.1017/S0007485300055449. [DOI] [Google Scholar]
- Haddow AJ. Observations on the biting habits of mosquitos in the forest canopy at Zika, Uganda, with special reference to the crepuscular periods. Bulletin of Entomological Research. 1964;55:589–608. doi: 10.1017/S0007485300049695. [DOI] [Google Scholar]
- Hall-Mendelin S, Jansen CC, Cheah WY, Montgomery BL, Hall RA, Ritchie SA, Van den Hurk AF. Culex annulirostris (Diptera: Culicidae) host feeding patterns and Japanese encephalitis virus ecology in northern Australia. Journal of Medical Entomology. 2012;49:371–377. doi: 10.1603/ME11148. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue virus-mosquito interactions. Annual Review of Entomology. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- Han BA, Schmidt JP, Alexander LW, Bowden SE, Hayman DT, Drake JM. Undiscovered bat hosts of filoviruses. PLOS Neglected Tropical Diseases. 2016;10:e0004815. doi: 10.1371/journal.pntd.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. PNAS. 2015;112:7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Novak RJ, Lampman RL, Vodkin MH. Notes on the biology of Orthopodomyia in Illinois. Journal of the American Mosquito Control Association. 1995;11:375–376. [PubMed] [Google Scholar]
- Harbach RE, Schnur HJ. Uranotaenia (Pseudoficalbia) mashonaensis, an afrotropical species found in northern israel. Journal of the American Mosquito Control Association. 2007;23:224–225. doi: 10.2987/8756-971X(2007)23[224:UPMAAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Harbach RE. The mosquitoes of the subgenus culex in southwestern Asia and Egypt (Diptera: culicidae) ResearchGate. 1988;24:1–240. [Google Scholar]
- Harbach RE. Mosquito taxonomic inventory. [28 Feb 2016];2015 http://mosquito-taxonomic-inventory.info/
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annual Review of Entomology. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of west nile virus disease. Emerging Infectious Diseases. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearnden MN, Kay BH. Changes in mosquito populations with expansion of the ross river reservoir, Australia, from stage 1 to stage 2A. Journal of the American Mosquito Control Association. 1995;11:211–224. [PubMed] [Google Scholar]
- Heinemann SJ, Aitken THG, Belkin JN. Collection records of the project "Mosquitoes of Middle America". Mosquito Systematics. 1980;12:179–284. [Google Scholar]
- Herve JP, Germain M, Geoffroy B. Bioecologie comparee d’Aedes opok Corbet et Van Someren et A. africanus Theobald dans une galerie forestiere du sud de la Republique Centrafricaine. Cah ORSTOM Ser Ent Med Et Parasitol. 1975;14:235–244. [Google Scholar]
- Hervy J, Legros F, Ferrara L. Influence de la clarte lunaire sur l’activite trophique d’Aedes taylori (Diptera, Culicidae) Cah ORSTOM Ser Ent Med Et Parasitol. 1986;24:59–65. [Google Scholar]
- Hickman R, Brown J. Culiseta Melanura Biology. 2013. [Google Scholar]
- Hoogstraal H, KNIGHT K. Observations on Eretmapodites silvestris Conchobius Edwards (Culicidae) in the Anglo-Egyptian Sudan. The American Journal of Tropical Medicine and Hygiene. 1951;31:659–664. doi: 10.4269/ajtmh.1951.s1-31.659. [DOI] [PubMed] [Google Scholar]
- Hopkins GHE. Mosquitoes of the ethiopian region I. larval bionomics of mosquitoes and taxonomy of culicine larvae. Adlard & Son, Ltd, British Museum of Natural History 1952 [Google Scholar]
- Huang YJ, Ayers VB, Lyons AC, Unlu I, Alto BW, Cohnstaedt LW, Higgs S, Vanlandingham DL. Culex species mosquitoes and zika virus. Vector-Borne and Zoonotic Diseases. 2016;16:673–676. doi: 10.1089/vbz.2016.2058. [DOI] [PubMed] [Google Scholar]
- Huho B, Briët O, Seyoum A, Sikaala C, Bayoh N, Gimnig J, Okumu F, Diallo D, Abdulla S, Smith T, Killeen G. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural africa. International Journal of Epidemiology. 2013;42:235–247. doi: 10.1093/ije/dys214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwuala M. Peri-Domestic ecology of Dry-Season populations of aedes (stegomyia) Mosquitos (diptera, Culicidae) in Uyo, Cross-River state, Nigeria. Environmental Entomology. 1981;10:592–599. doi: 10.1093/ee/10.5.592. [DOI] [Google Scholar]
- Jansen CC, Williams CR, van den Hurk AF. The usual suspects: comparison of the relative roles of potential urban chikungunya virus vectors in Australia. PLoS One. 2015;10:e0134975. doi: 10.1371/journal.pone.0134975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen CC, Zborowski P, Ritchie SA, van den Hurk AF. Efficacy of bird-baited traps placed at different heights for collecting ornithophilic mosquitoes in eastern Queensland, Australia. Australian Journal of Entomology. 2009;48:53–59. doi: 10.1111/j.1440-6055.2008.00671.x. [DOI] [Google Scholar]
- Johansen CA, Power SL, Broom AK. Determination of mosquito (Diptera: culicidae) bloodmeal sources in western Australia: implications for arbovirus transmission. Journal of Medical Entomology. 2009;46:1167–1175. doi: 10.1603/033.046.0527. [DOI] [PubMed] [Google Scholar]
- Jupp PG, Brown RG. The laboratory colonization of culex (Culex) univittatus theobald (Diptera: culicidae) from material collected in the highveld region of south Africa. Journal of the Entomological Society of Southern Africa. 1967;30:34–39. [Google Scholar]
- Jupp PG, Kemp A. Studies on an outbreak of wesselsbron virus in the free state province, South Africa. Journal of the American Mosquito Control Association. 1998;14:40–45. [PubMed] [Google Scholar]
- Jupp PG, Kemp A. Laboratory vector competence experiments with yellow fever virus and five south african mosquito species including aedes aegypti. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:493–498. doi: 10.1016/S0035-9203(02)90417-7. [DOI] [PubMed] [Google Scholar]
- Jupp PG, McIntosh BM, Anderson D. Culex (Eumelanomyia) rubinotus theobald as vector of banzi, Germiston and witwatersrand viruses. Journal of Medical Entomology. 1976;12:647–651. doi: 10.1093/jmedent/12.6.647. [DOI] [PubMed] [Google Scholar]
- Jupp PG, McIntosh BM. A bionomic study of adult aedes (Neomelaniconion) circumluteolus in northern Kwazulu, South Africa. Journal of the American Mosquito Control Association. 1987;3:131–136. [PubMed] [Google Scholar]
- Jupp PG. Larval habitats of culicine mosquitoes (Diptera: culcidae) in a sewage effluent disposal area in the south african high veld. Journal of Entomology of South Africa. 1967;30:243–250. [Google Scholar]
- Kampen H, Werner D. Out of the bush: the asian bush mosquito aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasites & Vectors. 2014;7:59. doi: 10.1186/1756-3305-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanojia PC, Geevarghese G. First report on high-degree endophilism in culex tritaeniorhynchus (Diptera: culicidae) in an area endemic for japanese encephalitis. Journal of Medical Entomology. 2004;41:994–996. doi: 10.1603/0022-2585-41.5.994. [DOI] [PubMed] [Google Scholar]
- Kanojia PC. Bionomics of culex epidesmus associated with japanese encephalitis virus in India. Journal of the American Mosquito Control Association. 2003;19:151–154. [PubMed] [Google Scholar]
- Karabatsos N. International catalog of arboviruses including certain other viruses of vertebrates. The American Journal of Tropical Medicine and Hygiene. 1985;27:372–440. doi: 10.4269/ajtmh.1978.27.372. [DOI] [PubMed] [Google Scholar]
- Karch S, Asidi N, Manzambi ZM, Salaun JJ. [The culicidian fauna and its nuisance in Kinshasha (Zaire)] Bulletin De La Société De Pathologie Exotique. 1993;86:68–75. [PubMed] [Google Scholar]
- Karch S, Mouchet J. [Anopheles paludis: important vector of malaria in zaire] Bulletin De La Société De Pathologie Exotique. 1992;85:388–389. [PubMed] [Google Scholar]
- Kaufman MG, Fonseca DM. Invasion biology of aedes japonicus japonicus (Diptera: culicidae) Annual Review of Entomology. 2014;59:31–49. doi: 10.1146/annurev-ento-011613-162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay BH, Ryan PA, Russell BM, Holt JS, Lyons SA, Foley PN. The importance of subterranean mosquito habitat to arbovirus vector control strategies in North Queensland, Australia. Journal of Medical Entomology. 2000;37:846–853. doi: 10.1603/0022-2585-37.6.846. [DOI] [PubMed] [Google Scholar]
- Kenawy MA, Beier JC, Zimmerman JH, el Said S, Abbassy MM. Host-feeding patterns of the mosquito community (Diptera: culicidae) in Aswan Governorate, Egypt. Journal of Medical Entomology. 1987;24:35–39. doi: 10.1093/jmedent/24.1.35. [DOI] [PubMed] [Google Scholar]
- Kenawy MA, Rashed SS, Teleb SS. Characterization of rice field mosquito habitats in Sharkia Governorate, Egypt. Journal of the Egyptian Society of Parasitology. 1998;28:449–459. [PubMed] [Google Scholar]
- Kerr JA. Studies on the transmission of experimental yellow fever by culex thalassius and mansonia uniformis. Ann Trop Med and Parasitol [Liverpool] 1932;26:119–127. doi: 10.1080/00034983.1932.11684709. [DOI] [Google Scholar]
- Khoshdel-Nezamiha F, Vatandoost H, Azari-Hamidian S, Bavani MM, Dabiri F, Entezar-Mahdi R, Chavshin AR. Fauna and larval habitats of mosquitoes (Diptera: culicidae) of west Azerbaijan province, northwestern iran. Journal of Arthropod-Borne Diseases. 2014;8:163–173. [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West nile virus risk assessment and the bridge vector paradigm. Emerging Infectious Diseases. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WV, Hoogstraal H. Two new species of mosquitoes of the genus Ficalbia from netherlands new guinea. Proceedings of the Entomological Society of Washington. 1946;48:186–190. [Google Scholar]
- Kirby MJ, West P, Green C, Jasseh M, Lindsay SW. Risk factors for house-entry by culicine mosquitoes in a rural town and satellite villages in the Gambia. Parasites & Vectors. 2008;1:41–47. doi: 10.1186/1756-3305-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J, Griffin L, Dale P, Phinn S. Oviposition and larval habitat preferences of the saltwater mosquito, aedes vigilax, in a subtropical mangrove forest in Queensland, Australia. Journal of Insect Science. 2012;12:6. doi: 10.1673/031.012.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KL, Hull WB. The aedes mosquitoes of the philippine islands. Pacific Science. 1953;7:453–481. [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of north american birds with the New York 1999 strain of west nile virus. Emerging Infectious Diseases. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SM, Rajput K. Day-Time resting habitats of culicine mosquitoes and their preponderance. Journal of Communicable Diseases. 1988;20:280–286. [PubMed] [Google Scholar]
- Kumar NP, Sabesan S, Panicker KN. Biting rhythm of the vectors of malayan filariasis, mansonia annulifera, M. uniformis & M. indiana in shertallai (Kerala state), India. The Indian Journal of Medical Research. 1989;89:52–55. [PubMed] [Google Scholar]
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. Journal of Virology. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmert HW, Hughes TP. The virus of IlhéUs encephalitis; isolation, serological specificity and transmission. journal of immunology (Baltimore, md. 1950) 1947;55:61–67. [PubMed] [Google Scholar]
- Lane RP, Crosskey RW. Medical Insects and Arachnids. Springer Science & Business Media; 2012. [Google Scholar]
- Laporta GZ, Crivelaro TB, Vicentin EC, Amaro P, Branquinho MS, Sallum MAM. Culex nigripalpus theobald (Diptera, Culicidae) feeding habit at the parque ecologico do Tiete, Sao Paulo, brazil. Revista Brasileira De Entomologia. 2008;52:663–668. doi: 10.1590/S0085-56262008000400019. [DOI] [Google Scholar]
- Le Berre R, Hamon J. Description of the larva, pupa and female of aedes (N.) jemoti and revision of the keys to the subgenus neomelaniconion in Africa south of the Sahara. Bulletin De La Société De Pathologie Exotique. 1961;53:1054–1064. [PubMed] [Google Scholar]
- Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, Pretrick M, Marfel M, Griggs A, Bel M, Duffy MR, Hancock WT, Ho-Chen T, Powers AM. Aedes hensilli as a potential vector of Chikungunya and zika viruses. PLoS Neglected Tropical Diseases. 2014;8:e3188. doi: 10.1371/journal.pntd.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hong HK. [Seasonal prevalence and behaviour of aedes togoi] The Korean Journal of Parasitology. 1995;33:19–26. doi: 10.3347/kjp.1995.33.1.19. [DOI] [PubMed] [Google Scholar]
- Lequime S, Lambrechts L. Vertical transmission of arboviruses in mosquitoes: a historical perspective. Infection, Genetics and Evolution : Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases. 2014;28:681–690. doi: 10.1016/j.meegid.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Bailey CL, Davies FG, Kairo A. Observations on the dispersal and survival of a population of aedes lineatopennis (Ludlow) (Diptera: culicidae) in Kenya. Bulletin of Entomological Research. 1985;75:661. doi: 10.1017/S0007485300015923. [DOI] [Google Scholar]
- Liu C, Newell G, White M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecology and Evolution. 2016;6:337–348. doi: 10.1002/ece3.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence-only data. Journal of Biogeography. 2013;40:778–789. doi: 10.1111/jbi.12058. [DOI] [Google Scholar]
- Liu LB, Wong KC, Chen KK, Wu SM. One year’s observations on the development and habits of A. sinensis and C. fatigans in Foochow. Acta Ent Sinica. 1960;10:86–95. [Google Scholar]
- Llorente F, Pérez-Ramírez E, Fernández-Pinero J, Elizalde M, Figuerola J, Soriguer RC, Jiménez-Clavero MÁ. Bagaza virus is pathogenic and transmitted by direct contact in experimentally infected partridges, but is not infectious in house sparrows and adult mice. Veterinary Research. 2015;46:93. doi: 10.1186/s13567-015-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography. 2008;17:145–151. doi: 10.1111/j.1466-8238.2007.00358.x. [DOI] [Google Scholar]
- Logan TM, Linthicum KJ, Thande PC, Wagateh JN, Roberts CR. Mosquito species collected from a marsh in western Kenya during the long rains. Journal of the American Mosquito Control Association. 1991;7:395–399. [PubMed] [Google Scholar]
- Lopes J. Ecology of mosquitoes (Diptera: culicidae) in natural and artificial breeding sites of rural area in the north of Parana state, Brazil. 4. wild species breeding in artificial reservoirs. Arquivos De Biologia E Tecnologia. 1996;39:671–676. [Google Scholar]
- Lopes J. Ecology of mosquitoes (Diptera, Culicidae) in natural and artificial rural breeding in places in northern Parana state, brazil. VI. Larvae Collections in the Home Surroundings. Revista Brasileira De Zoologia. 1997;14:571–578. doi: 10.1590/S0101-81751997000300007. [DOI] [Google Scholar]
- Lounibos LP. The bionomics of three sympatric Eretmapodites (Diptera: culicidae) at the Kenya coast. Bulletin of Entomological Research. 1980;70:309–320. doi: 10.1017/S0007485300007598. [DOI] [Google Scholar]
- Lutomiah J, Omondi D, Masiga D, Mutai C, Mireji PO, Ongus J, Linthicum KJ, Sang R. Blood meal analysis and virus detection in blood-fed mosquitoes collected during the 2006-2007 rift valley fever outbreak in Kenya. Vector Borne and Zoonotic Diseases. 2014;14:656–664. doi: 10.1089/vbz.2013.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald WW, Smith CE, Webb HE. Arbovirus infections in Sarawak: observations on the mosquitoes. Journal of Medical Entomology. 1965;1:335–347. doi: 10.1093/jmedent/1.4.335. [DOI] [PubMed] [Google Scholar]
- Mackay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. Host feeding patterns of culex mosquitoes (Diptera: culicidae) in east baton rouge parish, Louisiana. Journal of Medical Entomology. 2010;47:238–248. doi: 10.1093/jmedent/47.2.238. [DOI] [PubMed] [Google Scholar]
- Mackenzie J, Barrett ADT, Deubel V. Japanese Encephalitis and West Nile Viruses. Springer Science & Business Media; 2012. [Google Scholar]
- Maestre-Serrano R, Cochero S, Bello B, Ferro C. [Registry and distribution update of the species of the haemagogus (Diptera: culicidae) genus in the Caribbean region of Colombia] Biomedica : Revista Del Instituto Nacional De Salud. 2013;33 Suppl 1:185–189. [PubMed] [Google Scholar]
- Mahmood F, Crans WJ. Effect of temperature on the development of Culiseta melanura (Diptera: culicidae) and its impact on the amplification of eastern equine encephalomyelitis virus in birds. Journal of Medical Entomology. 1998;35:1007–1012. doi: 10.1093/jmedent/35.6.1007. [DOI] [PubMed] [Google Scholar]
- Mahy BWJ. The Dictionary of Virology. Academic Press; 2009. [Google Scholar]
- Martin DH, Chaniotis BN, Tesh RB. Host preferences of deinocerites pseudes dyar & knab. Journal of Medical Entomology. 1973;10:206–208. doi: 10.1093/jmedent/10.2.206. [DOI] [PubMed] [Google Scholar]
- Mcclelland GA, Weitz B. Further observations on the natural hosts of three species of mansonia blanchard (Diptera, Culicidae) in Uganda. Annals of Tropical Medicine & Parasitology. 1960;54:300–304. doi: 10.1080/00034983.1960.11685990. [DOI] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, Hendrickx G, Zeller H, Van Bortel W, Schaffner F. An entomological review of invasive mosquitoes in Europe. Bulletin of Entomological Research. 2015;105:637–663. doi: 10.1017/S0007485315000103. [DOI] [PubMed] [Google Scholar]
- Messina JP, Kraemer MU, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, Golding N, Ruktanonchai CW, Gething PW, Cohn E, Brownstein JS, Khan K, Tatem AJ, Jaenisch T, Murray CJ, Marinho F, Scott TW, Hay SI. Mapping global environmental suitability for zika virus. eLife. 2016;5:1–22. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi I, Toma T, Suzuki H, Okazawa T. Mosquitoes of the Takara archipelago, Japan. Mosquito Systematics. 1983;15:18–27. [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. Host-Feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals from 23 species of aedes, anopheles, culex, Coquillettidia, psorophora, and uranotaenia. Journal of Medical Entomology. 2008;45:1143–1151. doi: 10.1093/jmedent/45.6.1143. [DOI] [PubMed] [Google Scholar]
- Molaei G, Oliver J, Andreadis TG, Armstrong PM, Howard JJ. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. The American Journal of Tropical Medicine and Hygiene. 2006;75:1140–1147. [PubMed] [Google Scholar]
- Montarsi F, Martini S, Dal Pont M, Delai N, Ferro Milone N, Mazzucato M, Soppelsa F, Cazzola L, Cazzin S, Ravagnan S, Ciocchetta S, Russo F, Capelli G. Distribution and habitat characterization of the recently introduced invasive mosquito aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in north-eastern italy. Parasites & Vectors. 2013;6:292. doi: 10.1186/1756-3305-6-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Madriñán MJ, Turell M. Factors of concern regarding zika and other aedes aegypti-Transmitted viruses in the United States. Journal of Medical Entomology. 2017:tjw212. doi: 10.1093/jme/tjw212. [DOI] [PubMed] [Google Scholar]
- Mores CN, Turell MJ, Dohm DJ, Blow JA, Carranza MT, Quintana M. Experimental transmission of west nile virus by culex nigripalpus from honduras. Vector Borne and Zoonotic Diseases. 2007;7:279–284. doi: 10.1089/vbz.2006.0557. [DOI] [PubMed] [Google Scholar]
- Morrison A, Andreadis TG. Larval population dynamics in a community of nearctic aedes inhabiting a temporary vernal pool. Journal of the American Mosquito Control Association. 1992;8:52–57. [PubMed] [Google Scholar]
- Morsy TA, el Okbi LM, Kamal AM, Ahmed MM, Boshara EF. Mosquitoes of the genus culex in the suez canal governorates. Journal of the Egyptian Society of Parasitology. 1990;20:265–268. [PubMed] [Google Scholar]
- Mouchet J. Observations sur quelques anophèles exophiles au cameroun. Bulletin De La Société De Pathologie Exotique. 1957;50:378–381. [PubMed] [Google Scholar]
- Muñoz J, Ruiz S, Soriguer R, Alcaide M, Viana DS, Roiz D, Vázquez A, Figuerola J. Feeding patterns of potential west nile virus vectors in south-west spain. PLoS ONE. 2012;7:e39549. doi: 10.1371/journal.pone.0039549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multini LC, Marrelli MT, Wilke AB. Microsatellite loci cross-species transferability in aedes fluviatilis (Diptera:culicidae): a cost-effective approach for population genetics studies. Parasites & Vectors. 2015;8:635. doi: 10.1186/s13071-015-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Olival KJ, Perkins SL. Molecular identification of host feeding patterns of snow-melt mosquitoes (Diptera: culicidae): potential implications for the transmission ecology of Jamestown canyon virus. Journal of Medical Entomology. 2010;47:226–229. doi: 10.1093/jmedent/47.2.226. [DOI] [PubMed] [Google Scholar]
- Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI, Novak RJ. Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in mwea rice scheme, Kenya. Malaria Journal. 2008;7:43. doi: 10.1186/1475-2875-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ, Alto BW. Larval environmental temperature and insecticide exposure alter aedes aegypti competence for arboviruses. Vector-Borne and Zoonotic Diseases. 2011;11:1157–1163. doi: 10.1089/vbz.2010.0209. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Muriu S, Shililu J, Mwangangi JM, Jacob BG, Mbogo C, Githure J, Novak RJ. Blood-feeding patterns of culex quinquefasciatus and other culicines and implications for disease transmission in mwea rice scheme, Kenya. Parasitology Research. 2008;102:1329–1335. doi: 10.1007/s00436-008-0914-7. [DOI] [PubMed] [Google Scholar]
- Mwandawiro C, Tsuda Y, Tuno N, Higa Y, Urakawa E, Sugiyama A, Yanagi T, Takagi M. Host-feeding patterns of culex tritaeniorhynchus and anopheles sinensis (Diptera: culicidae) in a ricefield agroecosystem. Medical Entomology and Zoology. 1999;50:267–273. doi: 10.7601/mez.50.267. [DOI] [Google Scholar]
- Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Githure JI, Yan G, Minakawa N, Novak R, Beier JC. Spatial distribution and habitat characterisation of anopheles larvae along the kenyan coast. Journal of Vector Borne Diseases. 2007;44:44–51. [PMC free article] [PubMed] [Google Scholar]
- Mwangangi JM, Muturi EJ, Mbogo CM. Seasonal mosquito larval abundance and composition in Kibwezi, lower eastern Kenya. Journal of Vector Borne Diseases. 2009;46:65–71. [PubMed] [Google Scholar]
- Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of anopheles arabiensis and anopheles coustani in indoor and outdoor malaria transmission in Taveta district, Kenya. Parasites & Vectors. 2013;6:114. doi: 10.1186/1756-3305-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natal D, Barata EAMDF, Urbinatti PR, Barata JMS, Paula MBD. On the adult mosquito fauna (Diptera, Culicidae) in an hydroelectric project area in the Parana river basin, Brazil. Revista Brasileira De Entomologia. 1998;41:213–216. [Google Scholar]
- Navarro JC, Enriquez S, Duque P, Campana Y, Benitez-Ortiz W. New Sabethes (Diptera: culicidae) species records for Ecuador, from Colonso-Chalupas biological reserve, province of napo (Amazon) Journal of Entomology and Zoology Studies. 2015;3:169–172. [Google Scholar]
- Nicholson J, Ritchie SA, Russell RC, Webb CE, Cook A, Zalucki MP, Williams CR, Ward P, van den Hurk AF. Effects of cohabitation on the population performance and survivorship of the invasive mosquito aedes albopictus and the resident mosquito aedes notoscriptus (Diptera: culicidae) in Australia. Journal of Medical Entomology. 2015;52:375–385. doi: 10.1093/jme/tjv004. [DOI] [PubMed] [Google Scholar]
- Nikolay B, Diallo M, Faye O, Boye CS, Sall AA. Vector competence of culex neavei (Diptera: culicidae) for usutu virus. The American Journal of Tropical Medicine and Hygiene. 2012;86:993–996. doi: 10.4269/ajtmh.2012.11-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y. Some characteristics of Israel turkey virus. Archiv Fur Die Gesamte Virusforschung. 1972;36:105–114. doi: 10.1007/BF01250300. [DOI] [PubMed] [Google Scholar]
- Nisbet DJ, Lee KJ, van den Hurk AF, Johansen CA, Kuno G, Chang GJ, Mackenzie JS, Ritchie SA, Hall RA. Identification of new flaviviruses in the kokobera virus complex. The Journal of General Virology. 2005;86:121–124. doi: 10.1099/vir.0.80381-0. [DOI] [PubMed] [Google Scholar]
- Njabo KY, Cornel AJ, Sehgal RN, Loiseau C, Buermann W, Harrigan RJ, Pollinger J, Valkiūnas G, Smith TB. Coquillettidia (Culicidae, diptera) mosquitoes are natural vectors of avian malaria in africa. Malaria Journal. 2009;8:193. doi: 10.1186/1475-2875-8-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njogu A, Kinoti G. Observations on breeding sites of mosquitoes in lake Manyara, a saline lake in east african rift valley. Bulletin of Entomological Research. 1971;60:473. doi: 10.1017/S0007485300040426. [DOI] [Google Scholar]
- NSW Health NSW arbovirus surveillance and vector monitoring program. [29 Feb 2016];2016 http://medent.usyd.edu.au/arbovirus/mosquit/othermosq.htm
- Ohba SY, Van Soai N, Van Anh DT, Nguyen YT, Takagi M. Study of mosquito fauna in rice ecosystems around Hanoi, northern vietnam. Acta Tropica. 2015;142:89–95. doi: 10.1016/j.actatropica.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Okorie TG. The flight activity of mosquitoes in Ibadan Nigeria. Nigerian Journal of Entomology. 1978;3:81–92. [Google Scholar]
- Omondi D, Masiga DK, Ajamma YU, Fielding BC, Njoroge L, Villinger J. Unraveling Host-Vector-Arbovirus interactions by Two-Gene high resolution melting mosquito bloodmeal analysis in a kenyan Wildlife-Livestock interface. Plos One. 2015;10:e0134375. doi: 10.1371/journal.pone.0134375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramasivan R, Philip SP, Selvaraj PR. Biting rhythm of vector mosquitoes in a rural ecosystem of south India. International Journal of Mosquito Research. 2015;2:106–113. [Google Scholar]
- Parascandola M. Skepticism, statistical methods, and the cigarette: a historical analysis of a methodological debate. Perspectives in Biology and Medicine. 2004;47:244–261. doi: 10.1353/pbm.2004.0032. [DOI] [PubMed] [Google Scholar]
- Paterson HE, Bronsden P, Levitt J, Worth CB. Some culicine mosquitoes (Diptera, Culicidae) at Ndumu, republic of south Africa. Medical Proceedings. 1964;10:188–192. [Google Scholar]
- Pedro PM, Sallum MA, Butlin RK. Forest-obligate Sabethes mosquitoes suggest palaeoecological perturbations. Heredity. 2008;101:186–195. doi: 10.1038/hdy.2008.45. [DOI] [PubMed] [Google Scholar]
- Peiris JS, Amerasinghe FP, Amerasinghe PH, Ratnayake CB, Karunaratne SH, Tsai TF. Japanese encephalitis in Sri Lanka--the study of an epidemic: vector incrimination, porcine infection and human disease. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:307–313. doi: 10.1016/0035-9203(92)90325-7. [DOI] [PubMed] [Google Scholar]
- Penn GH. The larval development and ecology of aedes (Stegomyia) scutellaris (Walker, 1859) in New Guinea. The Journal of Parasitology. 1947;33:43–50. doi: 10.2307/3273619. [DOI] [PubMed] [Google Scholar]
- Petersen E, Wilson ME, Touch S, McCloskey B, Mwaba P, Bates M, Dar O, Mattes F, Kidd M, Ippolito G, Azhar EI, Zumla A. Rapid spread of zika virus in the americas--implications for public health preparedness for mass gatherings at the 2016 Brazil olympic games. International Journal of Infectious Diseases. 2016;44:11–15. doi: 10.1016/j.ijid.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Peyton EL, Reinert JF, Peterson NE. The occurrence of deinocerites pseudes dyar and knab in the united states, with additional notes on the biology of deinocerites species of Texas. Mosquito News. 1964;24:449–458. [Google Scholar]
- Pinto CS, Confalonieri UEC, Mascarenhas BM. Ecology of Haemagogus sp. and Sabethes sp. (Diptera: Culicidae) in relation to the microclimates of the Caxiuanã National Forest, Pará, Brazil. Memórias do Instituto Oswaldo Cruz. 2009;104:592–598. doi: 10.1590/S0074-02762009000400010. [DOI] [PubMed] [Google Scholar]
- Ponçon N, Balenghien T, Toty C, Baptiste Ferré J, Thomas C, Dervieux A, L'ambert G, Schaffner F, Bardin O, Fontenille D. Effects of local anthropogenic changes on potential malaria vector anopheles hyrcanus and west nile virus vector culex modestus, camargue, France. Emerging Infectious Diseases. 2007;13:1810–1815. doi: 10.3201/eid1312.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JR, Tabachnick WJ. History of domestication and spread of aedes aegypti--a review. Memórias Do Instituto Oswaldo Cruz. 2013;108 Suppl 1:11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prummongkol S, Panasoponkul C, Apiwathnasorn C, Lek-Uthai U. Biology of culex sitiens, a predominant mosquito in Phang Nga, Thailand after a tsunami. Journal of Insect Science. 2012;12:1–8. doi: 10.1673/031.012.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls WA, Smith ML, Muller GC, Zhao TY, Xue RD. Field evaluation of a large-scale barrier application of bifenthrin on a golf course to control floodwater mosquitoes. Journal of the American Mosquito Control Association. 2012;28:219–224. doi: 10.2987/12-6255R.1. [DOI] [PubMed] [Google Scholar]
- Radrova J, Seblova V, Votypka J. Feeding behavior and spatial distribution of culex mosquitoes (Diptera: culicidae) in wetland areas of the czech republic. Journal of Medical Entomology. 2013;50:1097–1104. doi: 10.1603/ME13029. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Surendran SN, Jude PJ, Dharshini S, Vinobaba M. Larval development of aedes aegypti and aedes albopictus in peri-urban brackish water and its implications for transmission of arboviral diseases. PLoS Neglected Tropical Diseases. 2011;5:e1369. doi: 10.1371/journal.pntd.0001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdale CD, Snow KR. Distribution of the genera Coquillettidia, orthopodomyia and uranotaenia in Europe. European Mosquito Bulletin. 2001;10:25–29. [Google Scholar]
- Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of ochlerotatus and allied taxa (Diptera: culicidae: aedini) based on morphological data from all life stages. Zoological Journal of the Linnean Society. 2008;153:29–114. doi: 10.1111/j.1096-3642.2008.00382.x. [DOI] [Google Scholar]
- Reinert JF. Contributions to the mosquito fauna of southeast asia. Contributions of the American Entomological Institute. 1970;5:1–44. [Google Scholar]
- Reinert JF. Albuginosus, a new subgenus of aedes Meigem (Diptera: Culicidae) described from the afrotropical region. Mosquito Systematics. 1986;3:307–326. [Google Scholar]
- Reisen W. The western encephalitis mosquito, culex tarsalis. Wing Beats. 1993;4:16. [Google Scholar]
- Reisen WK, Aslamkhan M, Suleman M, Naqvi ZH. Observations on the diel activity patterns of some punjab Pakistan mosquitoes diptera Culicidae. Biologia. 1976;22:67–78. [Google Scholar]
- Renshaw M, Service MW, Birley MH. Host finding, feeding patterns and evidence for a memorized Home-Range. Medical and Veterinary Entomology. 1994;8:187–193. doi: 10.1111/j.1365-2915.1994.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Silver JB, Service MW, Birley MH. Spatial-Dispersion patterns of larval aedes cantans (diptera, Culicidae) Bulletin of Entomological Research. 1995;85:125–133. doi: 10.1017/s0007485300052081. [DOI] [Google Scholar]
- Reuben R, Thenmozhi V, Samuel PP, Gajanana A, Mani TR. Mosquito blood feeding patterns as a factor in the epidemiology of japanese encephalitis in southern India. The American Journal of Tropical Medicine and Hygiene. 1992;46:654–663. doi: 10.4269/ajtmh.1992.46.654. [DOI] [PubMed] [Google Scholar]
- Reuben R. Studies on the mosquitoes of north arcot district, madras state, India. 5. breeding places of the culex vishnui group of species. Journal of Medical Entomology. 1971;8:363–366. doi: 10.1093/jmedent/8.4.363. [DOI] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MA, O'Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. Journal of Medical Entomology. 2006;43:1134–1141. doi: 10.1093/jmedent/43.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway G. Gbm: generalized boosted regression models. R package version 2.1.12015
- Robert V, Awono-Ambene HP, Thioulouse J. Ecology of larval mosquitoes, with special reference to anopheles arabiensis (Diptera: culcidae) in market-garden wells in urban Dakar, Senegal. Journal of Medical Entomology. 1998;35:948–955. doi: 10.1093/jmedent/35.6.948. [DOI] [PubMed] [Google Scholar]
- Robinson WH. Urban insects and arachnids: a handbook of urban entomology. Cambridge University Press. 2005 doi: 10.1017/CBO9780511542718. [DOI] [Google Scholar]
- Rochlin I, Dempsey ME, Campbell SR, Ninivaggi DV. Salt marsh as culex salinarius larval habitat in coastal New York. Journal of the American Mosquito Control Association. 2008;24:359–367. doi: 10.2987/5748.1. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Iwakami M, O'Guinn M, Mogi M, Prendergast BE, Miyagi I, Toma T, Pecor JE, Wilkerson RC. Habitats and distribution of anopheles sinensis and associated Anopheles hyrcanus group in Japan. Journal of the American Mosquito Control Association. 2005;21:458–463. doi: 10.2987/8756-971X(2006)21[458:HADOAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rueda LM, Kim HC, Klein TA, Pecor JE, Li C, Sithiprasasna R, Debboun M, Wilkerson RC. Distribution and larval habitat characteristics of anopheles hyrcanus group and related mosquito species (Diptera: culicidae) in South Korea. Journal of Vector Ecology. 2006;31:198–205. doi: 10.3376/1081-1710(2006)31[198:DALHCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rueger ME, Price RD, Olson TA. Larval habitats of culex tarsalis (Diptera: culicidae) in Minnesota. Mosquito News. 1964;24:39–42. [Google Scholar]
- Russell RC, Otranto D, Wall RL. The Encyclopedia of Medical and Veterinary Entomology. CABI; 2013. [Google Scholar]
- Russell RC. Seasonal abundance of mosquitoes in a native forest of the murray valley of Victoria, 1979?1985. Australian Journal of Entomology. 1986;25:235–240. doi: 10.1111/j.1440-6055.1986.tb01109.x. [DOI] [Google Scholar]
- Russell RC. A review of the status and significance of the species within the culex pipiens group in Australia. Journal of the American Mosquito Control Association. 2012;28:24–27. doi: 10.2987/8756-971X-28.4s.24. [DOI] [PubMed] [Google Scholar]
- Ryan PA, Kay BH. Emergence trapping of mosquitoes (Diptera: culicidae) in brackish forest habitats in Maroochy Shire, south-east Queensland, Australia, and a management option for verrallina funerea (Theobald) and aedes procax (Skuse) Australian Journal of Entomology. 2000;39:212–218. doi: 10.1046/j.1440-6055.2000.00169.x. [DOI] [Google Scholar]
- Sabesan S, Kumar NP, Krishnamoorthy K, Panicker KN. Seasonal abundance & biting behaviour of mansonia annulifera, M. uniformis & M. indiana & their relative role in the transmission of malayan filariasis in shertallai (Kerala state) The Indian Journal of Medical Research. 1991;93:253–258. [PubMed] [Google Scholar]
- Sallum MA, Forattini OP. Revision of the spissipes section of culex (Melanoconion) (Diptera:culicidae) Journal of the American Mosquito Control Association. 1996;12:517–600. [PubMed] [Google Scholar]
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, Brazilian Medical Genetics Society–Zika Embryopathy Task Force Possible association between zika virus infection and microcephaly - Brazil, 2015. MMWR. Morbidity and Mortality Weekly Report. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- Schwetz J. Contributions a l’etude de la Biologie de Taeniorhynchus (Mansonoides) africanus et de Taeniorhynchus (Coquillettidia) aurites au Congo Beige. Revue De Zoologie Et De Botanique Africaines. 1930;4:311–330. [Google Scholar]
- Sebesta O, Halouzka J, Hubálek Z, Juricová Z, Rudolf I, Sikutová S, Svobodová P, Reiter P. Mosquito (Diptera: culicidae) fauna in an area endemic for west nile virus. Journal of Vector Ecology. 2010;35:156–162. doi: 10.1111/j.1948-7134.2010.00072.x. [DOI] [PubMed] [Google Scholar]
- Selvaraj PR, Dwarakanath SK. The biting activity rhythm in aedini mosquitoes of Madurai. Comparative Physiological Ecology. 1992;17:66–70. [Google Scholar]
- Serandour J, Rey D, Raveton M. Behavioural adaptation of coquillettidia (Coquillettidia) richiardii larvae to underwater life: environmental cues governing plant–insect interaction. Entomologia Experimentalis Et Applicata. 2006;120:195–200. doi: 10.1111/j.1570-7458.2006.00444.x. [DOI] [Google Scholar]
- Service MW. The ecology of the Tree-Hole breeding mosquitoes in the northern guinea savanna of Nigeria. The Journal of Applied Ecology. 1965a;2:1–16. doi: 10.2307/2401689. [DOI] [Google Scholar]
- Service MW. The identification of blood-meals from culicine mosquitos from northern Nigeria. Bulletin of Entomological Research. 1965b;55:637–643. doi: 10.1017/S0007485300049749. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. Springer Science & Business Media. 1993 doi: 10.1007/978-94-015-8113-4. [DOI] [Google Scholar]
- Shililu J, Ghebremeskel T, Seulu F, Mengistu S, Fekadu H, Zerom M, Ghebregziabiher A, Sintasath D, Bretas G, Mbogo C, Githure J, Brantly E, Novak R, Beier JC. Larval habitat diversity and ecology of anopheline larvae in Eritrea. Journal of Medical Entomology. 2003;40:921–929. doi: 10.1603/0022-2585-40.6.921. [DOI] [PubMed] [Google Scholar]
- Silver JB. Mosquito Ecology: Field Sampling Methods. Springer Science & Business Media; 2007. [Google Scholar]
- Simsek FM. Seasonal larval and adult population dynamics and breeding habitat diversity of culex theileri theobald, 1903 (Diptera: culicidae) in the golbasi district, Ankara, turkey. Turkish Journal of Zoology. 2004;28:337–344. [Google Scholar]
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR, Kabaria CW, Harbach RE, Hay SI. The dominant anopheles vectors of human malaria in the Asia-Pacific Region: occurrence data, distribution maps and bionomic précis. Parasites & Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirivanakarn S, Galindo P. Culex-adamesi new-species from panama diptera culicidae. Mosquito Systematics. 1980;12:25–34. [Google Scholar]
- Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. American Journal of Epidemiology. 2014;180:318–324. doi: 10.1093/aje/kwu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Mountain mosquitoes of the gothic, Colorado, area. American Midland Naturalist. 1966;76:125–150. doi: 10.2307/2423238. [DOI] [Google Scholar]
- Snow WF, Boreham PFL. The feeding habits of some west african culex (Dipt., Culicidae) mosquitoes. Bulletin of Entomological Research. 1973;62:517–526. doi: 10.1017/S0007485300004041. [DOI] [Google Scholar]
- Snow WF, Boreham PFL. The host-feeding patterns of some culicine mosquitoes (Diptera: culicidae) in the Gambia. Bulletin of Entomological Research. 1978;68:695–706. doi: 10.1017/S0007485300009652. [DOI] [Google Scholar]
- Someren ECC. The female and early stages of culex (Culex) nakuruensis mattingly, with a description of a new subspecies of culex (Culex) shoae hamon & ovazza; Proceedings of the Royal Entomological Society of London. Series B, Taxonomy; Taxonomy. 1967. pp. 11–16. [DOI] [Google Scholar]
- Sommerman KM. Notes on activities of Alaskan Culiseta adults (Diptera: culicidae) Mosquito News. 1964;24:60–64. [Google Scholar]
- Sriwichai P, Samung Y, Sumruayphol S, Kiattibutr K, Kumpitak C, Payakkapol A, Kaewkungwal J, Yan G, Cui L, Sattabongkot J. Natural human plasmodium infections in major anopheles mosquitoes in western Thailand. Parasites & Vectors. 2016;9:17. doi: 10.1186/s13071-016-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Zalazar L, Willener JA, Almeida FL, Almirón WR. Culicidae (Diptera) selection of humans, chickens and rabbits in three different environments in the province of chaco, Argentina. Memórias Do Instituto Oswaldo Cruz. 2013;108:563–571. doi: 10.1590/S0074-02762013000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn JJ, Schulz KH. Aëdes (Ochlerotatus) caballus theobald, the south african vector of rift valley fever. South African Medical Journal = Suid-Afrikaanse Tydskrif Vir Geneeskunde. 1955;29:1114–1120. [PubMed] [Google Scholar]
- Suárez-Mutis MC, Fe NF, Alecrim W, Coura JR. Night and crepuscular mosquitoes and risk of vector-borne diseases in areas of piassaba extraction in the middle negro river basin, state of amazonas, brazil. Memórias Do Instituto Oswaldo Cruz. 2009;104:11–17. doi: 10.1590/S0074-02762009000100002. [DOI] [PubMed] [Google Scholar]
- Sudeep AB. Culex gelidus: an emerging mosquito vector with potential to transmit multiple virus infections. Journal of Vector Borne Diseases. 2014;51:251–258. [PubMed] [Google Scholar]
- Sylla M, Ndiaye M, Black WC. Aedes species in treeholes and fruit husks between dry and wet seasons in southeastern senegal. Journal of Vector Ecology. 2013;38:237–244. doi: 10.1111/j.1948-7134.2013.12036.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Taxonomic and ecological notes on culex (Melanoconion) Spissipes (Theobald) Journal of Medical Entomology. 1968;5:329–331. doi: 10.1093/jmedent/5.3.329. [DOI] [PubMed] [Google Scholar]
- Tang Y, Diao Y, Chen H, Ou Q, Liu X, Gao X, Yu C, Wang L. Isolation and genetic characterization of a tembusu virus strain isolated from mosquitoes in Shandong, China. Transboundary and Emerging Diseases. 2015;62:209–216. doi: 10.1111/tbed.12111. [DOI] [PubMed] [Google Scholar]
- Taye A, Hadis M, Adugna N, Tilahun D, Wirtz RA. Biting behavior and plasmodium infection rates of anopheles arabiensis from Sille, Ethiopia. Acta Tropica. 2006;97:50–54. doi: 10.1016/j.actatropica.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Thomson MC, Doblas-Reyes FJ, Mason SJ, Hagedorn R, Connor SJ, Phindela T, Morse AP, Palmer TN. Malaria early warnings based on seasonal climate forecasts from multi-model ensembles. Nature. 2006;439:576–579. doi: 10.1038/nature04503. [DOI] [PubMed] [Google Scholar]
- Toma T, Miyagi I, Okazawa T, Kobayashi J, Saita S, Tuzuki A, Keomanila H, Nambanya S, Phompida S, Uza M, Takakura M. Entomological surveys of malaria in Khammouane Province, lao PDR, in 1999 and 2000. The Southeast Asian Journal of Tropical Medicine and Public Health. 2002;33:532–546. [PubMed] [Google Scholar]
- Traoré-Lamizana M, Fontenille D, Diallo M, Ba Y, Zeller HG, Mondo M, Adam F, Thonon J, Maïga A. Arbovirus surveillance from 1990 to 1995 in the barkedji area (Ferlo) of Senegal, a possible natural focus of rift valley fever virus. Journal of Medical Entomology. 2001;38:480–492. doi: 10.1603/0022-2585-38.4.480. [DOI] [PubMed] [Google Scholar]
- Tsunoda T, Fukuchi A, Nanbara S, Higa Y, Takagi M. Aedes mosquito larvae collected from Ishigaki-jima and Taketomi-jima islands in southern Japan. The Southeast Asian Journal of Tropical Medicine and Public Health. 2012;43:1375–1379. [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of north american mosquitoes (Diptera: culicidae) to transmit west nile virus. Journal of Medical Entomology. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O'Guinn ML, Dohm DJ, Jones JW. Vector competence of north american mosquitoes (Diptera: culicidae) for west nile virus. Journal of Medical Entomology. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Van der Kuyp E. Notes on haemagogus anastasionis dyar of curaçao. Documenta Neerlandica Et Indonesica De Morbis Tropicis; Quarterly Journal of Tropical Medicine and Hygiene. 1949;1:142–144. [PubMed] [Google Scholar]
- Van Regenmortel MHV, Fauquet CM, Bishop DHL. Virus Taxonomy : Classification and Nomenclature of Viruses : Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press; 2000. [Google Scholar]
- Ventim R, Ramos JA, Osório H, Lopes RJ, Pérez-Tris J, Mendes L. Avian malaria infections in western european mosquitoes. Parasitology Research. 2012;111:637–645. doi: 10.1007/s00436-012-2880-3. [DOI] [PubMed] [Google Scholar]
- Veronesi R, Gentile G, Carrieri M, Maccagnani B, Stermieri L, Bellini R. Seasonal pattern of daily activity of aedes caspius, aedes detritus, culex modestus, and culex pipiens in the po Delta of northern Italy and significance for vector-borne disease risk assessment. Journal of Vector Ecology. 2012;37:49–61. doi: 10.1111/j.1948-7134.2012.00199.x. [DOI] [PubMed] [Google Scholar]
- Versteirt V, Boyer S, Damiens D, De Clercq EM, Dekoninck W, Ducheyne E, Grootaert P, Garros C, Hance T, Hendrickx G, Coosemans M, Van Bortel W. Nationwide inventory of mosquito biodiversity (Diptera: culicidae) in Belgium, Europe. Bulletin of Entomological Research. 2013;103:193–203. doi: 10.1017/S0007485312000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LA, Soares P, Paiva F, Lourenço-De-Oliveira R. Caiman-biting mosquitoes and the natural vectors of Hepatozoon caimani in brazil. Journal of Medical Entomology. 2010;47:670–676. doi: 10.1093/jmedent/47.4.670. [DOI] [PubMed] [Google Scholar]
- Waddell MB, Taylor RM. Studies on cyclic passage of yellow fever virus in south american mammals and mosquitoes - Marmosets (callithrix-Aurita) and Cebus monkeys (cebus-Versutus) in combination with Aedes-Aegypti and Haemagogus-Equinus. American Journal of Tropical Medicine. 1945;25:225–230. doi: 10.4269/ajtmh.1946.s1-26.455. [DOI] [PubMed] [Google Scholar]
- Walter Reed Biosystematics Unit, W.D Walter Reed biosystematics unit systematic catalog of Culicidae. [02, May 2016];2016 http://www.mosquitocatalog.org/
- Wang LY. Host preference of mosquito vectors of japanese encephalitis. Zhonghua Minguo Wei Sheng Wu Xue Za Zhi = Chinese Journal of Microbiology. 1975;8:274–279. [PubMed] [Google Scholar]
- Wang ZM, Xing D, Wu ZM, Yao WJ, Gang W, Xin DS, Jiang YF, Xue RD, Dong YD, Li CX, Guo XX, Zhang YM, Zhao TY. Biting activity and host attractancy of mosquitoes (Diptera: culicidae) in Manzhouli, China. Journal of Medical Entomology. 2012;49:1283–1288. doi: 10.1603/ME11131. [DOI] [PubMed] [Google Scholar]
- Wanson M, Lebred eB. L’habitat des Phlebotomes cavernicoles de Thys-ville (Congo Beige) Archives De l’Institute Pasteur d’Algerie. 1946;24:153–156. [PubMed] [Google Scholar]
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika Virus: history, emergence, biology, and prospects for control. Antiviral Research. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Research. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Weaver SC. Host range, amplification and arboviral disease emergence. In: Peters CJ, Calisher CH, editors. Infectious Diseases From Nature: Mechanisms of Viral Emergence and Persistence. Springer-Verlag/Wien: Springer Vienna; 2005. pp. 33–44. [DOI] [Google Scholar]
- Webb C, Russell R, Doggett S. A Guide to Mosquitoes of Australia. Csiro Publishing; 2016. [Google Scholar]
- Wharton RH. The biology of mansonia mosquitoes in relation to the transmission of filariasis in Malaya. Bulletin - Institute for Medical Research, Kuala Lumpur. 1962;11:1–114. [PubMed] [Google Scholar]
- Williams CR, Kokkinn MJ. Daily patterns of locomotor and sugar-feeding activity of the mosquito culex annulirostris from geographically isolated populations. Physiological Entomology. 2005;30:309–316. doi: 10.1111/j.1365-3032.2005.00462.x. [DOI] [Google Scholar]
- Williams CR. Timing of host-seeking behaviour of the mosquitoes anopheles annulipes sensu lato walker and Coquillettidia linealis (Skuse) (Diptera: culicidae) in the Murray River Valley, South Australia. Australian Journal of Entomology. 2005;44:110–112. doi: 10.1111/j.1440-6055.2005.00463.x. [DOI] [Google Scholar]
- Wright AE, Anderson S, Stanley NF, Liehne PF, Britten DK. A preliminary investigation of the ecology of arboviruses in the Derby area of the Kimberley region, Western Australia. Australian Journal of Experimental Biology and Medical Science. 1981;59:357–367. doi: 10.1038/icb.1981.30. [DOI] [PubMed] [Google Scholar]
- Yamar BA, Diallo D, Kebe CM, Dia I, Diallo M. Aspects of bioecology of two rift valley fever virus vectors in Senegal (West africa): Aedes vexans and culex poicilipes (Diptera: culicidae) Journal of Medical Entomology. 2005;42:739–750. doi: 10.1093/jmedent/42.5.739. [DOI] [PubMed] [Google Scholar]
- Yee DA, Skiff JF. Interspecific competition of a new invasive mosquito, culex coronator, and two container mosquitoes, aedes albopictus and cx. quinquefasciatus (Diptera: culicidae), across different detritus environments. Journal of Medical Entomology. 2014;51:89–96. doi: 10.1603/ME13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EC. Mosquitoes of rarotonga, cook islands: a survey of breeding sites. New Zealand Journal of Zoology. 2007;34:57–61. doi: 10.1080/03014220709510064. [DOI] [Google Scholar]