Capsule summary
Patients with germline mutations in PIK3CD, immunodeficiency, lymphoproliferation, and autoimmunity show a distinct pattern of abnormal B-cell maturation in the bone marrow.
Keywords: PIK3CD, PI3K, PASLI, APDS, immunodeficiency, B cells, B-cell maturation, lymphoma
To the Editor
Class IA Phosphoinositide 3-Kinases (PI3Ks) are lipid kinases that transduce signals received from receptor tyrosine kinases, resulting in activation of downstream effectors including AKT, mTOR, and BTK and regulation of multiple cellular processes1. PI3K heterodimers are composed of a catalytic p110 subunit and a regulatory subunit p852. p110δ, one of three isoforms of the catalytic subunit, encoded by the PIK3CD gene, is preferentially expressed in leukocytes, and plays a critical role in signaling via the B-cell receptor, IL-4R and, TLRs and is therefore critical in B-cell development, activation, proliferation, differentiation and B-cell mediated immunity3. Overexpression of p110δ confers oncogenic potential4. Recently, patients were described with germline gain of function (GOF) mutations in PIK3CD leading to immunodeficiency, lymphoproliferation, and increased incidence of B-cell lymphomas; termed p110 delta-activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency (PASLI)5, or activated PI3Kdelta syndrome (APDS)6. Clinical and laboratory findings in these patients include recurrent sinopulmonary bacterial infections, bronchiectasis, CMV and EBV viremia, lymphoproliferation in tissues, progressive lymphopenia in peripheral blood, autoimmune cytopenias, and variable but mostly abnormal immunoglobulin levels with reduced total and class-switched memory B cells.
Herein, we report markedly abnormal bone marrow (BM) findings in 10 patients with PIK3CD germline mutations showing characteristic precursor B-cell (hematogone) hyperplasia with impaired maturation of B-cells. Patients were enrolled on IRB-approved protocols and had a median age of 12 years (range 4–23 years) at the time of bone marrow evaluation, and a male:female ratio of 1. One of the patients has a novel mutation (E1025G) that has not been previously reported (Patient 10, see Table E1 in this article’s Online Repository). Five of the patients were previously reported5. Relevant clinical and laboratory data are summarized in Table E1. Patient samples were obtained in accordance with the Declaration of Helsinki, with informed consent, and approval of the NIAID institutional review board. BM biopsies were obtained prior to treatment. Flow cytometric immunophenotyping of BM was performed in 7 patients, and the results were compared to 6 age-matched BM controls (see Methods in Online Repository and Table E1) using the Mann-Whitney test. B-cell maturation was assessed using immunophenotypic criteria for B-cell developmental stages previously described7, 8. A p value of <0.05 was considered significant. Morphologic, immunohistochemical (IHC) and in situ hybridization studies were performed on all 10 BM biopsies.
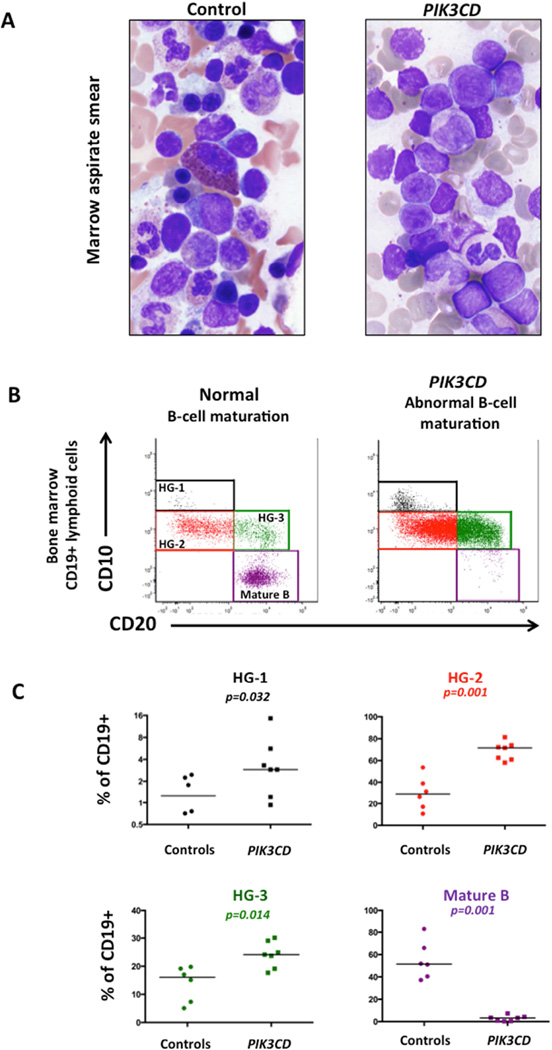
Morphologically, PIK3CD GOF BM aspirate smears showed increased immature lymphoid cells (Figure 1A), many of which morphologically resembled blasts. BM biopsies showed increased CD20+ and CD10+ lymphocytes for age (20–40% of all nucleated cells) by IHC (Figure E1 in Online Repository). TdT positive cells ranged from 1–8% (median 5.3%) (Table E2) and CD34− expressing early progenitors represented 2.6–3.3% (median 2.9%) of all cells by IHC. Plasma cells were present in the BM highlighted by immunohistochemistry for CD138 (Figure E1), and showed polytypic light chain expression by in situ hybridization (ISH) for kappa and lambda light chains. ISH for EBV was positive in one case. IGH PCR of the bone marrow aspirates demonstrated polyclonality.
Figure 1.
Comparison of bone marrow in PIK3CD and healthy pediatric controls. A. Aspirate smears. B. Flow cytometry analysis of B-cell maturation in CD19+ lymphocytes: early B-cell precursor “hematogone” stage I (HG-1, black); stage II (HG-2, red); stage III (HG-3, green), and mature B-cells (purple). C. Scatter plots showing percentage of CD19+ lymphocytes in HG-1, HG-2, HG-3, and mature B-cell stages.
Flow cytometric analysis of BM aspirates showed significant abnormalities within the B-cell compartment in PIK3CD GOF marrows. Overall, the total number of CD19+ B lymphocytes of all BM cells was not significantly different in PIK3CD GOF patients (median 5.1%, interquartile range [IQR] 3.4–17.6%) from age-matched controls (median 7.7%, IQR 3.7–24.0%) (p=0.52). However, the distribution of B lymphocytes within distinct maturation stages was markedly abnormal in PIK3CD GOF marrows. Among all B-lymphoid cells, the median percentages of all three developmental stages of CD10+ B cell precursors (hematogones) were significantly higher in PIK3CD GOF marrows than in controls (Figure 1B–C). Stage I hematogones (HG-1: CD10+[bright], CD20−), represented 2.90% (IQR 1.2–5.6%) in PIK3CD GOF vs. 1.27% (IQR 0.54–2.3%) in controls (p=0.032). Stage II hematogones (HG-2: CD10+, CD20−) represented 71.56% (IQR 60.9–73.9%) in PIK3CD GOF vs 29.05% (IQR 15.5–42.6%) in controls, (p=0.0012). Stage III hematogones (HG-3: CD10+, CD20+), represented 24.10% (IQR 19.1–29.6%) in PIK3CD GOF vs 16.09% (IQR 6.8–19.3%) in controls (p=0.014). Most strikingly, the PIK3CD GOF marrows showed a marked reduction in mature B-cells (CD10−, CD20+) (median 3.3%; IQR 0.45–4.3%) compared to controls (median 51.49%, IQR 39.6–70.2%) (p=0.0012), suggesting impaired maturation beyond the late hematogone or transitional B-cell (CD10+/CD20+) stage (Figure 1B). Surface immunoglobulin light chain expression was polytypic by flow cytometry analysis and similar among the two groups. Of note, the increase in CD10 positive B-cell precursors in the marrow coincided with increased CD10+ transitional B13 cells in the peripheral blood (Table E1).
The increase in B-cell precursors and nearly absent mature B-cells seen in PIK3CD GOF marrows can mimic flow cytometry profiles seen in acute B lymphoblastic leukemia (B-ALL). However, in addition to the maturation block, B-ALL commonly shows expression of aberrant markers or phenotypic asynchrony7 which was not seen in PIK3CD GOF marrows. Other benign conditions, which can demonstrate transient overlapping features include immune reconstitution following BM transplantation, chemotherapy, or treatment with rituximab7, 8.
Cytopenias were common with anemia in 8/10, thrombocytopenia in 6/10, and leukopenia in 3/10 patients. The cytopenias may be multifactorial in etiology given the presence of splenomegaly (9/10), positivity for direct antibody test (DAT) in 5 out of 7 tested, and presence of other autoantibodies in 4 out of 10 (Table E1). Of note, previous studies in mice demonstrated a loss of anergy and the presence of self-reactive B cells after sustained increase in PI3K signaling due to PTEN deficiency9. Circulating B-lymphocytes were abnormal, demonstrating increased CD10+ transitional B-cells and decreased CD20+CD27+ memory B cells5 in the peripheral blood (Table E1). Serum immunoglobulin levels showed elevated IgM in 7/10 cases, and decreased IgA in 5 patients. All but 3 patients had diminished IgG levels prior to initiation of IVIG replacement. Impaired humoral immune response was suggested by low titers of protective antibodies post vaccination observed in a subset of patients tested (Table E1).
Of note, 3/10 patients developed B-cell lymphomas (Hodgkin lymphoma, diffuse large B-cell lymphoma, and marginal zone lymphoma), underscoring the risk of malignant transformation in the setting of sustained PI3K signaling, B-cell hyperplasia, and abnormal B-cell maturation5. In summary, the B-cell maturation pattern in BM of patients with germline GOF PIK3CD mutations is markedly abnormal with characteristic hyperplasia of B-cell precursors (CD10+) with impaired maturation beyond the late hematogone or transitional B-cell stage. These findings suggest that the increased circulating transitional B-cells characteristic of PIK3CD5 are related to abnormal B-cell maturation in the bone marrow. This pattern is readily detected by flow cytometry and may help identify patients who may benefit from genetic testing for mutations in PIK3CD.
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Cancer Institute, and National Institutes of Health Division: Clinical Center. The content of this letter does not necessarily reflect the views or policies of the Department of Health and Human Services.
Abbreviations
- APDS
activated PI3Kdelta syndrome
- BM
bone marrow
- GOF
gain of function
- IHC
immunohistochemistry
- ISH
in situ hybridization
- PASLE
p110 delta-activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency
- PI3K
phosphoinositide 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: ADF, RCB, and KRC, performed flow cytometry, analyzed data, created the figure, and wrote the manuscript. KW, RKG, JMP, JD, VKR, SMH and GU provided clinical care, patient samples, and reviewed data. KW and GU helped write the paper and the Table. ADF, RCB, SP, KTS and KRC reviewed pathology and flow cytometry data. All authors read, critically reviewed, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Werner M, Hobeika E, Jumaa H. Role of PI3K in the generation and survival of B cells. Immunol Rev. 2010;237:55–71. doi: 10.1111/j.1600-065X.2010.00934.x. [DOI] [PubMed] [Google Scholar]
- 2.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, Stoddard J, Ouyang W, Frucht DM, Rao VK, Atkinson TP, Agharahimi A, Hussey AA, Folio LR, Olivier KN, Fleisher TA, Pittaluga S, Holland SM, Cohen JI, Oliveira JB, Tangye SG, Schwartzberg PL, Lenardo MJ, Uzel G. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debre M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers ER, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4-color flow cytometry. Leuk Lymphoma. 2004;45:277–285. doi: 10.1080/1042819031000151950. [DOI] [PubMed] [Google Scholar]
- 8.Carulli G, Ottaviano V, Sammuri P, Domenichini C, Guerri V, Rousseau M, Ciancia E, Ciabatti E, Petrini M. Kinetics of hematogones in bone marrow samples from patients with non-Hodgkin lymphomas treated with rituximab-containing regimens: a flow cytometric study. International Journal of Hematology. 2015;102:59–66. doi: 10.1007/s12185-015-1798-9. [DOI] [PubMed] [Google Scholar]
- 9.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.