Abstract
Clinical and preclinical studies suggest that dysfunction of the glutamatergic system is implicated in mood disorders such as major depressive disorder (MDD) and bipolar depression. In clinical studies of individuals with MDD and bipolar depression, rapid reductions in depressive symptoms have been observed in response to subanesthetic-dose ketamine, an agent whose mechanism of action involves the modulation of glutamatergic signaling. The findings from these studies have prompted the repurposing and/or development of other glutamatergic modulators for antidepressant efficacy, both as monotherapy or as an adjunct to conventional monoaminergic antidepressants. This review will highlight the evidence supporting the antidepressant effects of subanesthetic-dose ketamine as well as other glutamatergic modulators, such as D-cycloserine (DCS), riluzole, CP-101,606, CERC-301 (previously known as MK-0657), basimglurant, JNJ-40411813, dextromethorphan, nitrous oxide (N2O), GLYX-13, and esketamine.
1. Introduction
Recent preclinical and clinical evidence implicates glutamatergic system impairments in mood disorders such as major depressive disorder (MDD) and bipolar depression. In particular, results from clinical studies have consistently found that subanesthetic intravenous (IV) doses of the glutamatergic modulator ketamine [1] exert rapid antidepressant effects [2–4]. In contrast to conventional monoaminergic antidepressants—which are associated with a 40–47% response rate and a lag time of weeks to months before onset of clinical effects [5]—ketamine has been associated with a 65–70% response rate and a significant antidepressant response that occurs within 24 hours and lasts for up to one week of single [2, 3, 6] and repeated [7–9] infusions. These findings have shifted our conceptualization of the pathophysiology of depression, extended the focus of research towards the glutamatergic system for identifying potential novel biomarkers for depression [10, 11], and urged new treatment paradigms in depression [6, 12–14]. Indeed, over the last decade, there has been a significant per annum increase in the rate of published clinical trials investigating the effectiveness of glutamatergic agents as rapid antidepressants, leading to a reawakening of a psychopharmacologic dormancy in this research area. Here, we provide a summary and update of published clinical studies that have examined the safety and antidepressant efficacy of glutamatergic agents, highlighting studies of subanesthetic-dose ketamine as a model for glutamatergic modulation and offering new directions based on emerging evidence from these studies.
2. Ketamine as a Prototypic Glutamatergic Antidepressant Agent
A glutamatergic hypothesis of depression has been posited, in large part due to the growing clinical evidence that a single subanesthetic dose (0.5 mg/kg) of IV ketamine—an Nmethyl-D-aspartate (NMDA) receptor antagonist and synaptic glutamatergic modulator—exerts antidepressant effects as rapidly as 24 hours after administration; these effects have been found to last up to seven days [15, 16].
2.1 Preclinical Studies of Stress, Depression, and Glutamate
In preclinical ketamine studies, alterations in rodent cortical glutamate levels have been associated with pharmacologic- and stress-induced depressive-like behaviors [17–19]. Animal models of stress-induced depression have shown that changes in the pattern of neuronal functioning affects both prefrontal cortical and hippocampal areas [20–25]. While acute stress appears to enhance glutamate transmission in the prefrontal cortex [26–28], chronic stress has been shown to induce dysfunction in glutamatergic neurotransmission coupled with changes at synaptic activity in the prefrontal cortex [25, 29] and hippocampus [24]. These changes have been associated with increased long-term depression as well as deficits in the ability to induce or maintain long-term potentiation [22, 29]. In association with chronic administration of traditional monoaminergic antidepressants, many of these stress-induced alterations—including glutamatergic neurotransmission [30] and pyramidal neuronal morphology and function [25]—have been reversed. As a result, reversal of stress-induced changes has been linked to an antidepressant-like response to monoaminergic modulation, as well as to electroconvulsive therapy (ECT) [31, 32].
Interestingly, preclinical studies have demonstrated that behavioral changes seen in response to the rapid-acting antidepressant effects associated with ketamine may be more directly linked to direct modulation of glutamate in affected brain regions [20, 21, 23, 27, 33]. These functional changes in glutamatergic neurotransmission have been associated with neuronal morphological remodeling, dendritic retraction, and synaptic reorganization, particularly within cortical areas [33, 34]. Although data drawn from clinical studies are more controversial, alterations in glutamatergic transmission and neuronal morphology have also been reported in patients suffering from mood disorders [35–37].
Notably, a recent series of preclinical bio-behavioral and pharmacologic studies found that the antidepressant actions of ketamine may be due to ketamine’s metabolites rather than from the R,S-ketamine molecule alone [38]. In this preclinical study series, the metabolism of (R,S)-ketamine to (2S,6S;2R,6R)-hydroxynorketamine (HNK) was found to be necessary for ketamine’s antidepressant effects; furthermore, the (2R,6R)-HNK enantiomer was found to exert antidepressant effects via early and sustained activation of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors independent of NMDA receptor inhibition. These findings shift the conceptualization of ketamine’s potential mechanism of action as an antidepressant agent and also open opportunities towards a more direct means of reducing depressive symptoms via glutamatergic modulation.
2.2 The Discovery of Ketamine’s Antidepressant Effect
For nearly half a century, ketamine has been safely and effectively used to induce anesthesia [39] and, more recently, for procedural sedation in children in emergency medical settings [40, 41]. The earliest clinical evidence of ketamine’s potential antidepressant properties was demonstrated over a decade ago in a small, double-blind study of eight depressed patients (seven with MDD, one with bipolar depression) randomized to receive either a subanesthetic (0.5 mg/kg) dose of IV ketamine or saline placebo. Four out of eight patients (n=7 completers) experienced an antidepressant response to ketamine, as defined by a reduction of 50% or greater on the Hamilton Depression Rating Scale (HAM-D) [2]. Subsequent to this study, our group and others replicated this ketamine-associated antidepressant response in clinical trials with single and repeated administrations under open-label, double-blind, placebo-controlled, and double-blind active comparator conditions via parallel arm or crossover treatment paradigms, but notably in treatment-resistant depression [15] (see also Table 1). In the sections that follow, we highlight ketamine as the model glutamatergic agent, particularly because it is the best studied and—to date—the most efficacious of the glutamatergic agents [15, 42]. Unfortunately, other glutamatergic modulating agents that have been developed and investigated in small clinical trials [15, 16] have not yielded such promising results. However, recent studies examining the properties of ketamine [43] and its metabolites [38] have begun to reveal the molecular mechanisms underlying ketamine’s antidepressant effects.
Table 1.
Clinical Studies of Ketamine in Depression in Chronological Order
| Authors, Date | Sample | Diagnostic Groups | Dosing and Treatment Groups | Clinical Trial Design | Primary Outcome Measure | Significance and Findings |
|---|---|---|---|---|---|---|
| Berman et al., 2000 [2] | N = 8 | MDD, BD | Single-dose ketamine IV (0.5 mg/kg) vs placebo | Randomized, double-blind, crossover | HAM-D25, BDI | Improvement in depressive symptoms within 72 hours after ketamine compared to placebo; HAM-D scores decreased by 14±10 vs. 0 ±12. |
| Zarate et al., 2006 [3] | N = 17 | MDD (TRD) | Two doses (one week apart) of ketamine IV (0.5 mg/kg) vs placebo | Randomized, double-blind, crossover | HAM-D21 | Improvement in depressive symptoms greater in ketamine than in placebo group within 110 minutes; effect remained significant throughout following week. Effect size for drug difference, d=1.46 (95% CI, 0.91–2.01) after 24 hours and d=0.68 (95% CI, 0.13–1.23) after one week. 71% of subjects met response and 29% met remission criteria the day following ketamine infusion. 35% of subjects maintained response for at least one week. |
| Price et al., 2009 [56] | N = 26 | MDD (TRD) | Single-dose and repeated dose (N = 9) ketamine IV (0.5 mg/kg) | Open-label | MADRS, IAT | Improvement in suicidality on average by 2.08 points on a 0 to 6 scale (p<.001; d=1.37), and 81% of patients received a rating of 0 or 1 post-infusion on the MADRS suicide item. Implicit suicidal associations reduced following ketamine (p< .003; d = 1.36), with reductions correlated across implicit and explicit measures. Reductions in suicidality were sustained for 12 days by repeated-dose ketamine (p < .001; d = 2.42). |
| Diazgranados et al., 2010a [50] | N = 16 | BD (receiving lithium or VPA) | Single-dose ketamine IV (0.5 mg/kg) vs placebo | Randomized, double-blind, crossover | MADRS | Improvement in depressive symptoms at 40 minutes in ketamine group compared with placebo (d = 0.52, 95% CI, 0.28–0.76), remaining significant through day 3. Drug difference effect size was largest at day 2 (d = 0.80, 95% CI, 0.55–1.04). 71% of subjects responded to ketamine and 6% responded to placebo at some point during the trial. One subject receiving ketamine and one receiving placebo developed manic symptoms. |
| Diazgranados et al., 2010b [57] | N = 33 | MDD (TRD) | Single-dose ketamine IV (0.5 mg/kg) | Open-label | MADRS, HAM-D, BDI, SSI | Suicidal ideation scores decreased significantly on the SSI within 40 minutes, remaining significant through the first four hours post-infusion (p < .001). Ten subjects (30%) had an SSI score ≥ 4 at baseline; all these scores dropped below 4 (nine dropped by 40 minutes and one by 80 minutes). Depression, anxiety, and hopelessness improved at all time points (P < .001). |
| Aan het Rot et al., 2010 [49] | N = 10 | MDD (TRD) | Repeated-dose (six in 12 days) ketamine IV (0.5 mg/kg) | Open-label | MADRS | Improvement in depressive symptoms with an 85% (12%) mean SD reduction in MADRS scores after the sixth infusion. Eight of nine patients relapsed, on average, 19 days after the sixth infusion (range six to 45 days). One patient remained antidepressant-free with minimal depressive symptoms for more than three months. |
| Ibrahim et al., 2011 [100] | N = 17 N = 23 |
MDD (TRD, with and without history of ECT) | Single-dose ketamine IV (0.5 mg/kg) | Open-label | MADRS | Improvement in depressive symptoms in ECT-resistant group at 230 minutes with a moderate effect size (p<.001, d=0.50, 95% CI: 0.21–0.80). At 230 minutes, the non-ECT exposed group showed significant improvement with a large effect size (p<.001, d=1.00, 95% CI: 0.71–1.29) |
| Larkin and Beautrais, 2011 [58] | N = 14 | Suicidal ideation in Emergency Department | Single-dose ketamine IV (0.2 mg/kg) bolus | Open-label | MADRS | Improvement in depressive symptom scores at 240 minutes. Improvement in suicidality scores from 3.9 (S.E.M.=0.4) at baseline to 0.6 (S.E.M.=0.2) after 40 minutes post-administration with sustained improvements over 10 days |
| Zarate et al., 2012 [48] | N = 14 | BD (taking lithium or VPA) | Two doses (two weeks apart) ketamine IV (0.5 mg/kg) vs placebo | Randomized, double-blind, crossover | MADRS | Improvement in depressive symptoms and suicidal ideation within 40 minutes in ketamine versus placebo group (d=0.89, 95% CI = 0.61–1.16 and 0.98, 95% CI = 0.64–1.33, respectively), which remained significant through day 3. 79% of subjects responded to ketamine and 0% responded to placebo at some point during the trial. The most common side effects were dissociative symptoms, which occurred only at 40 minutes. |
| Abdallah et al., 2012 [52] | N = 16 | MDD, BD | Single-dose pre-ECT ketamine IV (0.5 mg/kg) + thiopental vs thiopental only | Randomized, double-blind, parallel | HAM-D25 | ECT improved depressive symptoms in both groups (F(2,24) = 14.35, p < .001). No significant group effect or group-by-time interaction on HAM-D scores. Post-hoc analyses of the time effect on HAM-D showed no significant HAM-D reduction after the first ECT session for either group. |
| Loo et al., 2012 [53] | N = 46 | MDD, BD | Single-dose pre-ECT ketamine IV (0.5 mg/kg) + thiopental vs thiopental only | Randomized, double-blind, parallel | HAM-D25 | The ECT-ketamine group had a slightly greater improvement in depressive symptoms over the first week of treatment and at one week. No difference in efficacy at end of ECT course. No psychotomimetic effects. |
| Wang et al., 2012 [88] | N = 48 | MDD (pre-ECT, ECT) | Single-dose pre-ECT ketamine IV 0.5 mg/kg + propofol vs ketamine only and propofol only | Randomized, double-blind, parallel | HAM-D17 | Improvement in depressive symptoms found in all groups from one to seven days after ECT. Greatest decrease in depression in ketamine and propofol plus ketamine groups (p<0.01) compared with propofol group one, two, and three days after ECT. |
| Jarventausta et al., 2013 [87] | N = 32 | MDD (psychotic and nonpsychotic) | Single-dose pre-ECT ketamine IV (0.4 mg/kg bolus) + propofol vs ketamine only vs propofol only | Randomized, double-blind, parallel | MADRS | Improvement in depressive symptoms found in both study groups during ECT. There was no difference in the magnitude or speed of response between the groups. |
| Murrough et al., 2013 [7] | N = 72 | MDD (TRD) | Single-dose ketamine IV (0.5 mg/kg) vs midazolam | Randomized, double-blind, parallel | MADRS | Improvement in depressive symptoms greater in ketamine than in midazolam group 24 hours post-treatment. After adjustment for baseline scores and site, MADRS score was lower in ketamine group than in midazolam group by 7.95 points (95% CI, 3.20 to 12.71). Likelihood of response at 24 hours was greater with ketamine than with midazolam (odds ratio, 2.18; 95% CI, 1.21 to 4.14), with response rates of 64% and 28%, respectively. |
| Sos et al., 2013 [45] | N = 27 | MDD | Single-dose ketamine IV (0.54 mg/kg) vs placebo | Randomized, double-blind, crossover | MADRS | Improvement in depressive symptoms greater in ketamine than in placebo group at all visits (day 1, 4, and 7) with effect size (Cohen’s d) of 0.62, 0.57, and 0.44, respectively. Higher intensity of psychotomimetic symptoms during ketamine administration correlated with alleviation in mood ratings. |
| Lapidus et al., 2014 [85] | N = 18 | MDD | Single-dose ketamine intranasal (0.5 mg/kg) vs placebo | Randomized, double-blind, crossover | MADRS | Improvement in depressive symptoms at 24 hours after ketamine compared to placebo (t = 4.39, p < .001). Intranasal ketamine well tolerated. Minimal psychotomimetic or dissociative effects. No clinically significant changes in hemodynamic parameters |
| Lai et al., 2014 [76] | N = 4 | MDD, Comorbid Axis I or II disorders | Single-dose ketamine IV (0.1, 0.2, 0.3, or 0.4 mg/kg) | Randomized, double-blind, crossover | MADRS | Three subjects achieved antidepressant response (≥ 50% decrease in MADRS scores), two subjects at the minimum 0.1 mg/kg dose, though all relapsed within a week. For two subjects, the greatest improvement occurred at 0.4 mg/kg. |
| Yoosefi et al., 2014 [89] | N = 29 | MDD | Three doses pre-ECT 1, 2, 6 ketamine IV (0.5 mg/kg) vs thiopental | Randomized, double-blind, parallel | HAM-D | Improvement in depressive symptoms found in both groups. A significant difference in depressive symptom improvement noted only before the second ECT + ketamine compared with thiopental group. |
| Ionescu et al., 2014 [61] | N = 26 | MDD (TRD, subgrouped into anxious and nonanxious) | Single-dose ketamine IV (0.5 mg/kg) vs placebo | Randomized, double-blind | MADRS, HAM-D | Improvement in depressive symptoms seen in both groups with a significant group main effect (p = .03) and group-by-time interaction (p = .01). Patients with anxious depression relapsed significantly later than those with nonanxious depression (median ± SE = 19.0 ± 17.9 vs 1.0 ± 0.0 days to relapse, respectively; χ2 = 9.30; p = .002). |
| Ballard et al., 2014 [60] | N = 133 | MDD and BD (both TRD) | Single-dose ketamine IV (0.5 mg/kg) | Mixed: open-label, double-blind | SSI, HAM-D, BDI, HAM-A | Improvement in suicidal ideation was associated with ketamine administration compared with placebo. At 230 minutes post-infusion, changes in suicidal ideation correlated with changes in depression and anxiety. |
| Price et al., 2014 [62] | N = 57 | MDD (TRD) | Single-dose ketamine IV (0.5 mg/kg) vs midazolam IV (0.045 mg/kg) | Randomized, double-blind, parallel | SSI, MADRS, QIDS, IAT | On all three explicit suicide measures, 53% of ketamine-treated patients scored zero at 24 hours, compared with 24% of the midazolam group (χ2 = 4.6; p = .03). |
| Kantrowitz et al., 2015 [51] | N = 12 | BD (on olanzapine + fluoxetine, lurasidone, or quetiapine) | Single-dose ketamine IV (0.5 mg/kg), then DCS (titrated to 1,000 mg/day from starting dose of 250 mg over three weeks) | Open-label | MADRS | Improvement in depressive symptoms from baseline to all rating points except at two weeks, with a large effect size seen at day 1 (Cohen d = 2.0 SD) and at eight weeks (Cohen d = 1.1 SD). |
| Shams Alizadeh et al., 2015 [90] | N = 42 | MDD | Single-dose pre-ECT ketamine IV (0.3 mg/kg) + propofol vs ketamine only and propofol only | Randomized, double-blind, parallel | HAM-D | Similar levels of improvement in depression severity found in both groups, with no difference between the groups in the recovery process (p = 0.92). Cognitive performance recovery time was lower in ketamine group than in control group (p= 0.042). |
| Murrough et al., 2015 [63] | N = 24 | Mood and Anxiety Spectrum | Single-dose ketamine IV (0.5 mg/kg) vs midazolam IV 0.045 mg/kg | Randomized, double-blind, parallel | SSI, MADRS | Improvement in suicidality greater in ketamine than midazolam group at 48 hours (p = 0.047) but not at 24 hours (p = 0.32). MADRS suicidal ideation score was lower in the ketamine group compared to midazolam group at 24 hours (p = 0.05). Treatment effect no longer reached significance at day 7. |
| Kuscu et al., 2015 [97] | N = 58 | MDD | Eight sessions of pre-ECT ketamine IV (1 mg/kg) vs thiopental IV (4 mg/kg) vs both | Randomized, double-blind, parallel | HAM-D25 HAM-A |
No statistical significant difference in HAM-D score between the groups. HAM-A scores were higher in both groups receiving thiopental. |
| Salehi et al., 2015 [95] | N = 160 | MDD | Single-dose ketamine IV (0.8 mg/kg) vs sodium thiopental IV (1.5 mg/kg) | Randomized, double-blind, parallel | HAM-D17 | Both groups showed significant improvement in depressive symptoms. Improvement in depressive symptoms was mildly greater in the ketamine group than the thiopental group (p = 0.049). |
| Hu et al., 2016 [91] | N = 30 | MDD | Single-dose ketamine IV (0.5 mg/kg) with and without escitalopram 10 mg | Randomized, double-blind, parallel | MADRS, QIDS-SR | Improvement in depressive symptoms and remission greater in escitalopram + ketamine than escitalopram + placebo group (92.3% v. 57.1%, p = 0.04) and (76.9% v. 14.3%, p = 0.001), with significantly shorter time to response (HR: 0.04, 95% CI: 0.01–0.22, p < 0.001) and remission (HR 0.11, 95% CI 0.02–0.63, p = 0.01). Lower MADRS scores found in ketamine group from two hours to two weeks (peak = 3 days to two weeks; effect size = 1.08–1.18), QIDS-SR scores from two hours to two weeks (maximum effect size = 1.27), and QIDS-SR suicidality at 2–72 hours (maximum effect size = 2.24). |
| Zhong et al., 2016 [98] | N = 90 | MDD | Eight sessions of pre-ECT ketamine IV (1 mg/kg) vs ketamine IV (0.5 mg/kg) + propofol IV (0.5 mg/kg) vs propofol IV (0.8 mg/kg) | Randomized, double-blind, parallel | HAM-D17 | The ketamine group had an earlier reduction in depressive symptoms, most pronounced after the completion of the second treatment and lasting through the last treatment compared to the ketamine+propofol and propofol groups. |
| Rybakowski et al., 2016 [96] | N = 45 | MDD | Pre-ECT ketamine IV (1–1.5 mg/kg at ECT sessions 2 and 3 vs ketamine IV (1–1.5 mg/kg at ECT sessions 2, 4, 6, 8, 10) vs thiopental IV (2–3 mg/kg) for all ECT sessions | Randomized, double-blind, parallel | HAM-D17 | Depressive symptom severity was reduced after the last ECT session in the group that received ketamine prior to the five ECT sessions compared with the group that received thiopental only for all ECT sessions. |
| Singh et al., 2016 [9] | N = 30 | MDD (TRD) | Single-dose esketamine IV (0.4 mg/kg, 0.2 mg/kg, or placebo). Those who received placebo received an additional infusion of esketamine 0.2 mg/kg or 0.4 mg/kg | Randomized, double-blind, parallel | MADRS | Improvement in depressive symptoms greater in esketamine group at both doses compared to placebo group. The least squares mean changes from baseline to day 2 in MADRS total score for the esketamine .20 mg/kg and .40 mg/kg dose groups were −16.8 (3.00) and −16.9 (2.61), respectively, and showed significant improvement (one-sided p = .001 for both groups) compared with placebo (−3.8 (2.97)). |
Abbreviations. BD: bipolar disorder; BDI: Beck Depression Inventory; DCS: D-cycloserine; ECT: electroconvulsive therapy; HAM-A: Hamilton Anxiety Rating Scale; HAM-D: Hamilton Depression Rating Scale; IAT: implicit association task; MADRS: Montgomery-Asberg Depression Rating Scale; MDD: major depressive disorder; QIDS: Quick Inventory of Depressive Symptomatology; SSI: Scale for Suicide Ideation; VPA: valproate
2.3 The Characteristics of Ketamine’s Antidepressant Effect in MDD and Bipolar Depression
Since the initial clinical study by Berman and colleagues [2], our group and others have consistently demonstrated that a single IV infusion of 0.5 mg/kg of ketamine produces an antidepressant response in individuals with treatment-resistant MDD [3, 7, 44, 45]. Depressive symptoms and severity in these studies have traditionally been measured with either the HAM-D [46] (at least 17-item versions) or the Montgomery-Asberg Depression Rating Scale (MADRS) [47], with antidepressant response defined as a ≥ 50% reduction in total score. Furthermore, in most of these studies, antidepressant response was observed in subjects who had previously not responded to at least two antidepressant medications (i.e., subjects with treatment-resistant depression (TRD)) [3, 7, 48], suggesting that ketamine may be equally effective in treating patients with a more severe type of depression. The time course of antidepressant response to ketamine is characterized by an initial reduction in depressive symptoms within two hours, a maximal reduction in depressive symptoms within 24 hours, and a sustained response for up to one week after administration [3, 15]. Independent single-site and multi-site collaborations [3, 7] have noted that one day after administration, response rates ranged between 66 to 77%, and remission rates (defined as a MADRS score ≤ 7 or 9) hovered at around 31% (reviewed in [49]).
It is important to note that, within four hours to one day, a single infusion of ketamine in TRD patients achieved response rates comparable to that seen following eight weeks of treatment with monoaminergic-based antidepressants in non-TRD patients. The fact that ketamine is capable of inducing remission in approximately one-third of TRD patients within a single day is in stark contrast to the effectiveness of monoaminergic-based approaches, which usually require 10–14 weeks of chronic use to produce similar remission rates [5]. These results introduced a new paradigm for research and development of antidepressants with a rapid onset of action.
The potential antidepressant effects of ketamine have also been evaluated in patients with bipolar depression [2, 48, 50, 51]; please note that clinical studies examining whether ketamine affects response to ECT are discussed in greater detail in Section 3, below ([52, 53], see also Table 1). Our group tested ketamine in a double-blind, add-on (to lithium or valproate (VPA)), placebo-controlled, crossover study of subjects with treatment-resistant bipolar depression [50]. Similar to patients with MDD, depressive symptoms significantly improved in bipolar depression subjects receiving ketamine compared with those who received placebo. This antidepressant effect was seen most robustly within the first hour after administration (at 40 minutes) and remained significant through day 3. Seventy-one percent of patients responded to ketamine and 6% responded to placebo at some point during the trial. This finding was replicated in an independent cohort of patients with treatment-resistant bipolar depression [48]. In the replication study, 79% of subjects responded to ketamine and none responded to placebo at some point during the trial.
Notably, a recent meta-analysis of seven clinical trials examining antidepressant response to ketamine found that the odds ratio for an antidepressant response was 8.42 (95% CI=3.47–20.39; p<0.001) in MDD, and 24.05 (95% CI=2.96–195.56; p=0.003) in bipolar depression. In the bipolar depression studies included in the meta-analysis, a statistically significant rate of symptom remission was found on day 1 (odds ratio=14.01 (95% CI=1.73–111.70), p=0.013) but not on day 7 (odds ratio=1.51 (95% CI=0.22–10.49), p=0.674); it should be noted that all of the bipolar depression studies used ketamine to augment therapy with a conventional mood stabilizer [15]. In contrast, another meta-analysis of five randomized control trials (two of which were conducted in patients with bipolar depression) examined the utility of ketamine administration as an adjunct to ECT and found significantly smaller standardized mean differences in response in MDD patients versus those with bipolar depression [54]. These two meta-analyses were based on only a few existing published studies of ketamine administration in patients with bipolar depression; thus, it remains unclear whether response and remission rates differ between patients with MDD and those with bipolar depression. In addition, it is important to note that despite the hypothetically increased risk of developing mania or psychosis in response to an NMDA receptor antagonist, the risk of developing mania in response to a single ketamine infusion does not appear to be greater in patients with bipolar depression who are concurrently taking lithium or VPA [55].
2.4 Ketamine’s Potential Anti-Suicidal and Anxiolytic Effects
In an open-label clinical ketamine trial, Price and colleagues found that suicidality measures were reduced on average by 2.08 points on a 0- to 6-point scale (p < 0.001; d=1.37), with 81% of patients receiving a rating of 0 or 1 24 hours post-infusion on the suicidality item of the MADRS (MADRS-SI) [56]. Given that the primary outcome measure took place at the 24-hour time point, it is uncertain how long the anti-suicidal effect was maintained. This finding was later replicated by our group in an open-label study of 33 TRD patients that found decreased Scale for Suicide Ideation (SSI) scores within 40 minutes of ketamine infusion (0.5 mg/kg); this measure remained significantly reduced throughout the four-hour post-infusion period [57]. In an emergency department setting, Larkin and Beautrais found that suicidal ideation resolved in 14 actively suicidal patients following an IV bolus of ketamine (0.2 mg/kg) administered over one to two minutes. In that study, antidepressant effects were seen at 40 minutes post-administration, and sustained improvements in suicidality scores lasted for over 10 days [58].
A meta-analysis of seven clinical trials providing unpublished data on the suicide item component of either the HAM-D or the MADRS noted a significant reduction in suicidality severity score for the ketamine group at days 1 and 3 [59]. Interestingly, the reduction in suicidality associated with ketamine administration may occur independently of its antidepressant effects [7]. This underscores the challenges of characterizing the clinical relationship between depression and suicidality as well as identifying the unique biological signatures linking depression and suicidality, to the extent that this can be achieved by examining the effects of ketamine. In a post-hoc analysis of 133 patients with treatment-resistant MDD and bipolar depression using the HAM-D, SSI, Beck Depression Inventory (BDI), and Hamilton Anxiety Rating Scale (HAM-A), improvement in suicidal ideation was observed 230 minutes after a single dose of ketamine (0.5 mg/kg). These improvements correlated with change in depressive symptoms (range: 0.23 to 0.44 (p < .05)) as well as with anxiety (range: 0.23 to 0.40 (p < .05)), each accounting for up to 19% of the variance in change in suicidal ideation [60]. This suggests that ketamine may have variable effects on anxiety and depression that act through different mechanisms, and that anxiety and depression may have a modulating—but not causative effect—on suicidality. This line of thinking, however, does not take into account the impact of anxiety on depression. For example, in a randomized, double-blind, placebo-controlled study, our group found that in association with ketamine administration, patients with anxious and non-anxious depression experienced a reduction in depressive symptoms; however, those with anxious depression relapsed significantly later than those with nonanxious depression (median ± SE = 19.0 ± 17.9 vs 1.0 ± 0.0 days to relapse, respectively; χ2 = 9.30; P = .002) [61].
In the first double-blind, randomized, placebo-controlled study to investigate an association between subanesthetic-dose ketamine and suicide, 15 patients with bipolar depression were maintained on therapeutic levels of lithium or valproate and administered a single IV ketamine infusion (0.5 mg/kg) or placebo on two test days two weeks apart. Compared with placebo, those who received ketamine experienced a significant reduction in suicidal ideation (as measured by the suicide item of the MADRS, HAM-D, and BDI) at 40 minutes that persisted three days post-administration [48]. The limitation in this study was that suicidality was not specifically selected for within the patient group. In two subsequent clinical studies of the association between IV ketamine administration and suicidality [56, 62], aspects of suicidality were measured via the Implicit Association Test (IAT), a scale that categorizes suicidal ideation into discrete cognitive categories. In the first study, open-label IV ketamine administration was associated with rapid reductions in explicit and implicit suicidal cognition within the first 24 hours. This effect persisted for patients who received up to five additional infusions over two weeks [62]. In a randomized, double-blind, controlled active comparator study of 57 MDD patients who were randomized to receive either a single infusion of ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg), 53% of ketamine-treated patients had no explicit suicidal ideation at 24 hours post-infusion compared with 24% of the midazolam group (χ2 = 4.6; P = .03) [62]. Although this was the first randomized clinical study to examine the association between ketamine administration and suicidality using an anesthetic control condition [62], the patients were not selected based on the presence or absence of suicidality. In a follow-up study from the same group, patients with clinically significant suicidal ideation were randomized to receive either ketamine or midazolam with a seven-day assessment of suicidality via the SSI [63]. Forty-eight hours post-infusion, patients who had received ketamine had significantly lower SSI scores than those who had received midazolam, but this difference was not seen at 24 hours or at any other time point throughout the seven-day assessment period. Although promising, this follow-up study was small (n=24). Thus, additional and larger randomized clinical trials are needed to confirm the validity of both an anti-suicidal and anxiolytic effect for ketamine.
2.5 Safety Profile of Subanesthetic Ketamine in Depression
Across ketamine studies, the most common side effects have been perceptual disturbances, confusion, elevations in systolic blood pressure, euphoria, dizziness, and increased libido [15, 16]. Although concerns of cognitive decline, neuronal injury, and physical dependence associated with the use of ketamine, or other NMDA antagonists, have been raised by some researchers [64–66], it is important to note that many of these concerns center around studies conducted only in animals [67, 68] or in human substance abuse populations, where ketamine use was uncontrolled and higher doses were used over a longer period of time [69–71]. In a controlled study of a single administration of IV ketamine (0.5 mg/kg) to 19 healthy subjects [72], immediate psychotomimetic symptoms and delayed recall effects returned to baseline within two hours of initiation of infusion. Perseverative errors (as noted by the Wisconsin Card Sorting Test (WCST)) were observed at the time of infusion, but there was no indication that these effects persisted and there was no significant change on the Mini Mental Status Exam (MMSE). Another study used a battery of cognitive tests (MATRICS)—including processing speed, attention/vigilance, working memory (nonverbal and verbal), verbal learning, visual learning, reasoning, problem solving, and social cognition—to assess function in individuals with MDD and found that ketamine was associated with selective impairments in memory recall [73]. Another two-site, double-blind clinical trial by the same group randomized subjects 2:1 to receive ketamine or midazolam; the study found that post-treatment neurocognitive performance (as measured by MATRICS) improved following treatment regardless of treatment condition. Interestingly, although there was no differential effect of treatment on neurocognitive performance and no association with antidepressant response, lower processing speed at baseline predicted greater improvement in depression at 24 hours following ketamine [74].
As noted above, in both single- [3, 7, 48, 50] and repeated-dose administration (six to 12 doses) [9, 75], no severe or long-term side effects have yet been noted with subanesthetic-dose ketamine. However, it remains unclear whether cognitive changes, alterations to brain tissue, or increased risk of substance abuse can develop in association with repeated doses in humans. Furthermore, the aforementioned studies used cognitive assessment measures that were relatively insensitive, of short duration, and not specifically designed to detect cognitive changes in association with drug intervention. Towards this end, large, controlled clinical studies with long-term follow-up using neurocognitive and neuroimaging assessments are warranted to address these issues. The results from such studies could help support or reject safe administration of subanesthetic-dose ketamine as an antidepressant in clinical settings.
2.6 Dosing Optimization and Dose Frequency of Subanesthetic Ketamine for Depression
IV ketamine has been used safely for procedural sedation in adults in doses as high as 2 mg/kg. In the only dose-finding study to date of IV ketamine, four subjects with TRD each received up to four IV doses of ketamine at 0.1 mg/kg, 0.2 mg/kg, 0.3 mg/kg, or 0.4 mg/kg (given over two to five minutes, one week apart); a placebo treatment was randomly inserted in a double-blind, placebo-controlled, crossover fashion. Among the three subjects achieving antidepressant response (≥ 50% decrease in MADRS score), two subjects responded at the 0.1 mg/kg dose; a clear dose-response relationship was demonstrated in only one subject, with the highest improvement occurring at 0.4 mg/kg [76]. In a randomized, crossover, double-blind study, 15 subjects with TRD were randomized to receive ketamine or midazolam in an ascending dose design with random insertion of a placebo comparator treatment. Patients received ketamine via one of three routes: IV (n = 4), intramuscular injection (n = 5), or subcutaneous injection (n = 6), starting at 0.1 mg/kg and increasing by 0.1 mg/kg until a maximum final dose of 0.5 mg/kg was reached. Ketamine doses were administered > one week apart, with one placebo control treatment randomly inserted. Twelve patients who received ketamine achieved response and met remission criteria at doses as low as 0.1 mg/kg regardless of route of administration. Indeed, all three routes of ketamine administration resulted in comparable antidepressant effects in a dose-related fashion, with the fewest adverse effects seen via the subcutaneous route [77]. Larger sample dose optimization studies are actively being conducted (NCT01558063).
With regard to dosing frequency, it should be noted that although antidepressant response rates to a single ketamine infusion (0.5 mg/kg) have been reported to be 50–71% in individuals with MDD [2, 3], depressive symptoms typically return within seven days after cessation of drug administration if subsequent treatments are not administered [15]; this is consistent with single administrations of other antidepressant interventions (e.g. ECT or sleep deprivation). Thus, repeated dose administration of IV ketamine to prolong its antidepressant and potential anti-suicidal effects has been studied in limited situations. In small, open-label studies of repeated ketamine infusions administered over a two-week interval, relapse rates have been reported to range between 55%–89% in the month following treatment [49, 78, 79].
In an open-label study, Murrough and colleagues found that the use of up to six IV infusions of ketamine administered over a 12-day period resulted in rapid antidepressant effects in patients with TRD [75]. They noted that the overall response rate at the end of the trial was 71%, and that, among responders, median time to relapse after the last ketamine infusion was 18 days; 80% of subjects relapsed within 28 days, but 20% maintained response to 78 days, though all subjects did eventually relapse. However, the treatments were well tolerated; no serious adverse events were noted, and no sustained increase in psychotomimetic/dissociative side effects was observed.
In a randomized, placebo-controlled, parallel design study that investigated the use of IV ketamine (0.5 mg/kg infused over 40 minutes either two or three times per week), subjects who received eight or 12 IV ketamine administrations over the course of the study demonstrated a statistically significant reduction in MADRS scores from Day 1 to Day 15 compared to those receiving placebo [9]. In another double-blind, randomized study, 18 patients with MDD received either three IV ketamine infusions (0.5 mg/kg) or ECT on three test days (two days apart). Within 24 hours, depressive symptoms improved in subjects receiving the first dose of ketamine compared with the ECT group and this improvement remained significant throughout the study [80]. In addition, Price and colleagues found that reductions in suicidality observed after a single dose of ketamine were sustained for 12 days by repeated doses (0.5 mg/kg, thrice weekly; p < .001; d = 2.42) [56].
As noted above, repeated ketamine infusions have been shown to be well-tolerated with no associated severe adverse events. During IV ketamine administration at 0.5 mg/kg, the occurrence of perceptual symptoms (as measured by the Brief Psychiatric Rating Scale (BPRS) and Clinician-Administered Dissociative States Scale (CADSS)) resolves within three hours of initiation of the infusion. The most common physical symptoms include dizziness, headache, hypoaesthesia, paresthesia, and nausea.
With regard to physical dependence, to date no evidence suggests that subanesthetic-dose ketamine causes physical dependence in humans [81]. Relatedly, a recent study found no evidence that repeated but limited exposure to ketamine increased the risk of more severe or more protracted psychosis, perceptual changes resembling dissociation, severe emotional distress, or euphoria in healthy subjects [82]. Although the existing data do not indicate prolonged cognitive or dissociative side effects, the evidence is drawn from only a few studies, and these specific issues have not yet been systematically examined. Furthermore, given that ketamine is considered an illicit substance in the US, a higher level of scrutiny must be considered, and large, long-term follow-up studies are needed to definitively assess possible associations between ketamine use and issues of abuse and/or dependence [83, 84].
2.7 Route of Administration for Subanesthetic Ketamine for Depression
Investigators are also exploring alternative—and perhaps more convenient—routes of ketamine administration, including intranasal and sublingual. One randomized, double-blind, crossover, placebo-controlled study found that 50mg intranasal ketamine improved depressive symptoms within 24 hours compared to placebo in 20 patients with MDD (t = 4.39, p < .001) [85]. Although there may be considerable variability associated with intranasal ketamine administration in terms of bioavailability, this means of administration was well tolerated with minimal psychotomimetic or dissociative effects and no significant hemodynamic changes. In addition, Lara and colleagues evaluated the tolerability and efficacy of a sublingual formulation of racemic ketamine (10 mg from a 100 mg/ml solution for five minutes, then swallowed), whose estimated bioavailability is greater than orally administered ketamine [86]. In this case series, 20 of 26 (77%) patients with bipolar depression or MDD who received sublingual racemic ketamine every two to three days or weekly reported improved and stable mood, cognition, and sleep with only mild and transient light-headedness as a common side effect (no euphoria, psychotic, or dissociative symptoms). In a small, placebo-controlled, crossover trial, subcutaneous and intramuscular injections of subanesthetic-dose ketamine were found to be associated with antidepressant response in a similar fashion to IV ketamine administrations; in that study, subcutaneous injection was associated with the fewest adverse effects [77]. Clearly, replication of both findings through larger randomized clinical trials is warranted.
3. Glutamatergic Synergism and the Maintenance of Ketamine’s Antidepressant Effects
Several studies (see Table 1) have examined the possibility of augmenting ketamine’s rapid antidepressant effects using other well-studied antidepressant strategies, including mood stabilizing medications [48, 50], ECT [52, 53, 87–90], monoaminergic antidepressants [91], and agents with glutamatergic modulating effects [6, 51, 92]; the latter are discussed in Section 4, below.
Our laboratory conducted two studies in which patients with bipolar depression received ketamine after being stabilized on VPA or lithium. A single ketamine infusion (0.5 mg/kg) had rapid antidepressant effects that lasted for three days [48, 50]. Although concomitant use of a mood stabilizing medication does not appear to maintain antidepressant effects in patients with bipolar depression, these findings have roused interest in examining possible lithium augmentation of ketamine in MDD patients. Interestingly, animal models suggest that both ketamine and lithium may inhibit glycogen synthase kinase-3 (GSK-3) in a synergistic manner, leading to both antidepressant and antimanic effects [93, 94]
Ketamine has also been investigated in conjunction with ECT. In double-blind studies in which depressed patients were randomized to receive an infusion of subanesthetic-dose ketamine in addition to or separate from infusion with another anesthetic agent (thiopental or propofol), both groups saw similar levels of improvement in their depressive symptoms [52, 53, 87]. Another study found that individuals who received ketamine anesthesia prior to a single ECT administration experienced a slightly greater improvement in depressive symptoms a few days later [88]. Another randomized, double-blind, single-infusion, pre-ECT study comparing ketamine and thiopental found that both groups showed significant improvement in depressive symptoms; however, improvement in depressive symptoms was mildly greater in the ketamine than the thiopental group (p = 0.049) [95]. Given that ECT’s antidepressant effects occur after a series of treatments, it is worth noting that depressive symptoms in this study as well as that of Wang and colleagues [88] were assessed after only a single administration of ketamine given prior to ECT.
Another study investigated a series of ketamine administrations pre-ECT and found a significant difference in improvement of depressive symptoms only before the second ECT + ketamine administration compared with the thiopental group [89]. It is worth noting that although significant reductions in depressive symptoms were associated with pre-ECT infusions of ketamine, these improvements were minimal. In addition, a randomized, controlled trial of MDD patients who received pre-ECT ketamine (1–1.5 mg/kg at ECT sessions 2 and 3 or 1–1.5 mg/kg at ECT sessions 2, 4, 6, 8, 10) or thiopental (2–3 mg/kg for all ECT sessions) found that depressive symptom severity was lower after the last ECT session in the group that received ketamine prior to the five ECT sessions than in the group that received only thiopental for all ECT sessions [96], suggesting that a greater frequency of paired ketamine and ECT may offer more clinical benefit than a single pre-ECT ketamine infusion. In contrast, another study series compared pre-ECT infusions of ketamine (1 mg/kg), thiopental (4 mg/kg), or both agents; the investigators found that depression symptom scores improved for all groups (as assessed by the HAM-D) and no statistically significant differences were observed between groups [97]. In another study of 90 patients with TRD randomly assigned to receive either ketamine (0.8mg/kg), subanesthetic ketamine (0.5mg/kg) plus propofol (0.5mg/kg), or propofol (0.8mg/kg) prior to eight ECT sessions, those in the ketamine group had an earlier reduction in depressive symptoms; compared to the other two groups, this effect was most pronounced after completion of the second treatment and lasted until the last treatment [98]. Finally, a recent meta-analysis of 14 double-blind, randomized, controlled trials examining the efficacy and tolerability of six anesthetic agents prior to ECT for depressive disorders found that ketamine and methohexital were more beneficial than propofol or thiopental [99]. The findings suggest that co-administration of ketamine and ECT may offer some additional benefit for alleviating depressive symptoms in patients. Interestingly, ketamine has also been shown to benefit patients who had not previously responded to an adequate trial of ECT [100]. A clinical trial aimed at determining ketamine’s efficacy for preventing relapse after ECT is underway [101], underscoring the need to identify subgroups of depressed patients who may respond to specific antidepressant treatments.
Ketamine has also been used in conjunction with selective serotonin reuptake inhibitors (SSRIs). In a double-blind study, 30 MDD patients were randomized to receive either a single administration of ketamine or placebo concomitant to daily treatment with escitalopram (10 mg) [91]. Improvement in depressive symptoms was greater, and remission rates were higher, in those that received escitalopram plus ketamine versus those that received escitalopram plus placebo for up to two weeks (92.3% v. 57.1%, p = 0.04 and 76.9% v. 14.3%, p = 0.001, respectively), with a significantly shorter time to response (HR: 0.04, 95% CI: 0.01–0.22, p < 0.001) and remission (HR 0.11, 95% CI 0.02–0.63, p = 0.01). This suggests that ketamine administration may be used to offer more immediate antidepressant relief during the lag time to monoaminergic antidepressant response.
Interestingly, a small open-label study of patients with obsessive compulsive disorder (OCD), found that when a single ketamine infusion (0.5 mg/kg) was added to exposure-based cognitive behavioral therapy (CBT), an initial reduction in OCD symptoms was observed; this was accompanied by sustained response over two weeks [102]. Although this was an open-label study of OCD patients, it suggests that emotion dysregulation may be rapidly and effectively targeted using synergistic approaches.
4. Non-Ketamine Glutamate Modulators for Depression
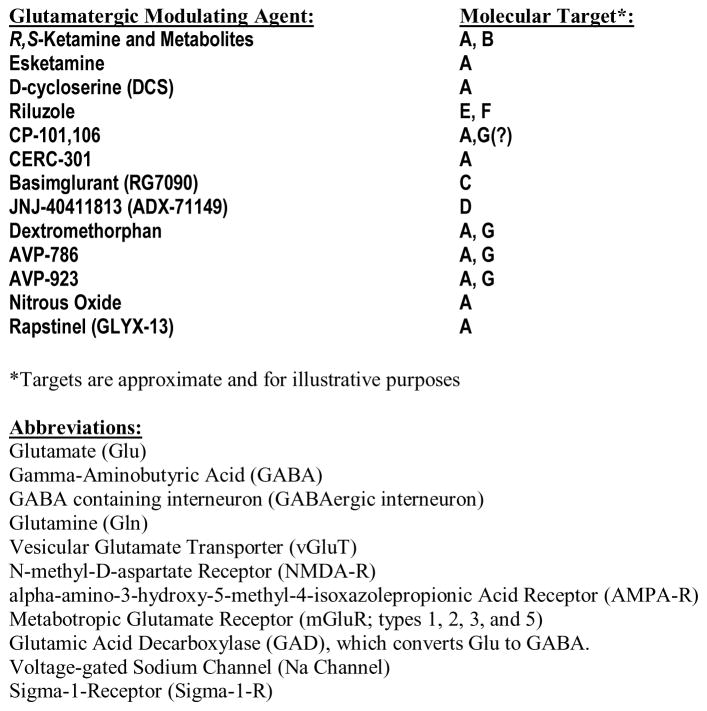
A variety of agents with direct and indirect effects on the glutamate system (see Figure 1) have been evaluated in small clinical trials [15, 16]. In lieu of an exhaustive review of all glutamatergic modulating agents that have been investigated in phase II clinical trials for the treatment of depression, here we highlight those agents that have shown preliminary evidence of an antidepressant effect and that may be particularly fruitful for developing novel glutamatergic modulating agents for the treatment of depression (see Table 2).
Figure 1.
Glutamatergic Synapse and Major Mechanisms of Action of Potential Glutamatergic Antidepressant Agents
Table 2.
Select Glutamatergic Modulating Agents with Potential Antidepressant Effects
| Glutamatergic Modulator | Target | Major Mechanism(s) of Action | Potential Indication(s) | Evidence of Efficacy |
|---|---|---|---|---|
| Ketamine (+ Metabolites?) | NMDA receptor, AMPA receptor (?) | NMDA receptor antagonist, AMPA receptor agonist | MDD, BD, Suicidality, Anxiety, PTSD, OCD | Moderate to strong evidence of efficacy (see Table 1); most clinical trials in MDD patients |
| Esketamine (Intranasal Ketamine) | NMDA receptor | NMDA receptor antagonist, AMPA receptor agonist | MDD, Suicidality, PTSD | Moderate evidence of efficacy (see Table 1); most clinical trials in MDD patients |
| D-cycloserine (DCS) | NMDA receptor | Partial agonist at glycine site; functional NMDA receptor antagonist | MDD, BD | Moderate evidence for antidepressant effect as adjunct to mood stabilizer in BD [15, 51, 103, 104] |
| Riluzole | Voltage-gated sodium channels | Increased synaptic glutamate reuptake, blockade of voltage-gated sodium channels | MDD, BD, OCD | Weak evidence of efficacy for MDD [6, 92, 106–110] |
| CP 101,606 | NMDA-NR2B subunit | NMDA-NR2B non-selective antagonist | MDD | Clinical trials ceased due to association with cardiac conduction abnormalities [111, 112] |
| CERC-301 | NMDA-NR2B subunit | NMDA-NR2B selective antagonist | MDD | Weak evidence of efficacy for MDD [113] |
| Basimglurant (RG7090) | mGluR5 | mGluR5 NAM | MDD | Weak evidence for antidepressant effects as adjunct to monoamine antidepressants; weak evidence for monotherapy as antidepressant [118] |
| JNJ-40411813 (ADX71149) | mGluR2 | mGluR2 PAM | MDD | Weak evidence as adjunct to monoamine antidepressants for anxiety [119] |
| Dextromethorphan | NMDA receptor, Sigma-1 receptor, SERT, NET | NMDA receptor antagonist, Sigma-1 receptor antagonist, SERT and NET inhibitor | BD | Weak evidence for antidepressant effects as adjunct to mood stabilizer in BD [120, 121] |
| AVP-786 | NMDA receptor, Sigma-1 receptor, SERT, NET | NMDA receptor antagonist, Sigma-1 receptor antagonist, SERT and NET inhibitor | MDD | Ongoing Phase II clinical trial in MDD (NCT02153502) as adjunct to monoamine antidepressants |
| AVP-923 (Nuedexta) | NMDA receptor, Sigma-1 receptor, SERT, NET | NMDA receptor antagonist, Sigma-1 receptor antagonist, SERT and NET inhibitor | MDD | Ongoing Phase II clinical trial in MDD (NCT01882829) |
| Nitrous Oxide (N2O) | NMDA receptor | NMDA receptor antagonist | MDD | Preliminary evidence of efficacy for MDD [122] |
| Rapastinel (GLYX-13) | NMDA receptor | NMDA receptor glycine site NAM | MDD | Moderate evidence of efficacy for MDD [123125] |
NMDA: N-methyl-D-aspartate; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; mGluR: metabotropic glutamate receptor; MDD: major depressive disorder; BD: bipolar disorder; PTSD: post-traumatic stress disorder; OCD: obsessive compulsive disorder; NAM: negative allosteric modulator; PAM: positive allosteric modulator; SERT: serotonin transporter; NET: norepinephrine transporter
4.1 D-cyloserine (DCS)
D-cycloserine (DCS) is an orally administered antibiotic medication that is FDA-approved for the treatment of tuberculosis [51]. It has been the most promising glutamatergic modulating agent, after ketamine, to show an acute antidepressant response. Through its effects on glycine binding sites, DCS’s mechanism of action at higher doses prevents the opening of the NMDA receptor channel, thereby acting as a functional NMDA receptor antagonist.
In 22 subjects with TRD, a six-week, crossover, placebo-controlled trial of adjunctive DCS (250mg/day) reduced depressive symptoms but did not separate from placebo due to the high placebo response rate [103]. A slightly larger study (n=26) of individuals with TRD over the same six-week period by the same group assessed the efficacy of escalating dose (up to 1000mg/day) adjunctive DCS [104] and found that higher-dose DCS had significant antidepressant effects as measured by the clinician-administered HAM-D and self-reported BDI; more than half of the patients randomized to high-dose DCS had a greater than 50% reduction in HAM-D scores by the end of the study.
With regard to DCS as an adjunctive therapy in bipolar depression, one open-label study investigated 12 patients (n = 7 completers) with bipolar depression who were stabilized on lurasidone, quetiapine, or a fluoxetine and olanzapine combination and who received ketamine (0.5 mg/kg) prior to titration with DCS [51]. In this study, improvement in depressive symptoms was seen from baseline to all rating points (except at two weeks) throughout the three-week trial, with a large effect size seen at day 1 (Cohen d = 2.0). Four of the seven patients with bipolar depression remained in remission after eight weeks, and clinical improvement at that time point correlated with the magnitude of improvement 24 hours post-ketamine. It should be noted that this study did not have a control group, which may limit interpretation of its efficacy.
In addition, a recent meta-analysis [15] found that among the agents that act directly on the NMDA receptor, DCS has been associated with an acute antidepressant response at high doses (1,000 mg) but not at low doses (250 mg); data were drawn from the studies cited above.
4.2 Riluzole
Riluzole, which is FDA-approved for the treatment of amyotrophic lateral sclerosis, has been repurposed and investigated as a potential glutamatergic antidepressant agent. An animal model of depression found that chronic unpredictable stress led to glial pathology that was reversed after riluzole administration [105]; this finding led to a small collection of open-label studies examining antidepressant response to riluzole monotherapy in patients with MDD or bipolar depression. In the first open-label study of 19 TRD patients, depressive symptoms improved within three to six weeks (the study endpoint) of riluzole administration [106]. Consistent with a case report of antidepressant response to riluzole as an augmentation strategy in patients concurrently treated with traditional antidepressants [107], a second open-label study of 10 patients with MDD showed a reduction in both depressive and anxiety symptoms after the first week and throughout the 12 weeks of the study when riluzole was added to their antidepressant regimen [108]. Another open-label study of riluzole added to ongoing treatment with lithium in 14 patients with bipolar depression similarly observed a reduction in depressive symptoms during weeks five through eight of the 12-week study [109].
Riluzole was also tested in two randomized, double-blind, placebo-controlled trials in individuals with MDD as a possible augmentation strategy to maintain ketamine’s antidepressant effects. In order to examine the efficacy of riluzole in preventing depressive relapse after experiencing an antidepressant response to ketamine, a randomized, double-blind, placebo-controlled, flexible-dose continuation trial of riluzole (100–200 mg/d) was performed over the course of a 32-day study period [6]. In this study, 14 (out of 26) patients with TRD who received open-label ketamine met response criteria; however, an interim analysis found no significant differences in time-to-relapse between the riluzole and placebo groups. In another randomized, double-blind, placebo-controlled, add-on study, 42 patients with TRD received a single IV ketamine infusion (0.5 mg/kg) and were then randomized, four to six hours post-infusion, to receive either riluzole (100–200 mg/day; n=21) or placebo (n=21) for four weeks [92]. Although a significant improvement in depressive symptoms (as measured by MADRS) remained throughout the entirety of the 28 day trial, the difference between the riluzole and placebo treatment groups was not significant, suggesting that riluzole did not impact antidepressant response to ketamine. Moreover, when the subgroup of ketamine non-responders was examined, an antidepressant response was still not observed in association with riluzole [110]. Taken together, the current data do not yet support the use of riluzole as an antidepressant agent in patients with TRD.
4.3 CP-101,606 and CERC-301 (MK-0657)
Two investigative agents that act on the NR2B subunit of the NMDA receptor as antagonists—CP-101, 606 and CERC-301 (previously known as MK-0657)—have been studied in small clinical trials of depressed patients. A rapid antidepressant effect was shown in association with a single IV infusion of CP-101,606 as monotherapy in a randomized, double-blind, placebo-controlled study of 30 patients with TRD. In the first treatment period, participants received a six-week open-label trial of paroxetine as well as a single-blind, IV placebo infusion of CP-101,606. Those who did not respond then received a randomized, double-blind single infusion of CP-101,606 or placebo plus continued treatment with paroxetine for up to an additional four weeks. Although CP-101,606 reduced depressive symptoms more effectively than placebo, and 78% of CP-101,606-treated responders maintained response status for at least one week post-infusion, development of this agent was stopped due to its resulting effect on cardiac conduction (i.e., QTc prolongation) [111]; thus, replication has not been performed. Furthermore, this compound may have off-site effects at sigma receptors [112], suggesting that any signal of efficacy may not be directly attributable to NR2B antagonism.
CERC-301 (previously known as MK-0657) is an orally administered NR2B-selective antagonist. A small, randomized, double-blind, placebo-controlled, crossover clinical trial that examined depressive symptoms after daily administration of CERC-301 as monotherapy over 12 days in 21 patients with TRD obtained mixed results [113]. Although a reduction in depression scores was not observed when assessed by the MADRS—the primary outcome measure—reductions in depression scale scores were observed via the clinician-administered HAM-D and the self-reported BDI [113]. In contrast to CP-101,106, no serious or dissociative adverse effects were observed with this orally administered agent. Most recently, a Phase 2 placebo-controlled trial (NCT02459236) using higher doses of CERC-301 (12mg and 20mg) did not meet the primary endpoint of mean improvement on subset scores of the HAM-D17, despite significant improvement in depressive symptoms at day 2 with the 20 mg dose (http://ir.cerecor.com/pressreleases/detail/30/cerecor-reports-top-line-data-from-cerc-301-phase-2-study).
4.4 Negative and Positive Allosteric Modulators: Basimglurant and JNJ-40411813/ADX71149
Agents that act as a negative or positive allosteric modulator (NAM or PAM) on the metabotropic glutamate receptor 5 (mGluR5) have shown preliminary modest evidence as antidepressant agents. Found in the postsynaptic density [114–116] and in glial cells [117], mGluRs are expressed widely throughout the brain and are an additional glutamate signaling pathway outside of the NMDA receptor and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor pathways. Basimglurant (RO4917523, RG7090), an mGluR5 NAM, is currently in clinical development for depression (NCT00809562, NCT01437657). In a multicenter nine-week, double-blind, placebo-controlled study of 333 patients with MDD, basimglurant was administrated orally at two dose levels (0.5 mg or 1.5 mg daily) adjunctive to ongoing treatment with SSRIs or serotonin-noradrenaline reuptake inhibitors (SNRIs) [118]. No difference was observed on the clinician-rated MADRS—the primary outcome measure—compared to placebo from baseline to endpoint. However, at a dose of 1.5 mg daily (but not at 0.5 mg), an antidepressant effect was observed at multiple time points as assessed by patient-rated scales (MADRS and the Quick Inventory of Depressive Symptomatology Self Report (QIDS-SR16)).
The efficacy, safety, and tolerability of PAMs have also been evaluated in individuals with MDD. In a phase IIa, randomized, multicenter, double-blind, proof-of-concept study, JNJ-40411813/ADX71149, a novel mGluR2 PAM, was administered adjunctively to MDD patients with significant anxiety (as measured by the HAM-D and HAM-A) who were already taking an SSRI or SNRI. The first study period, lasting four weeks, comprised randomization to either flexibly-dosed JNJ-40411813 or placebo, followed by a re-randomization and another four-week study period for those who received placebo and continued to meet entry severity criteria. Among the 100 patients who completed the study, no differences were observed in depression or anxiety rating scale scores via primary outcome measures, although some reductions in anxiety symptoms, as assessed by secondary measures, were noted [119].
Taken together, the evidence suggests that neither mGluR NAMs nor PAMs have yet demonstrated a strong antidepressant effect.
4.5 Dextromethorphan
Dextromethorphan is the active agent in over-the-counter antitussive medications. Although it acts on opioid receptors, at higher doses dextromethorphan acts as a sigma-1 receptor agonist and inhibitor of the serotonin (SERT) and norepinephrine (NET) transporters, as well as a non-competitive NMDA receptor antagonist. To date, no randomized clinical trials have explored dextromethorphan as monotherapy in mood disorders. A 12-week, placebo-controlled study of 250 patients with bipolar depression added dextromethorphan to treatment with VPA and found that this combination reduced depressive symptoms; however, this difference in treatment groups did not reach statistical significance, potentially due to metabolism-related reductions in drug concentration [120]. A retrospective chart review of 22 subjects with bipolar disorder (BD)-II or BD not otherwise specified (BD-NOS) found that adding 20 mg dextromethorphan and 10 mg quinidine (a cytochrome 2D6 inhibitor) once or twice daily to a current medication regimen over a 90-day treatment period significantly improved Clinical Global Impression (CGI) scale scores [121]. This dextromethorphan-quinidine combination—Nuedexta (AVP-923), which is FDA-approved for the treatment of pseudobulbar affect—is currently under investigation as a potential antidepressant agent in patients with MDD (NCT01882829). In addition, AVP 786, a combination of deuterated (d6)-dextromethorphan and an ultra-low dose of quinidine, received fast-track designation for agitation in Alzheimer’s Disease; it is currently under investigation for use in depression (NCT02153502).
4.6 Indirect glutamatergic modulators: Nitrous Oxide (N2O) and GLYX-13
Indirect glutamatergic modulation as a potential mechanism of antidepressant effects has been investigated in clinical trials using nitrous oxide (N2O) [122] and the glycine partial agonist GLYX-13 (rapastinel) [123, 124]. N2O, a non-competitive NMDA receptor inhibitor, has been used as an inhaled general anesthetic in medical and dental settings. In a placebo-controlled, double-blind, crossover study examining 20 patients with TRD who received an inhalation of 50% N2O or 50% nitrogen (placebo), both over one hour, those who received N2O experienced a reduction in depressive symptoms (as measured by the HAM-D) at both two hours and 24 hours post-inhalation compared to placebo [122]. Adverse effects included anxiety, headache, and nausea/vomiting, but there were no psychotomimetic effects associated with N2O inhalation.
In a clinical trial of GLYX-13 (rapastinel)—an NMDA receptor glycine site functional partial agonist—as monotherapy, 116 unmedicated patients with TRD were randomized to receive a single IV GLYX-13 dose of 1mg/kg, 5mg/kg, 10 mg/kg, 30 mg/kg, or placebo over three to 15 minutes and then followed for seven days. At one week post-infusion, those who received 5 and 10 mg/kg of GLYX-13 had experienced a significant antidepressant response compared to placebo [124]. The same investigators subsequently performed a randomized, double-blind, clinical trial of adjunctive GLYX-13 in 116 patients with TRD (concurrently maintained on a psychotropic medication regimen). Subjects were randomized to receive weekly infusions of IV GLYX-13 (at doses of 1mg/kg, 5mg/kg, or 10 mg/kg) or placebo, with follow-up on days 3, 7, and 14. After an interim safety and efficacy analysis was performed, an additional cohort was added and randomized to receive IV GLYX-13 (30 mg/kg) or placebo, with follow-up at days 3, 7, 14, 21, and 28. Those who received IV GLYX-13 at doses of 5 or 10 mg/kg IV showed a reduction in HAM-D scores on days 1 through 7, but no antidepressant effects were observed thereafter [123]. The authors suggested that the U-shaped dose response curve observed for GLYX-13 may be due to the unique molecular interactions of GLYX-13 as a partial agonist on the glycine site of the NMDA receptor. GLYX-13 infusion at any dose was not associated with psychotomimetic properties, and no serious adverse events were reported in either study.
Interestingly, in a recently published series of preclinical studies, a single infusion of GLYX-13 led to increased dendritic arborization within the medial prefrontal cortex, presumably in part via the mTORC1 pathway, as well as increased synaptic responses to hypocretin in thalamocortical synapses [125]. Thus, it appears that GLYX-13 may induce specific intracellular molecular pathways that can lead to plastic changes within specific brain regions, providing a possible link between a drug action, brain function, and antidepressant response.
5. Ketamine’s Mechanism of Action Revisited
Despite the preponderance of the evidence supporting ketamine’s antidepressant effects, the active molecule(s) as well as the molecular and intracellular pathways responsible for the mechanism of action has not yet been fully elucidated. In this context, it is interesting to note that there are currently six ongoing, Phase 3 clinical trials studying the efficacy of esketamine (the S-enantiomer of ketamine) in TRD. A recent proof-of-concept clinical trial examining the antidepressant efficacy and safety of 0.20 mg/kg and 0.40 mg/kg IV esketamine compared with IV placebo in 30 patients with TRD found that subjects who received either dose demonstrated an improvement in depressive symptoms as measured by the MADRS [43]. Another double-blind, placebo-controlled, multi-center study (SYNAPSE) evaluated the antidepressant efficacy and dose response of intranasal esketamine in 67 individuals with TRD [126]. Over a one-week period, change in MADRS total scores on Day 8 in all three esketamine treatment groups (28mg, 56mg, or 84mg) was statistically superior to placebo. Dissociative symptoms appeared to diminish with repeated dosing. Finally, a 12-week, randomized, placebo-controlled, double-blind, multicenter study investigated the efficacy of intranasal esketamine (84mg) in 68 adults with MDD and active suicidal ideation. Intranasal esketamine significantly reduced depressive symptoms (measured by the MADRS) and thoughts of suicide (measured by the SSI) [127]. Taken together, these results suggested that the “S” enantiomer of ketamine may contribute to its antidepressant effect.
In contrast, and as mentioned earlier in this review, a recent study series that sought to identify which—if any—of the major ketamine metabolites were responsible for ketamine’s antidepressant effects found that metabolism of (R,S)-ketamine to (2S,6S; 2R,6R)-HNK is essential to ketamine’s antidepressant effects. Furthermore, the (2R,6R)-HNK enantiomer—which is one of the direct metabolites of (R,S)-ketamine—is the primary agent involved in exerting ketamine’s antidepressant effects [38]. In addition, mice treated with a single dose of (2R,6R)-HNK showed behavioral, cellular, and electrophysiological improvements in their depression-related symptoms that lasted up to three days. (2R,6R)-HNK’s significant antidepressant effects were not associated with any dissociative effects, suggesting that the antidepressant actions exerted by this metabolite occurred independently of NMDA receptor inhibition. Notably, these specific ketamine metabolites induced early and sustained activation of AMPA receptors, contrary to prior suggestions that ketamine’s antidepressant effect is exerted primarily through the NMDA receptor.
6. Conclusions
As the evidence reviewed above has underscored, a number of clinical avenues are currently being investigated in the quest to develop an equally effective, more convenient alternative to ketamine that is not associated with psychotomimetic and dissociative side effects. Although some have suggested that ketamine’s antidepressant effects may be related to mechanisms other than glutamate [64]—suggesting, for instance, AMPA throughput as a potential venue—mechanistic confirmatory studies are lacking at this stage. Similar to subanesthetic-dose ketamine, pre-clinical studies of GLYX-13 (rapastinel) [125] have emphasized the importance of further investigating mechanisms of action that can allow for continued work towards developing more effective and safe treatments for mood disorders.
Given that our current armamentarium of psychotropic medications shown to be the most effective for psychiatric disorders act on multiple receptors and molecules (e.g. clozapine for schizophrenia), we may need to find the right combination of molecular actions and cellular effects to develop more effective medications for mood disorders. The most recent evidence suggests that—as observed in recent ketamine studies—other molecular and cellular actions in addition to primary NMDA receptor effects may need to converge to induce antidepressant action. Therefore, linking biological to clinical effects requires simultaneous preclinical and clinical investigations, with each paving the way towards the other. Finally, with regard to clinical investigations, an integrated approach using different functional brain imaging techniques, psychophysiological methods, and reconceptualization of clinical phenomenology may be essential to disentangling clinical biological heterogeneity of patients with depression [13].
Key Points.
This review highlights evidence supporting the antidepressant effects of subanesthetic-dose ketamine as a prototypical glutamatergic-modulating agent from which other glutamatergic agents have been investigated. Evidence suggests that ketamine may have broad biological effects on the glutamatergic system and may exert its clinical efficacy by altering other symptom domains related to depression, such as anxiety and suicidality. This review also highlights important safety concerns regarding ketamine and selected glutamatergic agents in order to promote the continued development of novel, effective, and safe antidepressant drug treatments.
Acknowledgments
Funding.
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA-MH002857), by a NARSAD Independent Investigator to Dr. Zarate, and by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate.
The authors thank the 7SE research unit and staff for their support. We also thank Ioline Henter (NIMH) for providing invaluable editorial assistance.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest.
Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. Dr. Zarate has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Drs. Lener and Kadriu have no conflict of interest to disclose, financial or otherwise.
References
- 1.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998 Nov;87(5):1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 2.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000 Feb 15;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 3.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006 Aug;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Du J, Quiroz J, Gray NA, Denicoff KD, Singh J, et al. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Ann N Y Acad Sci. 2003 Nov;1003:273–91. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006 Jan;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 6.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010 Feb;13(1):71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. The American journal of psychiatry. 2013 Oct 1;170(10):1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messer M, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010 Fall;22(4):442–4. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- 9.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. The American journal of psychiatry. 2016 Aug 1;173(8):816–26. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 10.Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, et al. Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety. 2014 Apr;31(4):297–307. doi: 10.1002/da.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015 May;1344:50–65. doi: 10.1111/nyas.12759. [DOI] [PubMed] [Google Scholar]
- 12.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014 Sep;19(9):978–85. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014 Apr;171(4):395–7. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 14.Park M, Niciu MJ, Zarate CA., Jr Novel Glutamatergic Treatments for Severe Mood Disorders. Curr Behav Neurosci Rep. 2015 Dec;2(4):198–208. doi: 10.1007/s40473-015-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015 Oct 1;172(10):950–66. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 16.Niciu MJ, Ionescu DF, Richards EM, Zarate CA., Jr Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014 Aug;121(8):907–24. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990 Aug 21;185(1):1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 18.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008 Feb 15;63(4):349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Musazzi L, Racagni G, Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011 Aug;59(2):138–49. doi: 10.1016/j.neuint.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016 Mar;21(3):454–64. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan TF, Hou G. The effects of stress on glutamatergic transmission in the brain. Mol Neurobiol. 2015;51(3):1139–43. doi: 10.1007/s12035-014-8783-9. [DOI] [PubMed] [Google Scholar]
- 22.Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012 Oct;64(4):901–38. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- 23.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011 Nov 30;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller HK, Wegener G, Liebenberg N, Zarate CA, Jr, Popoli M, Elfving B. Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res. 2013 Jul;47(7):892–9. doi: 10.1016/j.jpsychires.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009 Jun;10(6):410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joels M, Pasricha N, Karst H. The interplay between rapid and slow corticosteroid actions in brain. Eur J Pharmacol. 2013 Nov 05;719(1–3):44–52. doi: 10.1016/j.ejphar.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012 Jan;62(1):63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groeneweg FL, Karst H, de Kloet ER, Joels M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol Cell Endocrinol. 2012 Mar 24;350(2):299–309. doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Radley J, Morilak D, Viau V, Campeau S. Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev. 2015 Nov;58:79–91. doi: 10.1016/j.neubiorev.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaveri E, Kelly F, Mallei A, Harris K, Taylor A, Reid J, et al. Expression profiling of a genetic animal model of depression reveals novel molecular pathways underlying depressive-like behaviours. PLoS One. 2010 Sep 07;5(9):e12596. doi: 10.1371/journal.pone.0012596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996 Jan;29(1):23–6. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 32.Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993 Jun;265(3):1380–6. [PubMed] [Google Scholar]
- 33.Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett. 2015 Aug 05;601:20–9. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011 Apr 15;69(8):754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–9. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012 Sep;18(9):1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007 Feb;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 38.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016 May 26;533(7604):481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989 Mar;36(2):186–97. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 40.Green SM, Rothrock SG, Lynch EL, Ho M, Harris T, Hestdalen R, et al. Intramuscular ketamine for pediatric sedation in the emergency department: safety profile in 1,022 cases. Ann Emerg Med. 1998 Jun;31(6):688–97. doi: 10.1016/s0196-0644(98)70226-4. [DOI] [PubMed] [Google Scholar]
- 41.Green SM, Rothrock SG, Harris T, Hopkins GA, Garrett W, Sherwin T. Intravenous ketamine for pediatric sedation in the emergency department: safety profile with 156 cases. Acad Emerg Med. 1998 Oct;5(10):971–6. doi: 10.1111/j.1553-2712.1998.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 42.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016 May;46(7):1459–72. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2015 Nov 3;80:424–31. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA., Jr A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord. 2015 Jun;17(4):438–43. doi: 10.1111/bdi.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34(4):287–93. [PubMed] [Google Scholar]
- 46.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979 Apr;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 48.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012 Jun 1;71(11):939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010 Jan 15;67(2):139–45. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 50.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010 Aug;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kantrowitz JT, Halberstam B, Gangwisch J. Single-dose ketamine followed by daily D-Cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry. 2015 Jun;76(6):737–8. doi: 10.4088/JCP.14l09527. [DOI] [PubMed] [Google Scholar]
- 52.Abdallah CG, Fasula M, Kelmendi B, Sanacora G, Ostroff R. Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT. 2012 Sep;28(3):157–61. doi: 10.1097/YCT.0b013e31824f8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loo CK, Katalinic N, Garfield JB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J Affect Disord. 2012 Dec 15;142(1–3):233–40. doi: 10.1016/j.jad.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 54.McGirr A, Berlim MT, Bond DJ, Neufeld NH, Chan PY, Yatham LN, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J Psychiatr Res. 2015 Mar;62:23–30. doi: 10.1016/j.jpsychires.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, Zarate CA., Jr Subanesthetic dose ketamine does not induce an affective switch in three independent samples of treatment-resistant major depression. Biol Psychiatry. 2013 Nov 15;74(10):e23–4. doi: 10.1016/j.biopsych.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009 Sep 1;66(5):522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010 Dec;71(12):1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011 Sep;14(8):1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 59.Xu Y, Hackett M, Carter G, Loo C, Galvez V, Glozier N, et al. Effects of Low-Dose and Very Low-Dose Ketamine among Patients with Major Depression: a Systematic Review and Meta-Analysis. Int J Neuropsychopharmacol. 2016 Apr;19(4) doi: 10.1093/ijnp/pyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014 Nov;58:161–6. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Slonena EE, Vande Voort JL, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry. 2014 Sep;75(9):e932–8. doi: 10.4088/JCP.14m09049. [DOI] [PubMed] [Google Scholar]
- 62.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014 Apr;31(4):335–43. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015 Dec;45(16):3571–80. doi: 10.1017/S0033291715001506. [DOI] [PubMed] [Google Scholar]
- 64.Sanacora G, Schatzberg AF. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology. 2015 Apr;40(5):1307. doi: 10.1038/npp.2014.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newport DJ, Schatzberg AF, Nemeroff CB. Whither Ketamine as an Antidepressant: Panacea or Toxin? Depress Anxiety. 2016 Aug;33(8):685–8. doi: 10.1002/da.22535. [DOI] [PubMed] [Google Scholar]
- 66.Schatzberg AF. A word to the wise about ketamine. Am J Psychiatry. 2014 Mar;171(3):262–4. doi: 10.1176/appi.ajp.2014.13101434. [DOI] [PubMed] [Google Scholar]
- 67.Ellison G, Switzer RC., 3rd Dissimilar patterns of degeneration in brain following four different addictive stimulants. Neuroreport. 1993 Oct 25;5(1):17–20. doi: 10.1097/00001756-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Horvath ZC, Czopf J, Buzsaki G. MK-801-induced neuronal damage in rats. Brain research. 1997 Apr 11;753(2):181–95. doi: 10.1016/s0006-8993(96)01290-5. [DOI] [PubMed] [Google Scholar]
- 69.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004 Jan;29(1):208–18. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- 70.Morgan CJ, Monaghan L, Curran HV. Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction. 2004 Nov;99(11):1450–61. doi: 10.1111/j.1360-0443.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 71.Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug and alcohol dependence. 2004 Sep 6;75(3):301–8. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994 Mar;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 73.Murrough JW, Wan LB, Iacoviello B, Collins KA, Solon C, Glicksberg B, et al. Neurocognitive effects of ketamine in treatment-resistant major depression: association with antidepressant response. Psychopharmacology (Berl) 2013 Sep 11; doi: 10.1007/s00213-013-3255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murrough JW, Burdick KE, Levitch CF, Perez AM, Brallier JW, Chang LC, et al. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: a randomized controlled trial. Neuropsychopharmacology. 2015 Mar 13;40(5):1084–90. doi: 10.1038/npp.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013 Aug 15;74(4):250–6. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, et al. Pilot dose-response trial of i.v. ketamine in treatment-resistant depression. World J Biol Psychiatry. 2014 Sep;15(7):579–84. doi: 10.3109/15622975.2014.922697. [DOI] [PubMed] [Google Scholar]
- 77.Loo CK, Galvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016 Jul;134(1):48–56. doi: 10.1111/acps.12572. [DOI] [PubMed] [Google Scholar]
- 78.Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014 Feb;155:123–9. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 79.Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013 May;27(5):444–50. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 80.Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, et al. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res. 2014 Feb 28;215(2):355–61. doi: 10.1016/j.psychres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Britt GC, McCance-Katz EF. A brief overview of the clinical pharmacology of “club drugs”. Subst Use Misuse. 2005;40(9–10):1189–201. doi: 10.1081/JA-200066730. [DOI] [PubMed] [Google Scholar]
- 82.Cho HS, D’Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, et al. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005 Apr;179(1):136–43. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- 83.Bachhuber MA, Maughan BC, Mitra N, Feingold J, Starrels JL. Prescription monitoring programs and emergency department visits involving benzodiazepine misuse: Early evidence from 11 United States metropolitan areas. Int J Drug Policy. 2016 Feb;28:120–3. doi: 10.1016/j.drugpo.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014 May 29;370(22):2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 85.Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014 Dec 15;76(12):970–6. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. 2013 Oct;16(9):2111–7. doi: 10.1017/S1461145713000485. [DOI] [PubMed] [Google Scholar]
- 87.Jarventausta K, Chrapek W, Kampman O, Tuohimaa K, Bjorkqvist M, Hakkinen H, et al. Effects of Sketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. J ECT. 2013 Sep;29(3):158–61. doi: 10.1097/YCT.0b013e318283b7e9. [DOI] [PubMed] [Google Scholar]
- 88.Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT. 2012 Jun;28(2):128–32. doi: 10.1097/YCT.0b013e31824d1d02. [DOI] [PubMed] [Google Scholar]
- 89.Yoosefi A, Sepehri AS, Kargar M, Akhondzadeh S, Sadeghi M, Rafei A, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder: a randomized, double-blind study. J ECT. 2014 Mar;30(1):15–21. doi: 10.1097/YCT.0b013e3182a4b4c6. [DOI] [PubMed] [Google Scholar]
- 90.Shams Alizadeh N, Maroufi A, Nasseri K, Sadeghi Najafabadi SH, Mousavi Taghiabad A, Gharibi F, et al. Antidepressant Effect of Combined Ketamine and Electroconvulsive Therapy on Patients With Major Depressive Disorder: A Randomized Trial. Iran J Psychiatry Behav Sci. 2015 Sep;9(3):e1578. doi: 10.17795/ijpbs-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, et al. Single i.v ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016 Feb;46(3):623–35. doi: 10.1017/S0033291715002159. [DOI] [PubMed] [Google Scholar]
- 92.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012 May;37(6):1526–33. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011 Nov;16(11):1068–70. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zarate CA, Jr, Machado-Vieira R. GSK-3: a key regulatory target for ketamine’s rapid antidepressant effects mediated by enhanced AMPA to NMDA throughput. Bipolar Disord. 2016 Nov 29; doi: 10.1111/bdi.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salehi B, Mohammadbeigi A, Kamali AR, Taheri-Nejad MR, Moshiri I. Impact comparison of ketamine and sodium thiopental on anesthesia during electroconvulsive therapy in major depression patients with drug-resistant; a double-blind randomized clinical trial. Ann Card Anaesth. 2015 Oct-Dec;18(4):486–90. doi: 10.4103/0971-9784.166444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rybakowski JK, Bodnar A, Krzywotulski M, Chlopocka-Wozniak M, Michalak M, Rosada-Kurasinska J, et al. Ketamine Anesthesia, Efficacy of Electroconvulsive Therapy, and Cognitive Functions in Treatment-Resistant Depression. J ECT. 2016 Sep;32(3):164–8. doi: 10.1097/YCT.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 97.Kuscu OO, Karacaer F, Biricik E, Gulec E, Tamam L, Gunes Y. Effect of Ketamine, Thiopental and Ketamine-Thiopental Combination during Electroconvulsive Therapy for Depression. Turk J Anaesthesiol Reanim. 2015 Oct;43(5):313–7. doi: 10.5152/TJAR.2015.92668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong X, He H, Zhang C, Wang Z, Jiang M, Li Q, et al. Mood and neuropsychological effects of different doses of ketamine in electroconvulsive therapy for treatment-resistant depression. J Affect Disord. 2016 Sep 01;201:124–30. doi: 10.1016/j.jad.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 99.Fond G, Bennabi D, Haffen E, Brunel L, Micoulaud-Franchi JA, Loundou A, et al. A Bayesian framework systematic review and meta-analysis of anesthetic agents effectiveness/tolerability profile in electroconvulsive therapy for major depression. Sci Rep. 2016 Jan 25;6:19847. doi: 10.1038/srep19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Jun 1;35(4):1155–9. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finnegan M, Ryan K, Shanahan E, Harkin A, Daly L, McLoughlin DM. Ketamine for depression relapse prevention following electroconvulsive therapy: protocol for a randomised pilot trial (the KEEP-WELL trial) Pilot Feasibility Stud. 2016;2:38. doi: 10.1186/s40814-016-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez CI, Wheaton M, Zwerling J, Steinman SA, Sonnenfeld D, Galfalvy H, et al. Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? an open-label trial. J Clin Psychiatry. 2016 Mar;77(3):408–9. doi: 10.4088/JCP.15l10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, et al. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord. 2006 Jul;93(1–3):239–43. doi: 10.1016/j.jad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 104.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013 Apr;16(3):501–6. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- 105.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010 May;15(5):501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004 Jan;161(1):171–4. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 107.Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. Riluzole augmentation for treatment-resistant depression. Am J Psychiatry. 2004 Nov;161(11):2132. doi: 10.1176/appi.ajp.161.11.2132. [DOI] [PubMed] [Google Scholar]
- 108.Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007 Mar 15;61(6):822–5. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005 Feb 15;57(4):430–2. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 110.Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, et al. Riluzole likely lacks antidepressant efficacy in ketamine non-responders. J Psychiatr Res. 2014 Nov;58:197–9. doi: 10.1016/j.jpsychires.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008 Dec;28(6):631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto K. Comments on “An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606 in patients with treatment-refractory major depressive disorder”. J Clin Psychopharmacol. 2009 Aug;29(4):411–2. doi: 10.1097/JCP.0b013e3181ace848. author reply 2. [DOI] [PubMed] [Google Scholar]
- 113.Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012 Aug;32(4):551–7. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997 Oct;13(4):219–41. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 115.Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol. 2004 Jul 5;474(4):589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- 116.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997 Oct 1;17(19):7503–22. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003 May;17(10):2106–18. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- 118.Quiroz JA, Tamburri P, Deptula D, Banken L, Beyer U, Rabbia M, et al. Efficacy and Safety of Basimglurant as Adjunctive Therapy for Major Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2016 Jul 1;73(7):675–84. doi: 10.1001/jamapsychiatry.2016.0838. [DOI] [PubMed] [Google Scholar]
- 119.Kent JM, Daly E, Kezic I, Lane R, Lim P, De Smedt H, et al. Efficacy and safety of an adjunctive mGlu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Jun 3;67:66–73. doi: 10.1016/j.pnpbp.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 120.Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, et al. The DRD2/ANKK1 gene is associated with response to add-on dextromethorphan treatment in bipolar disorder. J Affect Disord. 2012 May;138(3):295–300. doi: 10.1016/j.jad.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 121.Kelly TF, Lieberman DZ. The utility of the combination of dextromethorphan and quinidine in the treatment of bipolar II and bipolar NOS. J Affect Disord. 2014;167:333–5. doi: 10.1016/j.jad.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 122.Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, et al. Nitrous Oxide for Treatment-Resistant Major Depression: A Proof-of-Concept Trial. Biol Psychiatry. 2015 Jul 1;78(1):10–8. doi: 10.1016/j.biopsych.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 123.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015 Mar;21(2):140–9. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 124.Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014 Feb;23(2):243–54. doi: 10.1517/13543784.2014.852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology. 2016 Oct 19; doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Daly E, Singh JB, Fedgchin M, Cooper K, Lim P, Melman C, et al. Intranasal esketamine in treatment-resistant depression, a dose response study – double-blind and open-label extension data. 54th Annual Meeting of the American College of Neuropsychopharmacology; 2015 December 6th – 10th, 2015; Hollywood, Florida. 2015. p. 74. [Google Scholar]
- 127.Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. PeRSEVERe: a study of esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in patients assessed to be at imminent risk for sucide. American Society of Clinical Psychopharmacology (ACSP); 2016 May 30th – June 3rd; Scottsdale, Arizona. 2016. [Google Scholar]