Abstract
Patients with inflammatory bone disease or cancer exhibit an increased risk of fractures and delayed bone healing. The S100A4 protein is a member of the calcium-binding S100 protein family, which is abundantly expressed in inflammatory diseases and cancers. We investigated the effects of extracellular S100A4 on osteoblasts, which are cells responsible for bone formation. Treating primary calvarial osteoblasts with recombinant S100A4 resulted in matrix mineralization reductions. The expression of osteoblast marker genes including osteocalcin and osterix was also suppressed. Interestingly, S100A4 stimulated the nuclear factor-kappaB (NF-κB) signaling pathway in osteoblasts. More importantly, the ex vivo organ culture of mouse calvariae with recombinant S100A4 decreased the expression levels of osteocalcin, supporting the results of our in vitro experiments. This suggests that extracellular S100A4 is important for the regulation of bone formation by activating the NF-κB signaling pathway in osteoblasts.
Keywords: Inflammation, Mineralization, NF-κB, Osteoblast, S100A4
INTRODUCTION
S100 proteins are calcium-binding proteins comprised of more than 20 members and they play important roles in inflammation, cytoskeleton dynamics, enzyme activity, as well as cell growth and differentiation (1–3). Many studies have revealed that S100 proteins are involved in pathological conditions, especially with regard to tumor progression and arthritis (3). For instance, S100A2, S100A4, S100A6, S100A7, and S100B were found to be differentially expressed in cancer cells, while S100A12 was detected at a high level in synovial tissues of arthritis patients (4). However, each kind of the S100 proteins is thought to play a specific part or an, as yet, unidentified critical role under certain circumstances. Notably, S100A8 has been shown to activate osteoclasts, cells responsible for bone resorption, by interacting with the toll-like receptor 4 (TLR4) (5).
Over the last decade, S100A4 has been linked to rheumatoid arthritis pathogenesis. Klingelhofer and colleagues reported that synovial tissues of rheumatoid arthritis patients express S100A4 (6). In addition, S100A4 was reported to stimulate matrix metalloproteinase (MMP)-13 secretion via the receptor for advanced glycation end products (RAGE) in chondrocytes, resulting in cartilage degradation (7). S100A4 also plays important roles in tumor progression by increasing tumor cell migration and invasion as well as MMP secretion (8). Both intracellular and extracellular functions of S100A4 have been studied. Intracellularly, S100A4 binds to the tumor suppressor protein p53, where it aids in tumor survival; tumor cell migration is regulated through interactions with non-muscle myosin II. Extracellularly, S100A4 binds to cell surface receptors such as RAGE, activating nuclear factor-kappaB (NF-κB) and mitogen-activated protein kinase pathways for tumor cell invasion and survival (9).
Recently, the NF-κB signaling pathway was reported to be a negative regulator of osteogenesis (10, 11). NF-κB activation by proinflammatory cytokines such as the tumor necrosis factor (TNF)-α in osteoblast precursors inhibits osteogenic differentiation (12). RAGE, a receptor of S100A4, was shown to mediate activation of NF-κB in endothelial cells, macrophages, and lymphocytes (13). A study by Ogawa et al. reported that osteoblastic cells expressed RAGE and that the addition of high glucose with AGE, one of the ligands of RAGE, inhibited mineralization (14). Therefore, we postulated that S100A4 signals through RAGE to activate the NF-κB pathway and to suppress osteoblast function.
In this study, we demonstrated that extracellular S100A4 did not affect early osteoblast differentiation. However, it inhibited mineralization activity due to activation of the NF-κB signaling pathway in osteoblasts. Also, the ex vivo culture of neonatal mice calvariae with recombinant S100A4 decreased the expression of osteocalcin.
RESULTS
S100A4 does not affect early stage of osteoblast differentiation
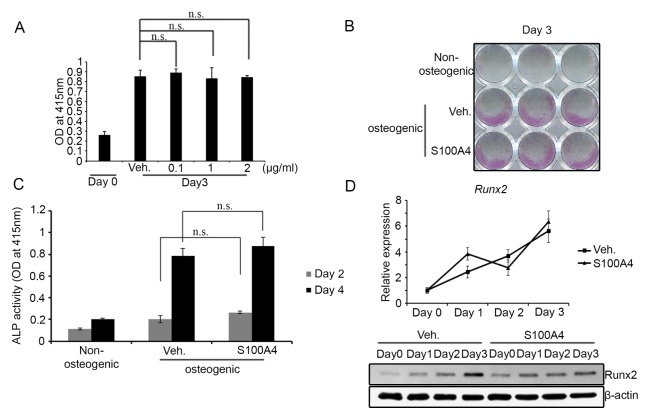
To test the effect of S100A4 on bone formation, we first investigated its influence on an in vitro culture of primary osteoblasts. Preosteoblasts derived from mouse calvarial tissues were treated with recombinant mouse S100A4 protein or an control vehicle of an osteogenic differentiation medium. S100A4 protein did not affect the viability of calvarial preosteoblasts at concentrations up to 2 μg/ml (Fig. 1A). Cells became positive for ALP, an early stage marker of osteoblast differentiation, within three days of culture in osteogenic media (Fig. 1B). S100A4 treatment did not alter the extent of ALP staining compared to the vehicle-treated culture (Fig. 1B). Consistent differences in ALP activity by S100A4 treatment at the second or fourth day of the culture could not be observed (Fig. 1C). We next assessed mRNA and protein levels of Runx2, the key transcription factor of early osteoblast differentiation. The mRNA expression of Runx2 in S100A4-treated cultures was comparable to that in the vehicle-treated group (Fig. 1D, upper part). On the other hand, a slight decrease in Runx2 protein level by S100A4 treatment at day 3 of the culture could be observed (Fig. 1D, lower part). Taken together, these results indicate that S100A4 does hardly influences the early stage of osteoblast differentiation.
Fig. 1.
Extracellular S100A4 did not influence the early differentiation of osteoblasts. (A) Mouse calvarial preosteoblasts cultured with osteogenic medium containing vehicle (Veh.) or indicated concentration of recombinant mouse S100A4 for three days were subjected to cell viability assay. (B) Mouse calvarial cells cultured with non-osteogenic or osteogenic medium containing vehicle or S100A4 (1 μg/ml) were ALP-stained after three days of culture. (C) ALP activities were measured at days 2 and 4. (D) Calvarial preosteoblasts were cultured with a vehicle or S100A4 in an osteogenic medium for the indicated days and subjected to real-time PCR for Runx2 expression. HPRT was employed as an internal control (upper part). The protein levels of Runx2 were assessed by Western blotting. β-Actin shown as a loading control (lower part). Error bars represent the SD of mean values. n.s.: not significant.
S100A4 inhibits mineralization and the expression of late-stage osteoblast markers
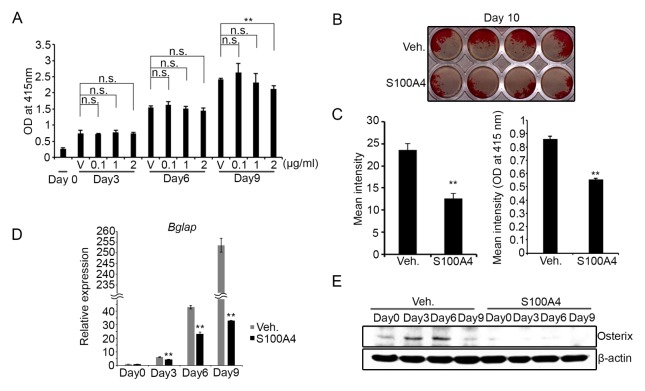
We examined the effect of S100A4 on matrix mineralization, a crucial function of osteoblasts during bone formation. Calvarial preosteoblasts were cultured in osteogenic media for ten days and the assessment of mineralization was performed by Alizarin-red staining. The cell proliferation and survival was not affected by the presence of S100A4 at concentrations up to 2 μg/ml until day 6 of the culture and only slightly decreased at 2 μg/ml in 9-day cultures (Fig. 2A). Interestingly, the addition of S100A4 at 1 μg/ml in osteogenic media significantly delayed matrix mineralization by calvarial osteoblasts (Figs. 2B and 2C). The expression level of osteocalcin (Bglap), a critical component of the bone matrix, was also lowered by the S100A4 treatment (Fig. 2D). Induction of the osterix protein level, a transcription factor necessary for mineralization, was also suppressed (Fig. 2E). In overall, our results demonstrate that an excessive amount of extracellular S100A4 impairs the mineralization activity of osteoblasts and delays induction of late osteoblast markers.
Fig. 2.
Extracellular S100A4 inhibited matrix mineralization. (A) Mouse calvarial preosteoblasts cultured with osteogenic medium containing vehicle (V) or indicated concentration of recombinant mouse S100A4 for the indicated days were subjected to viability assay. (B) Mouse calvarial preosteoblasts were cultured with vehicle (Veh.) or S100A4 (1 μg/ml) in an osteogenic medium for 10 days. Alizarin-red staining was performed, and a representative image is shown. (C) The intensity of the image from (B) was measured using ImageJ program (left). The intensity of Alizarin-red stain solubilized with cetylpyridium chloride was also quantified (right). (D) Mouse calvarial preosteoblasts were cultured with a vehicle or S100A4 (1 μg/ml) in osteogenic medium for the indicated days and subjected to real-time PCR for Bglap (osteocalcin) expression. HPRT was used as an internal control. (E) Mouse calvarial preosteoblasts were cultured with a vehicle or S100A4 (1 μg/ml) in osteogenic medium for the indicated days. The osterix protein levels were assessed by Western blotting and β-Actin was included as a loading control. Error bars represent the SD of mean values. **P < 0.01 versus Veh. n.s.: not significant.
S100A4 activates the NF-κB pathway
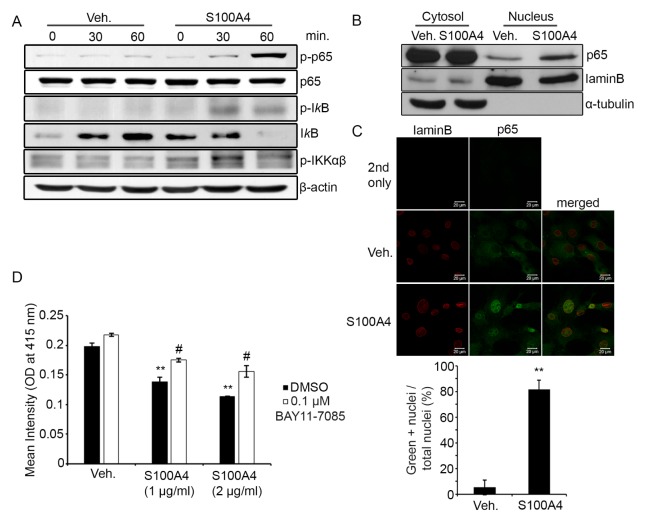
A number of studies have reported that activation of the NF-κB pathway in osteoblast precursors impairs osteogenic differentiation (10, 11). To investigate the mechanism of mineralization inhibition by S100A4, we tested whether S100A4 activated the NF-κB pathway in calvarial osteoblasts. Stimulating them with recombinant mouse S100A4-increased IKKα/β, IκB, and p65 phosphorylation within 60 minutes (Fig. 3A). We also assessed the protein level of p65 in the nucleus by fractionating the cytosol and nuclear proteins. Increased levels of p65 were observed in the nuclei of S100A4-treated cells (Fig. 3B). Consistent with our Western blotting results, immunofluorescence confocal microscopy also demonstrated localization of p65 in the nuclei after S100A4 stimulation (Fig. 3C). Finally, the decreases in mineralization caused by S100A4 were attenuated by treatment with BAY11-7085, a NF-κB inhibitor (Fig. 3D). These data suggest that S100A4 negatively regulates osteogenic differentiation by activating the NF-κB signaling pathway.
Fig. 3.
S100A4 induced NF-κB activation in osteoblasts. (A) Mouse calvarial preosteoblasts were serum-starved for five hours, stimulated with either a vehicle (Veh.) or recombinant mouse S100A4 (2 μg/ml) for the indicated time and subjected to Western blotting to detect protein levels of phosphorylated p65 (p-p65), total p65, phosphorylated IκB (p-IκB), total IκB, and phosphorylated IKKαβ. β-Actin is shown as a loading control. (B) Calvarial cells were serum-starved for five hours and stimulated with either vehicle or S100A4 (2 μg/ml) for one hour. Cytosolic proteins (30 μg) and nuclear proteins (8 μg) were separated and subjected to Western blotting to detect protein levels of p65, laminB, and α-tubulin. (C) Calvarial cells were serum-starved for five hours and stimulated with either a vehicle or S100A4 (2 μg/ml) for one hour. Cells were stained with anti-laminB (red) and anti-p65 (green). LaminB was labeled to locate the nuclear membrane. Cells were subjected to confocal microscopy and representative images displayed (top). Green positive (+) nuclei were counted and depicted as a graph (bottom). Primary antibodies were not added for 2nd-only samples. (D) Mouse calvarial preosteoblasts were cultured with a vehicle or S100A4 (1 or 2 μg/ml) in osteogenic medium for nine days, together with DMSO or indicated concentrations of BAY11-7085. Cells were subjected to Alizarin-red staining. The intensity of Alizarin-red stain was quantified after solubilization using cetylpyridium chloride. Error bars represent the SD of mean values. **P < .01 versus Veh.. #P < .01 versus DMSO.
S100A4 suppresses osteocalcin expression in ex vivo organ culture of calvariae
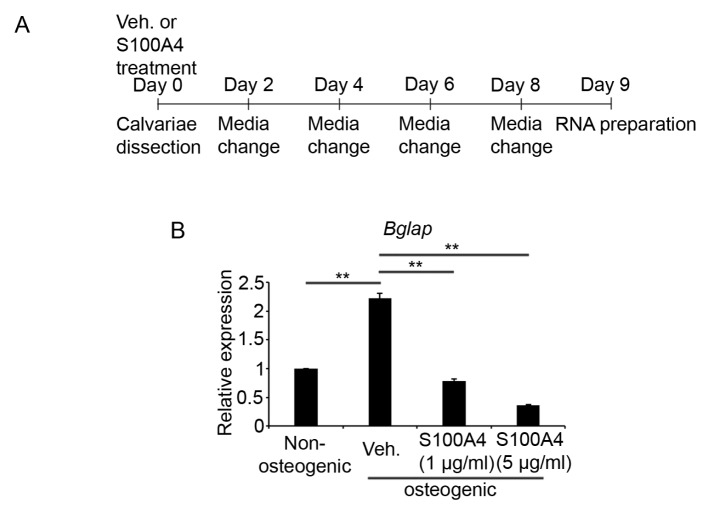
To obtain further evidence supporting the regulatory role of S100A4 on the bone mineralization activity of osteoblasts, we next utilized an ex vivo organ culture. Calvariae from four-day-old mice were cultured for nine days in osteogenic media with or without S100A4 protein. The osteogenic medium was changed in 2-day intervals and total RNAs were isolated for cDNA synthesis (Fig. 4A). Real-time PCR analyses revealed a strong suppression of Bglap (osteocalcin) gene expression by S100A4 treatment (Fig. 4B).
Fig. 4.
S100A4 treatment decreased Bglap expression in ex vivo calvaria culture. (A) A schematic timeline describing the experiment. Calvarie from 4-day-old mice were cultured in organ culture dishes with the osteogenic medium containing either mouse recombinant S100A4 (1 or 5 μg/ml) or vehicle for nine days. The medium was changed every other day. At the end of the culture, calvariae were frozen and ground for total RNA isolation. (B) After, reverse transcription, the mRNA levels of Bglap were determined by real-time PCR analyses. n = 3 to 4 per group. **P < .01 between the indicated groups.
DISCUSSION
In this study, we investigated the role of extracellular S100A4 on the inhibition of bone formation by activating the NF-κB pathway in osteoblasts. The early differentiation of osteoblast was not affected (Fig. 1). However, both late-stage differentiation of osteoblasts and the mineralization activity were reduced by S100A4 treatment (Fig. 2). Consistent with the results of our in vitro experiments, S100A4 addition to ex vivo culture of neonatal calvariae decreased the expression levels of osteocalcin (Fig. 4). The NF-κB activation in osteoblasts by S100A4 seems to be responsible for the inhibition of bone formation (Fig. 3).
Bone-loss under inflammatory conditions largely depends on enhanced bone resorption by osteoclasts and delayed bone formation by osteoblasts. Among the inflammatory cytokines, TNF-α was shown to directly inhibit the mineralized nodule formation and osteocalcin secretion from osteoblasts (18). Moreover, the expression of Runx2 was decreased after TNF-α treatment (19) as well as a TNF-α induced expression of the Smad ubiquitination regulatory factor 1/2 (Smurf 1/2), which ubiquitylates Runx2 for proteasomal degradation (20). Yamazaki et al. demonstrated that bone morphogenetic protein 2 (BMP2)-mediated activation of Smads, crucial transcription factors of osteoblast differentiation, abrogated by TNF-α via NF-κB activation (12). Moreover, the activation of NF-κB by TNF-α induced a cAMP response element-binding protein H (CREBH), which - in turn - up-regulated Smurf 1 to inactivate the Smad/Runx2 regulatory system (21). Mice that specifically overexpressed the dominant negative form of IKK in osteoblasts displayed increased bone mass (10). Such studies are in accordance with our finding of S100A4 mediating inhibition of mineralization through NF-κB activation and strongly support a negative relationship between bone anabolism and the activation of NF-κB in osteoblasts.
The NF-κB signaling pathway is also critical to the differentiation of osteoclasts by RANKL (receptor activator of NF-κB ligand) (22). Interestingly, mice lacking S100A4 showed a decrease in the number of osteoclasts (23). Therefore, it is reasonable to postulate that S100A4 also mediates the activation of NF-κB in osteoclasts, accelerating bone catabolism. We are currently investigating whether S100A4 directly regulates osteoclastogenesis via the NF-κB pathway and if this could be a useful therapeutic target to treat bone metabolic disorders.
It was previously demonstrated that the synovial fluids of rheumatoid arthritis patients had a mean concentration of 1.98 μg/ml of S100A4, significantly higher than 0.247 μg/ml in osteoarthritis patients (6). Since rheumatoid arthritis promotes and maintains a more inflammated condition near the articular joints than osteoarthritis, the effect of extracellular S100A4 on bone cells might be more pronounced in rheumatoid arthritis. In one of our previous studies, we determined that S100A4 was secreted from lipopolysaccharide-stimulated human periodontal ligament cells, supporting the possibility that S100A4 regulates bone cells in inflammatory diseases (24). The concentrations of recombinant S100A4 used in this study for the in vitro tests were 1 or 2 μg/ml, similar to the level found in arthritis patients.
We described the effect of extracellular S100A4 with respect to the NF-κB pathway’s activation. Detecting receptors that mediate activation in osteoblasts is an appealing subject for further studies. RAGE and TLR4 are among the two best characterized receptors for S100A4 in a variety of cell types (25–28). NF-κB signaling in peripheral blood mononuclear cells was shown to be activated by S100A4 via TLR4 (25), while a series of studies demonstrated that S100A4 mediates signals via RAGE, especially in cancer cells (26–28). Both receptors are critical components of the NF-κB signaling pathway.
In conclusion, our study demonstrates that extracellular S100A4 interrupts the mineralization function of osteoblasts and causes an imbalance in bone homeostasis by inhibiting new bone formation. We demonstrated for the first time that this phenomenon is mediated through NF-κB activation. Further investigations of S100A4 may open new avenues for developing novel therapies against inflammatory bone-destructive conditions.
MATERIALS AND METHODS
Animals
All animals were kept in a specific pathogen-free animal facility with a consistent temperature (22°C) and humidity (55%), as well as a 12-hour light/dark cycle. The facility was operated by experienced animal handlers who were responsible for changing bedding material as well as for the provision of food and sterilized water under the supervision of a veterinarian. One-day-old ICR mice were purchased from OrientBio (Sungnam, Korea) and used for both the in vitro calvarial osteoblast preparation and the ex vivo organ culture experiments. Animal experiments were approved by the Committee on the Care and Use of Animals in Research at Seoul National University (SNU-130311-4-3).
Reagents
Recombinant mouse S100A4 was purchased from Prospec (East Brunswick, NJ, USA). Phosphospecific antibodies against IκB kinase (IKK)α/β (Ser176/180), IκB (Ser32), p65 (Ser536), IKKα/β, IκB, and p65 were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies against osterix, laminB, and p65 were acquired from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-β-actin (AC-74) was purchased from Sigma-Aldrich (St. Louis, MO, USA), anti-Runx2 from MBL International (Woburn, MA, USA), the alkaline phosphatase (ALP) assay kit from Takara Bio Inc. (Fukui, Japan). β-Glycerophosphate, cetylpyridium chloride, and ascorbic acid from Sigma-Aldrich, Bay11-7082 from Alexis Biochemicals (Grunberg, Germany), and the Cell Counting Kit-8 (CCK-8) from Dojindo (Kumamoto, Japan).
Cell viability assay
Calvarial osteoblasts were seeded in 96-well plates at the density of 1 × 104 cells per well. Cells were cultured in the presence of 10 mM β-glycerophosphate and 100 μM ascorbic acid together with vehicle or recombinant mouse S100A4 (0.1–2 μg/ml) for 3–9 days. Cells at days 0, 3, 6, and 9 were subjected to cytotoxicity assay using the CCK-8 assay kit. After incubating cells with the reagents for one hour, the optical density was measured at 450 nm with an iMark Microplate Absorbance Reader (Biorad, Hercules, CA, USA).
Real-time PCR analysis
Quantification of the mRNA level by real-time PCR analysis was performed as previously described (15). Primers for real-time PCR analysis were as follows: Runx2 forward, 5′-CGCACGACAACCGCACCA-3′; Runx2 reverse, 5′-CAGCACG GAGCACAGGAAGTT-3′; Bglap forward, 5′-CCGGGAGCAG TGTGAGCTTA-3′; Bglap reverse, 5′-TAGATGCGTTTGTAGG CGGTC-3′; Hprt (hypoxanthine-guanine phosphoribosyltransferase) forward, 5′-CCTAAGATGAGCGCAAGTTGAA-3′; Hprt reverse, 5′-CCACAGGGACTAGAACACCTGCTAA-3′.
Calvarial preosteoblast preparation and differentiation
Calvarial preosteoblasts were prepared from 1-day-old mice as previously described (16). These were seeded onto 48-well or 6-well cell culture plates in alpha minimum essential medium (WELGENE, Daegu, Korea), supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA). Osteogenic differentiation was induced by supplementing the culture media with 10 mM β-glycerophosphate and 100 μM ascorbic acid. To evaluate the osteoblast differentiation, ALP activity was measured using the ALP assay kit, following the manufacturer’s protocol. Mineralization was assessed after 10 days of culture by Alizarin-red staining as described before (17) and the mean intensity was assessed using the ImageJ program. Alternatively, Alizarin-red stained wells were incubated with 100 mM cetylpyridium chloride for two hours at 37°C and the optical density of eluates was measured at 415 nm.
Western blotting
Cells were washed with cold PBS and lysed with RIPA buffer (10 mM Tris pH 7.2, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 1% sodium deoxycholate, 5 mM ethylenediaminetetraacetic acid). Whole cell lysates or nuclear lysates were subjected to Western blot analysis as previously described (16).
Ex vivo calvarial organ culture
Calvariae were dissected from 4-day-old ICR mice and cultured in 60 mm center well culture dishes (Corning, NY, USA). Osteogenic differentiation was induced by supplementing BGJb medium (Invitrogen) with 10 mM β-glycerophosphate and 100 μM ascorbic acid. The culture medium containing mouse recombinant S100A4 (1 or 5 μg/ml) or vehicle was changed every other day during nine days. For real-time PCR analysis, calvariae were snap-frozen in liquid nitrogen and ground to powder for total RNA isolation using TRIzol (Invitrogen).
Immunofluorescence microscopy
Calvarial osteoblasts seeded on 12-mm cover glass were fixed with 3.7% formaldehyde and made permeable with 0.1% Triton X-100 for 15 minutes. After blocking non-specific sites with 1% bovine serum albumin in PBS for 1.5 hours, the cells were incubated with anti-p65 (1/200 dilution) and anti-laminB (1/200 dilution) antibodies for two hours, followed by a 1-hour incubation with secondary mouse-FITC and goat-Cy3 (1/300 dilution) antibody. The prepared slides were viewed under a Zeiss LSM 700 laser-scanning microscope with the following requirements: Objective lenses; C-Apochromat 40x/1.20 W, detectors; PMT, filter model; green (BP 490–555), red (560 IF), and lasers; 488 nm, 555 nm. Pictures were taken and analyzed using the ZEN2010 program (version 6.0.0.320).
Statistics
All experiments, except the mice calvariae organ culture studies, were performed at least three times. All quantitative measurements had 3 or 4 replicates. Two-way ANOVA followed by a Bonferroni test and Student’s t test were performed to define differences between multiple and 2 groups, respectively. Quantitative data are presented as the mean ± SD. A P value < .05 was considered statistically significant.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation of Korea (NRF-2014R1A2A1A10050406) to H-HK.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting financial interests.
REFERENCES
- 1.Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 2.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/S1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 3.Emberley ED, Murphy LC, Watson PH. S100 proteins and their influence on pro-survival pathways in cancer. Biochem Cell Biol. 2004;82:508–515. doi: 10.1139/o04-052. [DOI] [PubMed] [Google Scholar]
- 4.Foell D, Kane D, Bresnihan B, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42:1383–1389. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
- 5.Grevers LC, de Vries TJ, Vogl T, et al. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum. 2011;63:1365–1375. doi: 10.1002/art.30290. [DOI] [PubMed] [Google Scholar]
- 6.Klingelhofer J, Senolt L, Baslund B, et al. Up-regulation of metastasis-promoting S100A4 (Mts-1) in rheumatoid arthritis: putative involvement in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2007;56:779–789. doi: 10.1002/art.22398. [DOI] [PubMed] [Google Scholar]
- 7.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 8.Sherbet GV. Metastasis promoter S100A4 is a potentially valuable molecular target for cancer therapy. Cancer Lett. 2009;280:15–30. doi: 10.1016/j.canlet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang J, Liu F, Lee M, et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci USA. 2013;110:9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamazaki M, Fukushima H, Shin M, et al. Tumor necrosis factor alpha represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB. J Biol Chem. 2009;284:35987–35995. doi: 10.1074/jbc.M109.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/S0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto M. The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm Metab Res. 2007;39:871–875. doi: 10.1055/s-2007-991157. [DOI] [PubMed] [Google Scholar]
- 15.Choi SW, Lee KS, Lee JH, et al. Suppression of Akt-HIF-1α signaling axis by diacetyl atractylodiol inhibits hypoxia-induced angiogenesis. BMB Rep. 2016;49:508–513. doi: 10.5483/BMBRep.2016.49.9.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Qiu Q, Zhou N, et al. Dickkopf-1 is involved in BMP9-induced osteoblast differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2016;49:179–184. doi: 10.5483/BMBRep.2016.49.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert L, He X, Farmer P, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 20.Kaneki H, Guo R, Chen D, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281:4326–4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang WG, Jeong BC, Kim EJ, et al. Cyclic AMP Response Element-binding Protein H (CREBH) Mediates the Inhibitory Actions of Tumor Necrosis Factor alpha in Osteoblast Differentiation by Stimulating Smad1 Degradation. J Biol Chem. 2015;290:13556–13566. doi: 10.1074/jbc.M114.587923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–214. doi: 10.1016/S0006-291X(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 23.Erlandsson MC, Svensson MD, Jonsson IM, et al. Expression of metastasin S100A4 is essential for bone resorption and regulates osteoclast function. Biochim Biophys Acta. 20131833:2653–2663. doi: 10.1016/j.bbamcr.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Mah SJ, Lee J, Kim H, et al. Induction of S100A4 in periodontal ligament cells enhances osteoclast formation. Arch Oral Biol. 2015;60:1215–1221. doi: 10.1016/j.archoralbio.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Cerezo LA, Remakova M, Tomcik M, et al. The metastasis-associated protein S100A4 promotes the inflammatory response of mononuclear cells via the TLR4 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford) 2014;53:1520–1526. doi: 10.1093/rheumatology/keu031. [DOI] [PubMed] [Google Scholar]
- 26.Herwig N, Belter B, Wolf S, Haase-Kohn C, Pietzsch J. Interaction of extracellular S100A4 with RAGE prompts prometastatic activation of A375 melanoma cells. J Cell Mol Med. 2016;20:825–835. doi: 10.1111/jcmm.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medapati MR, Dahlmann M, Ghavami S, et al. RAGE Mediates the Pro-Migratory Response of Extracellular S100A4 in Human Thyroid Cancer Cells. Thyroid. 2015;25:514–527. doi: 10.1089/thy.2014.0257. [DOI] [PubMed] [Google Scholar]
- 28.Siddique HR, Adhami VM, Parray A, et al. The S100A4 Oncoprotein Promotes Prostate Tumorigenesis in a Transgenic Mouse Model: Regulating NFkappaB through the RAGE Receptor. Genes Cancer. 2013;4:224–234. doi: 10.1177/1947601913492420. [DOI] [PMC free article] [PubMed] [Google Scholar]