Abstract
Little is known about the immunomodulation by tick saliva during a natural tick bite in human skin, the site of the tick-host interaction. We examined the expression of chemokines, cytokines, and leukocyte markers on the mRNA levels and histopathologic changes in human skin biopsies of tick bites (n=37) compared to unaffected skin (n=9). Early tick bite skin lesions (<24 h of tick attachment) were characterized by a predominance of macrophages and dendritic cells, elevated mRNA levels of macrophage chemoattractants (CCL2, CCL3, CCL4) and neutrophil chemoattractants (CXCL1, CXCL8), of the pro-inflammatory cytokine, IL-1β and the anti-inflammatory cytokine, IL-5. In contrast, the numbers of lymphocytes and mRNA levels of lymphocyte cell markers (CD4, CD8, CD19), lymphocyte chemoattractants (CXCL9, CXCL10, CXCL11, CXCL13, CCL1, CCL22), dendritic cell chemoattractants (CCL20), and of other pro- (IL-6, IL-12p40, IFN-γ, TNFα) and anti-inflammatory cytokines (IL-4, IL-10, TGFβ) did not differ from normal skin. With longer tick attachment (>24 h), the numbers of innate immune cells and mediators (not significantly) declined, whereas the numbers of lymphocytes (not significantly) increased. Natural tick bites by Ixodes ricinus ticks initially elicit a strong local innate immune response in human skin. Beyond 24h of tick attachment, this response usually becomes less, perhaps because of immunomodulation by tick saliva.
Keywords: Tick bite, cytokine, chemokine, immune response, human skin
Introduction
Ticks of the Ixodes ricinus complex are the most significant vectors for human pathogens in the Northern hemisphere. Estimated 2 - 20% of the population in tick-endemic areas suffer from tick bites every year (1). The incidence of tick-transmitted diseases has steadily increased in recent years (2). Lyme disease, which is caused by infection with Borrelia burgdorferi sensu lato, is the most common tick-borne disease, and affects around 85,000 individuals in Europe (3) and 300,000 individuals in the United States (4) every year.
The skin as the tick-host interface is the site of the tick's blood meal, of a potential pathogen transmission, and of the host immune response against the tick and pathogens. For example, natural tick bites elicit an inflammatory response in human skin, including the accumulation of eosinophils at the tick bite site (5). Because Ixodes ticks feed for several days, they must overcome the host's hemostatic, inflammatory, and immunologic responses during this time. For this purpose, ticks inject a panel of pharmacologically active proteins with their saliva into the feeding site (6-10). Of importance, the immunomodulatory activities of tick saliva have been demonstrated to facilitate pathogen transmission from the tick to the host during the blood meal (11). For example, tick saliva inhibits activation of toll-like receptors 2 and 3 in primary human keratinocyte cell lines, which may facilitate pathogen transmission (12). However, little is known about the ability of tick saliva to modulate the immune system in human skin, since previous studies have been performed only in animal models and in vitro experiments.
In this study, we examined the histopathologic changes and the expression of 12 chemokines, 9 cytokines, and 5 leukocyte markers in human skin biopsy samples of natural tick bites that did not lead to infection to elucidate the influence of tick saliva on the immune system in human skin.
Methods
Study population
This study was approved by the Institutional Review Board of the Medical University of Graz and conducted according to Declaration of Helsinki Principles. All patients gave their written informed consent. We recruited healthy individuals who were seen at the Department of Dermatology in Graz for recent tick bites. The inclusion criteria were as follows: (i) Age of patient ≥ 18 years; (ii) An I. ricinus tick (identified by two of us, M.G. and R.R.M) had still to be attached to the skin; (iii) The tick bite was acquired in the area of Graz, Austria, which is a highly endemic area for tick-borne diseases; (iv) The patient has not applied topical medication to the tick bite site nor has taken antibiotic or anti-inflammatory drugs; (v) The patient could not recall a previous tick bite during the last six months.
Skin biopsy samples
From each individual, a 4-mm skin punch biopsy specimen was obtained from the center of the tick bite. Skin biopsies were then subjected either (i) to quantitative real-time polymerase chain reaction (qPCR), or (ii) to histopathology and immunohistochemistry (Figure 1). Samples for qPCR analyzes of chemokine-, cytokine-, and leukocyte antigen mRNA expression were immediately transferred into RNAlater (Ambion, Austin, TX), refrigerated overnight at 4°C and stored at –70°C until analyzes. Samples for histopathology and immunohistochemistry were immediately fixed in 4% formaldehyde/PBS and embedded in paraffin. The control group consisted of healthy subjects in whom clinically atypical melanocytic nevi were excised. A 4-6 mm skin sample from the unaffected edge of these excision specimens was immediately placed in RNAlater. Routine histopathology showed that all excised nevi were benign without any sign of inflammation.
Figure 1. Flowchart for the processing of skin samples.
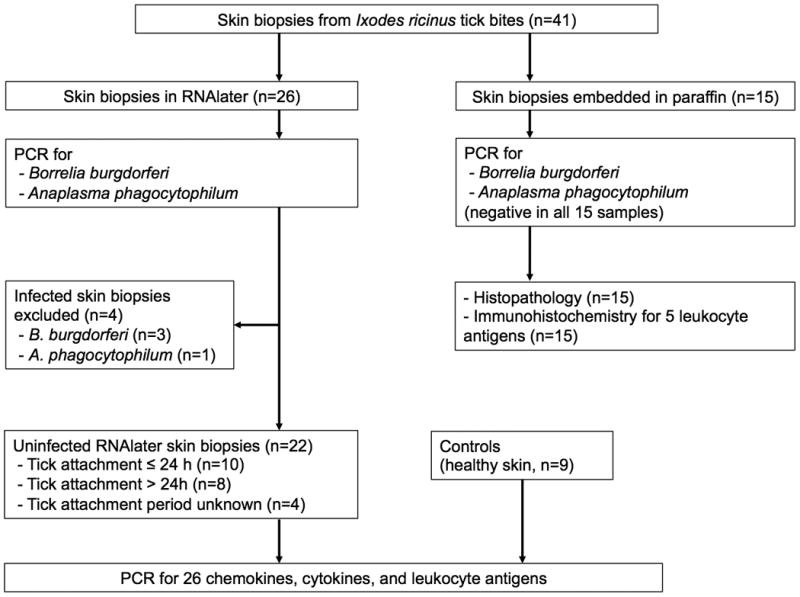
41 skin biopsy samples from tick bite lesions and 9 samples from healthy controls without tick bites were processed.
PCR for tick-transmitted pathogens
We aimed to exclude infection of biopsy samples with common tick-transmitted pathogens, which would potentially bias the expression of chemokine and cytokine mediators or leukocyte antigens. We therefore performed PCR examinations for DNA of B. burgdorferi, the pathogenic agent of Lyme disease, and Anaplasma phagocytophilum, the pathogenic agent of human granulocytic anaplasmosis, in all skin samples (except the control samples) as described previously (13-16).
Histopathology and immunohistochemistry
In the paraffin-embedded tick-bite samples, 4 μm serial sections were stained with hematoxylin and eosin for histopathologic examination. All specimens were subjected to immunohistochemical staining for the expression of cluster of differentiation (CD) 4, CD8, CD11c, CD20, and CD68 (Dako, Glostrup, DK or Novocastra, Newcastle, UK). For these analyzes, 5 μm serial sections of paraffin blocks were incubated with EDTA (pH 8.0) for CD4, with citrate (pH 6.0) for CD8, CD11c, and CD20 and with trypsin for CD68 to enhance immunoreactivity. After blocking of non-specific binding with peroxidase-blocking solution, sections were incubated with primary monoclonal mouse antibodies to CD4 (1:30 dilution), CD8 (1:25), CD11c (1:25), CD20 (1:400), and CD68 (1:25) for 30-60 min. Binding was detected by the universal biotinylated goat anti-mouse/anti-rabbit secondary antibody (Dako) diluted 1:50 in PBS (30 min). Following treatment with the avidin-biotin-horseradish peroxidase complex for 25 min, sections were developed in H2O2 for 15 min with 3-amino-9-ethylcarbazole as chromogenic substrate and counterstained with hematoxylin for 1 min. As negative controls sections were incubated with secondary antibodies only.
For evaluation, the entire tissue section was examined under ×400 magnification. The percentage of cells positive for a respective leukocyte marker was estimated relative to the total number of infiltrating cells. Grading from 0 to 3 was defined as follows: 0, all infiltrating cells negative; 1, up to 1/3 of all cells positive; 2, between 1/3 and 2/3 of all cells positive; 3, more than 2/3 of all cells positive.
RNA isolation, cDNA synthesis and qPCR for chemokines, cytokines and leukocyte markers
After removal of the attached tick, each of the RNAlater skin samples was ground mechanically in guanidine isothiocyanate lysis buffer (Quiagen, Valencia, CA). RNA was extracted with the RNeasy kit (Qiagen), treated with DNase I for 15 min and subjected to reverse transcription reaction as described previously (17). qPCR with previously published primers (18, 19) for macrophage (CCL2, CCL3, CCL4), neutrophil (CXCL8, CXCL1), dendritic cell (CCL20), Th1 lymphocyte (CXCL9, CXCL10, CXCL11), Th2 lymphocyte (CCL1, CCL22) and B lymphocyte (CXCL13) chemoattractants, proinflammatory (Interferon (IFN)-γ, Interleukin (IL)-1β, Tumor necrosis factor (TNF)-α, IL-6, IL-12p40) and anti-inflammatory (Transforming growth factor (TGF)-β, IL-4, IL-5) cytokines, macrophage (CD14), dendritic cell (CD11c), T lymphocyte (CD4, CD8) and B lymphocyte (CD19) markers, as well as gene expression analysis was performed as previously described (17).
Statistics
The primary outcome measures of the study were the amounts of cDNA specific for 26 cytokines, chemokines and leukocyte cell markers relative to the amount of cDNA of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (17). Each sample was tested in duplicate. The mean number of copies of cDNA of each duplicate was divided by the mean copies of GAPDH cDNA, termed ‘copies/GAPDH’.
Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, IBM Corp Armonk, NY). Normal distributions were tested with Kolmogorov-Smirnov tests and Q-Q-plots. Copies/GAPDH of all 26 parameters were compared between tick bite samples and control samples using Mann-Whitney test. To investigate the influence of the duration of tick attachment on the histopathologic changes, and the expression of chemokines, cytokines, and leukocyte markers, skin samples were allocated to two subgroups depending on the tick attachment period (<24 h vs. >24 h). We have chosen this threshold, because borrelial transmission seems to increase after 24 h after tick attachment (20). Copies/GAPDH were then compared between the two tick bite groups and the control group by analysis of variance with a Mann-Whitney test and a post-hoc Dunnett's test for multiple comparison correction. P-values were two-sided and values less than 0.05 were considered significant.
Results
Samples and patient characteristics
Forty-one skin biopsy samples of natural tick bites were processed as shown in Figure 1. Four of the RNAlater samples were infected with B. burgdorferi or A. phagocytophilum as demonstrated by PCR and therefore excluded from further analyses. The remaining 37 samples have been collected from 37 patients (females, 43%; median, 40 years; range 18-73 years). Tick bite sites in these patients were the lower extremities (n=17), the (lateral) chest / axillary folds (n=11), the abdomen / groin (n=6), the buttocks (n=2), or the neck (n=1). An erythema of 3-15mm (median, 5mm) around the tick-bite site was present in 33 individuals, irrespective of the duration of tick attachment. None of the individuals developed other cutaneous or extracutaneous symptoms during a follow-up period of 6 weeks. In the 22 patients for qPCR analysis and the 15 patients for histopathology the duration of tick attachment was obtained by history. In the 22 patients for qPCR analysis the tick attachment period was as follows: <24 h, n=10; >24 h, n=8; unknown, n=4. In the 15 patients for histopathology, the tick attachment period was as follows: <24h, n=11; >24h, n=1; unknown, n=3. Due to the low number of patients in the histopathology group with a tick attachment period >24h (n=1) we did not statistically analyze differences according the tick attachment period in these samples. There were no differences in demographic and clinical parameters between the 22 individuals in whom samples were examined for mRNA levels by qPCR and the 15 individuals in whom samples were analyzed for (immuno)histopathology. In addition, the clinical characteristics between patients with tick attachment for <24 h or >24 h did not differ significantly.
The control group consisted of 9 healthy subjects who had no actual history of a tick bite (females 44%; median, 36.5 years; range, 18-56 years).
Histopathologic and immunohistopathologic findings
In all 15 paraffin-embedded biopsy samples of tick bite lesions, we found a severe dermal infiltrate, composed of histiocytes, lymphocytes, and neutrophils, intermingled with eosinophils (Figure 2a). The infiltrate was located predominantly in perivascular areas and was more pronounced in the upper than the deep dermis. It extended into the subcutaneous tissue in 8 cases. A conspicuous edema in the papillary dermis and erythrocyte extravasation was present in all samples. Epidermal findings included vesicle formation with erythrocytes and nuclear debris or necrosis at the direct center and a moderate to severe spongiosis in the surrounding area of the tick bite lesion.
Figure 2. Histopathology and immunohistochemistry of natural tick bites in human skin.
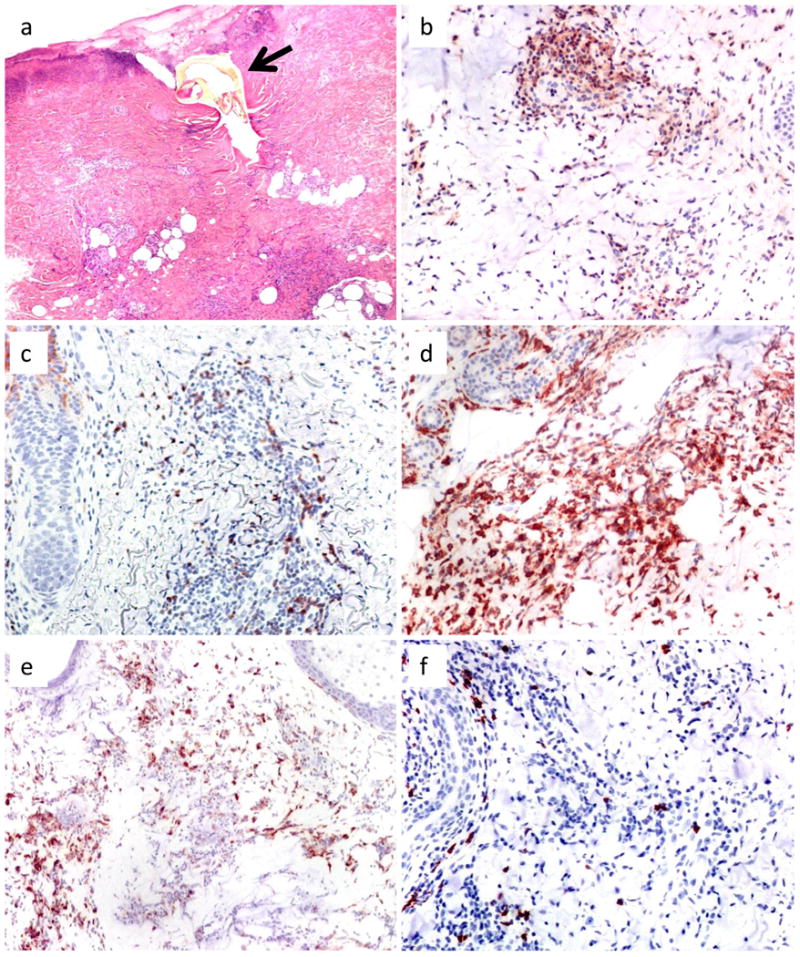
(a) The tick remnant (arrow) is surrounded by necrosis and epidermal vesicle formation with erythrocytes. The dermal infiltrate consists of histiocytes, lymphocytes, and neutrophils in predominantly perivascular arrangement and extends into the subcutis. (b-f) Immunohistochemistry reveals a moderate number of (b) CD4+ and (c) CD8+ T cells, whereas (d) CD11c+ dendritic cells and (e) CD68+ macrophages are the predominant cell types. (f) CD20+ B cells are infrequent. Magnifications, (a) and (e) ×100; (b-d) and (f) ×200.
The semi-quantitative analysis of immunohistochemical stainings revealed an inter-individual variation in the quantity of particular cell types. Altogether, CD11c+ dendritic cells (mean score, 1.9; range 0-3) and CD68+ macrophages (1.7; 0-3) were the predominant cell types in most samples. The numbers of CD4+ (1.5; 1-3) and CD8+ (1.2; 0-2) lymphocytes was lower, and CD20+ B cells were infrequent (0.5; 0-1). B cells more than T cells were clustered in primarily perivascular location throughout the dermis, whereas dendritic cells and macrophages were more scattered with predominance for the deep dermis (Figure 2b-f).
Chemokine expression in the skin of tick-bite sites
In the 22 skin biopsy samples from tick-bite sites, we found high-to-intermediate mRNA levels of the macrophage chemoattractants CCL2, CCL3, and CCL4, and of the neutrophil chemoattractants CXCL1 and CXCL8 (Figure 3a), showing strong stimulation of the innate immune system. Accordingly, high amounts of the CD14 macrophage marker were identified. For each of these chemokines and the CD14 marker, mRNA expression was significantly greater in lesional skin than in normal skin. Although mRNA expression of the dendritic cell chemoattractant CCL20 was low, the mRNA levels of the dendritic cell marker CD11c was significantly above that in normal skin (Figure 3a).
Figure 3. mRNA expression in tick bite skin lesions (n=22) compared to normal skin (n=9).
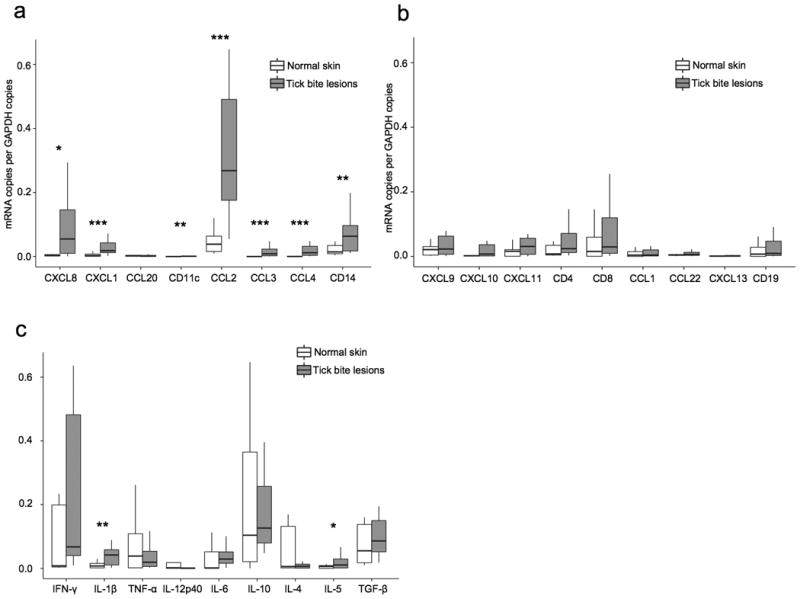
mRNA expression was measured by quantitative real-time PCR and expressed relative to the copies of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (a) Neutrophil chemoattractants (CXCL8, CXCL1), the dendritic cell chemoattractant CCL20, and the dendritic cell marker CD11c, macrophage chemoattractants (CCL2, CCL3, CCL4) and the macrophage cell marker CD14.. (b) Th1 cell chemoattractants (CXCL9, CXCL10, CXCL11), T cell markers (CD4, CD8), Th2 cell chemoattractants (CCL1, CCL22), the B cell chemoattractant CXCL13, and the B cell marker CD19. (c) Pro-inflammatory cytokines (IFN-γ, IL-1β, TNF-α, IL-12p40, IL-6) and anti-inflammatory cytokines (IL-10, IL-4, IL-5, and TGF-β). * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
The adaptive immune system was found to be less activated. Compared with healthy control subjects, the 22 individuals with tick bites had higher median mRNA levels for CD4+ and CD8+ T cells and for the chemoattractants for these cells, CXCL9, CXCL10, and CXCL11 (Figure 3b). However, none of the differences between case and control subjects were statistically significant, though the levels of CXCL10 in the tick-bite group approached statistical significance (p=0.086). mRNA expression for the Th2-cell chemokines, CCL1 and CCL22, as well as of the B-cell chemoattractant CXCL13 and the CD19 B-cell marker were low to very low (Figure 3b).
Cytokine expression in the skin of tick-bite sites
In the 22 skin biopsy samples from tick-bite sites, only the mRNA levels of the pro-inflammatory macrophage-derived cytokine IL-1β and the anti-inflammatory Th2 lymphocyte-derived cytokine IL-5 were significantly higher than in normal skin (Figure 3c). Although intermediate-to-high levels of mRNA were found for the other pro-inflammatory cytokines, IFN-γ, TNFα, and IL-6 as well as for the anti-inflammatory cytokines, IL-10 and TGFβ, they did not differ significantly from values in normal skin. Only very small amounts of mRNA were found for the pro-inflammatory cytokine IL12P40 and the anti-inflammatory cytokine IL-4 in lesional skin.
Expression of chemokines, cytokines, and leukocytes antigens according to the duration of tick attachment
To determine whether the duration of tick attachment influenced the local inflammatory and immune response, we compared chemokine, cytokine, and leukocyte antigen mRNA levels between the 10 individuals, in whom tick attachment was reported to be <24 hours, with those in the 8 individuals in whom tick attachment was reported to be >24 hours. Although none of the parameters differed significantly between shorter and longer tick attachment (Figure 4a-c), patients with a longer attachment generally had lower mRNA levels for innate immune cells and mediators than patients with shorter attachment (Figure 4a). In contrast, a general increase of the activity of the adaptive immune response was observed over time. Individuals with tick attachment of >24 hours tended to have greater numbers of lymphocytes and higher levels of their respective chemokines and cytokines (Figure 4b). The median mRNA level for IL-4, a prototypic Th2 cytokine, was slightly higher in the group with longer tick attachment than in the group with shorter attachment, but in both groups, the amounts were less than in normal control subjects (Figure 4c). Conversely, the median levels of mRNA for IL-5, another Th2 cytokine, were higher in the group with shorter tick attachment (Figure 4c).
Figure 4. mRNA expression in skin lesions with a tick attachment period of <24 h (n=10) versus >24 h (n=8) compared to normal skin (n=9).
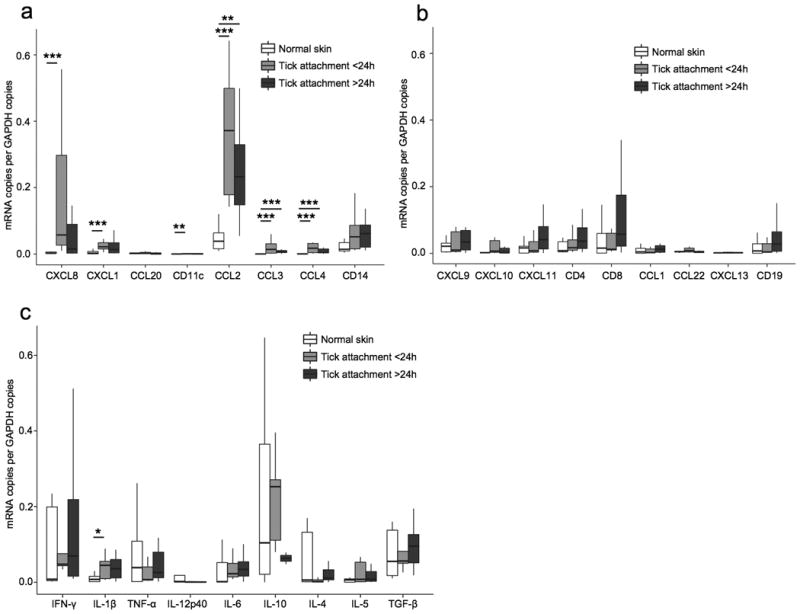
The same samples as in figure 3 are depicted. mRNA expression was measured by quantitative real-time PCR and expressed relative to the copies of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (a) Neutrophil chemoattractants (CXCL8, CXCL1), the dendritic cell chemoattractant CCL20, the dendritic cell marker CD11c, macrophage chemoattractants (CCL2, CCL3, CCL4) and the macrophage cell marker CD14. (b) Th1 cell chemoattractants (CXCL9, CXCL10, CXCL11), T cell markers (CD4, CD8), Th2 cell chemoattractants (CCL1, CCL22), the B cell chemoattractant CXCL13, and the B cell marker CD19. (c) Pro-inflammatory cytokines (IFN-γ, IL-1β, TNF-α, IL-12p40, IL-6) and anti-inflammatory cytokines (IL-10, IL-4, IL-5, and TGF-β). * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Discussion
In this study, we found activation of the innate human immune system in Ixodes ricinus tick bite lesions as reflected by a significantly increased mRNA expression of markers and/or chemoattractants for macrophages, neutrophils, and dendritic cells, particularly in lesions of less than 24 hours duration. Although less pronounced, activation of an adaptive immune response was apparent after 24 hours, including elevated mRNA levels for CD4+ and CD8+ T cells and lymphocyte chemoattractants for these cells, particularly CXCL10. The tick bite lesions had increased amounts of proinflammatory cytokines, particularly IL-1β and a compensatory expression of anti-inflammatory cytokines, particularly of the Th2 cytokine IL-5.
Previous experimental studies demonstrated the influence of tick bites, tick saliva or its components on the innate and adaptive immunity of the host. (9, 21, 22) However, these studies used ex vivo or in vitro models, and may therefore not reflect the events of a natural tick infestation in the skin, in which the composition of the tick saliva proteome changes during time (23). Only a few studies of the immune reaction to natural tick bites have been performed in mouse skin in vivo. In BALB/c mice, very early (6 – 12 h post infestation) tick-bite lesions are characterized histologically by a strong innate immune reaction with a predominance of neutrophils and mononuclear cells, intermingled with CD4+ and CD8+ T cells in a ratio of 2.2:1 (24). Consistent with these results, molecular investigations of early lesions in mice found upregulation of the chemoattractants for monocytes, neutrophils, and dendritic cells CXCL1, CXCL5, CCL2, and CXCL14 (25, 26). These early alterations in tick bite lesions in murine skin are comparable with our findings in human skin, which showed an initial strong, transient innate immune response.
After 24 to 96 hours of incubation with tick saliva, T cells produce increased amounts of the Th2 cytokines IL-4, IL-5, and IL-10, but decreased quantities of the Th1 cytokines IL-2 and IFN-γ (21, 27, 28). This Th2-promoting effect appears to become markedly greater with longer exposure to tick saliva (28, 29). This facilitates transmission of pathogens such as B. burgdorferi (11). Vice versa, the suppression of IL-4 and IL-5 (30) as well as the application of IFN-γ, IL-2 and TNF-α, or of cells releasing those cytokines significantly reduces the borrelial load in susceptible mice (31). In our study on human skin, the adaptive immune response as a whole increased after 24 hours of tick feeding. However, we were not able to show a predominant Th2 response at that time. This may be due to several reasons. First, since only 8 patients reported a tick attachment >24 hours, we may not have investigated a large enough group to demonstrate this immunological shift. Second, the development of a Th2 response may require a longer period than the maximum duration of tick attachment of 48 hours in our patients. Third, other methods, such as intracellular cytokine determinations from T cells, are likely to be a more sensitive method than measuring mRNA levels of cytokines in skin biopsy samples. Fourth, it is possible that humans do not have as pronounced a shift toward a Th2 response to tick bites as mice. In humans, the principal effect of immune modulators from tick saliva may be the gradual down-regulation of the initial innate immune response to the tick bite. This may be a factor why B. burgdorferi spirochetes, which are controlled by an innate and Th1-dominated adaptive immune response (18), are usually not transmitted before a tick attachment period of 24 - 48 hours (32). This critical window of time for tick saliva to exert its pathogen-transmission promoting modulatory effects on the host immune response underlines the importance of tick removal as early as possible.
We excluded infection of our ticks with B. burgdorferi and A. phagocytophilum, which are the most common pathogens transmitted by I. ricinus ticks (1, 2). It is still possible that undetected pathogens influenced the immune response to the tick bites. However, it is unlikely that the ticks were infected with other pathogens that might have migrated into the host skin, because none of the study individuals reported any cutaneous or extracutaneous symptoms during a follow-up period of 6 weeks after the tick bite.
In conclusion, natural tick bites produce a strong innate immune response with an infiltrate of mononuclear cells, neutrophils, and dendritic cells with an upregulation of pro-inflammatory cytokines in the skin early on. After an attachment period of 24 hours, this primary inflammatory reaction appears to be weakened and an adaptive immune response starts to develop. This presumably facilitates blood intake by the tick and transmission of pathogens, such as B. burgdorferi, into the skin.
Acknowledgments
We are grateful to Jenny Shin, Kathryn Jones and Gail McHugh at the Center for Immunology and Inflammatory Diseases, Division of Rheumatology, Allergy and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA, and to Ulrike Schmidbauer at the Department of Dermatology, Medical University of Graz, Austria for technical support.
Funding sources: Research reported in this publication was supported by a grant from the National Institutes of Health (R01 AI-101175), the English, Bonter, Mitchell Foundation, the Eshe Fund, and the Lyme Disease and Arthritis Research Fund at Massachusetts General Hospital (To A.C.S.).
Footnotes
Author contributions: Martin Glatz performed the research, analysed the data and wrote the paper
Terry Means contributed essential reagents and tools and critically revised the paper
Josef Haas analysed the data and critically revised the paper
Allen C. Steere designed the research study, contributed essential reagents and tools and critically revised the paper
Robert R. Müllegger designed the research study, performed sample collection, analysed the data and wrote the paper
Conflict of interests: All authors state no conflicts of interest.
References
- 1.Altpeter E, Zimmermann H, Oberreich J, et al. Tick related diseases in Switzerland, 2008 to 2011. Swiss Med Wkly. 2013;143:w13725. doi: 10.4414/smw.2013.13725. [DOI] [PubMed] [Google Scholar]
- 2.Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudenko N, Golovchenko M, Grubhoffer L, et al. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011;2:123–128. doi: 10.1016/j.ttbdis.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention C. Summary of notifiable diseases--United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–111. [PubMed] [Google Scholar]
- 5.Krause PJ, Grant-Kels JM, Tahan SR, et al. Dermatologic changes induced by repeated Ixodes scapularis bites and implications for prevention of tick-borne infection. Vector Borne Zoonotic Dis. 2009;9:603–610. doi: 10.1089/vbz.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Booth CJ, Paley MA, et al. Inhibition of neutrophil function by two tick salivary proteins. Infect Immun. 2009;77:2320–2329. doi: 10.1128/IAI.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannier S, Liversidge J, Sternberg JM, et al. Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: a potential role in enhanced Borrelia burgdoferi transmission. Immunology. 2004;113:401–408. doi: 10.1111/j.1365-2567.2004.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieskovska J, Kopecky J. Effect of tick saliva on signalling pathways activated by TLR-2 ligand and Borrelia afzelii in dendritic cells. Parasite Immunol. 2012;34:421–429. doi: 10.1111/j.1365-3024.2012.01375.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Renneker S, Beyer D, et al. Identification and partial characterization of a Salp15 homolog from Ixodes ricinus. Ticks Tick Borne Dis. 2014;5:318–322. doi: 10.1016/j.ttbdis.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Marchal C, Schramm F, Kern A, et al. Antialarmin effect of tick saliva during the transmission of Lyme disease. Infect Immun. 2011;79:774–785. doi: 10.1128/IAI.00482-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuttall PA, Labuda M. Tick-host interactions: saliva-activated transmission. Parasitology. 2004;129(Suppl):S177–189. doi: 10.1017/s0031182004005633. [DOI] [PubMed] [Google Scholar]
- 12.Bernard Q, Gallo RL, Jaulhac B, et al. Ixodes tick saliva suppresses the keratinocyte cytokine response to TLR2/TLR3 ligands during early exposure to Lyme borreliosis. Exp Dermatol. 2016;25:26–31. doi: 10.1111/exd.12853. [DOI] [PubMed] [Google Scholar]
- 13.Michel H, Wilske B, Hettche G, et al. An ospA-polymerase chain reaction/restriction fragment length polymorphism-based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med Microbiol Immunol. 2004;193:219–226. doi: 10.1007/s00430-003-0196-8. [DOI] [PubMed] [Google Scholar]
- 14.Morrison TB, Ma Y, Weis JH, et al. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pancholi P, Kolbert CP, Mitchell PD, et al. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 16.Rauter C, Oehme R, Diterich I, et al. Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. J Clin Microbiol. 2002;40:36–43. doi: 10.1128/JCM.40.1.36-43.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Means TK, Hayashi F, Smith KD, et al. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 18.Mullegger RR, Means TK, Shin JJ, et al. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–4628. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 20.Huegli D, Moret J, Rais O, et al. Prospective study on the incidence of infection by Borrelia burgdorferi sensu lato after a tick bite in a highly endemic area of Switzerland. Ticks Tick Borne Dis. 2011;2:129–136. doi: 10.1016/j.ttbdis.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Leboulle G, Crippa M, Decrem Y, et al. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem. 2002;277:10083–10089. doi: 10.1074/jbc.M111391200. [DOI] [PubMed] [Google Scholar]
- 22.Vancova I, Hajnicka V, Slovak M, et al. Anti-chemokine activities of ixodid ticks depend on tick species, developmental stage, and duration of feeding. Vet Parasitol. 2010;167:274–278. doi: 10.1016/j.vetpar.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Narasimhan S, Deponte K, Marcantonio N, et al. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One. 2007;2:e451. doi: 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbow ML, Rutti B, Brossard M. Infiltration of CD4+ CD8+ T cells, and expression of ICAM-1, Ia antigens, IL-1 alpha and TNF-alpha in the skin lesion of BALB/c mice undergoing repeated infestations with nymphal Ixodes ricinus ticks. Immunology. 1994;82:596–602. [PMC free article] [PubMed] [Google Scholar]
- 25.Heinze DM, Carmical JR, Aronson JF, et al. Early immunologic events at the tick-host interface. PLoS One. 2012;7:e47301. doi: 10.1371/journal.pone.0047301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinze DM, Wikel SK, Thangamani S, et al. Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit Vectors. 2012;5:26. doi: 10.1186/1756-3305-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhansova H, Bopp T, Schmitt E, et al. Tick saliva increases production of three chemokines including monocyte chemoattractant protein-1, a histamine-releasing cytokine. Parasite Immunol. 2015;37:92–96. doi: 10.1111/pim.12168. [DOI] [PubMed] [Google Scholar]
- 28.Mejri N, Brossard M. Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naive CD4+T to induce Th2 cell differentiation in vitro and in vivo. Int Immunol. 2007;19:535–543. doi: 10.1093/intimm/dxm019. [DOI] [PubMed] [Google Scholar]
- 29.Zeidner N, Mbow ML, Dolan M, et al. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeidner NS, Schneider BS, Rutherford JS, et al. Suppression of Th2 cytokines reduces tick-transmitted Borrelia burgdorferi load in mice. J Parasitol. 2008;94:767–769. doi: 10.1645/GE-1416.1. [DOI] [PubMed] [Google Scholar]
- 31.Zeidner N, Dreitz M, Belasco D, et al. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-alpha, interleukin-2, and interferon-gamma. J Infect Dis. 1996;173:187–195. doi: 10.1093/infdis/173.1.187. [DOI] [PubMed] [Google Scholar]
- 32.Fikrig E, Narasimhan S. Borrelia burgdorferi--traveling incognito? Microbes Infect. 2006;8:1390–1399. doi: 10.1016/j.micinf.2005.12.022. [DOI] [PubMed] [Google Scholar]


