Abstract
Recently, we demonstrated that superoxide dismutase 3 (SOD3) is a strong candidate for biomedicine. Anti-oxidant function of SOD3 was accomplished without cell penetration, and it inhibited the inflammatory responses via non-enzymatic functions. SOD3 has the heparin binding domain associating cell surface. Interestingly, we found that Zn2+ promotes transduction effects of recombinant human SOD3 (rhSOD3) by increasing uptake via the heparin binding domain (HBD). We demonstrated an uptake of rhSOD3 from media to cell lysate via HBD, resulting in an accumulation of rhSOD3 in the nucleus, which was promoted by the presence of Zn2+. This resulted in increased inhibitory effects of rhSOD3 on NF-kB and STAT3 signals in the presence of Zn2+, which shows elevated association of rhSOD3 into the cells. These results suggest that an optimized procedure can help to enhance the inflammatory efficacy of rhSOD3, as a novel biomedicine.
Keywords: Anti-inflammatory, Cytopermeability, Heparin Binding Domain, Superoxide dismutase 3, Zinc (II) ion
INTRODUCTION
Superoxide dismutases (SODs) are a group of anti-oxidant enzymes of which functions are removing reactive oxygen species (ROSs) from the cellular context and preserving cells from environmental stresses. SODs function to dismutate two superoxide radicals into a hydrogen peroxide and an oxygen molecule. Recently, Serra et al. reported that SOD3 inhibits telomere shortening, a cellular aging process, by extending the telomeric clock of human fibroblast (1). In the other hand, over-expression of SOD3 suppresses the growth of breast carcinoma and melanoma cells efficiently by the adenoviral transduction (2, 3). It has been also shown that gene therapy using SOD3 gene alleviates aorta restenosis, and mitigates collagen-induced arthritis in rodents (4). The presence of SOD3 in serum and even in extracellular matrices (ECM) plays an important role for the systemic defense mechanisms (5). Inflammatory cytokines, like IL-4, IL-1α and IFN-γ up-regulate the level of expression of SOD3, but, TNF-α and TGF-β down-regulates SOD3 in vascular smooth muscle cells and human skin fibroblasts (6, 7). However, the underlying specific mechanisms remain to be exploited, particularly in regards with IFN-γ-induced SOD3 expression.
SODs can be further classified as: SOD1, containing Cu and Zn atoms; SOD2, containing Mn atom; and SOD3, located in the extracellular fluid or on the cell surface. It is important to note that, as like SOD1, SOD3 contains Cu and Zn atoms, however, a heparin-binding domain (HBD) has been characterized to exist in the C-terminal end, unlike SOD1. It has been reported that the HBD of SOD3 acts as a nuclear localization signal, and which protects genomic DNA from oxidative stress and regulates the DNA transcription which is sensitive to the detox reaction within the nuclei of testis and thymus cells (8).
Certain proteins enter cells through the plasma membranes, effectively. Several lines of studies are now actively performed to utilize such proteins to transduce with beneficial substances into cytoplasm. Typical examples include VP22 protein, HIV Tat protein, PEP-1 peptide, and ANTP (9). It has been shown that the cell-transduction property of proteins is the function of protein transduction domain (PTD), capable of crossing phospholipid bilayers of plasma membrane (10). The HBD of SOD3 has been shown to have similar properties as the other cell-penetrating peptides having highly positive charged residues.
Here, we compared rhSOD3 and 209E; a proteolytic variant of SOD3 with a missing HBD at the C-terminal end, to investigate the role of HBD in the cellular uptake. We found that Zn2+, which is one of the major cofactors of SOD3, promoted the cellular uptake and anti-inflammatory effects of rhSOD3. Therefore, we expect further optimization processes could help developing rhSOD3 and its variants as novel biomedicines.
RESULTS
Effect of cellular uptake efficiency by metal 2+ ions in HaCaT and 293T cells
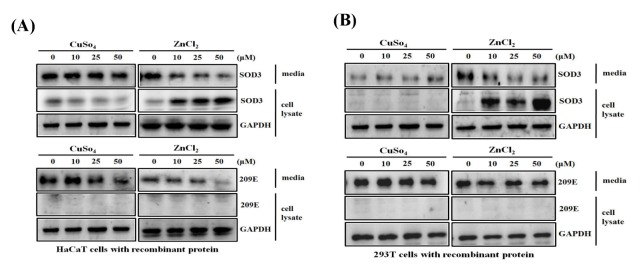
Since SOD3 shows its anti-inflammatory effects by scavenging extra cellular superoxide molecules, we elevated the enzymatic activity of SOD by adding its catalytic cofactors, zinc and copper ions. To examine the potential effect of metal 2+ ions, HaCaT or 293T cells were exposed to recombinant human SOD3 (rhSOD3), and incubated for 1 h in the presence of CuSO4 or ZnCl2. Interestingly, when ZnCl2 was added to the HaCaT or 293T cells, the residual rhSOD3 in the media was decreased, while rhSOD3 associated to the cell increased in a dose-dependent manner (Fig. 1A, left), indicating that zinc ions increase the cellular association or uptake of rhSOD3. However, rhSOD3 levels in media and cells remained unchanged on addition of CuSO4. Under similar conditions, rh209E, which is missing the HBD, remained unchanged in the cell lysates of both HaCaT and 293T cells (Fig. 1B, lower, right), indicating that increase of cellular association or uptake of rhSOD3 by zinc ions is mediated by HBD.
Fig. 1.
Zn2+ induces uptake of rhSOD3 via heparin binding domain. HaCaT (A) and 293T cells (B) were cultured with purified rhSOD3 or rh209E at various concentrations (0, 10, 25, 50 μM) of CuSO4/ZnCl2. Amounts of rhSOD3 or rh209E in HaCaT and 293T cells were compared between media and cell lysates by western blot analysis.
Localization of exogenous rhSOD3 in the HaCaT
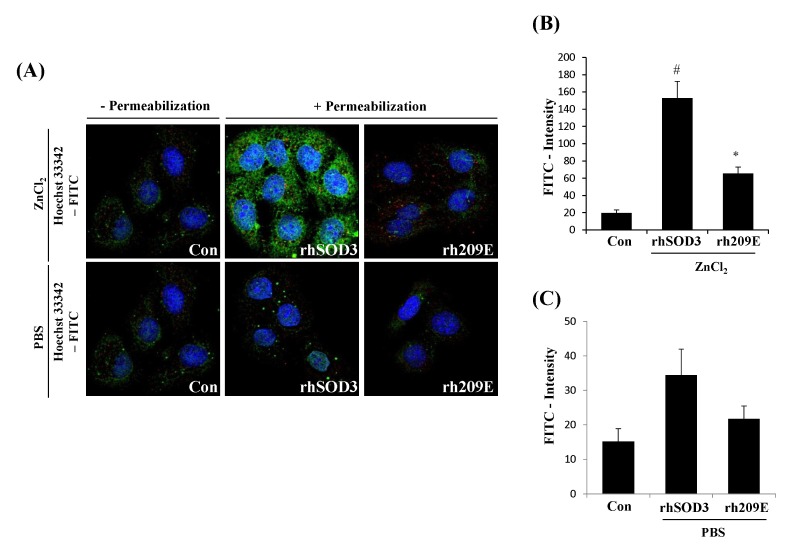
In order to investigate the transduction effect of zinc ions between HBD and cell line, we studied the internalization of exogenously added fusion proteins using HaCaT, which expresses cell surface GAGs such as heparin sulfates. Using fluorescence microscopy, we identified surface-bound and internalized rhSOD3 in HaCaT. The punctate was a typical membrane signal by fluorescence microscopy. rhSOD3 were found to be internalized, and strikingly increased the internalization of SOD3 in the presence of Zn2+ (Fig. 2A, middle lane). These results suggest that Zn2+ enhance the internalization of rhSOD3. However, rh209E, which is deficient of HBD, failed to internalize in the presence or absence of Zn2+ (Fig. 2A, right lane), supporting that HBD is critical for cellular internalization. Also, rh209E could be not internalized in the cell line lacking the ability to interact with cell surface heparin sulfate proteoglycans (HSPGs) (11), suggesting that Zn2+ plays a crucial and essential role in the uptake of rhSOD3 into the HaCaT, which is expressed on the cell surface such as heparin sulfates.
Fig. 2.
Zn2+ promotes nuclear accumulation by rhSOD3, but not rh209E, in HaCaT. After incubation with/without ZnCl2, HaCaT were washed and stained with secondary antibodies labeled with FITC, prior to and after cell permeabilization, respectively. Green labeled surface-bound fraction of rhSOD3 was evident in intercellular contacts, whereas intracellular fraction of rh209E manifests that did not locate in cytosol and nuclear region. Counterstaining was labeled with nucleic acid stain, Hoechst33342 (A). The data are represented as mean ± SEM of fluorescent intensity (B, C). Statistical comparisons between groups are made with the unpaired two-sided t-test. All experiments were performed at least three times. Differences with #P < 0.005 versus Control group, and *P < 0.05 versus rhSOD3 treatment group were considered statistically significant.
Zn2+ promotes inflammatory responses of SOD3 by inhibiting the phosphorylation of NF-kB and STAT3 in HaCaT
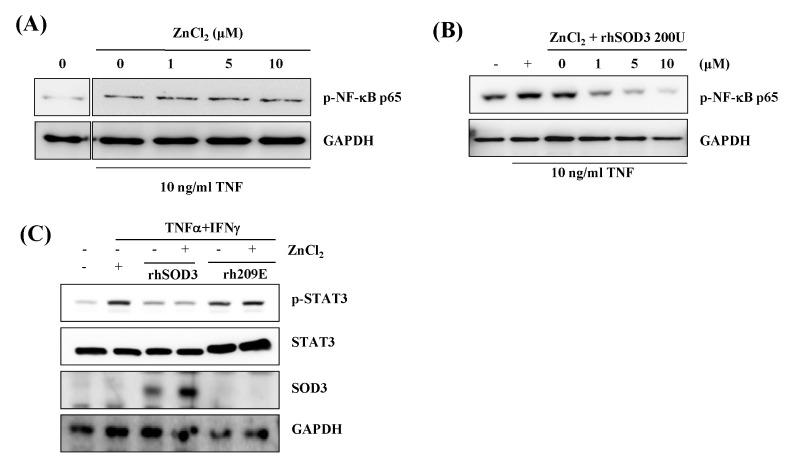
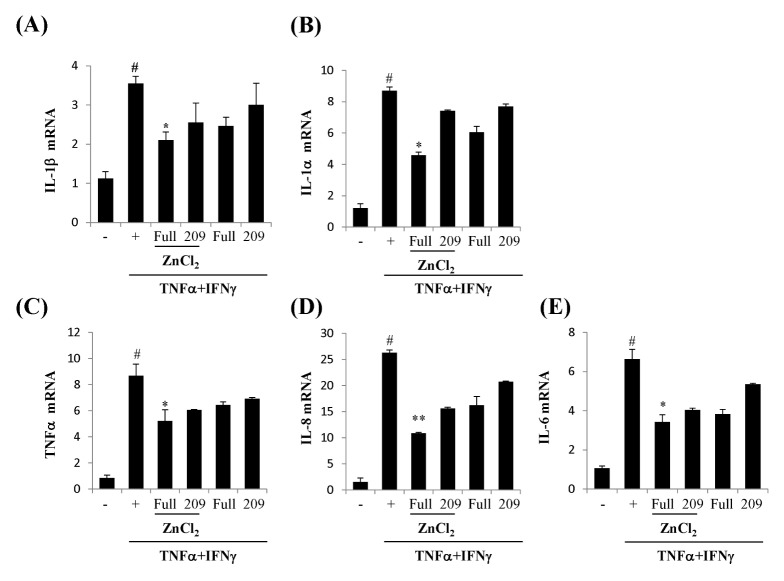
We first examined whether Zn2+ can promote cellular efficacy of rhSOD3 to regulate NF-kB signal in HaCaT. NF-kB is one of the important signals in the inflammatory responses. The phosphorylation of NF-kB p65 was strongly inhibited by incubation with rhSOD3 and ZnCl2, whereas ZnCl2 itself exhibited no inhibitory effect (Fig. 3A, 3B). When the cells were treated with ZnCl2 and rhSOD3, p-NF-kBp65 was inhibited in a dose-dependent manner (Fig. 3B). These results suggested that Zn2+ promotes the anti-inflammatory activity of SOD3 through the inhibition of the p-NF-kB65. After HaCaT were stimulated by TNFα and IFNγ, we investigated whether STAT3 is induced by tyrosine phosphorylation by cytokines. The pSTAT3 was strongly inhibited by treatment with rhSOD3, whereas the pSTAT3 was unaffected by rh209E (Fig. 3C- lane 1). Compared to the levels of tyrosine phosphorylation, the total levels of these proteins were not altered (Fig. 3C- lane 2). To determine the effect of ZnCl2, we analyzed mRNA expression level of IL-1β, IL-1α, TNFα, IL-8 and IL-6 in HaCaT. After stimulation by TNFα and IFNγ, the HaCaT cells were treated with rhSOD3 or rh209E, with or without ZnCl2. As shown in Fig. 4, in combination with rhSOD3 and ZnCl2, the levels of inflammatory cytokines are effectively reduced by ZnCl2. However, the combination of rh209E and ZnCl2 was ineffective and did not reduce the levels of inflammatory cytokines. Quantitative analysis showed that Zn2+ inhibited inflammatory responses of SOD3 caused in response to IL-1β, IL-1α, TNFα, IL-8 and IL-6 altered (Fig. 4).
Fig. 3.
Inhibition of NF-kB p65 and STAT3 signal through transduction effect rhSOD3, but not rh209E, by Zn2+ in HaCaT. The cells were treated both TNF-α (10 ng/ml) and IFNγ (100 U/ml) and various concentration of ZnCl2 (0–10 μM) (A). Purified rhSOD3 was added to cells at 200 U/ml (B). Amounts of p-NF-kB P65 in the cytosol was analyzed by western blot analysis. After purified rhSOD3 and rh209E were added to cells (with/without 200 U/mL), and added with/without ZnCl2 (10 μM) (C). All proteins were analyzed by western blot analysis.
Fig. 4.
Zn2+ reduces inflammatory cytokine by treating with rhSOD3 or rh209E in HaCaT. HaCaT were treated with 10 ng/ml TNF-α and 100 U/ml IFN-γ. Purified rhSOD3 or rh209E were added to cells at 200 U/ml, and various concentration of ZnCl2 (0–10 μM). Quantitative real-time PCR was performed using the KAPA SYBR fast qPCR Kit (KAPA biosystems, Woburn, MA, USA) as previously described (24). The data are represented as mean ± SEM. Statistical comparisons between groups were made with the unpaired two-sided t-test. All experiments were performed at least three times. Differences with #P < 0.005 versus control group (−), and *P < 0.05 and **P < 0.001 versus TNFα + IFNγ treatment group (+) were considered statistically significant.
Zn2+ enables penetration of rhSOD3 into epidermis of mouse skin
To evaluate effects of Zn2+, rhSOD3 is translocated to dorsal skin, sections of 30 μm thickness were made with cryotome, and were then observed under confocal microscope (Supplementary 1). When applied to the skin with FITC-conjugated rhSOD3 and ZnCl2, the signals of rhSOD3 were found in the epidermis. However, application of FITC-conjugated rhSOD3 alone did not reveal any signals of rhSOD3 (Supplementary 1A). The same dose of FITC-conjugated rhSOD3 was further analyzed and confirmed by Western blot (Supplementary 1B). We found that the presence of Zn2+ enhanced the skin permeation. This property of Zn2+ might therefore increase the penetration of rhSOD3 into viable layers of the skin, indicating that Zn2+ elevates the delivery of HBD proteins into the skin.
DISCUSSION
We have evaluated the potentiality of SOD3 as an important therapeutic agent for curing diverse diseases. However, diverse restraints prevented the product of homogenous, an active form of hSOD3 (12–14). The anti-oxidative effect of SOD3 has been well founded (15–18). Even though the role of SOD3 in immune responses remains undeveloped, we recently have reported a specific role of SOD3 in leading immune response (19).
SOD3 is a secretory protein and is composed of two subunits. One is a full length SOD3, and the other is 209E which lacks HBD. HBD of SOD3 could be eliminated by intracellular proteolytic cleavage before secretion (20). When we treated rh209E to the cells, no signals of 209E signal were found in the cell lysate. However, full length rhSOD3 was found in the cell lysate in the presence of Zn2+, in a dose dependent manner. These results suggest that HBD of SOD3 played a role in the internalization of SOD3 under the presence of Zn2+; in particular, Zn2+ enhanced the internalization of SOD3 from the media to cell lysate. HBD of SOD3 is likely to chelate Zn2+ to permit interaction with the negatively charged oligosaccharides.
Interestingly, the expression of cytokines such as IL-1β, IL-1α, TNF-α, IL-8, and IL-6 decreased with rhSOD3 and rh209E. These recombinant proteins are effective enzymes which reduce the mRNA level of cytokines after treatment with TNFα and IFNγ to HaCaT. However, rhSOD3 significantly reduces the inflammatory cytokines in the presence of Zn2+. The inhibitory effect of rhSOD3 on inflammatory cytokines is dependent on the presence of Zn2+ which elevates the internalization of rhSOD into the cell and translocation into the nuclear region. These results suggest that Zn2+ promotes anti-inflammatory effects of rhSOD3 by increasing cellular association via HBD of rhSOD3. In addition, Zn2+ elevates the delivery of HBD proteins into the skin, similar to SOD3. This property would be a useful enhancer in the delivery of small and large therapeutics having poor skin penetration, especially proteins possessing HBD. Optimization of the procedure will help in overcoming fundamental barriers in the development of HBD proteins, including rhSOD3, as a novel biomedicine.
MATERIALS AND METHODS
Preparation of recombinant SOD3
The recombinant SOD3 was prepared as described previously (21). 293T cells were transiently transfected with SOD3 construct for 48 h. The supernatant was collected, and subjected to purification using a column containing Ni-NTA agarose (Qiagen), and dialysis. The purified SOD3 activity was measured with a SOD assay kit (Dojindo) as described previously (22). For injection in mice or treatment in vitro, SOD3 was filtered to eliminate endotoxins. SOD3 has two isoforms, the full length and the proteolytic variant 209E (which is devoid of HBD at the C-terminal end). The full length human SOD3 and proteolytic cleaved form 209E variant (from Met1 to Glu227) containing a C-terminal His6 tag, were inserted into pcDNA3.1 (Invitrogen) using HindIII and EcoRI or HindIII and XbaI, respectively. Plasmids encoding hSOD3 and 209E variants were transfected into 293T-EBNA cells with Attractene (Qiagen), based on the manufacturer’s instructions. One day after transfection, the media was replaced with serum-free Dulbecco’s Modified Eagle Medium (DMEM).
Protein expression and purification
Five days after transfection, culture media containing rhSOD3 were collected, filtered, and loaded onto HiTrap Chelating HPcolumn (GE Healthcare). After loading, the column was washed with more than 50 column volumes of washing buffer, 50 mM NaPO4, 500 mM NaCl, and 30 mM imidazole. Next, the rhSOD3 and rh209E were eluted by increasing the elution buffer containing 500 mM imidazole, followed by dialysis in PBS. The concentration of purified rhSOD3 was determined based on a bovine serum albumin standard curve, with a protein assay dye (Bio-Rad).
Activity assay for SOD
To measure the enzymatic activity of rhSOD3, the rate of superoxide radical formation was quantified by spectrophotometer. A 20 μl sample was mixed with 200 μl of 200 μM xanthine (Sigma) and 50 μM WST-1 (Dojindo) in PBS. After adding 0.0005 unit XOD (Sigma), the increase in the formazan dye was immediately recorded using a colorimetric method, at A450. The generation of a formazan dye was determined kinetically, and absolute SOD activity was determined from the dilution factor exhibiting 50% inhibition (IC50) on the inhibition curve. The purified SOD3 activity was measured with a SOD assay kit (Dojindo), as described previously (22).
Western blot analysis
Western blot was performed as previously described (23). Briefly, the cells were lysed with radio-immuno-precipitation assay buffer (2 mM EDTA, 137 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM sodium vanadate, 10 mM NaF, 1 mM PMSF, 1% Triton X-100, 10% glycerol, and a protease inhibitor cocktail) and harvested immediately. The samples were loaded onto sodium dodecyl sulfate-polyacrylamide gels for electrophoresis and subsequently transferred onto polyvinylidene fluoride membranes obtained from Millipore (Bedford, MA, USA). After membranes were blocked, they were incubated with specific primary antibodies overnight at 4°C, with gentle agitation. The membranes were washed and incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Bands were detected using ECL Plus western blotting detection reagents from Amersham Biosciences Co (Piscataway, NJ, USA).
Measurements of nucleus accumulation for rhSOD3
In order to visualize the rhSOD3 and rh209E distribution within the cell, two kinds of the SOD3 proteins were labeled with FITC (Fluorescein isothiocyanate). Specifically, PBS phosphate buffered saline (PBS) was labeled by reaction for 2 h at room temperature with FITC-labeled rhSOD3 protein in 1 mg to 100 mg of the buffer solution. rhSOD3 or rh209E were prepared with FITC-labeled protein solution, in order to remove the unreacted FITC by high-speed liquid chromatography devices for protein purification (FPLC, Fast Protein Purification Liquid Chromatography), and were then purified by desalting columns. HaCaT was cultured on 8 mm cover slip to a 1 × 104 cells/well in dispensing a rear, in IMDM (Isocove’s modified Dulbecco’s medium, using the GIBCO) medium supplemented with 10% FBS, 100 units/ml penicillin, 100 g/ml streptomycin and incubated under conditions of 5% CO2, 37°C. Once the cells attached to the coverslip and attained 30 to 40% confluency, the cells were starved in serum free media and cultured for 24 h. At 50–60% confluency, the cells were treated with isolated and purified rhSOD3 or rh209E of 10 μg protein labeled with FITC and Hoechst33342 fluorescence staining, under same culture conditions mentioned above. After the requisite incubation, the cells were viewed under confocal fluorescence microscopy (confocal laser scanning microscope, Carl Zeiss LSM 510).
Anti-inflammatory effects of rhSOD3
At 70% confluency, HaCaT were starved in serum-free DMEM for 6 h prior to treatment with 10 ng/ml TNF-α and 100 U/ml IFN-γ. Purified rhSOD3 and 209E were added to cells (200 U/mL concentration), followed by treatment with various concentration of ZnCl2 (0–10 μM). Cells were harvested after 24 h incubation by directly adding SDS sample buffer containing protease inhibitors. p-STAT3, STAT3, GAPDH, and rhSOD3 were analyzed by western blot analysis, using anti-p-STAT3, p-STAT3, GAPDH (Santa Cruz Biotechnology, CA, USA) and anti-hSOD3 (AbCam, Cambridge, UK) antibodies.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from cells or tissue using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Complementary DNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). Quantitative real-time PCR was performed using the KAPA SYBR fast qPCR Kit (KAPA biosystems, Woburn, MA, USA), as previously described (24). The results were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression. The PCR conditions were 1 cycle at 95°C for 5 min, followed by 35 cycles at 96°C for 20 s, 60°C for 20 s and 72°C for 20 s, and ending with one cycle at 72°C for 5 min. Primers used in this experiment were purchased from Qiagen.
Histological analysis
After shaving the mouse dorsal skin, 50 μl of a mixture of rhSOD3 (2000 unit) and ZnCl2 (50 mM) was treated. Only rhSOD3 (2000 unit) was applied to the mice, and this group was designated as the rhSOD3 treatment group. After 1 h exposure, the skin was fixed with 4% paraformaldehyde in phosphate buffered saline for 24 h, washed with tap-water, dehydrated with grade ethanol, and then embedded in paraffin. The paraffin blocks were cut in 4-μm thick sections, mounted on glass slides, dewaxed, rehydrated with grade ethanol, and stained with FITC and Hoechst33342 fluorescence staining. Analysis was carried out using a fluorescence attached microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data were presented as means ± SEM, and statistical comparisons between groups were made with the unpaired two-sided t-test. All experiments were performed at least three times.
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by the Industrial Technology Innovation program (10063322, Development of Animal Cell Culture based hEC-SOD for Management of Atopic Dermatitis) funded By the Ministry of Trade, industry & Energy.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting financial interests.
REFERENCES
- 1.Serra V, von Zglinicki T, Lorenz M, Saretzki G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J Biol Chem. 2003;278:6824–6830. doi: 10.1074/jbc.M207939200. [DOI] [PubMed] [Google Scholar]
- 2.Teoh ML, Fitzgerald MP, Oberley LW, Domann FE. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 2009;69:6355–6363. doi: 10.1158/0008-5472.CAN-09-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler MD, Smutney OM, Samulski RJ. Secretion of extracellular superoxide dismutase from muscle transduced with recombinant adenovirus inhibits the growth of B16 melanomas in mice. Mol Cancer Res. 2003;1:871–881. [PubMed] [Google Scholar]
- 4.Laukkanen MO, Kivelä A, Rissanen T, et al. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2002;106:1999–2003. doi: 10.1161/01.CIR.0000031331.05368.9D. [DOI] [PubMed] [Google Scholar]
- 5.Marklund SL. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990;266:213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992;267:6696–6701. [PubMed] [Google Scholar]
- 7.Stralin P, Marklund SL. Multiple cytokines regulate the expression of extracellular superoxide dismutase in human vascular smooth muscle cells. Atherosclerosis. 2000;151:433–441. doi: 10.1016/S0021-9150(99)00427-X. [DOI] [PubMed] [Google Scholar]
- 8.Ookawara T, Kizaki T, Takayama E, et al. Nuclear translocation of extracellular superoxide dismutase. Biochem Biophys Res Commun. 2002;296:54–61. doi: 10.1016/S0006-291X(02)00804-5. [DOI] [PubMed] [Google Scholar]
- 9.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 10.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 11.Dixon JE, Osman G, Morris GE, et al. Highly efficient delivery of functional cargoes by the synergistic effect of GAG binding motifs and cell-penetrating peptides. Proc Natl Acad Sci U S A. 2016;113:E291–299. doi: 10.1073/pnas.1518634113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha P, Yun JH, Kim WT, Kim TY, Lee W. Cloning, purification, and characterization of recombinant human extracellular superoxide dismutase in SF9 insect cells. Mol Cells. 2016;39:242–249. doi: 10.14348/molcells.2016.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park KY, Kim EY, Lee W, Kim TY, Kim WT. Expression, subcellular localization, and enzyme activity of a recombinant human extra-cellular superoxide dismutase in tobacco (Nicotiana benthamiana L.) Protein Expr Purif. 2016;119:69–74. doi: 10.1016/j.pep.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Ryu K, Kim YH, Kim Y, Lee JS, Jeon B, Kim TY. Increased yield of high-purity and active tetrameric recombinant human EC-SOD by solid phase refolding. J Microbiol Biotechnol. 2008;18:1648–1654. [PubMed] [Google Scholar]
- 15.Auten RL, O’Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L32–40. doi: 10.1152/ajplung.00133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gongora MC, Lob HE, Landmesser U, et al. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juul K, Tybjaerg-Hansen A, Marklund S, Lange P, Nordestgaard BG. Genetically increased antioxidative protection and decreased chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:858–864. doi: 10.1164/rccm.200509-1387OC. [DOI] [PubMed] [Google Scholar]
- 18.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 19.Kwon MJ, Jeon YJ, Lee KY, Kim TY. Superoxide dismutase 3 controls adaptive immune responses and contributes to the inhibition of ovalbumin-induced allergic airway inflammation in mice. Antioxid Redox Signal. 2012;17:1376–1392. doi: 10.1089/ars.2012.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Kim HY, Kim JH, et al. Enhancement of potency and stability of human extracellular superoxide dismutase. BMB Rep. 2015;48:91–96. doi: 10.5483/BMBRep.2015.48.2.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon MJ, Han J, Kim BH, Lee YS, Kim TY. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: superoxide dismutase 3 inhibits reactive oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid Redox Signal. 2012;16:297–313. doi: 10.1089/ars.2011.4066. [DOI] [PubMed] [Google Scholar]
- 22.Jeon B, Kim BH, Lee YS, Kim S, Yoon JB, Kim TY. Inactive extracellular superoxide dismutase disrupts secretion and function of active extracellular superoxide dismutase. BMB Rep. 2011;44:40–45. doi: 10.5483/BMBRep.2011.44.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Kim BH, Lee H, et al. Regulation of skin inflammation and angiogenesis by EC-SOD via HIF-1a and NF-kB pathways. Free Radic Biol Med. 2011;51:1985–1995. doi: 10.1016/j.freeradbiomed.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu T, Nagashima S, Umemura K, Kanamaru M, Nakashima M. Pharmacokinetics and safety of intravenous recombinant human superoxide dismutase (NK341) in healthy subjects. Int J Clin Pharmacol Ther. 1994;32:638–641. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.