Abstract
The National Health and Nutrition Examination Survey (NHANES) collects information on human papillomavirus (HPV) vaccination history as well as sexual activity. We evaluated data from NHANES to assess report of HPV vaccination with ≥1 dose and 3 doses among females and males aged 11 to 26 years during 2007–2014. We also examined age at first vaccine dose and age at first sexual activity among females aged 14 to 26 years. Vaccination significantly increased in females aged 13 to 26 years, but not among 11- to 12-year-old girls, and remained low for both females and males. In NHANES 2011–2014, among females with known age at first vaccine dose, 43.1% reported having had sex before or in the same year they received their first HPV vaccine, and this varied by race/ethnicity. Clinicians should provide strong recommendations consistent with guidelines, including routine vaccination of girls and boys at age 11 or 12 years.
Keywords: human papillomavirus (HPV), HPV vaccine, National Health and Nutrition Examination Survey, vaccine initiation, sexual activity
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States,1 occurring in approximately 39.5 million girls and women aged 14 to 59 years with a prevalence of 32.9% among 14- to 19-year-olds and 53.8% among 20- to 24-year-olds.2 Since 2006, the Advisory Committee on Immunization Practices (ACIP) has recommended HPV vaccine for routine vaccination of girls aged 11 or 12 years and through age 26 years for those not previously vaccinated.3 This recommendation was extended in 2011 to include routine vaccination of boys aged 11 or 12 years and through age 21 years for those not previously vaccinated.4 The vaccine is also recommended for men who have sex with men and immunocompromised men through age 26 years.4
Despite ACIP recommendations, the uptake of the HPV vaccine has been lower than other adolescent vaccines. In 2014, data from the National Immunization Survey (NIS)–Teen found that receipt of ≥1 HPV vaccine dose among girls and boys aged 13 to 17 years was 60.0% and 41.7%, respectively; both estimates lagged behind those of tetanus, diphtheria, and acellular pertussis (Tdap) vaccine and meningococcal conjugate (Men ACWY) vaccine (87.6% and 79.3%, respectively).5 Findings from NIS-Teen also showed that ≥1 HPV vaccine dose coverage was lower among 13-year-old girls than 14- to 17-year-old girls.5 Other studies suggest HPV vaccine initiation is delayed beyond the recommended ages of 11 or 12 years.6
HPV incidence is high among young persons within the first few years after initiating sexual activity,7 and delaying HPV vaccine initiation leaves individuals vulnerable to vaccine-type HPV infection. In a recent study of a large urban population of females aged 11 to 19 years receiving their first dose of HPV vaccine, more than one-third had already undergone STD (sexually transmitted diseases) screening, suggesting prior sexual experience, and nearly one-third of those with cervical cancer screening already had an abnormal result, suggesting prior exposure to HPV.6
The National Health and Nutrition Examination Survey (NHANES) collects information on HPV vaccination history as well as sexual activity across birth cohorts eligible for HPV vaccination. We analyzed NHANES to describe HPV vaccination in females and males aged 11 to 26 years and explore changes in vaccination among females during 2007–2014. We also assessed the timing of HPV vaccine initiation relative to age at first sexual activity among females during 2011–2014, the first years in which age at vaccination was collected in NHANES. We restricted our assessment of HPV vaccine initiation relative to age at first sexual activity among females because few males were vaccinated during the NHANES cycles we analyzed.
Methods
Study Population and Design
NHANES is an ongoing series of cross-sectional surveys conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The survey is designed to be nationally representative of the civilian, noninstitutionalized US population. Participants have a household interview followed by a physical examination in a mobile examination center (MEC). NHANES data are released in 2-year cycles. Since NHANES 2011–2012, Asians have been oversam-pled in addition to the ongoing oversampling of Hispanics, non-Hispanic blacks, older adults, and low-income whites/others.8 Written informed consent was obtained from all participants and parental permission for participants <18 years. The survey was approved by the NCHS/CDC Research Ethics Review Board.
We analyzed NHANES 2007–2014 data. During 2007–2010, a total of 2155 females aged 11 to 26 years were interviewed and 2103 (97.6%) were examined in the MEC. During 2011–2014, a total of 2298 females and 2262 males aged 11 to 26 years were interviewed and 2210 (96.2%) females and 2194 (97.0%) males were examined in the MEC.
Sociodemographic, Behavioral, and HPV Vaccination History
Sociodemographic information and report of HPV vaccination were obtained during the household interview. Participants aged ≥16 years and emancipated minors were interviewed directly while information for participants aged <16 years was provided by a parent or guardian. Race/ethnicity in NHANES 2007–2014 was self-reported and included non-Hispanic black, non-Hispanic white, Mexican American, and Asian. Poverty index was calculated according to the US Health and Human Services poverty guideline by dividing total family income by the poverty guideline and adjusting for family size and geographic location. We categorized responses about health insurance as public/government, private, or no insurance. Sexual history was obtained directly from participants aged 14 to 69 years through Audio-Computer-Assisted Self Interview conducted in the MEC. Sex was defined as vaginal, anal, or oral sex. Respondents who reported ever having sex were asked how old they were the first time they had sex.
Report of HPV vaccination has been collected from females aged 9 to 59 years since 2007 and males aged 9 to 59 years since 2011. The question to assess vaccination was “Human papillomavirus (HPV) vaccine is given to prevent cervical cancer in girls and women [HPV infection and genital warts in boys and men] … It is given in 3 separate doses over a 6-month period. {Have you/has SP [selected participant]} ever received one or more doses of the HPV vaccine?” Answers included “yes,” “no,” “refuse,” and “don’t know.” Persons with missing data or who answered “refuse” or “don’t know” were excluded (3.3% of females during 2007–2014 and 10.1% of males during 2011–2014). Those who answered “yes” to HPV vaccination were asked the number of doses received. Age at first dose of HPV vaccine has been collected since 2011 for both sexes.
Statistical Analysis
Our analysis included female and male participants aged 11 to 26 years. We evaluated HPV vaccination (receipt of ≥1 and 3 doses of HPV vaccine) by age group for females (11–12, 13–17, and 18–26 years) during 2007–2014 and males (11–12, 13–17, 18–21, and 22–26 years) during 2011–2014. We assessed vaccination across NHANES cycles among females to compare changes in vaccination by age group from the first 4 years (2007–2010) to the last 4 years (2011–2014). Sociodemographic characteristics among females were assessed in three age categories: 11–12 years, selected to include the recommended age group for routine HPV vaccination; 13–17 years, selected to include the age group assessed by NIS-Teen;9 and 18–26 years, selected to include the remaining ages eligible for HPV vaccination and for closely corresponding to the age group of 19–26 years analyzed in the National Health Interview Survey (NHIS).10 We calculated P values based on an F statistic derived from a Wald χ2 test. We examined differences in mean age at first dose of HPV vaccine and mean age at first sexual activity among females aged 14–26 years by race/ethnicity and determined the percentage of vaccinated females who reported having had sex for the first time before or in the same year as their first vaccine dose. We combined those reporting first sexual activity in the same year as their first dose of HPV vaccine with those reporting sex before their first dose of HPV vaccine as we were unable to determine if the first vaccine dose was received before first sexual activity if in the same year.
Statistical analyses were conducted using SAS 9.3 (SAS, Inc, Cary, NC) and SAS-callable SUDAAN 11.0 (Research Triangle Institute, Research Triangle Park, NC). Standard errors accounted for the complex survey design. Estimates were weighted using the weights provided by NCHS for survey years 2007–2014 to be nationally representative. Sample weights accounted for nonresponse to the interview and medical examination and oversampling of certain populations.8,11 Statistical significance was set at a 2-tailed P value of .05.
Results
History of ≥1 and 3 HPV Vaccine Doses by Age Group, 2007–2014
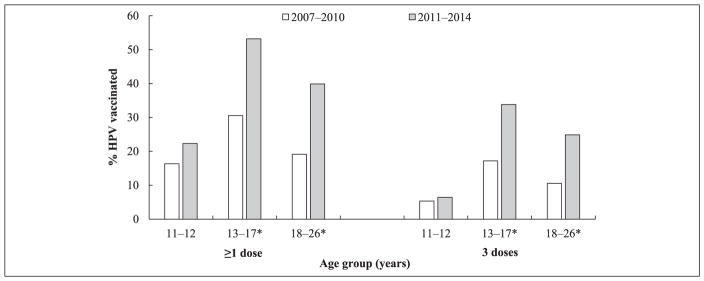
A total of 4276 (96.9%) females and 2046 (89.9%) males aged 11–26 years responded to the question on HPV vaccine receipt. Vaccination among females significantly increased between the 2 time periods among the older age groups but not among 11- to 12-year-olds (Figure 1 and Table 1). Vaccination with ≥1 dose among 11- to 12-year-olds was 16.4% in 2007–2010 and 22.3% in 2011–2014 (P = .2). For the older age groups, vaccination with ≥1 dose significantly increased from 30.6% to 53.2% in 13- to 17-year-olds and 19.2% to 39.9% in 18- to 26-year-olds (P < .01). A similar pattern was observed for 3 doses, with 5.4% (95% confidence interval [CI] = 3.3–8.6) of 11- to 12-year-olds having completed the vaccine series in 2007–2010 and 6.4% (95% CI = 3.3–12.3) in 2011–2014 (P = .2). For the older age groups, HPV vaccination with 3 doses significantly increased from 17.2% (95% CI = 13.5–22.2) to 33.8% (95% CI = 28.9–39.1) in 13- to 17-year-olds and 10.6% (95% CI = 7.3–15.1) to 24.9% (95% CI = 21.1–29.1) in 18- to 26-year-olds (P < .01).
Figure 1.
History of ≥1 and 3 human papillomavirus (HPV) vaccine doses among females aged 11 to 26 yearsa,b by age group, National Health and Nutrition Examination Survey, 2007–2014.
aAt the time of the survey.
bIncludes participants who responded “Yes” or “No” to the HPV vaccination question.
* Significantly increased among females aged 13 to 17 and 18 to 26 years (P < .01).
Table 1.
History of ≥1 Human Papillomavirus (HPV) Vaccine Dose Among Females Aged 11 to 26 Yearsa,b by Age Group and Sociodemographic Characteristics, National Health and Nutrition Examination Survey, 2007–2014.
| Characteristics | 2007–2010
|
2011–2014
|
2007–2010 to 2011–2014
|
||
|---|---|---|---|---|---|
| n | Weighted % (95% CI) | n | Weighted % (95% CI) | Prevalence Difference % (SE) | |
| A: Females aged 11–12 yearsa | |||||
| Overall | 375 | 16.4 (12.6–20.9) | 381 | 22.3 (16.7–29.2) | 6.0 (3.8) |
| Race/Ethnicity | P < .001 | P = .050 | |||
| White, non-Hispanic | 116 | 9.0 (5.3–14.9) | 96 | 17.3 (9.5–29.4) | 8.3 (5.5) |
| Black, non-Hispanic | 97 | 30.5 (20.3–43.2) | 99 | 37.3 (27.2–48.5) | 6.8 (7.9) |
| Mexican American | 101 | 22.7 (15.6–31.8) | 82 | 28.8 (19.1–41.0)c | 6.1 (6.8) |
| Asian | — | — | 29 | 20.6 (8.2–42.9)c | — |
| Health insurance and type | P = .116 | P = .013 | |||
| Public/Government | 147 | 25.7 (17.7–35.7) | 176 | 31.9 (23.3–42.0) | 6.3 (6.6) |
| Private | 175 | 13.0 (8.7–18.9) | 162 | 16.1 (10.1–24.7) | 3.1 (4.4) |
| None | 46 | 7.2 (2.8–17.4)c | 30 | 14.8 (5.2–35.7)c | 7.6 (8.1) |
| Poverty index | P = .005 | P = .034 | |||
| Below poverty | 103 | 22.4 (13.7–34.4) | 122 | 31.1 (22.3–41.4) | 8.6 (7.1) |
| At or above poverty | 239 | 15.1 (11.5–19.6) | 240 | 18.6 (13.0–25.9) | 3.5 (3.8) |
| B: Females aged 13–17 yearsa | |||||
| Overall | 763 | 30.6 (25.7–35.9) | 816 | 53.2 (46.7–59.6) | 22.6 (4.1)* |
| Race/Ethnicity | P = .306 | P = .041 | |||
| White, non-Hispanic | 249 | 32.2 (25.0–40.5) | 192 | 55.4 (45.0–65.3) | 23.1 (6.5)* |
| Black, non-Hispanic | 189 | 23.4 (16.6–32.0) | 213 | 47.0 (38.5–55.7) | 23.6 (5.8)* |
| Mexican American | 189 | 28.2 (19.6–38.6) | 165 | 60.0 (50.0–69.2) | 31.8 (6.8)* |
| Asian | — | — | 116 | 40.0 (30.0–50.9) | — |
| Health insurance and type | P = .008 | P = .217 | |||
| Public/Government | 301 | 33.4 (24.4–43.8) | 347 | 56.5 (50.0–62.8) | 23.1 (5.9)* |
| Private | 335 | 32.1 (25.8–39.1) | 363 | 50.6 (42.4–58.8) | 18.5 (5.3)* |
| None | 110 | 11.9 (5.6–23.8)c | 85 | 56.7 (37.7–73.9) | 44.8 (10.4)* |
| Poverty index | P = .645 | P = .651 | |||
| Below poverty | 194 | 28.8 (20.6–38.7) | 226 | 56.0 (45.7–65.7) | 27.2 (6.8)* |
| At or above poverty | 503 | 31.4 (25.5–38.1) | 522 | 53.5 (45.6–61.2) | 22.0 (5.0)* |
| C: Females aged 18–26 yearsa | |||||
| Overall | 964 | 19.2 (15.2–23.8) | 977 | 39.9 (35.3–44.7) | 20.7 (3.2)* |
| Race/Ethnicity | P = .222 | P = .156 | |||
| White, non-Hispanic | 358 | 22.2 (16.1–29.7) | 306 | 43.9 (36.6–51.5) | 21.7 (5.1)* |
| Black, non-Hispanic | 208 | 14.3 (9.7–20.5) | 272 | 37.0 (30.5–44.0) | 22.7 (4.3)* |
| Mexican American | 219 | 11.7 (6.7–19.8) | 140 | 30.9 (24.5–38.2) | 19.2 (4.7)* |
| Asian | — | — | 109 | 38.2 (25.6–52.6) | — |
| Health insurance and type | P = .003 | P = .011 | |||
| Public/Government | 242 | 17.6 (11.3–26.4) | 277 | 43.2 (32.9–54.1) | 25.6 (6.6)* |
| Private | 367 | 25.2 (19.0–32.7) | 422 | 43.0 (35.8–50.6) | 17.8 (5.1)* |
| None | 337 | 10.0 (6.5–15.1) | 262 | 29.8 (23.1–37.5) | 19.8 (4.2)* |
| Poverty index | P = .409 | P = .256 | |||
| Below poverty | 331 | 17.4 (12.2–24.3) | 395 | 38.4 (32.4–44.7) | 21.0 (4.3)* |
| At or above poverty | 536 | 20.3 (15.4–26.4) | 511 | 42.3 (36.8–48.1) | 22.0 (4.0)* |
At the time of the survey.
Includes participants who responded “Yes” or “No” to the HPV vaccination question.
Relative standard error (RSE) >30%.
Significantly increased in females aged 13–17 and 18–26 years (P < .01).
Among males, HPV vaccination with ≥1 dose in 2011–2014 was 19.4% (95% CI = 15.0–24.6) in 11- to 12-year-olds, 20.2% (95% CI = 16.4–24.6) in 13- to 17-year-olds, 15.3% (95% CI = 11.7–19.7) in 18- to 21-year-old, and 7.5% (95% CI = 5.7–10.0) in 22- to 26-year-olds. Vaccination with 3 doses was 5.8% (95% CI = 3.8–8.9) in 11- to 12-year-olds, 9.5% (95% CI = 6.9–13.0) in 13- to 17-year-olds, 7.3% (95% CI = 4.9–10.7) in 18- to 21-year-olds, and 3.2% (95% CI = 1.4–7.4) in 22- to 26-year-olds.
History of ≥1 HPV Vaccine Dose Among Females by Sociodemographic Characteristics and Age Group
HPV vaccination with ≥1 dose among girls aged 11 to 12 years varied by race/ethnicity in 2007–2010 (Table 1, panel A); coverage was highest among non-Hispanic blacks (30.5%) and lowest among non-Hispanic whites (9.0%) (P < .001). There was variation by race/ethnicity during 2011–2014, but differences were not statistically significant. History of ≥1 dose did not differ by health insurance type in 2007–2010, but differed during the later period. In 2011–2014, this was highest among those with public/government insurance (31.9%) and lowest among those with no insurance (14.8%) (P = .013). There were differences by poverty level in both time periods, with a larger proportion of those below poverty reporting vaccination with ≥1 dose compared with those at or above poverty.
Among girls aged 13 to 17 years, history of ≥1 HPV vaccine dose significantly increased between the 2 time periods in all subgroups (Table 1, panel B). There was variation by race/ethnicity during 2007–2010, but differences were not statistically significant. In 2011–2014, history of ≥1 dose was highest among Mexican Americans (60.0%) and lowest among Asians (40.0%) (P = .04). History of ≥1 dose varied significantly by health insurance type in 2007–2010 and was highest among those with public/government insurance (33.4%) and lowest among those with no insurance (11.9%) (P < .01). There were no differences by health insurance in 2011–2014 and no difference by poverty level in either of the time periods examined.
Among women aged 18 to 26 years, history of ≥1 vaccine dose significantly increased between the 2 time periods in all subgroups (Table 1, panel C). There was variation by race/ethnicity in both time periods, but differences were not statistically significant. History of ≥1 vaccine dose significantly differed by health insurance in 2007–2010 and was highest among those with private insurance (25.2%) and lowest among those with no insurance (10.0%) (P < .01). In 2011–2014 there were no differences between those with public/government insurance (43.2%) or private insurance (43.0%), but history of ≥1 vaccine dose was lower in those with no insurance (29.8%) (P = .01). There was no difference by poverty level in either of the time periods examined. We further examined age at vaccination among women aged 18 to 26 years in 2011–2014; the median age at first vaccine dose was 16.9 years, and 34.8% (95% CI = 27.5–43.0) reported that they had received the first dose at age 19 years or older.
Age at First HPV Vaccine Dose and Age at First Sexual Activity Among Females Aged 14–26 Years, 2011–2014
Among 1069 females aged 14 to 26 years who reported having received HPV vaccine, 95.3% provided age at first dose. Among 953 females who reported ever having sex, 99.8% provided age at first sexual activity. Among females with known age at first HPV vaccine dose, 43.1% reported having had sex before or in the same year as their first HPV vaccination. This varied by race/ethnicity, and was higher among non-Hispanic blacks (52.5%) than non-Hispanic whites (42.2%), Mexican Americans (33.3%), and Asians (30.9%) (P < .001). Mean age at first HPV vaccine dose and mean age at first sexual activity by race/ethnicity are shown in Table 2. Mean age at first HPV vaccine dose was older than mean age at first sex among non-Hispanic blacks.
Table 2.
Age at First Human Papillomavirus (HPV) Vaccine Dose and Age at First Sexual Activity Among Females Aged 14–26 Yearsa by Race/Ethnicity, National Health and Nutrition Examination Survey, 2011–2014.
| Age (Years) at First HPV Vaccine Dose, Mean (SD) | Age (Years) at First Sexual Activity, Mean (SD) | |
|---|---|---|
| Overall | 15.9 (0.2) | 16.2 (0.1) |
| Race/Ethnicity | ||
| White, non-Hispanic | 15.8 (0.3) | 16.2 (0.1) |
| Black, non-Hispanic | 16.2 (0.2) | 15.8 (0.1) |
| Mexican American | 15.1 (0.4) | 16.0 (0.2) |
| Asian | 16.7 (0.5) | 17.9 (0.4) |
At the time of the survey.
Discussion
Using self- or parent-reported data from a nationally representative survey, we found HPV vaccination with ≥1 dose and 3 doses among females aged 13 to 26 years significantly increased between 2007–2010 and 2011–2014. However, there was no change in vaccination among girls aged 11 to 12 years, and coverage remained low for both females and males. More than 40% of females with known age at first vaccine dose reported having had sex for the first time before or in the same year they received their first HPV vaccination, which is important because exposure to HPV prior to vaccination may reduce vaccine effectiveness.
Our data on coverage with ≥1 HPV vaccine dose are generally consistent with those of other national surveys for those aged 13 to 17 years and 18 to 26 years. In NHANES 2011–2014, HPV vaccine initiation among girls aged 13 to 17 years was 53%, similar to estimates from NIS-Teen, which ranged from 53% in 201112 to 60% in 2014.5 Variation between these 2 surveys could be due to the use of self- or parent-report of HPV vaccination in NHANES11 compared with provider-verified records in NIS-Teen.9 Among women aged 18 to 26 years in NHANES 2011–2014, 40% reported having received at least one HPV vaccine dose. Among 19- to 26-year-olds in NHIS, which also uses self-reported vaccination history, estimates ranged from 29.5% in 201113 to 40% in 2014.10 However, among 11- to 12-year-olds, our data show lower coverage than a recent study using a birth cohort analysis.14 That analysis reported 47% of girls aged 13 years in 2013 had received ≥1 dose of HPV vaccine before they reached age 13.
We found that coverage with ≥1 HPV vaccine dose by sociodemographic factors and insurance differed by age group. In 11- to 12-year-old girls, coverage with ≥1 dose was highest among non-Hispanic blacks and lowest among non-Hispanic whites in 2007–2010; a similar pattern, although not statistically significant, was observed in 2011–2014. Among girls aged 13 to 17 years, coverage with ≥1 dose in 2011–2014 was highest among Mexican Americans (60%) and lowest among Asians (40%). NIS-Teen 2014 similarly found ≥1 dose coverage among girls aged 13 to 17 years to be lowest in Asians and high among Hispanics (66.3%) and non-Hispanic blacks (66.4%).5 Among 18- to 26-year-olds, variation by race/ethnicity was not statistically significant in either time period we examined whereas HPV vaccine initiation among females was significantly higher among non-Hispanic whites than other racial/ethnic groups in NHIS 2008–2012.15
Report of ≥1 dose among 11- to 12-year-old girls with public/government insurance was nearly twice as high compared with those with private or no insurance, but there was no difference by type of health insurance for girls aged 13 to 17 years. In both time periods, a significantly higher proportion of 11- to 12-year-olds below poverty reported ≥1 dose coverage compared with those at or above poverty. Among girls aged 13 to 17 years and women aged 18 to 26 years, differences by poverty were not observed. Our findings for girls aged 13 to 17 years differ from NIS-Teen, which found ≥1 dose to be higher among those below poverty,5 but are similar to findings from other studies in adult women of comparable age groups.13,16,17
Our findings as well as other studies indicate that black and Hispanic, low-income, and publicly insured adolescents initiate the HPV vaccine at equivalent or higher rates than white, higher income adolescents.15,18,19 These findings may be due to the availability of the vaccine through the Vaccines for Children (VFC) program and/or to lower refusal rates in these groups.20 VFC provides vaccines for uninsured, Medicaid-eligible children aged 18 years and younger.21 While higher vaccine coverage in these groups will help decrease disparities in HPV-associated disease, increases in vaccine coverage are needed in all sociodemographic groups.
In our study, report of ≥1 HPV vaccine dose among women aged 18 to 26 years increased regardless of health insurance type. While the increase in vaccination in this age group was due, in part, to girls vaccinated when they were younger entering this age group, approximately one-third of women who had initiated the vaccine series reported they received the first vaccine at age 19 years or older. The provisions of the Affordable Care Act (ACA) passed in 2010 require insurance plans to offer dependent coverage through age 25 years and provide targeted preventive services with zero cost sharing.22 Other studies have reported that ACA was associated with a significant increase in the percentage of young adult women aged 19 to 25 years initiating HPV vaccination.23
We were able to examine the timing of first dose of HPV vaccine and first sexual activity using data from NHANES 2011–2014. Among females aged 14 to 26 years with known age at first HPV vaccine dose, 43% reported first sexual activity before or in the same year they received their first HPV vaccine, and this varied by race/ethnicity, being highest in non-Hispanic blacks. Vaccination after first sexual activity could decrease the benefit of vaccination and lower estimates of vaccine effectiveness in the early vaccine era. Many of the vaccinated individuals in the survey were older than the recommended age for vaccination when HPV vaccine became available in the United States. As more girls are vaccinated at the recommended age for routine vaccination, the proportion of females already sexually active at the time they receive their first HPV vaccine dose should decrease.
Parents may decline or defer HPV vaccination due to concerns over vaccine safety, beliefs about the age-related need for vaccination, and perceived low-infection risk.24,25 Studies indicate that both parents and providers may underestimate adolescent sexual experience,26–28 highlighting the importance of vaccinating by the recommended ages when adolescents are less likely to have initiated sexual activity.29 Some parents may worry that HPV vaccination could lead to behavioral disinhibition and risky sexual behaviors,30 although studies have not found this to occur.31 Strong provider recommendations are highly influential for vaccination uptake,32 but providers may prefer to recommend HPV vaccination in older rather than younger adolescents as they may perceive younger adolescents to be at low risk for HPV infection and may be concerned about negative parental reactions.33–35 To facilitate timely vaccination, clinicians should provide strong recommendations consistent with guidelines, including administration of the vaccine the same day as other adolescent vaccines, and emphasize the vaccine’s protection against cancer and vaccine safety.
This study has some limitations. First, HPV vaccination was self- or parent-reported and subject to reporting bias. However, our findings were similar to other surveys.9,10 Second, we analyzed vaccination with ≥1 dose at age 11 or 12 years; therefore, our estimate will be lower than estimates of vaccine initiation by age 13 years.14 Third, our estimates for mean age at first sexual activity may have been lower than the true mean age at first sex because we could only include data from those who had sex at the time of the survey. Fourth, we were unable to determine if sex occurred before vaccination for those reporting first sexual activity in the same year as their first dose of HPV vaccine, and we chose to combine this subgroup with those reporting sex before their first dose of HPV vaccine. Last, the small sample size in some groups limited our ability to conduct further analyses: we were unable to assess if HPV vaccination before having sex varied by age and there was an insufficient number of Asians to form a separate race category during 2007–2010 because this group was not oversampled.
Conclusion
This study used data from a nationally representative survey that includes vaccination history across a wider age range than available from other national surveys. There were significant increases in HPV vaccination among females aged 13 to 26 years in the 8 years following vaccine licensure. However, there was no change in vaccination among girls aged 11 to 12 years. Coverage remained low for both females and males in all age groups we examined. Among females with known age at first vaccine dose, 43% in 2011–2014 reported having had sex before or the same year they received their first HPV vaccine, which may decrease the initial population level impact of HPV vaccination. Clinicians should provide strong recommendations consistent with guidelines, including routine vaccination of girls and boys at age 11 or 12 years.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Author Contributions
All authors participated in the study conception and design, data synthesis and analysis, and interpretation of the results. EYP drafted the manuscript. All authors reviewed and edited the manuscript and approved the final version.
Authors’ Note
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC). Mention of company names or products does not mean endorsement by CDC.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204:566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 5.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofstetter AM, Stockwell MS, Al-Husayni N, et al. HPV vaccination: are we initiating too late? Vaccine. 2014;32:1939–1945. doi: 10.1016/j.vaccine.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 7.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. [Accessed March 18, 2016];National Health and Nutrition Examination Survey: analytic guidelines, 2011–2012. http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf.
- 9.Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591–595. [PMC free article] [PubMed] [Google Scholar]
- 10.Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. doi: 10.15585/mmwr.ss6501a1. [DOI] [PubMed] [Google Scholar]
- 11.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013;(161):1–24. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–693. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Noninfluenza vaccination coverage among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62:66–72. [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyarajah J, Elam-Evans LD, Stokley S, Smith PJ, Singleton JA. Human papillomavirus vaccination coverage among girls before 13 years: a birth year cohort analysis of the National Immunization Survey–Teen, 2008–2013 [published online November 24, 2015] Clin Pediatr (Phila) doi: 10.1177/0009922815616245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt S, Parsons HM. Vaccination interest and trends in human papillomavirus vaccine uptake in young adult women aged 18 to 26 years in the United States: an analysis using the 2008–2012 National Health Interview Survey. Am J Public Health. 2014;104:946–953. doi: 10.2105/AJPH.2013.301828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bednarczyk RA, Birkhead GS, Morse DL, Doleyres H, McNutt LA. Human papillomavirus vaccine uptake and barriers: association with perceived risk, actual risk and race/ethnicity among female students at a New York State university, 2010. Vaccine. 2011;29:3138–3143. doi: 10.1016/j.vaccine.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 17.Patel DA, Zochowski M, Peterman S, Dempsey AF, Ernst S, Dalton VK. Human papillomavirus vaccine intent and uptake among female college students. J Am Coll Health. 2012;60:151–161. doi: 10.1080/07448481.2011.580028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeudin P, Liveright E, Del Carmen MG, Perkins RB. Race, ethnicity, and income factors impacting human papillomavirus vaccination rates. Clin Ther. 2014;36:24–37. doi: 10.1016/j.clinthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Pierce JY, Korte JE, Carr LA, Gasper CB, Modesitt SC. Post approval human papillomavirus vaccine uptake is higher in minorities compared to whites in girls presenting for well-child care. Vaccines. 2013;1:250–261. doi: 10.3390/vaccines1030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorell C, Yankey D, Jeyarajah J, et al. Delay and refusal of human papillomavirus vaccine for girls, National Immunization Survey–Teen, 2010. Clin Pediatr (Phila) 2014;53:261–269. doi: 10.1177/0009922813520070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [Accessed March 18, 2016];Vaccines for Children Program (VFC) http://www.cdc.gov/vaccines/programs/vfc/index.html.
- 22.Cogan JA. The Affordable Care Act’s preventive services mandate: breaking down the barriers to nationwide access to preventive services. J Law Med Ethics. 2011;39:355–365. doi: 10.1111/j.1748-720X.2011.00605.x. [DOI] [PubMed] [Google Scholar]
- 23.Lipton BJ, Decker SL. ACA provisions associated with increase in percentage of young adult women initiating and completing the HPV vaccine. Health Aff (Millwood) 2015;34:757–764. doi: 10.1377/hlthaff.2014.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol. 2009;19:531–538. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins RB, Clark JA, Apte G, et al. Missed opportunities for HPV vaccination in adolescent girls: a qualitative study. Pediatrics. 2014;134:e666–e674. doi: 10.1542/peds.2014-0442. [DOI] [PubMed] [Google Scholar]
- 26.Jaccard J, Dittus PJ, Gordon VV. Parent-adolescent congruency in reports of adolescent sexual behavior and in communications about sexual behavior. Child Dev. 1998;69:247–261. [PubMed] [Google Scholar]
- 27.Mollborn S, Everett B. Correlates and consequences of parent-teen incongruence in reports of teens’ sexual experience. J Sex Res. 2010;47:314–329. doi: 10.1080/00224490902954315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr. 2011;11:74. doi: 10.1186/1471-2431-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kann L, Kinchen S, Shanklin SL, et al. Youth Risk Behavior Surveillance—United States, 2013. MMWR Surveill Summ. 2014;63(suppl 4):1–168. [PubMed] [Google Scholar]
- 30.Marlow LA, Forster AS, Wardle J, Waller J. Mothers’ and adolescents’ beliefs about risk compensation following HPV vaccination. J Adolesc Health. 2009;44:446–451. doi: 10.1016/j.jadohealth.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Bednarczyk RA, Davis R, Ault K, Orenstein W, Omer SB. Sexual activity–related outcomes after human papillomavirus vaccination of 11- to 12-year-olds. Pediatrics. 2012;130:798–805. doi: 10.1542/peds.2012-1516. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890–895. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 33.Kahn JA, Zimet GD, Bernstein DI, et al. Pediatricians’ intention to administer human papillomavirus vaccine: the role of practice characteristics, knowledge, and attitudes. J Adolesc Health. 2005;37:502–510. doi: 10.1016/j.jadohealth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Allison MA, Hurley LP, Markowitz L, et al. Primary care physicians’ perspectives about HPV vaccine. Pediatrics. 2016;137:1–9. doi: 10.1542/peds.2015-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010;126:425–433. doi: 10.1542/peds.2009-3500. [DOI] [PubMed] [Google Scholar]