Abstract
Vascularized composite allotransplantation represents a potential shift in approaches to reconstruction of complex defects resulting from congenital differences as well as trauma and other acquired pathology. Given the highly specialized function of the eye and its unique anatomical components, vascularized composite allotransplantation of the eye is an appealing method for restoration, replacement, and reconstruction of the nonfunctioning eye. Herein, we describe conventional treatments for eye restoration and their shortcomings as well as recent research and events that have brought eye transplantation closer to a potential clinical reality. In this article, we outline some potential considerations in patient selection, donor facial tissue procurement, eye tissue implantation, surgical procedure, and potential for functional outcomes.
Keywords: Composite tissue allotransplantation, eye transplant, face transplant, reconstructive transplantation, vascularized composite allotransplantation
Vascularized composite allotransplantation (VCA) is an established approach to reconstruction of complex defects resulting from trauma and congenital pathology. Face, abdominal wall, lower extremity, and hand and upper extremity transplants have now successfully been performed in a combined total of >100 known patients in 5 countries.1 As this treatment continues to grow in application, so does the scope to which it might be applied.
Given the highly specialized function of the eye and its unique anatomical components, VCA of the eye is appealing for restoration, replacement and reconstruction of the nonfunctioning eye. With the global prevalence of blindness estimated to be nearly 40 million, the potential for such treatment is vast.2 Furthermore, given that at least 3 patients known to have received face transplants to date have had loss of at least 1 functioning eye, concomitant eye transplant is an obvious adjunct in the reconstructive armamentarium for similar clinical scenarios in the future.3
The concept of eye transplant is not entirely novel but dates to 1885 when Chibret unsuccessfully transplanted a rabbit eye into a blind girl.4 In 1977, the advisory council for the National Eye Institute called for a ‘‘limited and thoughtful laboratory effort’’ in eye transplantation, concluding that ‘‘at present, any effort to transplant a mammalian eye is doomed to failure by the ganglion cell axons’ inability to withstand cutting, by the difficulty of insuring adequate circulation of blood to the transplanted eye.. .and and lastly by immune rejection of foreign tissue.’’4 Now with the advances in microsurgery and immunomodulation that have heralded successful VCA, together with new therapies in neuroregeneration, strategies for overcoming the challenges previously thought to undermine the possibility of eye transplantation are emerging.
Herein, we describe conventional treatments for eye restoration and their shortcomings as well as recent research and events that have brought eye transplantation closer to clinical reality. We discuss the clinical considerations in patient selection, donor facial tissue procurement, eye tissue implantation, and a potential surgical procedure for a functional outcome.
CONVENTIONAL TREATMENTS FOR EYE RESTORATION
Surgical solutions to blindness are lacking. Reported attempts to preserve the eye following avulsion of the globe lack the return of even light perception.5 In such situations successful management of reimplantation is measured by retrieval of extraoccular muscle function and an eye that appears cosmetically normal. Return of any visual function is exceedingly rare and with the associated complications surgical enucleation is most commonly the ultimate outcome.6
Management goals and outcomes in anophthalmia, congenital or degenerative absence of the eye, are similarly modest. Typically, for a child, plastic conformers of increasing size are serially fitted to the orbit to maintain growth with expanders also used to promote any necessary expansion. At skeletal maturity a painted permanent prosthesis can be fitted. Furthermore, in microphthlamia, current practice is actually to remove the affected nonfunctioning eye.7
For composite defects in which there is partial or whole absence of the orbit as well as surrounding structures, standard care is either free tissue transfer to obliterate or reconstruct the orbit to enable the use of an ocular prosthesis or using osseointegrated implant-based composite prostheses with their inherent cosmetic and functional limitations.8,9
These approaches all compromise the fundamental reconstructive principle of ‘‘replacing like with like,’’ which VCA could potentially afford.
ADVANCES POTENTIATING VASCULARIZED COMPOSITE ALLOTRANSPLANTATION OF THE EYE
Microsurgery and Vascularized Composite Allotransplantion
Since Carrel’s Nobel Prize winning description of vascular anastomosis techniques, there has been an explosion in our ability to revascularize and reinnervate tissue; first with replantation, organ transplantation, and free tissue transfer, then more recently with vascularized composite allotrasplantation.10,11
Specifically, transplantation of the eye necessitates revascularization of the ocular and nonocular, or orbital structures of the eye, supplied through their respective branches of the ophthalmic artery.12 Using fluorescein angiography Sher demonstrated revascularization of the retina after microsurgical anastamosis of autotransplanted ovine eyes and in canine eyes following anastomosis of the ciliary artery of dogs to the femoral artery in rats.13,14 Ultimately, the technical success of vascularized eye allotransplantation demands full understanding of ophthalmic circulatory patterns and this likely necessitates cadaveric studies similar to those conducted ahead of face transplantation.15
Evolution of Immunomodulation Therapy
Since its inception, skepticism for VCA has been centered around the long-term burden related to immunosuppression and the shift in the risk-to-benefit ratio from the acceptable in lifesaving solid organ transplantation to the questionable in VCA.16 Rather than having to answer the question of whether the benefit of vision is worth the current risk, which is clearly a subjective matter for debate, a better answer lies in recent advances in understanding of transplant immunology that mitigate immunosuppressive risk.
Chimerism-induced tolerance is a promising strategy for reducing or eliminating the need for long-term immunosuppression as well as overcoming chronic rejection. 16,17 Establishing chimerism through bone marrow transplantation is particularly appealing given its safety in producing mixed chimerism, a mixture of recipient and donor hematopoietic cells, limiting the reduction of immunocompetence and risk of graft-versus-host disease as seen with myeloablative methodologies.17 – 22 In ongoing investigations, T regulatory cells, dendritic cells and mesenchymal stem cells are emerging as potential adjuncts to bone marrow transplantation for tolerance induction.23 – 30
Paralleling attempts to reduce the risk of immunosuppression through induction of tolerance are attempts to limit the systemic effects of immunosuppressive therapy by mechanisms directed at more specific local delivery of agents.31,32 Biomimetic sustained release formulations for suppressing composite tissue transplant rejection are particularly appealing for eye transplantation given the precedent for successful similar practices in clinical ophthalmology using dexamethasone intravitreal implants in the treatment of vitreoretinal eye disease.33
A further interesting twist in the complexity of transplant immunology pertaining to VCA of the eye is the existence of immune privilege in the eye. Discovered by Peter Medawar34 when he studied the fates of allogeneic skin grafts placed heterotopically in the anterior chamber of the eye and found that they survived, immune privilege and its mechanisms are still not fully understood. Nonetheless, the ocular compartments as well as the cornea, retinal pigment epithelium, and neuronal retina function as immune privileged sites that resist immune rejection when implanted as allografts.35 The potential clinical ramifications for this phenomenon in highlights the need for further research in this arena.
Neuroregeneration
Arguably, the greatest impediment to VCA of the eye is the ability to ensure maintenance of donor eye viability, optic nerve regeneration, and restoration of topographic organization that are fundamental to recovery of visual function.
Animal models studies of ocular viability after enucleation with or without reanastomosis have demonstrated at least partial maintenance of donor ocular viability in cold-blooded vertebrates and mammals to varying degrees.4,36
Strategies for nerve regeneration encompass an overwhelming body of emerging research far beyond the scope of the present article but include the modulation of phosphatase and tensin homolog pathway, peripheral nerve grafts, intravitreal growth factor injection, and inhibition of glial scarring. The task remains of streamlining of these possibilities into a workable framework to optimize outcomes in VCA of the eye.37 – 45 The most promising treatment modality specifically targeting optic nerve regeneration with implications for eye transplantation is that of de Lima et al46. The ability to regenerate the full length of the optic nerve into the brain visual centers has been demonstrated in a small animal model. With adequate stimulation, using a coninjection of an inflammatory stimulator, cyclic adenosine monophosphate analogue and targeted gene deletion, retinal ganglion cells are able to regenerate axons the full length of the visual pathway and on into the lateral geniculate nucleus, superior colliculus, and other visual centers following a crush injury to the optic nerve in mice. They were also able to demonstrate partial restoration of the optomotor response, depth perception and circadian entrainment, and partial restoration of pupillary light reflex.
The plasticity of the visual cortex to topographically reorganize sensory input from a transplanted eye remains to be tested. The effect on the processing axis from the optic nerve through optic chiasm, optic tract, lateral geniculate nucleus, and optic radiation to the cortex is also unknown. Neuronal circuits in the brain are not static, however, and connectivity can change after altered sensory input or following specific experiences, thereby providing neurons with new response properties, tailored to the new environment.47 Although it is established that the thalamocortical projection is physically rearranged as a consequence of monocular deprivation, the extent to which it is again rearranged in the event of restoration of binocular vision is uncertain.48 Work in rodents has revealed residual potential for restoration of vision despite the existence of amblyopia or ‘‘permanent’’ vision loss after the critical period of development; resetting excitatory-inhibitory balance or removing molecular ‘‘brakes’’ on structural plasticity may unmask the potential for recovery of function in adulthood.49
Integration of these 3 aspects of neuroregeneration—maintenance of donor eye viability, optic nerve regeneration, and restoration of topographic organization—demands further exploration in the context of allotransplantation.
Animal Models of Eye Transplantation
Optimization of surgical revascularization technique, immunomodulation, and neuroregeneration therapies, as described above requires the genesis of reliable animal models that most closely recapitulate true physiology and anatomy.
Polat et al50 have described a rat model for heterotopic transplantation of orbit, globe, periorbital soft tissues, and optic nerve, based on the carotid artery and jugular vein, heterotopically to the anterior neck of recipients. This model has utility in demonstrating viability of tissue but is limited by failing to not recapitulate true anatomy.
Our group has established the first viable orthotopic model for vascularized eye transplantation in the rat. Maintenance of structural integrity and viability were confirmed by slit lamp examination, optical coherence tomography, and histology. This model is ideal for examining viability, functional return and immunology in whole eye transplantation (Fig. 1).51
FIGURE 1.
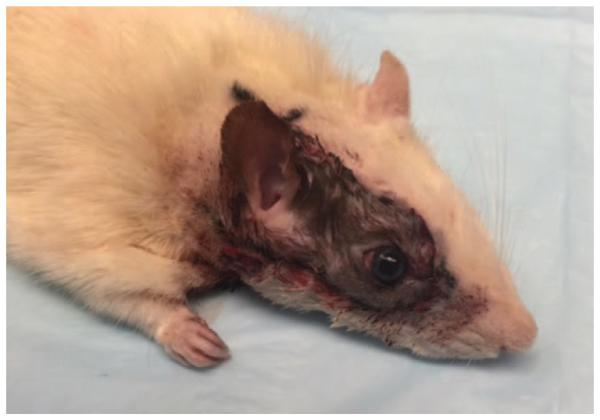
The orthotopic model for vascularized eye transplantation in the rat.
PATIENT CONSIDERATIONS
Identifying those patients optimally suited to receiving vascularized eye transplantation is a challenge. Practical considerations include defining goals of treatment. If primarily the goal is restoration of vision, then this renders only those with bilateral nonfunctioning eyes as candidates. Given the likely difficulties of achieving synchrony of muscle balancing, this might further suggest only unilateral transplants should be performed. Realizing the full potential of VCA of the eye extends the indications for the treatment to include cosmesis. This then renders the need to pursue possibilities to explore the use of VCA of the eye bilaterally in cases of bilateral functional eye deficiencies and unilateral VCA in cases of unilateral functional eye deficiency. This in turn raises the surgical challenge of achieving functional synchrony.
It is likely VCA of the eye will take the form of globe only, globe and soft tissue (eg, eyelids), and globe and orbit (with or without soft tissue) transplants as well as those concomitantly with more expansive facial transplant procedures. The latter, as previously stated, is a clear choice to first offer eye transplantation given the limited extra risk of including the eye if already planning for a face transplant.
Identifying the optimal candidates requires development of an effective tool for objective evaluation of the ocular deficit for the allocation of limited donor eyes, analogous to the functional status, aesthetic deficit, comorbidities, exposed tissue, surgical history (FACES) score assessment tool for identifying the optimal face transplant candidate as well as the model for end-stage liver disease score in liver transplantation, for example.52 Similar to the FACES score, the assessment tool for identifying the optimal eye transplant candidate would assign numerical score stratified in categories conceptualized to be relevant in evaluating patients including functional status, aesthetic deficit, comorbidities, exposed tissue, and surgical history/recipient vessel status.
DONOR PROCUREMENT AND TISSUE TRANSFER
Principles of donor tissue procurement and tissue handling during transportation and transfer are largely shared by other transplantation practices, namely appropriate donor screening, rapid harvest from a donor with a beating heart, restoration of donor appearance, cooling of the graft, and expeditious transfer.53 – 57
Donor selection criteria, as with facial transplantation, should include ABO compatibility, suitable human leukocyte antigen typing and negative cross-match. The near-universal proportions of the globe potentially obviate the need for sex- and age-match as well as any anthropometric analysis.58 Skin-, and potentially, age-match do become relevant if the eye transplant is to include soft tissue that is, eyelids in combination with the globe.
It is intuitive that premorbid donor visual acuity be uncompromised to confer the optimal restoration of vision possible though the effect of acuity being compromised on transplant outcomes is unclear. Iris color match to a contralateral native eye in unilateral transplant, though admittedly a relatively low priority, could be matched using the Martin-Schulz classification.59 An alternative option is the use of colored contact lenses to achieve a match artificially. The natural history of iris color, and any potential for this to morph, is unknown.
The need for expeditious harvest and transfer to reduce ischemia time remains true in this transplant modality as with others. Retinal function of an enucleated eye is greatly decreased or absent within 5 minutes. Maintenance and restoration of retinal function as measured by electroretinogram has been demonstrated ex vivo in isolated perfused eyes for up to 10 hours. In these studies, eyes were enucleated, and perfusion was initiated shortly thereafter. The eyes were perfused under positive pressure with various buffered perfusates.36,60 – 63 The ideal perfusion fluid for the eye remains to be identified.
The actual surgical procedure for donor procurement is still to undergo cadaveric testing but one can hypothesize the following based on our animal studies and the work of others.51,64 Essentially, donor tissue harvest involves performing an exenteration to include globe, muscles, fat, suspensory ligaments, and trochlea within the periosteum of the orbit. Superior and inferior ophthalmic veins would also be dissected with this approach (Fig. 2). An endonasal endoscopic approach would be used to clip the ophthalmic artery at the suprasellar cistern, for transection of the optic nerve at the optic chiasm and for transection of occulomotor, trochlear, and abducens nerves at the cavernous sinus (Fig. 3). Superior ophthalmic vein and inferior ophthalmic vein branch would also be harvested endoscopically at the cavernous sinus, with the inferior branch having been ligated at the inferior orbital fissure and harvested during exenteration.
FIGURE 2.
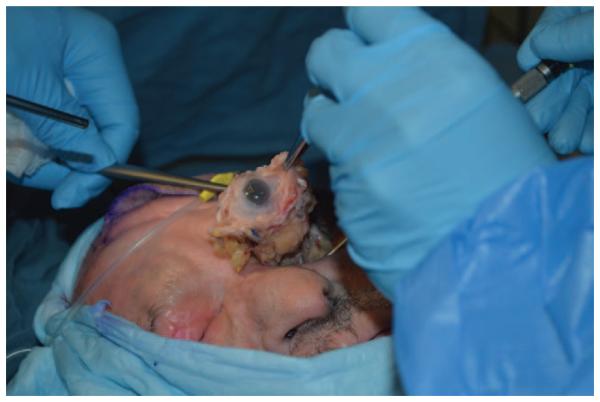
Preliminary donor cadaveric dissection demonstrating orbital exenteration including globe, muscles, fat, suspensory ligaments, and trochlea within the periosteum of the orbit. Superior and inferior ophthalmic veins.
FIGURE 3.
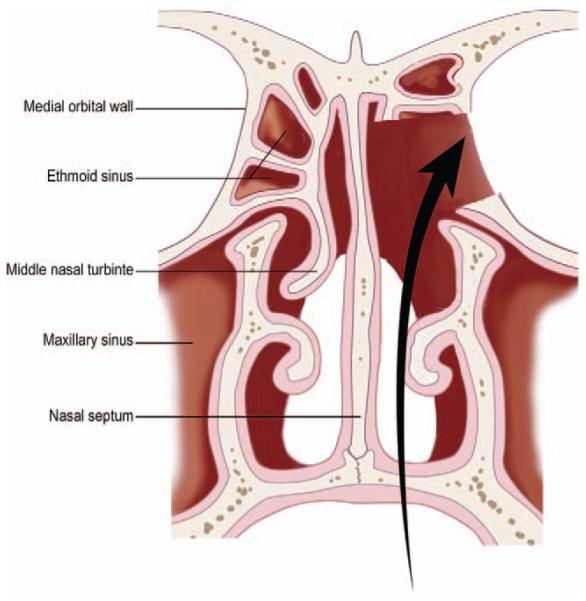
Schematic illustration of endonasal approach to decompress orbital apex and access the cavernous sinus.
To approach the optic chiasm, suprasellar cistern and cavernous sinus, endonasal optic nerve decompression would be performed in conjunction with an endonasal transplanum approach with lateral extension to allow for the harvest of the entire optic nerve and the maximum length of the ophthalmic artery as well as occulomotor, trochlear and abducens nerves.
Using standard endoscopic surgical techniques, the ipsilateral middle turbinate is partially resected and a complete ipsilateral sphenoethmoidectomy is performed. The skull base and lamina papyracea are then skeletonized in a posterior to anterior manner. A maxillary antrostomy is performed to aid in identification of the orbital floor. Key landmarks within the sphenoid sinus are then identified including the carotid artery and optic nerve prominence, the lateral opticocarotid recess, and sella turcica. A medial orbital decompression can then be performed while maintaining intact periorbita. The medial inferior orbital floor can also be decompressed if necessary. The thicker bone surrounding the orbital apex is drilled thin enough to be dissected free. Drilling of bone overlying the optic canal and orbital apex is then undertaken. The bone overlying the optic canal is thinned and carefully dissected free up to the tuberculum and carotid artery. The dissection can then proceed medially to identify the ophthalmic artery and the optic chiasm.
An endoscopic endonasal transplanum approach is undertaken to identify the ophthalmic artery and optic chiasm in the suprasellar cistern (Fig. 4). In brief, a binarial technique is used by performing a wide sphenoidotomy on the contralateral side in conjunction with a small posterior septectomy. The intervening bony septations and sphenoid rostrum are removed. The sella tursica is decompressed while preserving the dura overlying the pituitary gland. Osteotomies are created superior to the bilateral optic nerves and anteriorly on the planum sphenoidale. The bone of the planum and tuberculum are decompressed, exposing the suprasellar dura. Dural incisions are created in the suprasellar dura and carefully extended laterally to expose both the intracisternal segment of the optic nerve and the takeoff of the ophthalmic artery from the internal carotid artery (Fig. 5). By expanding the exposure lateral to the sella, the anterior wall of the cavernous sinus is exposed and removed to access the lateral cavernous sinus and the occulomotor, trochlear, and abducens nerves (Fig. 6).
FIGURE 4.
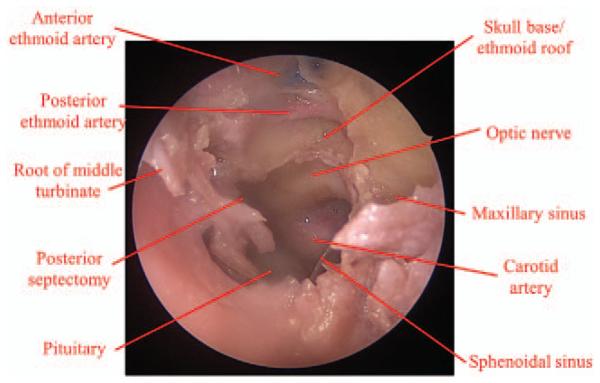
Preliminary donor cadaveric dissection demonstrating endonasal approach to decompress orbital apex and access the cavernous sinus.
FIGURE 5.
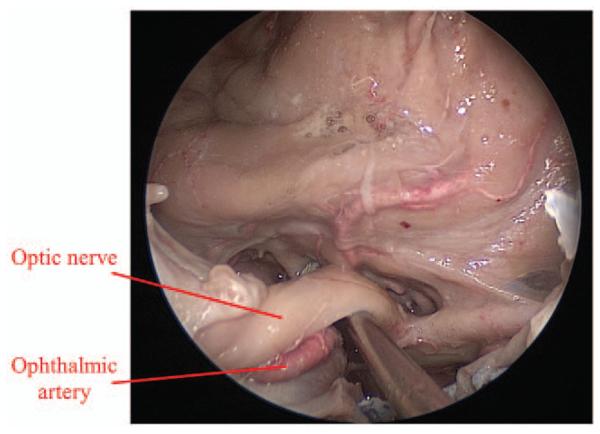
Preliminary donor cadaveric dissection demonstrating exposure of optic nerve (ON) and ophthalmic artery (OA) after endoscopic optic nerve decompression.
FIGURE 6.
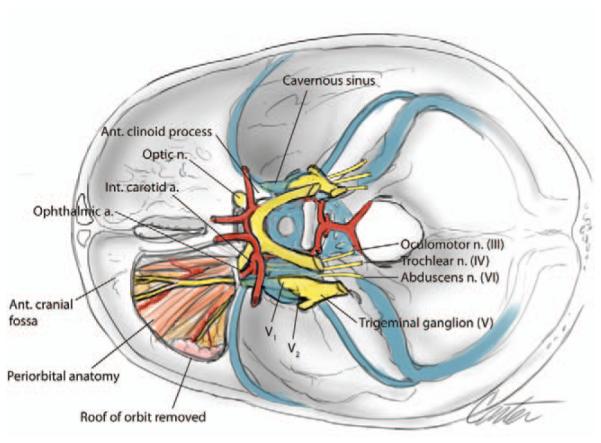
Schematic illustration of superior view of cavernous sinus demonstrating inferior access window created endonasally.
Sequential transection of occulomotor, trochlear, abducens nerves, optic nerve, ophthalmic artery, and veins is then performed.
RECIPIENT SURGICAL PROCEDURE
A tentative surgical flight plan for the recipient surgery follows. Prior to introduction of this in to the clinical realm, this would first need to be tested, validated, and optimized through cadaveric studies. This work is ongoing at our institution. Given the challenge of an anastomosis to the recipient ophthalmic vessels due to limited exposure and need to escape the zone of injury or pathology, the angular or internal maxillary artery would be ideal recipient vessels. The former could be achieved through a Lynch incision. The latter would probably be preferred given the larger caliber of the vessel. The internal maxillary artery could be exposed by a bicoronal incision with turndown of the temporalis muscle and extended lateral orbitotomy approach as in middle cerebral artery bypass (Fig. 7).65 The superficial temporal artery if patent is a further alternative recipient artery. Options for venous recipient vessels include the superficial temporal vein if intact, the facial vein or contralateral superior ophthalmic vein, all of which would require interposition vein grafting (Fig. 8). Coaptation of oculomotor, trochlear, and abducens nerves at the cavernous sinus and optic nerve at the optic chiasm would be achieved endoscopically (via the same approach to the donor procedure described above) using adhesive fibrin glue with or without nerve guidance conduits.
FIGURE 7.
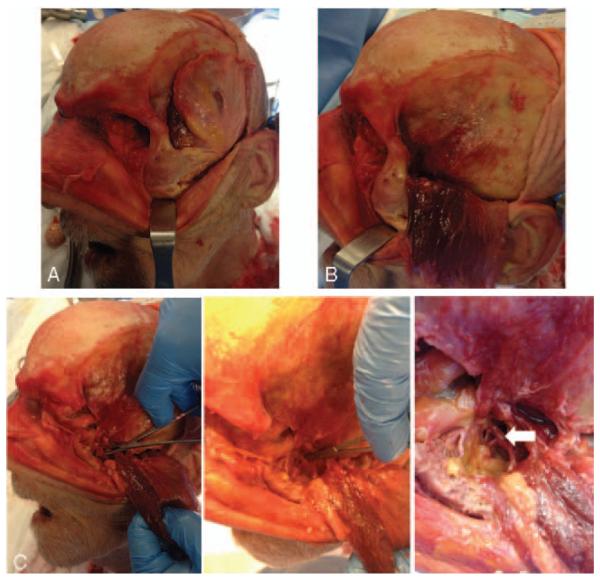
Preliminary recipient cadaveric dissection demonstrating the internal maxillary artery via bicoronal incision (A), temporalis turndown (B), and extended lateral orbitotomy (C).
FIGURE 8.
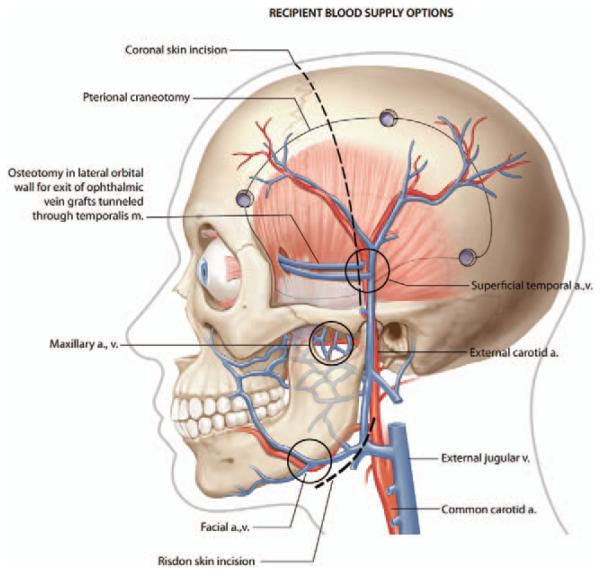
Schematic illustration of candidate recipient vessels with vein grafted superficial temporal vessels as an example.
POSTOPERATIVE CONSIDERATIONS
Vision therapy or vision training is already practiced to improve vision skills such as eye movement control and eye coordination in numerous conditions of ocular pathology including problems of eye strain, visually induced headaches, strabismus, and diplopia. Orthoptic vision therapy addresses eye movements with exercises such as near point of convergence exercises, base-out prism reading, stereogram cards, computerized training programs are used to improve fusional vergence, and the wearing of convex/concave lenses. Behavioral vision therapy aims to treat problems including difficulties of visual attention and concentration.66 Secondary adjunctive procedures in the form of strabismus surgery may also be necessary in some cases.
Clinical ophthalmological examination in conjunction with optical coherence tomography and electroretinography would form the foundation of an integrated tool for outcome measures.
CONCLUSIONS
Ushering in the era of vascularized composite allotransplantion of the eye necessitates the amalgamation of recent advances in transplant immunology medicine and neuroregenerative solutions with new animal models of eye transplantation as well as testing in cadaveric studies to address optimal adjunctive therapies and practical surgical protocols. For the first time the component parts necessary for such an endeavor are being realized and the possibility of clinical vascularized transplantation of the eye now looms large.
Acknowledgments
This study was supported in part by National Institutes of Health P30-EY008098 (Bethesda, MD); The Louis J. Fox Center for Vision Restoration of UPMC and the University of Pittsburgh; The Eye and Ear Foundation (Pittsburgh, PA); an unrestricted grant from Research to Prevent Blindness (New York, NY), the VA Pittsburgh Healthcare System, and the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-14-1-0421.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Petyruizzo P, Lanzetta M, Dubernard JM, et al. The International Registry on Hand and Composite Tissue transplantation. Transplantation. 2010;90:1590–1594. doi: 10.1097/TP.0b013e3181ff1472. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Carty MJ, Bueno EM, Lehmann LS, et al. A position paper in support of face transplantation in the blind. Plastic Reconstr Surg. 2012;130:319–324. doi: 10.1097/PRS.0b013e3182589b27. [DOI] [PubMed] [Google Scholar]
- 4.Ellenberg D, Shi J, Jain S, et al. Impediments to eye transplantation: ocular viability following optic-nerve transection or enucleation. Br J Ophthalmol. 2009;93:1134–1140. doi: 10.1136/bjo.2008.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirath H, Tümer B, Bilgicç S. Management of traumatic luxation of the globe. A case report. Acta Ophthalmol Scand. 1999;77:340–342. doi: 10.1034/j.1600-0420.1999.770319.x. [DOI] [PubMed] [Google Scholar]
- 6.Schneck M, Oshry T, Marcus M, et al. Attempted bilateral manual enucleation (gouging) during a physical assault. Ophthalmology. 2003;110:575–577. doi: 10.1016/S0161-6420(02)01768-2. [DOI] [PubMed] [Google Scholar]
- 7.Gundlach KKH, Guthoff RF, Hingst VHM, et al. Expansion of the socket and orbit for congenital clinical anophthalmia. Plastic Reconstr Surg. 2005;116:1214–1222. doi: 10.1097/01.prs.0000181653.38200.eb. [DOI] [PubMed] [Google Scholar]
- 8.Schusterman MA, Reece GP, Miller MJ. Osseous free flaps for orbit and midface reconstruction. Am J Surg. 1993;166:341–345. doi: 10.1016/s0002-9610(05)80328-9. [DOI] [PubMed] [Google Scholar]
- 9.Jackson IT, Tolman DE, Desjardins RP, et al. A new method for fixation of external prostheses. Plastic Reconstr Surg. 1986;77:668–672. doi: 10.1097/00006534-198604000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Sade RM. Transplantation at 100 years: Alexis Carrel, Pioneer Surgeon. Ann Thorac Surg. 2005;80:2415–2418. doi: 10.1016/j.athoracsur.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 11.Schneeberger S, Morelon E, Landin L, et al. Vascularized composite allotransplantation: a member of the transplant family? Transplantation. 2012;93:1088–1091. doi: 10.1097/TP.0b013e318254ad8a. [DOI] [PubMed] [Google Scholar]
- 12.Hedges TR. Ophthalmic artery blood flow in humans. Br J Ophthalmol. 2002;86:1197. doi: 10.1136/bjo.86.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sher H. Revascularization of autotransplanted ovine eyes by microsurgical anastomosis. J Microsurg. 1981;2:269–272. doi: 10.1002/micr.1920020408. [DOI] [PubMed] [Google Scholar]
- 14.Sher H, Cohen RJ. Revascularization of isolated extracorporeal canine eyes by direct microsurgical anastomosis. J Microsurg. 1980;1:399–402. doi: 10.1002/micr.1920010512. [DOI] [PubMed] [Google Scholar]
- 15.Banks N, Hui-Chou HG, Tripathi S, et al. An anatomical study of external carotic artery vascular territories in face and midface flaps for transplantation. Plastic Reconstr Surg. 2009;123:1677–1687. doi: 10.1097/PRS.0b013e3181a3f3ae. [DOI] [PubMed] [Google Scholar]
- 16.Ravindra KV, Ildstad ST. Vascularized composite allotransplantation at a crossroad. Transpl Proc. 2011;43:3501–3503. doi: 10.1016/j.transproceed.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 18.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathes DW, Randolph MA, Solari MG, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75:25–31. doi: 10.1097/00007890-200301150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Hettiaratchy S, Melendy E, Randolph MA, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77:514–521. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 22.Horner BM, Randolph MA, Duran-Struuck R, et al. Induction of tolerance to an allogeneic skin flap transplant in a preclinical large animal model. Transpl Proc. 2009;41:539–541. doi: 10.1016/j.transproceed.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issa F, Hester J, Goto R, et al. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90:1321–1327. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathes DW, Solari MG, Gazelle GS, et al. Stable mixed hemoatopoietic chimerism permits tolerance of vascularized composite allografts across a full major histocompatibility mismatch in swine. Transpl Int. 2014;27:1086–1096. doi: 10.1111/tri.12380. [DOI] [PubMed] [Google Scholar]
- 25.Plock JA, Schnider JT, Solari MG, et al. Perspectives on the use of mesenchymal stem cells in vascularized composite allotransplantation. Front Immunol. 2013;4:175. doi: 10.3389/fimmu.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AW, Sacks JM, Kuo YR, et al. Dendritic cell therapy in composite tissue allotransplantation. Transpl Proc. 2009;41:537–538. doi: 10.1016/j.transproceed.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan H, Zhao K, Wang L, et al. Mesenchymal stem cells enhance the induction of mixed chimerism and tolerance to rat hind-limb allografts after bone marrow transplantation. J Surg Res. 2010;160:315–324. doi: 10.1016/j.jss.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Kuo YR, Goto S, Shih HS, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87:1769–1777. doi: 10.1097/TP.0b013e3181a664f1. [DOI] [PubMed] [Google Scholar]
- 30.Schneeberger S, Gorantla VS, Brandacher G, et al. Upper extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnider JT, Weinstock M, Plock JA, et al. Site-specific immunosuppression in vascularized composite allotransplantation: prospects and potential. Clin Dev Immunol. 2013;2013:495212. doi: 10.1155/2013/495212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solari MG, Washington KM, Sacks JM, et al. Daily topical tacrolimus therapy prevents skin rejection in a rodent hind limb allograft model. Plastic Reconst Surg. 2009;123(2S):17S–25S. doi: 10.1097/PRS.0b013e318191bcbd. [DOI] [PubMed] [Google Scholar]
- 33.Arcinue CA, Cerón OM, Foster CS, et al. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013;29:501–507. doi: 10.1089/jop.2012.0180. [DOI] [PubMed] [Google Scholar]
- 34.Medawar P. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;129:58–69. [PMC free article] [PubMed] [Google Scholar]
- 35.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 36.Shi J, Washington KM, Sacks JM, et al. Restoration of electroretinogram activity in exenterated swine eyes following ophthalmic artery anastomosis. Restor Neurol Neurosci. 2009;27:351–357. doi: 10.3233/RNN-2009-0485. [DOI] [PubMed] [Google Scholar]
- 37.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmignoto G, Maffei L, Candeo P, et al. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9:1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CJ, Negra M, Levine A, et al. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- 40.Thanos S, Naskar R, Heiduschka P. Regenerating ganglion cell axons in the adult rat establish retinofugal topography and restore visual function. Exp Brain Res. 1997;114:483–491. doi: 10.1007/pl00005657. [DOI] [PubMed] [Google Scholar]
- 41.van Adel BA, Arnold JM, Phipps J, et al. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63:215–234. doi: 10.1002/neu.20117. [DOI] [PubMed] [Google Scholar]
- 42.van Adel BA, Kostic C, Déglon N, et al. Delivery of ciliary neurotrophic factor via lenti viral mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–115. doi: 10.1089/104303403321070801. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Tokita Y, Kato M, et al. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- 44.Wong EV, David S, Jacob MH, et al. Inactivation of myelin-associated glycoprotein enhances optic nerve regeneration. J Neurosci. 2003;23:3112–3117. doi: 10.1523/JNEUROSCI.23-08-03112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Q, Wang J, Matheson CR, et al. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF) J Neurobiol. 1999;38:382–390. doi: 10.1002/(sici)1097-4695(19990215)38:3<382::aid-neu7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 46.de Lima, Koriyama Y, Kurimoto T, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proce Natl Acad Sci. 2012;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keck T, Mrsic-Flogel TD, Afonso MV, et al. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci. 2008;11:1162–1167. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- 48.Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Polat RM, Zor F, Isik S, et al. Evaluation of the optic nerve regeneration in anew orbital composite tissue allotransplantation model. Plastic Reconstr Surg. 2012;130(5S):72–73. [Google Scholar]
- 51.Li Y, Komatsu C, Wang B, et al. Evaluation of Viability, Structural Integrity and Functional Outcome after Whole Eye Transplantation. Plastic and Reconstr Surg. 2015;135:82. [Google Scholar]
- 52.Gordon CR, Siemionow M, Coffman K, et al. The Cleveland Clinic FACES Score: a preliminary assessment tool for identifying the optimal face transplant candidate. J Craniofac Surg. 2009;20:1969–1974. doi: 10.1097/SCS.0b013e3181bd2c86. [DOI] [PubMed] [Google Scholar]
- 53.Rahmel A. Vascularized composite allografts: procurement, allocation and implementation. Curr Transpl Rep. 2014;1:173–182. doi: 10.1007/s40472-014-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meningaud JP, Paraskevas A, Ingallina F, et al. Face transplant graft procurement: a preclinical and clinical study. Plastic Reconst Surg. 2008;122:1383–1389. doi: 10.1097/PRS.0b013e3181882146. [DOI] [PubMed] [Google Scholar]
- 55.Meningaud JP, Benjoar MD, Hivelin M, et al. Procurement of total human face graft for allotransplantation: a preclinical study and the first clinical case. Plastic Reconstr Surg. 2010;126:1181–1190. doi: 10.1097/PRS.0b013e3181ec2089. [DOI] [PubMed] [Google Scholar]
- 56.Pomahac B, Papay F, Bueno EM, et al. Donor facial composite allograft recovery operation: Cleveland and Boston Experiences. Plastic Reconstr Surg. 2012;129:461e–467e. doi: 10.1097/PRS.0b013e31824129d7. [DOI] [PubMed] [Google Scholar]
- 57.Baccarni A, Follmar KE, Das RR, et al. A pilot study in sub-SMAS face transplantation: defining donor compatibility and assessing outcomes in a cadaver model. Plastic Reconstr Surg. 2007;119:121–129. doi: 10.1097/01.prs.0000245078.66513.c0. [DOI] [PubMed] [Google Scholar]
- 58.Riordan-Eva P. Vaughan & Asbury’s General Ophthalmology. 18th. McGraw-Hill Medical; New York: 2011. ISBN 978-007163420. [Google Scholar]
- 59.Sturm RA, Larsson M. Genetics of human iris colour and patterns. Pigment Cell Melanoma Res. 2009;22:544–556. doi: 10.1111/j.1755-148X.2009.00606.x. [DOI] [PubMed] [Google Scholar]
- 60.Gouras P, Hoff M. Retinal function in an isolated, perfused mammalian eye. Invest Ophthalmol. 1970;9:388–399. [PubMed] [Google Scholar]
- 61.Peachey NS, Green DJ, Ripps H. Ocular ischemia and the effects of allopurinol on functional recovery in the retina of the arterially perfused cat eye. Invest Ophthalmol Vis Sci. 1993:58–65. [PubMed] [Google Scholar]
- 62.Niemeyer G. The function of the retina in the perfused eye. Doc Ophthalmol. 1975;39:53–116. doi: 10.1007/BF00578759. [DOI] [PubMed] [Google Scholar]
- 63.Niemeyer G. Retinal research using the perfused mammalian eye. Prog Retin Eye Res. 2001;20:289–318. doi: 10.1016/s1350-9462(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 64.Canavero S. Total eye transplantation for the blind: a challenge for the future. Medical Hypotheses. 1992;39:201–211. doi: 10.1016/0306-9877(92)90111-o. [DOI] [PubMed] [Google Scholar]
- 65.Nossek E, Costantino PD, Eisenberg M, et al. Internal maxillary artery-middle cerebral artery bypass: infratemporal approach for subcranial-intracranial (SC-IC) Bypass. Neurosurgery. 2014;75:87–95. doi: 10.1227/NEU.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vision Therapy Information for Health Care and Other Allied Professionals a Joint Organizational Policy Statement of the American Academy of Optometry and the American Optometric Association (American Optometric Association) 1999 [PubMed] [Google Scholar]


