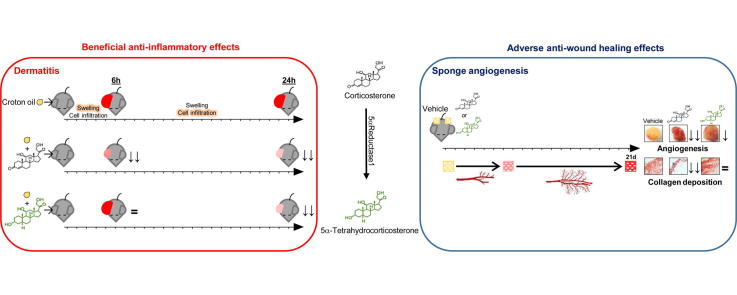
Graphical abstract
Keywords: Glucocorticoid, 5α-Tetrahydrocorticosterone, Dermatitis, Angiogenesis, Collagen, Wound healing
Abstract
Use of topical glucocorticoid for inflammatory skin conditions is limited by systemic and local side-effects. This investigation addressed the hypothesis that topical 5α-tetrahydrocorticosterone (5αTHB, a corticosterone metabolite) inhibits dermal inflammation without affecting processes responsible for skin thinning and impaired wound healing. The topical anti-inflammatory properties of 5αTHB were compared with those of corticosterone in C57Bl/6 male mice with irritant dermatitis induced by croton oil, whereas its effects on angiogenesis, inflammation, and collagen deposition were investigated by subcutaneous sponge implantation. 5αTHB decreased dermal swelling and total cell infiltration associated with dermatitis similarly to corticosterone after 24 h, although at a five fold higher dose, but in contrast did not have any effects after 6 h. Pre-treatment with the glucocorticoid receptor antagonist RU486 attenuated the effect of corticosterone on swelling at 24 h, but not that of 5αTHB. After 24 h 5αTHB reduced myeloperoxidase activity (representative of neutrophil infiltration) to a greater extent than corticosterone. At equipotent anti-inflammatory doses 5αTHB suppressed angiogenesis to a limited extent, unlike corticosterone which substantially decreased angiogenesis compared to vehicle. Furthermore, 5αTHB reduced only endothelial cell recruitment in sponges whereas corticosterone also inhibited smooth muscle cell recruitment and decreased transcripts of angiogenic and inflammatory genes. Strikingly, corticosterone, but not 5αTHB, reduced collagen deposition. However, both 5αTHB and corticosterone attenuated macrophage infiltration into sponges. In conclusion, 5αTHB displays the profile of a safer topical anti-inflammatory compound. With limited effects on angiogenesis and extracellular matrix, it is less likely to impair wound healing or cause skin thinning.
1. Introduction
Inflammatory skin disease is highly prevalent worldwide: according to estimates published in 2012, eczema affects approximately 230 million people globally, and topical anti-inflammatory glucocorticoids are the most common treatment [1]. However, the therapeutic benefits of these drugs are offset by severe side-effects [2].
Following application of glucocorticoids to the skin, adverse effects manifest locally (skin atrophy and impaired wound healing) and systemically (e.g. osteoporosis, abdominal obesity, and glaucoma) [3]. Skin atrophy is characterised by increased transparency and striae, due to suppression of cell proliferation and inhibition of collagen synthesis, whereas delayed wound healing is attributed to dysregulation of keratinocyte proliferation, fibroblastic activity, and angiogenesis causing delayed formation of granulation tissue [4], [5]. These factors restrict the use of more potent glucocorticoids and also the length of treatment, particularly in vulnerable patients such as infants and the elderly. Accordingly, novel drugs or delivery modes have been sought to improve the therapeutic index of topical steroids. This may be achieved by “dissociated glucocorticoids” which invoke trans-repression through the glucocorticoid receptor (GR) without trans-activation [6].
5α-Tetrahydrocorticosterone (5αTHB) is a naturally occurring glucocorticoid metabolite, formed by reduction of the A-ring of corticosterone, the principle endogenous glucocorticoid in rodents. Two 5α-reductase isozymes catalyse the rate-determining step in 5αTHB synthesis from corticosterone: the type I isozyme is expressed in the liver, kidney and skin; the type II isozyme is expressed principally in the liver, genital tract, prostate and skin. Recent studies have suggested that 5αTHB exhibits a dissociated profile of action, possibly acting through GR. 5αTHB displayed anti-inflammatory properties when administered subcutaneously in mice subjected to thioglycollate-induced peritonitis, but did not induce the chronic adverse metabolic changes (such as increased insulin and body mass) produced by corticosterone [7]. 5αTHB and corticosterone suppressed infiltration of neutrophils into the inflamed peritoneum to a similar extent, but 5αTHB had a lesser effect on macrophage recruitment, suggesting a different profile of action. When infused systemically, 5αTHB demonstrated rapid clearance from the systemic circulation [7], likely limiting its oral use, but the same characteristic would be beneficial in topical treatments in which systemic effects are unwanted.
This investigation addressed the hypothesis that topical 5αTHB can effectively reduce skin inflammation through a GR-dependent mechanism, but with limited effects on processes such as angiogenesis and collagen deposition, the inhibition of which underpin most common problems associated with topical glucocorticoid therapy. The efficacy of topical 5αTHB application for treating skin inflammation was compared with that of corticosterone in vivo using croton oil-induced dermatitis and the role of GR was assessed by pre-administration of the GR antagonist mifepristone (RU486). This is a steroid-responsive model of dermal inflammation, driven primarily by neutrophil accumulation, commonly used for testing anti-inflammatory treatments for irritant dermatitis [8], [9]. An in vivo model of sponge implantation allowed comparison of the effects of corticosterone and 5αTHB on inflammation, angiogenesis and collagen deposition.
2. Materials and methods
2.1. Materials
Chemicals were from Sigma Aldrich (Poole, UK) unless otherwise stated. Steroids (corticosterone, 5αTHB and RU486) were from Steraloids (Newport, RI, USA).
2.2. Animal welfare, models and ethical statements
Male mice (C57BL/6, 8–10 week old) were from Harlan Laboratories (Shardlow, UK). Animals were allowed to acclimatise for one week prior to experimentation, maintained under controlled conditions of light (lights on 0700–1900 h) and temperature (18–20 °C) and allowed free access to standard chow (Special Diet Services, Witham, UK) and drinking water. All experimental procedures were performed under UK Home Office guidelines.
2.2.1. In vivo model of irritant dermatitis
Mice were treated on the inner surface of the right ear with either croton oil only (CO mixture: 10 μL, 3% v/v in ethanol and isopropyl myristate 30:5) or with solutions of corticosterone or 5αTHB diluted in the CO mixture; the left ear was untreated. The EC50 dose of corticosterone to reduce swelling was determined performing dose-response experiments (0.3–30 μg; 6 h, n = 4/group; 24 h, 8–12/group) in which a “non-linear log(agonist) versus response (three parameters)” regression curve was fitted using GraphPad 6 software (La Jolla, CA, USA). Subsequently, the efficacy of 5αTHB was compared using doses corresponding to 1, 3 and 5 times the EC50 of corticosterone after 6 h (corticosterone, 10 μg; 5αTHB, 10–50 μg; n = 4–8/group) or 24 h (corticosterone, 5 μg; 5αTHB, 5–25 μg; n = 8–12/group). Mice were culled by asphyxiation with carbon dioxide and ears excised and wet weighed. Inflammatory swelling was evaluated as the wet weight difference between treated and untreated ears, and the effect of steroids is presented as a percentage of the response to CO alone (mean weight difference = 100%).
2.2.2. In vivo model of irritant dermatitis and treatment with RU486 in adrenalectomised mice
Male mice (n = 6–11/group) were injected, prior to treatment with steroids, with the GR antagonist RU486. Adrenalectomised mice were used to avoid the influence of increased physiological levels of glucocorticoids due to stress responses.
2.2.2.1. Surgery
Bilateral adrenalectomy surgery was performed through dorsal incisions, under isoflurane anaesthesia (Merial, Harlow, UK). After surgery, animals were maintained on 0.9% saline drinking water to maintain fluid and electrolyte balance and allowed to recover for one week before subsequent intervention.
2.2.2.2. RU486 treatments
RU486 (0.5 mg/mouse; 25 μL, 20 mg/mL in ethanol (vehicle)) was injected subcutaneously into mice 15 min prior to any topical treatment. Contact irritant dermatitis was induced and steroid treatment applied (24 h) as described in Section 2.2.1.
2.2.3. In vivo model of inflammatory angiogenesis
An autoclaved cubic polyurethane sponge (∼0.5 cm3; grade XE1700V, Caligen Foam Ltd., Accrington, U.K.) was implanted sub-cutaneously on each flank of mice anaesthetised with isoflurane (Merial, Harlow, UK). For the delivery of steroids, one silastic pellet (Silastic 20 medical grade, Dow Corning®, Midland, USA) impregnated with vehicle (silastic only), corticosterone (3 mg/pellet) or 5αTHB (3 or 15 mg/pellet) was inserted in each sponge. Validation of the preparation and release properties of these pellets has been reported [10]. Each animal in the treatment groups had an intervention-impregnated sponge (corticosterone or 5αTHB) implanted on the right side, and a vehicle sponge on the left. Animals in the control group had vehicle sponges implanted on each side. 21 days after surgery, mice were euthanized, sponges excised and pellets removed. Sponges were bisected and one half was preserved in 10% formalin (in PBS) and the other immersed in RNAlater solution.
2.3. Laboratory analysis
2.3.1. Histological analysis
Paraffin-embedded ear tissue and sponge slices (5 μm) were re-hydrated and stained with haematoxylin and eosin (H&E) or with Picrosirius red solution (PRS) for collagen content analysis (sponges only), prior to dehydration and mounting. Images were captured using the software QCapture Pro 7 (QImaging, Canada). On H&E stained ear sections the width of the dermis (in μm) and cell infiltration (number of nuclei) were assessed as further measurements of swelling and inflammation at both sides of the central cartilage layer. For each parameter three different areas were analysed and the mean value calculated.
2.3.2. Quantitation of neutrophils by myeloperoxidase activity
Myeloperoxidase (MPO) activity in ear tissue was quantified using a fluorometric detection kit (ENZO Life Sciences (Exeter, UK)).
2.3.3. Quantification of angiogenesis, macrophage infiltration and collagen content in sponges
For each sponge section vessels were counted in three different areas at magnification 100X. Vessels in sections stained with H&E were recognized by their round appearance and the presence of erythrocytes in the lumen. Vessels identified in sections immunostained for CD31 and αSMA marker were counted. The numbers of newly formed vessels and vessels positive for either CD31 or αSMA are represented as percentage of the vehicle group (mean value = 100%). Macrophages were counted as the number of cells positive for the marker F4/80 and expressed as a proportion of the total number of cells (blue nuclei; magnification 200X). PRS staining was quantified calculating the number of red pixels at a magnification of 100X using ImageJ software (NIH, USA).
2.3.4. Immunostaining for CD31, αSMA and F4/80 in sponges
Immunostaining of sponge slices was performed using a Leica Staining Robot with robotic antigen retrieval for F4/80 using trypsin solution (0.5 mg/mL in PBS, 10 min, 37 °C), or for αSMA manual antibody retrieval using a pressure cooker under standard conditions (citrate NCL pH 6 buffer); antigen retrieval was not performed for CD31. For F4/80 and αSMA, samples were blocked (5 min) with hydrogen peroxide solution from the Leica Refine Detection Kit (Leica Biosystems, Milton Keynes, UK) followed by serum block (30 min; F4/80, Immpress anti-rat (mouse absorbed) kit (Vector Laboratories, Peterborough, UK); αSMA, Mouse on Mouse Abcam Kit (Cambridge, UK)). Thereafter, samples were incubated with primary antibody (30 min; F4/80 1:300 (eBiosciences, Hatfield, UK); αSMA 1:4000 (Sigma-Aldrich, Dorset, UK)) prior to incubation with polymer (30 min, F4/80 Impress Kit as above; αSMA as above). For CD31 a Leica Refine Kit (as above) was used, which included a hydrogen peroxide block (5 min), primary antibody (120 min, 1:200 (Abcam)) and polymer incubation (15 min). Immunostaining was completed with incubation with 3,3′-diaminobenzidine (10 min) and counterstaining with haematoxylin (5 min), both from the Leica Refine Kit.
2.3.5. Total RNA extraction and PCR
Total RNA was extracted from ears and sponges using the RNeasy Fibrous Tissue Mini Kit, and first strand cDNA synthesis was performed using the QuantiTect Reverse Transcription kit (Qiagen Ltd, West Sussex, UK). Real-time quantitative PCR was performed using a LightCycler®480 (Roche Diagnostics, Mannheim, Germany) with primers (6 pmol each, Table 1), corresponding 5′ FAM-labelled probe (UPL, 2 pmol) and LightCycler®480 Probes Master [11]. Data were normalized for the mean of the transcript abundance of two housekeeping genes, TATA-binding protein and glyceraldehyde 3-phosphate dehydrogenase, the abundances of which did not differ between groups, and represented as percentage of the respective control group whose mean value was set to 100%.
Table 1.
Assay details for real-time PCR of murine samples. UPL denotes Universal Probe Library fluorescent probe number (Roche Diagnostics Ltd, Burgess Hill, UK). Gene names: Acta2 = actin, alpha 2, smooth muscle; Anxa1 = annexin A1; Ccl4 = chemokine (C-C motif) ligand 4; Col1a1, Col1a2, Col3a1, Col4a1 = collagen, type 1-3-4, alpha 1-2; Cxcl = chemokine (C-X-C motif) ligand; Dusp1 = dual specificity phosphatase 1; E-selectin = selectin, endothelial; Gapdh = glyceraldehyde 3-phosphate dehydrogenase; Icam = intercellular adhesion molecule; Il = interleukin; Ifnγ = interferon gamma; L-selectin = selectin, lymphocytes; Mcp1 = monocyte chemoattractant protein 1; Mmp = matrix metallopeptidase; Pecam1 = platelet/endothelial cell adhesion molecule 1; P-selectin = selectin, platelets; Tbp = TATA-binding protein: Timp2 = tissue inhibitor of metalloproteinase 2; Tnf α = tumour necrosis factor alpha; VE-cadherin = vascular-endothelial cadherin; Vcam1 = vascular cell adhesion molecule 1; Vegfα = vascular endothelial growth factor alpha; Vegfr2 = vascular endothelial growth factor receptor 2.
| Gene Symbol | Forward primer | Reverse Primer | UPL |
|---|---|---|---|
| Acta2 | ctctcttccagccatctttcat | tataggtggtttcgtggatgc | 58 |
| Anxa1 | ctttgccaagccatcctg | tgggatgtctagtttccacca | 21 |
| Ccl4 | ccagcagtctttgctccaa | gctcactggggttagcaca | 34 |
| Col1a1 | acctaagggtaccgctgga | tccagcttctccatctttgc | 19 |
| Col1a2 | cacctggtcctgttggaagt | caccagggaagccagtca | 9 |
| Col3a1 | tcccctggaatctgtgaatc | tgagtcgaattggggagaat | 49 |
| Col4a1 | agttggaggaatgggcttg | ccagggacaccctgtgag | 80 |
| Cxcl1 | gactccagccacactccaac | tgacagcgcagctcattg | 83 |
| Cxcl2 | ccctggttcagaaaatcatcc | cttccgttgagggacagc | 63 |
| Dusp1 | tggttcaacgaggctattgac | ggcaatgaacaaacactctcc | 89 |
| E-selectin | acagcagggcaacatgaaat | caactggacccattttggaa | 48 |
| FilaminA | tccctcagtcctttcaggtg | gcactttgacctgcagtgg | 78 |
| Gapdh | aggcaaaagacaccgtcaag | agaagatgcggctgtctctg | 52 |
| Icam1 | ttggagctagcggaccag | ccggagctgaaaagttgtaga | 80 |
| Icam2 | gcactcggagagtctcaaca | gctgcagcttcagtgtgact | 75 |
| Il1β | tgtaatgaaagacggcacacc | tcttctttgggtattgcttgg | 78 |
| Il6 | gctaccaaactggatataatcagga | ccaggtagctatggtactccagaa | 6 |
| Ifnγ | ctcaggaagcggaaaagga | aaaattcaaatagtgctggcaga | 60 |
| Keratin6 | gccaaggcagacagtctaaca | caggctacggttgttgtcc | 55 |
| L-selectin | tggtcatctccagagccaat | gcagtccatggtacccaact | 47 |
| Mcp1 | catccacgtgttggctca | gatcatcttgctggtgaatgagt | 62 |
| Mmp2 | tgcagggtggtggtcatag | tcacgctcttgagactttgg | 78 |
| Mmp9 | cagaggtaacccacgtcagc | gggatccaccttctgagactt | 7 |
| Mmp10 | gagtctggctcatgcctacc | caggaataagttggtccctga | 81 |
| Pecam1 | cggtgttcagcgagatcc | actcgacaggatggaaatcac | 45 |
| P-selectin | agcgttgcaatgtccagagt | ggatccgagcagttcacct | 48 |
| Tbp | gggagaatcatggaccagaa | gatgggaattccaggagtca | 97 |
| Timp2 | aggtaccagatgggctgtga | gtccatccagaggcactcat | 52 |
| Tnfα | ttgagatccatgccgttg | ctgtagcccacgtcgtagc | 25 |
| VE-cadherin | tcaccttctgtgaggagatgg | gatgatcagcaaggtaatcactgt | 6 |
| Vcam1 | tcttacctgtgcgctgtgac | gacctccacctgggttctct | 47 |
| Vegfα | aaaaacgaaagcgcaagaaa | tttctccgctctgaacaagg | 1 |
| Vegfr2 | accagagaccctcgttttca | catttgcttgcaggaggttt | 22 |
2.4. Data analysis
All data were analysed using GraphPad Prism6 software and presented as mean ± SEM, using statistical tests as indicated. Significant differences were reported when p ⩽ 0.05. For histological analysis, the operator was blinded to treatment.
3. Results
3.1. 5αTHB reduces croton oil-induced inflammation with a different time course than corticosterone
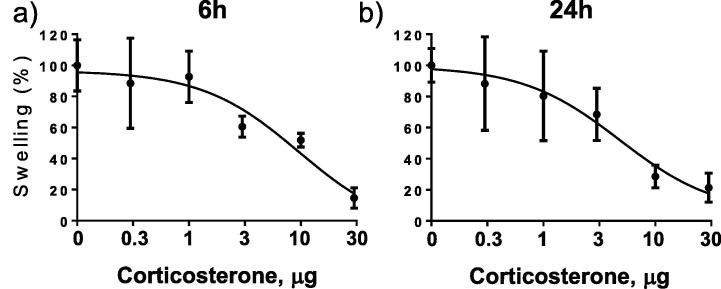
Increasing concentrations of corticosterone reduced croton oil-induced ear swelling in a dose-dependent manner after 6 and 24 h (Fig. 1a, b), with EC50 doses of 10 μg at 6 h and 5 μg at 24 h. 5αTHB reduced swelling at 24 h (EC50 dose: 25 μg) but not at 6 h (Fig. 2a–d). Only samples treated with the highest doses of 5αTHB (50 μg at 6 h and 25 μg at 24 h) were subject to further analysis.
Fig. 1.
Corticosterone reduced croton oil-induced swelling in mouse ears in a dose-dependent manner at 6 and 24 h in a model of irritant dermatitis. Reduction in inflammatory swelling by increasing dose (μg) of corticosterone after (a) 6 and (b) 24 h of treatment with croton oil (6 h, n = 4/group; 24 h, n = 8–12/group). Data are mean ± SEM. Corticosterone was applied to mouse ears in a volume of 10 μL vehicle.
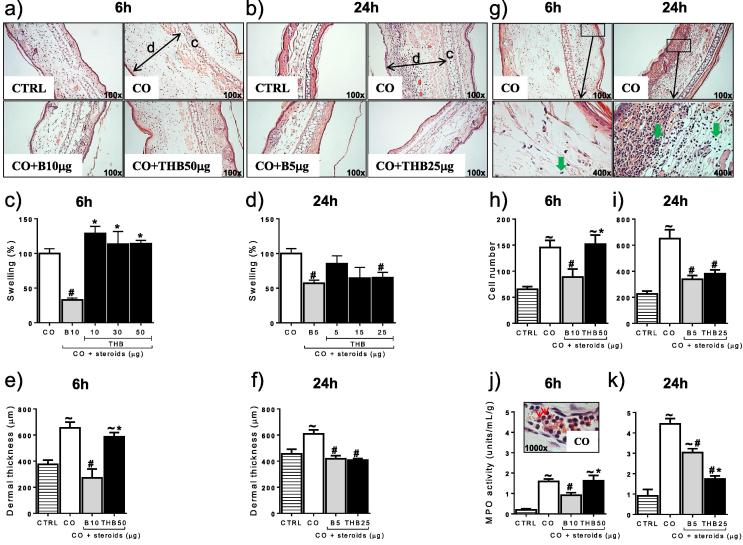
Fig. 2.
Corticosterone (B) and 5α-tetrahydrocoticosterone (THB) reduced swelling, dermal width, cell infiltration and myeloperoxidase (MPO) activity in mouse ears with a different time course in a model of croton oil (CO)-induced irritant dermatitis. Representative images of ear tissue stained with H&E showing inflammatory swelling and changes in dermal thickness (a and b) and cell infiltration (g) after treatment with CO alone or together with steroids for 6 or 24 h, as indicated; c = cartilage & d = dermis. Magnification = 100x and 400x as indicated on the images; green arrows in (g) point to cell nuclei of infiltrating cells. Quantification of swelling (c, d), dermal thickness (e, f), cell number (h, i) and MPO activity (j, k) in ears of mice after treatment for 6 or 24 h with CO alone or combined with steroids as indicated. CTRL = control ears. In (j) a representative high magnification (1000x) image of neutrophils (red arrows) in a blood vessel of a CO-treated ear is provided. The dose of each steroid in the figure refers to the total dose applied to the mouse ear in a volume of 10 μL; B 5/10 = B 5/10 μg, THB 5/10/15/25/30/50 = 5αTHB 5–50 μg; data (mean ± SEM) were analysed by one-way ANOVA followed by Tukey’s post hoc test; in (c) n = 4–8/group, in (d) n = 8–12/group; in (e), (f), (h), (i), (j) and (k) n = 6–10 tissue sections/group; p ⩽ 0.05, ∼ vs CTRL, # vs CO, * vs B.
Histological and biochemical analyses showed that croton oil-induced swelling was accompanied by enlargement of the dermal layer at both 6 and 24 h (Fig. 2a, b, e, f), cell infiltration (Fig. 2g–i) and increased MPO activity (Fig. 2j, k). Application of corticosterone reduced dermal width at 6 and 24 h but 5αTHB only had this effect at 24 h (Fig. 2a, b, e, f). Corticosterone and 5αTHB reduced cell accumulation to a similar extent at 24 h, although corticosterone also had this effect after only 6 h (Fig. 2h, i). Moreover, 5αTHB was more effective than corticosterone in suppressing MPO activity, an indicator of neutrophil activity, after 24 h, but did not have any effect at 6 h (Fig. 2j, k).
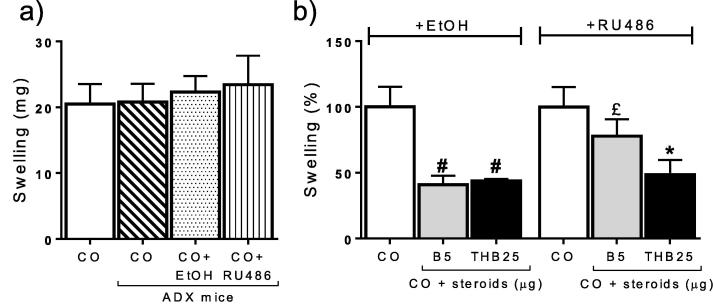
The involvement of GR in mediating the anti-inflammatory effects of corticosterone and 5αTHB was tested in adrenalectomised mice injected with the GR antagonist RU486. Neither adrenalectomy nor ethanol nor RU486 injection influenced the extent of CO-induced swelling (Fig. 3a). Injection of RU486 attenuated the anti-inflammatory effect of corticosterone but not that of 5αTHB (Fig. 3b).
Fig. 3.
The glucocorticoid receptor antagonist RU486 attenuated the anti-inflammatory effect of corticosterone (B) but not of 5α-tetrahydrocoticosterone (THB) on croton oil (CO)-induced ear swelling at 24 h in a model of irritant dermatitis in adrenalectomised (ADX) mice. (a) Comparable swelling (mg) induced by CO in ears of non-ADX versus ADX mice treated with CO alone or in combination with ethanol (vehicle, EtOH) or RU486. (b) CO-induced ear swelling in ADX mice treated either with CO alone or together with steroids after injection of either EtOH or RU486. Numbers after steroid refer to the total dose in μg applied to the ear in a volume of 10 μL. Data (mean ± SEM) were analysed by one-way ANOVA followed by Tukey’s post hoc test; n = 7–11/group; p ⩽ 0.05, # vs CO + EtOH, * vs CO + RU486, £ vs CO + B5 + EtOH.
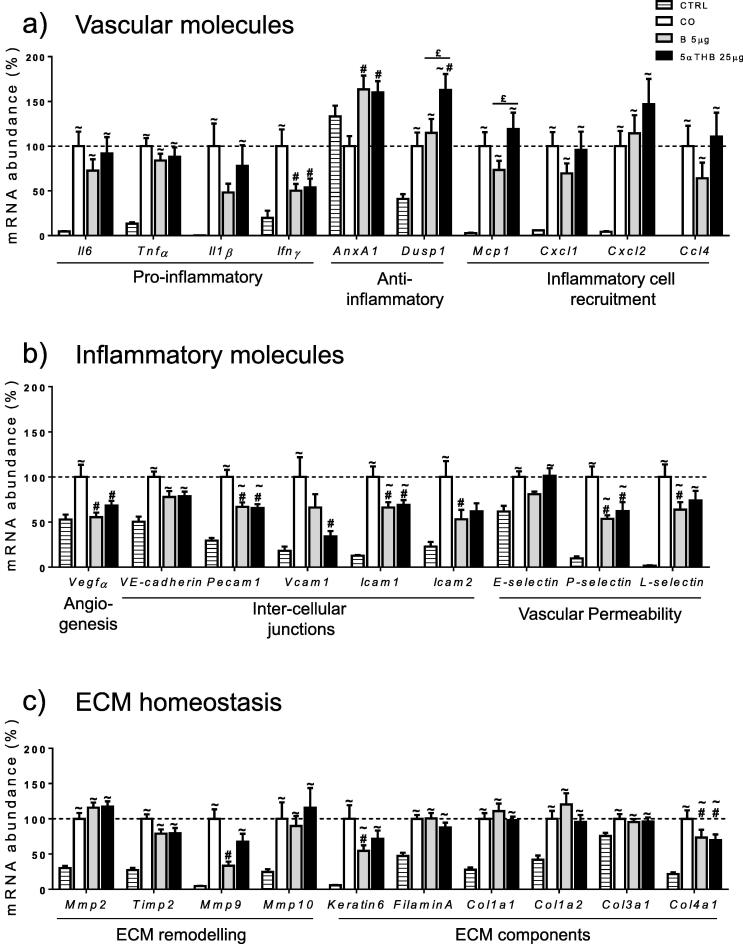
At 24 h, croton oil increased all transcripts characterising inflammatory responses, altered vascular permeability and extra-cellular matrix (ECM) regulation, with the exception of AnxA1 (Fig. 4a–c). Corticosterone reduced abundance of Ifnγ, Vegfα, Pecam1, Icam1, Icam2, P- and L-selectin, Mmp9, Keratin6 and Col4a1 and increased abundance of AnxA1 compared to the group receiving croton oil alone. 5αTHB caused similar changes to those of corticosterone, with the exception of Icam2, L-selectin, Mmp9 and Keratin6 which were not affected; additionally, 5αTHB increased transcripts of the anti-inflammatory gene Dusp1 and decreased those of Vcam1.
Fig. 4.
Corticosterone (B) and 5α-tetrahydrocoticosterone (5αTHB) had limited but similar effects on a variety of gene transcripts in mouse ears in a model of croton oil (CO)-induce irritant dermatitis after 24 h. Real-time PCR analysis of transcripts for (a) inflammatory, (b) vascular and (c) extra-cellular matrix (ECM) genes in control ears (CTRL) or ears treated for 24 h with either CO alone or together with B 5 μg or 5αTHB 25 μg. Data (mean ± SEM) were analysed with one-way ANOVA followed by Tukey’s post hoc test; n = 10/group; p ⩽ 0.05, # = vs CO, ∼ = vs CTRL, £ = B vs 5αTHB.
3.2. 5αTHB and corticosterone have discrepant effects on de novo angiogenesis in sponges
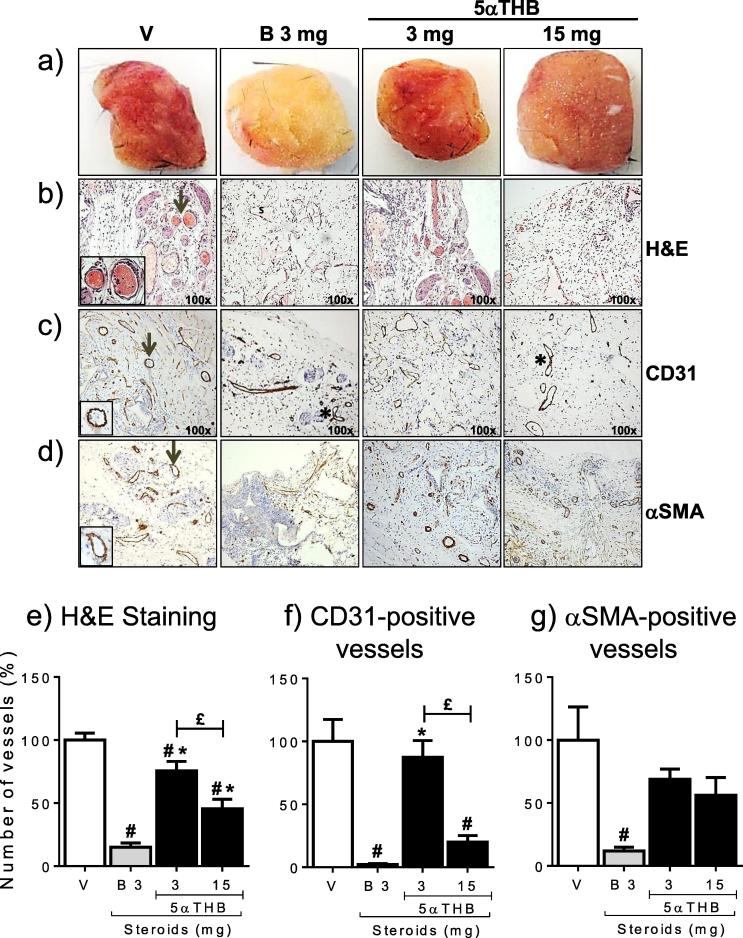
Sponges containing vehicle pellets (V) were bright red, suggesting extensive vascularization, whereas those with corticosterone pellets (3 mg) were pale (similar to pre-implantation sponges) indicating less vascularization (Fig. 5a). Sponges with 5αTHB (3 mg) had a similar appearance to vehicle-treated sponges, whereas those with 15 mg 5αTHB were somewhat paler, although the lack of vascularization was not as extensive as that shown by sponges containing corticosterone.
Fig. 5.
Corticosterone (B) but not 5α-tetrahydrocorticosterone (5αTHB) dramatically reduced vessel number in a model of in vivo angiogenesis. (a) Representative macroscopic pictures of sponges retrieved from mice after 20 days in situ, and containing either vehicle pellets (V) or pellets loaded with either B (3 mg) or 5αTHB (3 mg & 15 mg) as indicated. Representative microscopic images (magnification = 100x) of sections of sponges stained with (b) haematoxylin and eosin (H&E) or immunostained for (c) the endothelial marker CD31 or (d) for the smooth muscle cell marker αSMA. Black arrows point either to vessels recognisable by H&E staining or positive for the marker indicated and magnified in the corresponding inset (400x) of each image; * = examples of vessels undergoing sprouting angiogenesis. Quantification of newly formed vessels in sponges containing either vehicle (V), B or 5αTHB and stained with (e) H&E or positive for (f) CD31 or (g) αSMA. Numbers on the x axis refer to the amount (mg) of steroids in each sponge; data (mean ± SEM) were analysed with one-way ANOVA followed by Tukey’s post hoc test; n = 8–12 mice/group; p ⩽ 0.05, # = vs V, * = vs B, £ = 5αTHB 3 mg vs 15 mg.
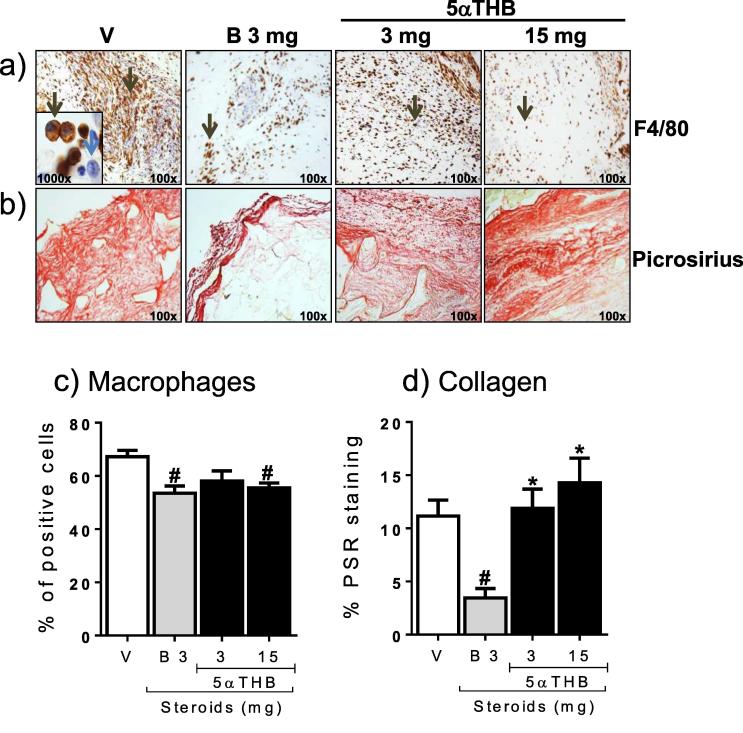
Vessels were identified by H&E staining (Fig. 5b), and immunoreactivity for CD31 (Fig. 5c) and αSMA (Fig. 5d) associated with vascular structures. Quantification showed that corticosterone dramatically reduced vessel density whether assessed by H&E (Fig. 5e), CD31 (Fig. 5f) or αSMA (Fig. 5g) staining. In contrast, an equivalent dose (3 mg) of 5αTHB in sponges had little effect on vascular density (Fig. 5e, f, g). The higher dose (15 mg: equipotent in anti-inflammatory model) reduced vascular density assessed by H&E (Fig. 5e) and CD31 (Fig. 5f) staining but less dramatically than with corticosterone. Strikingly, 5αTHB had little effect on the density of αSMA positive vessels (Fig. 5g). Macrophage infiltration of sponges was observed (Fig. 6a, c) and was reduced following treatment with corticosterone or 5αTHB, although this did not achieve significance at the lower dose of 5αTHB (Fig. 6c). Collagen staining (Fig. 6b, d) was lower in sponges treated with corticosterone, compared with vehicle-treated controls, whereas 5αTHB had no effect at either concentration.
Fig. 6.
Corticosterone (B) and 5α-tetrahydrocorticosterone (5αTHB) decreased macrophage infiltration in sponges but only B reduced collagen content. Representative microscopic images (magnification 100x) of sections of sponges stained for (a) the macrophage marker F4/80 or for (b) collagen with Picrosirius red and containing either vehicle (V), B (3 mg) or 5αTHB (3 and 15 mg) as indicated. Black arrows in (a) point to cells expressing F4/80; the blue arrow in the inset (1000x) identifies the nucleus of a cell negative for F4/80. Quantification of (c) cells positive for F4/80 and (d) collagen content in sponges containing either V, B or 5αTHB; numbers on the x axis refer to the amount (mg) of steroids in each sponge. Data (mean ± SEM) were analysed with one-way ANOVA followed by Tukey’s post hoc test; n = 8–12 mice/group; p ⩽ 0.05, # = vs V, * = B vs 5αTHB.
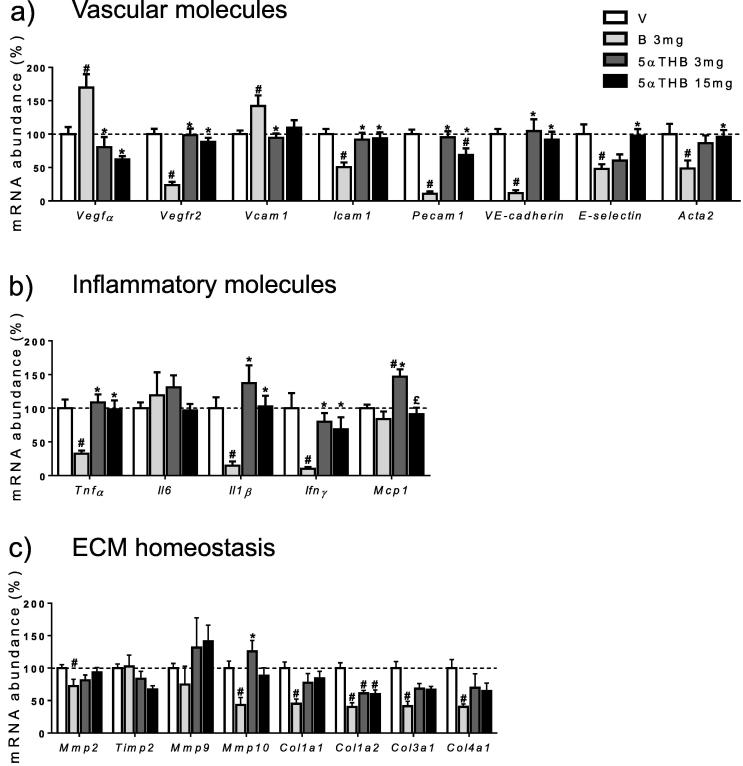
Corticosterone treatment was associated with increased transcripts for Vegfα and Vcam1 whereas transcripts for Vegfr2, Icam1, Pecam1 (CD31), VE-cadherin, E-selectin and Acta2 (αSMA) were decreased (Fig. 7a). In contrast, 5αTHB only decreased transcripts of Pecam1. Corticosterone reduced the transcript abundance for the inflammatory genes Tnfα, Il1β and Ifnγ but had no effect on Mcp1 or Il6 (Fig. 7b). In contrast, neither concentration of 5αTHB reduced any of the gene transcripts, with the lower concentration (3 mg) in fact increasing the abundance of transcripts for Mcp1 (Fig. 7b). In sponges containing corticosterone, transcripts for two matrix metalloproteinases (Mmp2; Mmp10) and four collagen types (Cola1a1, Col1a2, Col3a1, Col4a1) were reduced compared to vehicle (Fig. 7c) but tissue inhibitor of metalloproteinases (Timp1) and matrix metalloproteinase Mmp9 was unchanged. In contrast, 5αTHB (3 mg & 15 mg) had limited effects, only decreasing Col1a2.
Fig. 7.
Corticosterone (B) affected gene transcripts in sponges to a greater degree than 5α-tetrahydrocorticosterone (5αTHB). Real-time PCR analysis of transcripts of (a) vascular, (b) inflammatory and (c) extra-cellular matrix (ECM) genes in sponges containing either vehicle (V), B 3 mg or 5αTHB 3 and 15 mg as indicated. Data (mean ± SEM) were analysed with one-way ANOVA followed by Tukey’s post hoc test; n = 8–12 mice/group; p ⩽ 0.05, # = vs V, * = vs B, £ = 5αTHB 3 mg vs 15 mg.
4. Discussion
Anti-inflammatory properties, but a safer systemic side effect profile, of 5αTHB, an A-ring reduced metabolite of corticosterone, have been demonstrated previously in vivo in a murine model of thioglycollate-induced peritonitis [7]. This investigation addressed the hypotheses that 5αTHB has advantages over conventional glucocorticoids as a topical treatment for irritant dermatitis, and that its actions are mediated through GR. 5αTHB was demonstrated to be an effective topical anti-inflammatory agent in an in vivo model of irritant dermatitis induced by croton oil, with effects mainly on pathways regulating vascular permeability to cell infiltration rather than suppression of pro-inflammatory markers. However, experiments using the GR antagonist RU486 suggested these effects may not be mediated by occupation of GR. Furthermore, in comparison with corticosterone, use of 5αTHB may be associated with fewer adverse local effects (less inhibition of angiogenesis and collagen deposition).
The direct suppression of inflammation by topical 5αTHB was assessed using croton oil-induced irritant dermatitis, a well-characterised, steroid-responsive model, used extensively in the pharmaceutical industry [6], [12], [13], [14]. Croton oil promotes the release of cytokines and chemokines from keratinocytes and other cells, stimulating in turn the release of pro-inflammatory cytokines such as IL1β and IL6 from epidermal and dermal cells, causing swelling and infiltration of leukocytes [15], [16], [17], [18]. The predominant effect at the early stages of the process is tissue oedema which peaks 6 h-post treatment; this phase is followed by cell infiltration (mainly neutrophils) which is at its highest between 12 and 24 h [19], [20], [21], [22]. This time course of changes was recapitulated here: at 6 h croton oil produced a marked swelling accompanied by cell infiltration and increased MPO activity, whereas at 24 h swelling persisted but was reduced, while cell infiltration and MPO activity increased further.
This model has been used extensively to test novel anti-inflammatory drugs, including glucocorticoids (e.g. dexamethasone, betamethasone and hydrocortisone) [21], [23], [24], [25], [26]. Here, the effects of 5αTHB on skin inflammation were compared with those of corticosterone, the active rodent glucocorticoid. Previous reports of corticosterone efficacy were not available but our data suggest that at 6 h it is twice as potent as one of the most frequently employed topical glucocorticoids, hydrocortisone [21], [27]. Notably, the dose-dependent reduction of dermal inflammation by 5αTHB exhibited a slower time course than corticosterone with no effect at 6 h. Significant suppression of inflammation was, however, achieved at 24 h, albeit requiring a 3–5 fold higher dose than corticosterone to match efficacy. This suggests that 5αTHB may predominantly target cell recruitment, which is prominent at 24 h post CO-treatment, with neutrophils believed to be the most abundant cell type present [19], [20], [21], [22]. Analysis of MPO activity showed that both steroids reduced neutrophil infiltration; interestingly, this was suppressed more effectively by 5αTHB than by corticosterone at 24 h. The lack of response to 5αTHB to reduce swelling at 6 h was accompanied by a failure to reduce cell number.
Experiments with the GR antagonist RU486 suggested that the anti-inflammatory effect of B on swelling is mediated by GR. However, this seems not to be the case for 5αTHB. The fact that the anti-inflammatory actions of 5αTHB were not antagonised by RU486 was unexpected, albeit in keeping with its different time course of action to corticosterone. 5αTHB is a glucocorticoid metabolite, previously shown capable of displacing dexamethasone from rat GR in vitro [28]. RU486 acts as a GR antagonist by binding to the ligand-binding domain, blocking the recruitment of essential GR co-activators [29], [30], and if 5αTHB does operate through GR it does so by an unconventional mechanism, perhaps through allosteric means. Further genetic and pharmacological studies are now required to elucidate the mechanism of action of 5αTHB.
To start to address this, the profile of action of 5αTHB was explored further through expression profiling and compared with responses produced by corticosterone. At 24 h, the impact of 5αTHB on expression of genes involved in regulation of inflammation and vascular homeostasis in the CO model was strikingly similar to those of corticosterone. A noted difference was in the upregulation of Dusp1 by 5αTHB in contrast to corticosterone. DUSP1 is a negative regulator of the MAPK signal transduction pathway, and is increased in response to inflammatory and irritant stimuli, leading to decreased production of cytokines, chemokines and adhesion molecules [31], [32]. A glucocorticoid-mediated increase in its protein abundance has been reported previously in many cell types [33], [34], [35], [36], [37]. DUSP1 is also upregulated by non-steroidal anti-inflammatory compounds [32] and by new selective modulators of GR [38]. 5αTHB and corticosterone also increased transcripts of the anti-inflammatory gene AnxA1 (lipocortin). Lipocortin was originally described as being responsible for the anti-inflammatory properties of glucocorticoids [39], again diminishing neutrophil recruitment by suppressing adhesion to endothelial cells [39]. Both corticosterone and 5αTHB strongly suppressed many factors involved with tissue permeability and cell recruitment. The effects of 5αTHB on expression of ECM proteins were less marked than those of corticosterone, whereas both lacked effects on transcription of most collagen genes, only decreasing Col4a1 transcripts. Type IV collagen is one of the minor components of the skin (less than 5%) [5], mainly forming the basement membrane; as a consequence both steroids are likely to have little impact on skin thickness of croton-oil treated ears after 24 h. However accurate measures of skin thickness cannot be made in the inflamed skin in vivo. Previous in vitro microarray studies on cultures of primary human keratinocytes showed that dexamethasone, a glucocorticoid known to cause skin thinning, decreases transcripts of Mmp9, Mmp10, Keratin6, FilaminA and Col4a1 after 24 h incubation [40], a profile similar to that of corticosterone in our model.
Two of the major side effects of topical anti-inflammatory glucocorticoids are skin thinning and delayed wound healing, requiring careful assessment of potential adverse effects for any proposed topical steroid therapy. Angiogenesis and collagen turnover are important factors in skin homeostasis, with angiogenesis particularly important for dermal healing [41]. Following the demonstration that cortisone inhibits angiogenesis in vitro, a number of “angiostatic steroids” have been identified [42]. These include 5β-tetrahydrocortisol, one of the A-ring reduced metabolites of cortisol (the major glucocorticoid in humans). Here the impact of 5αTHB on angiogenesis, inflammation and collagen deposition was studied in response to implantation of subcutaneous sponges [43]. This model is steroid sensitive [44] and equipotent anti-inflammatory doses of corticosterone and 5αTHB (based on results from the croton-oil model) were administered in the sponges. 5αTHB reduced angiogenesis but to a lesser degree than, and with a different pattern to, corticosterone. It was notable that both compounds reduced vessel density, predominantly by reducing the number of CD31-positive vessels. However, unlike corticosterone, 5αTHB did not reduce the number of αSMA positive vessels. CD31-staining identifies endothelial cells lining vascular structures and can be used to detect small (capillaries) and larger vessels [45]. In contrast, αSMA, staining detects pericytes and smooth muscle cells, and can be used to identify larger/maturing vessels with a functional medial layer [46]. Thus, these results suggest that, although 5αTHB does reduce new vessel formation, it does not adversely affect the number of mature vessels and, hence, may be less likely to attenuate wound healing. Caveats do, however, apply in that retention of 5αTHB and corticosterone within sponges was not directly compared in this study.
In terms of local inflammation, both corticosterone and 5αTHB inhibited macrophage infiltration. Inflammatory cells, in particular monocytes/macrophages, are central players in angiogenesis as they are a source of pro-angiogenic cytokines [47]. Indeed, it is often difficult to distinguish between direct angiostatic properties and those that are a consequence of reduced inflammation. In the croton oil model there was a bigger effect of 5αTHB compared to corticosterone on neutrophil infiltration, whereas this is not true regarding the recruitment of macrophages analysed in the angiogenesis model. This suggests that neutrophils might be the preferential cellular target for 5αTHB in an inflammatory setting. Importantly, macrophages have a pivotal role in wound repair [41], and a compound with fewer inhibitory effects on their infiltration might be more desirable.
Relatively few changes in gene transcripts for factors that regulate aspects of vascular function were consistent with 5αTHB having a limited effect on the developing vasculature, with only a modest reduction in Pecam1 which support the immunostaining data for CD31. In contrast, corticosterone inhibited expression of most of the factors assessed (Icam1, Pecam1, VE-cadherin, E-selectin and Acta2), consistent with its potent anti-angiogenic properties [44], and again reinforce the data obtained with the immunostaining of CD31 and αSMA in sponge sections. These factors contribute to stabilisation of cell-cell contact between endothelial cells [45], and the dynamics of cell rearrangement central to vessel formation [48], [49]. The only exceptions were the increased expression of Vegfα and Vcam1. Vegfα has a key role in stimulating angiogenesis [50], [51], [52], so would be expected to be reduced by corticosterone (although it is possible that the increased expression is a consequence of insufficient angiogenesis in the sponges). However, this was accompanied by reduced Vegfr2 transcript number, consistent with inhibition of angiogenesis seen in previous studies with other glucocorticoids [53]. VEGFR2 is the main receptor responsible for the majority of the effects of VEGF on endothelial cells [53] and is associated with proliferation of new vessels during wound healing [53].
Intriguingly, despite its potent anti-inflammatory properties, corticosterone only produced a small reduction in transcripts of pro-inflammatory genes. 5αTHB, despite reducing macrophage infiltration, did not reduce expression of any of the transcripts analysed and, indeed, increased abundance of transcripts of Mcp1. A similar induction of Mcp1/MCP1 promoted vessel growth in infarcted hearts in mice [54], suggesting this may be a compensatory mechanism in response to an ischemic event. In previous studies of peritonitis 5αTHB also suppressed inflammatory cell recruitment, but with a more profound effect on neutrophils compared with macrophages [7].
The extra-cellular matrix (ECM) plays a vital role in wound healing and many studies show that glucocorticoids cause skin damage by depleting collagen isoforms [55]. This again could be modelled in the implanted sponges with consistent reductions in collagen deposition and expression of the four collagen isoform transcripts analysed in corticosterone-treated sponges. Importantly, 5αTHB had little effect on collagen staining, significantly reducing only Col1a2 expression. Skin collagen fibrils are made mostly of type 1 collagen, a heterodimer composed by three alpha chains, two encoded by Col1a1 and one by Col1a2 [5]. The lack of effects on Col1a1 transcripts by 5αTHB might explain why this compound did not affect the overall collagen content in sponges. Furthermore, the absence of effect on Col4a1 transcripts suggest a reduced impact, compared to corticosterone, on angiogenesis, as type IV collagen is a major component of the basement membrane (facilitating the differentiation of endothelial cells during formation of capillary-like structures) [56]. This contrasts with the dermatitis model where both steroids had a small effect to reduce Col4a1, but over a shorter time course. Metalloproteinase enzymes (MMPs) influence wound healing, predominantly by promoting ECM degradation and cell migration [57], whereas tissue inhibitors of MMPs (TIMPs) oppose these actions. Corticosterone, but not 5αTHB, decreased expression of MMPs (Mmp10 and Mmp2) within the sponges, which may contribute to alterations in matrix composition and to inhibition of angiogenesis [58], [59].
Studies of mechanism in the croton oil and sponge model were mainly limited to analysis of transcript abundance, due to paucity of tissue. While complementary analysis confirmed effects on CD31 (Pecam1), αSMA (Acta2) and collagen protein, GCs have been shown to inhibit translation rather than transcription for some molecules involved in inflammation [60], [61]. As a consequence, while no significant effect of 5αTHB or B was found on transcription of cytokines, it remains possible that they may be affecting the translation of those transcripts.
In conclusion, 5αTHB is an effective topical anti-inflammatory steroid, reducing acute skin inflammation possibly binding to a different receptor than GR. Its limited effects on angiogenesis and de novo deposition of collagen (combined with previous reports of its rapid systemic elimination and, therefore, reduced metabolic side-effects), suggest it may have potential as a novel topical anti-inflammatory treatment and careful dissection of its mechanism of action is now required.
Conflicts of interest
RA and BRW are inventors on a relevant patent held by the University of Edinburgh.
Acknowledgements
We thank the British Heart Foundation and Wellcome Trust for funding. BRW is a Wellcome Trust Senior Investigator. We would also like to thank the staff of the Biomedical Research Facility and the Histology Core of the Shared University Research Facilities (SuRF), Little France Campus, Edinburgh, for their help with in vivo experiments and histological analysis.
Contributor Information
Annalisa Gastaldello, Email: A.Gastaldello@soton.ac.uk.
Dawn E.W. Livingstone, Email: Dawn.Livingstone@ed.ac.uk.
Amber J. Abernethie, Email: s1358252@sms.ed.ac.uk.
Nicola Tsang, Email: nhyt3@cam.ac.uk.
Brian R. Walker, Email: B.Walker@ed.ac.uk.
Patrick W.F. Hadoke, Email: Patrick.Hadoke@ed.ac.uk.
Ruth Andrew, Email: Ruth.Andrew@ed.ac.uk.
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oakley R.H., Cidlowski J.A. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013;132(5):1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonough A.K., Curtis J.R., Saag K.G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 2008;20(2):131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 4.Hengge U.R., Ruzicka T., Schwartz R.A., Cork M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006;54(1):1–15. doi: 10.1016/j.jaad.2005.01.010. quiz 6-8. [DOI] [PubMed] [Google Scholar]
- 5.Schacke H., Docke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 6.Schacke H., Zollner T.M., Docke W.D., Rehwinkel H., Jaroch S., Skuballa W. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br. J. Pharmacol. 2009;158(4):1088–1103. doi: 10.1111/j.1476-5381.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C., Nixon M., Kenyon C.J., Livingstone D.E., Duffin R., Rossi A.G. 5alpha-Reduced glucocorticoids exhibit dissociated anti-inflammatory and metabolic effects. Br. J. Pharmacol. 2011;164(6):1661–1671. doi: 10.1111/j.1476-5381.2011.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Young L.M., Kheifets J.B., Ballaron S.J., Young J.M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989;26(3–4):335–341. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- 9.Cabrini D.A., Moresco H.H., Imazu P., da Silva C.D., Pietrovski E.F., Gasparin D.A. Analysis of the potential topical anti-inflammatory activity of Averrhoa carambola L. in mice. Evid. Based Complement. Alternat. Med. 2011;2011 doi: 10.1093/ecam/neq026. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soro A., Panarelli M., Holloway C.D., Fraser R., Kenyon C.J. In vivo and in vitro effects of carbenoxolone on glucocorticoid receptor binding and glucocorticoid activity. Steroids. 1997;62(4):388–394. doi: 10.1016/s0039-128x(96)00252-8. [DOI] [PubMed] [Google Scholar]
- 11.Livingstone D.E., Barat P., Di Rollo E.M., Rees G.A., Weldin B.A., Rog-Zielinska E.A. 5alpha-Reductase type 1 deficiency or inhibition predisposes to insulin resistance, hepatic steatosis, and liver fibrosis in rodents. Diabetes. 2015;64(2):447–458. doi: 10.2337/db14-0249. [DOI] [PubMed] [Google Scholar]
- 12.Mirshahpanah P., Docke W.D., Merbold U., Asadullah K., Rose L., Schacke H. Superior nuclear receptor selectivity and therapeutic index of methylprednisolone aceponate versus mometasone furoate. Exp. Dermatol. 2007;16(9):753–761. doi: 10.1111/j.1600-0625.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 13.Schacke H., Schottelius A., Docke W.D., Strehlke P., Jaroch S., Schmees N. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc. Natl. Acad. Sci. USA. 2004;101(1):227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vayssiere B.M., Dupont S., Choquart A., Petit F., Garcia T., Marchandeau C. Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol. Endocrinol. 1997;11(9):1245–1255. doi: 10.1210/mend.11.9.9979. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie R.C., Sauder D.N. The role of keratinocyte cytokines in inflammation and immunity. J. Invest. Dermatol. 1990;95(6 Suppl):105S–107S. doi: 10.1111/1523-1747.ep12874955. [DOI] [PubMed] [Google Scholar]
- 16.Lee H., Stieger M., Yawalkar N., Kakeda M. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013;2013:916497. doi: 10.1155/2013/916497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker J.N., Mitra R.S., Griffiths C.E., Dixit V.M., Nickoloff B.J. Keratinocytes as initiators of inflammation. Lancet. 1991;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 18.Effendy I., Loffler H., Maibach H.I. Epidermal cytokines in murine cutaneous irritant responses. J. Appl. Toxicol. 2000;20(4):335–341. doi: 10.1002/1099-1263(200007/08)20:4<335::aid-jat698>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli G., Thibault L., Ringler I. A bio-assay for the concomitant assessment of the antiphlogistic and thymolytic activities of topically applied corticoids. Endocrinology. 1965;77(4):625–634. doi: 10.1210/endo-77-4-625. [DOI] [PubMed] [Google Scholar]
- 20.Tubaro A., Dri P., Delbello G., Zilli C., Della Loggia R. The croton oil ear test revisited. Agents Actions. 1986;17(3–4):347–349. doi: 10.1007/BF01982641. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner L., Sosa S., Atanasov A.G., Bodensieck A., Fakhrudin N., Bauer J. Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. J. Nat. Prod. 2011;74(8):1779–1786. doi: 10.1021/np200343t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Towbin H., Pignat W., Wiesenberg I. Time-dependent cytokine production in the croton oil-induced mouse ear oedema and inhibition by prednisolone. Inflamm. Res. 1995;44(Suppl 2):S160–S161. doi: 10.1007/BF01778311. [DOI] [PubMed] [Google Scholar]
- 23.Muramoto K., Goto M., Inoue Y., Ishii N., Chiba K., Kuboi Y. E6201, a novel kinase inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-1 and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase-1: in vivo effects on cutaneous inflammatory responses by topical administration. J. Pharmacol. Exp. Ther. 2010;335(1):23–31. doi: 10.1124/jpet.110.168583. [DOI] [PubMed] [Google Scholar]
- 24.da Silva G.L., Sperotto N.D., Borges T.J., Bonorino C., Takyia C.M., Coutinho-Silva R. P2X7 receptor is required for neutrophil accumulation in a mouse model of irritant contact dermatitis. Exp. Dermatol. 2013;22(3):184–188. doi: 10.1111/exd.12094. [DOI] [PubMed] [Google Scholar]
- 25.Vassallo A., De Tommasi N., Merfort I., Sanogo R., Severino L., Pelin M. Steroids with anti-inflammatory activity from Vernonia nigritiana Oliv. & Hiern. Phytochemistry. 2013;96:288–298. doi: 10.1016/j.phytochem.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Pacheco N.R., Pinto Nde C., da Silva J.M., Mendes Rde F., da Costa Jde C., Aragao D.M. Cecropia pachystachya: a species with expressive in vivo topical anti-inflammatory and in vitro antioxidant effects. Biomed. Res. Int. 2014;2014:301294. doi: 10.1155/2014/301294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brantner A.H., Quehenberger F., Chakraborty A., Polligger J., Sosa S., Della Loggia R. HET-CAM bioassay as in vitro alternative to the croton oil test for investigating steroidal and non-steroidal compounds. Altex. 2002;19(2):51–56. [PubMed] [Google Scholar]
- 28.McInnes K.J., Kenyon C.J., Chapman K.E., Livingstone D.E., Macdonald L.J., Walker B.R. 5alpha-reduced glucocorticoids, novel endogenous activators of the glucocorticoid receptor. J. Biol. Chem. 2004;279(22):22908–22912. doi: 10.1074/jbc.M402822200. [DOI] [PubMed] [Google Scholar]
- 29.Kauppi B., Jakob C., Farnegardh M., Yang J., Ahola H., Alarcon M. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J. Biol. Chem. 2003;278(25):22748–22754. doi: 10.1074/jbc.M212711200. [DOI] [PubMed] [Google Scholar]
- 30.Clark R.D. Glucocorticoid receptor antagonists. Curr. Top. Med. Chem. 2008;8(9):813–838. doi: 10.2174/156802608784535011. [DOI] [PubMed] [Google Scholar]
- 31.Ryser S., Massiha A., Piuz I., Schlegel W. Stimulated initiation of mitogen-activated protein kinase phosphatase-1 (MKP-1) gene transcription involves the synergistic action of multiple cis-acting elements in the proximal promoter. Biochem. J. 2004;378(Pt 2):473–484. doi: 10.1042/BJ20031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayush O., Lee C.H., Kim H.K., Im S.Y., Cho B.H., Lee H.K. Glutamine suppresses DNFB-induced contact dermatitis by deactivating p38 mitogen-activated protein kinase via induction of MAPK phosphatase-1. J. Invest. Dermatol. 2012;133(3):723–731. doi: 10.1038/jid.2012.373. [DOI] [PubMed] [Google Scholar]
- 33.Kassel O., Sancono A., Kratzschmar J., Kreft B., Stassen M., Cato A.C. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20(24):7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham S.M., Clark A.R. Dual-specificity phosphatase 1: a critical regulator of innate immune responses. Biochem. Soc. Trans. 2006;34(Pt 6):1018–1023. doi: 10.1042/BST0341018. [DOI] [PubMed] [Google Scholar]
- 35.Abraham S.M., Lawrence T., Kleiman A., Warden P., Medghalchi M., Tuckermann J. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 2006;203(8):1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark A.R. Anti-inflammatory functions of glucocorticoid-induced genes. Mol. Cell. Endocrinol. 2007;275(1–2):79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Newton R., Holden N.S. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol. Pharmacol. 2007;72(4):799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 38.Joanny E., Ding Q., Gong L., Kong P., Saklatvala J., Clark A.R. Anti-inflammatory effects of selective glucocorticoid receptor modulators are partially dependent on up-regulation of dual specificity phosphatase 1. Br. J. Pharmacol. 2012;165(4b):1124–1136. doi: 10.1111/j.1476-5381.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliani S.M., Ciocca G.A., Pimentel T.A., Damazo A.S., Gibbs L., Perretti M. Fluctuation of annexin-A1 positive mast cells in chronic granulomatous inflammation. Inflamm. Res. 2008;57(10):450–456. doi: 10.1007/s00011-008-7222-7. [DOI] [PubMed] [Google Scholar]
- 40.Stojadinovic O., Lee B., Vouthounis C., Vukelic S., Pastar I., Blumenberg M. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J. Biol. Chem. 2007;282(6):4021–4034. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- 41.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 42.Folkman J., Ingber D.E. Angiostatic steroids. Method of discovery and mechanism of action. Ann. Surg. 1987;206(3):374–383. doi: 10.1097/00000658-198709000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira M.A., Barcelos L.S., Campos P.P., Vasconcelos A.C., Teixeira M.M., Andrade S.P. Sponge-induced angiogenesis and inflammation in PAF receptor-deficient mice (PAFR-KO) Br. J. Pharmacol. 2004;141(7):1185–1192. doi: 10.1038/sj.bjp.0705731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Small G.R., Hadoke P.W., Sharif I., Dover A.R., Armour D., Kenyon C.J. Preventing local regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1 enhances angiogenesis. Proc. Natl. Acad. Sci. USA. 2005;102(34):12165–12170. doi: 10.1073/pnas.0500641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson D.E. The unfolding tale of PECAM-1. FEBS Lett. 2003;540(1–3):7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 46.Bergers G., Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunderkotter C., Nikolic T., Dillon M.J., Van Rooijen N., Stehling M., Drevets D.A. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 48.Bentley K., Franco C.A., Philippides A., Blanco R., Dierkes M., Gebala V. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 2014;16(4):309–321. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 49.Goddard L.M., Iruela-Arispe M.L. Cellular and molecular regulation of vascular permeability. Thromb. Haemost. 2013;109(3):407–415. doi: 10.1160/TH12-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connolly D.T., Heuvelman D.M., Nelson R., Olander J.V., Eppley B.L., Delfino J.J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 1989;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoeben A., Landuyt B., Highley M.S., Wildiers H., Van Oosterom A.T., De Bruijn E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004;56(4):549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 53.Zhang N., Fang Z., Contag P.R., Purchio A.F., West D.B. Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood. 2004;103(2):617–626. doi: 10.1182/blood-2003-06-1820. [DOI] [PubMed] [Google Scholar]
- 54.Morimoto H., Takahashi M., Izawa A., Ise H., Hongo M., Kolattukudy P.E. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ. Res. 2006;99(8):891–899. doi: 10.1161/01.RES.0000246113.82111.2d. [DOI] [PubMed] [Google Scholar]
- 55.Oishi Y., Fu Z.W., Ohnuki Y., Kato H., Noguchi T. Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br. J. Dermatol. 2002;147(5):859–868. doi: 10.1046/j.1365-2133.2002.04949.x. [DOI] [PubMed] [Google Scholar]
- 56.Kubota Y., Kleinman H.K., Martin G.R., Lawley T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michopoulou A., Rousselle P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? Eur. J. Dermatol. 2015;25(Suppl. 1):33–42. doi: 10.1684/ejd.2015.2553. [DOI] [PubMed] [Google Scholar]
- 58.Shikatani E.A., Trifonova A., Mandel E.R., Liu S.T., Roudier E., Krylova A. Inhibition of proliferation, migration and proteolysis contribute to corticosterone-mediated inhibition of angiogenesis. PLoS One. 2012;7(10):e46625. doi: 10.1371/journal.pone.0046625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pross C., Farooq M.M., Angle N., Lane J.S., Cerveira J.J., Xavier A.E. Dexamethasone inhibits vascular smooth muscle cell migration via modulation of matrix metalloproteinase activity. J. Surg. Res. 2002;102(2):57–62. doi: 10.1006/jsre.2001.6220. [DOI] [PubMed] [Google Scholar]
- 60.Clark A.R. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J. Endocrinol. 2003;178(1):5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- 61.Clark A.R., Dean J.L., Saklatvala J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546(1):37–44. doi: 10.1016/s0014-5793(03)00439-3. [DOI] [PubMed] [Google Scholar]