Abstract
This pilot randomized controlled trial (RCT) investigated benefits of omega-3 fatty acid supplementation and Individual-Family Psychoeducational Psychotherapy (PEP; a family-focused, cognitive-behavioral therapy) for behavior problems among youth with depression. Participants aged 7–14 with DSM-IV-TR depressive disorders (N = 72; 56.9% male) were randomized to 1 of 4 treatment conditions: PEP + omega-3, PEP monotherapy (with pill placebo), omega-3 monotherapy, or placebo (without active intervention). At screen, baseline, and 2, 4, 6, 9, and 12 weeks post-baseline, parents completed the SNAP-IV, which assesses attention-deficit/hyperactivity disorder symptoms, oppositional defiant disorder symptoms, and overall behavior problems. At screen, baseline (randomization), 6 and 12 weeks, parents completed the Eyberg Child Behavior Inventory (ECBI), which includes Intensity and Problem scales for child behavior problems. Youth who had a completed SNAP-IV or ECBI for at least two assessments during treatment (n=48 and 38, respectively) were included in analyses of the respective outcome. ClinicalTrials.gov:NCT01341925. Linear mixed effects models indicated a significant effect of combined PEP + omega-3 on SNAP-IV Total (p = .022, d = 0.80) and Hyperactivity/Impulsivity trajectories (p = .008, d=0.80), such that youth in the combined group saw greater behavioral improvement than those receiving only placebo. Similarly, youth in combined treatment had more favorable ECBI Intensity trajectories than youth who received no active treatment (p = .012, d = 1.07). Results from this pilot RCT suggest that combined PEP + omega-3 is a promising treatment for co-occurring behavior symptoms in youth with depression.
Keywords: randomized controlled trial, depression, children, adolescents, psychotherapy, omega-3 supplementation
Depression affects 2.8% of children and 5.6% of adolescents (Costello, Erkanli, & Angold, 2006) and is associated with impairments in social, cognitive, school, and family functioning (Birmaher, Arbelaez, & Brent, 2002; Birmaher, Brent, & the AACAP Work Group on Quality Issues, 2007). Comorbidity occurs in 81–96% of youth with major depressive disorder (MDD; Kovacs, 1996). A review of epidemiological studies indicates that 21 – 83% of children and adolescents with depression also have conduct or oppositional-defiant disorder (ODD) and up to 57% have attention-deficit hyperactivity disorder (ADHD) (Angold &Costello, 1993). Comorbid behavior problems not only contribute to a more complex presentation but may also affect course and treatment response. Among adolescents with depression, those with comorbid behavior problems were more likely to experience depression recurrence after cognitive-behavioral therapy (CBT) (Rohde, Clarke, Lewinsohn, Seeley, & Kaufman, 2001). Longitudinal data indicate that disruptive behavior among youth with depression is associated with increased use of inpatient and criminal justice services and higher cost of care across a wide range of services in adulthood (Knapp, McCrone, Fombonne, Beecham, & Wostear, 2002). Thus, effectively treating comorbid behavior problems may help reduce depression relapse and later-life need for intensive services and high health-care costs.
Despite high rates of comorbidity and the association between disruptive behavior and depression onset, duration, and recurrence (Birmaher et al., 2002; Rohde et al., 2001), little research has investigated how depression interventions affect youth behavior problems. Using meta-analysis, Weisz, McCarty, and Valeri (2006) reported a small effect (ES = 0.34) of psychotherapy for youth depression overall among 35 randomized trials; among 11 studies that reported both depression and externalizing behavior outcomes, there was a nonsignificant effect (ES = 0.05) of therapy on externalizing problems (Weisz et al., 2006). However, only three of the 11 studies reported inclusion of a parent component in therapy (Clarke, Rohde, Lewinsohn, Hops, & Seeley, 1999; Diamond, Reis, Diamond, Siqueland, & Isaacs, 2002; Lewinsohn, Clarke, Hops, & Andrews, 1990), which is recommended in practice parameters concerning psychotherapy for youth depression (Birmaher et al., 2007) and behavioral disorders (Pliszka &AACAP Work Group on Quality Issues, 2007; Steiner, Remsing, & AACAP Work Group on Quality Issues, 2007). Additionally, a meta-analysis of CBT for child & adolescent anger revealed medium effects overall (d = 0.67), with skills training being the most effective (d=0.79) (Sukhodolsky, Kassinove, & Gorman, 2004); a recent meta-analysis of CBT for adolescent aggression found a medium effect (d=0.50) (Smeets et al., 2015). In a single-arm-trial of CBT for adolescents with ADHD, CBT improved treatment compliance, self-esteem, inattention, and academic functioning with greater improvements among youth with comorbid anxiety or depression (Antshel, Faraone, & Gordon, 2012), indicating that youth with behavior problems and comorbid internalizing problem may experience particular benefit from CBT.
Recent studies not included in Weisz and colleagues’ 2006 meta-analysis provide some support for psychotherapy effects in treating depression and comorbid behavior problems. Psychoeducational Psychotherapy (PEP) is a family-focused psychoeducation and CBT skills training intervention consisting of separate parent and child sessions. PEP parent sessions emphasize the role of parents as part of the treatment team and include parent stress management, identifying and improving negative family cycles (e.g., increasing positive reinforcement of desired behaviors; managing behaviors that are disruptive), family problem-solving, improving parent-child communication, and family preservation skills (Fristad, Goldberg-Arnold, & Leffler, 2011). Thus, while PEP was developed to treat mood symptoms, some of its principles overlap with those of evidence-based therapy for disruptive behavior (Pliszka &AACAP Work Group on Quality Issues, 2007; Steiner et al., 2007), which may facilitate improvements in comorbid disruptive behavior. Multifamily group PEP (MF-PEP) has demonstrated efficacy in treating mood symptoms (d = 0.53) compared to wait-list control (Fristad, Verducci, Walters, & Young, 2009), and small to medium with-in group effects on ADHD (d = 0.36), ODD (d = 0.53), and overall disruptive behavior (d = 0.37) symptoms in children with primary depression or bipolar disorder diagnoses (Boylan, MacPherson, & Fristad, 2013). Thus, PEP has demonstrated efficacy in treating primary mood and comorbid behaviors.
Effects of antidepressants on youth depression are fairly well-studied (Bridge et al., 2007), while antidepressants’ effects on disruptive behavior are less understood. In the Treatment for Adolescent Depression Study (TADS), CBT, fluoxetine, and their combination yielded reductions in adolescents’ depression and ODD symptoms, with combination and fluoxetine alone superior to CBT alone (Jacobs et al., 2010; TADS Team, 2004). No studies of combined treatment include children under age 12. Thus, antidepressants’ effects on school-aged children’s disruptive behavior remain unknown. Further, while fluoxetine has demonstrated positive effects, antidepressants in youth may be associated with increased risk of suicidal ideation (U.S. Food and Drug Administration, 2004). Therefore, complementary and integrative approaches to treating depression and comorbid disruptive behavior should be investigated.
Polyunsaturated fatty acids (PUFAs, including omega-3) are important for the development of healthy brain structure and functioning; PUFA deficiency is linked to psychiatric morbidity including mood disorders, ADHD, psychosis, and developmental disorders (Richardson, 2003). Omega-3 fatty acids, which have anti-inflammatory properties and affect serotonin and dopamine neurotransmission in the frontal cortex, may play a particularly important role in mental health (Bloch &Qawasmi, 2011; Owen, Rees, & Parker, 2008). Omega-3 supplementation has shown promise in the treatment of mood disorders in youth. In a small randomized controlled trial (RCT), children with MDD who were taking omega-3 supplements were more likely than those taking placebo to experience ≥ 50% reduction in depressive symptoms (Nemets, Nemets, Apter, Bracha, & Belmaker, 2006). Bipolar disorder trials offer further evidence for omega-3’s efficacy in treating youth depression symptoms; Clayton et al. (2009) not only found benefit for depression and mania symptoms but also for externalizing symptoms. Further, a 12-week RCT of vitamin, mineral, and omega-3 supplementation vs. placebo found that adolescents (N = 196) experienced greater improvement in disruptive behavior in the supplemented group (d = 0.35) (Tammam, Steinsaltz, Bester, Semb-Andenaes, & Stein, 2016); a similar RCT indicated that omega-3 yielded greater reduction in behavior problems than did placebo (mean d = 0.59) among 200 youth (Raine, Portnoy, Liu, Mahoomed, & Hibbeln, 2015). Results of trials investigating the effects of PUFA supplementation for ADHD symptoms specifically are fairly mixed (Ahn, Ahn, Cheong, & dela Pena, 2016). A meta-analysis (including trials with a variety of study designs) indicated that youth with ADHD who took omega-3 + omega-6 supplements vs. placebo were less likely to have clinically significant symptoms post-treatment (risk ratio = 2.19 across two trials). However, there were no significant treatment effects on parent- or teacher-rated ADHD symptoms (three - six trials per analysis) (Gillies, Sinn, Lad, Leach, & Ross, 2012). Another meta-analysis including only RCTs found small but significant placebo-controlled effects of PUFA supplementation on ADHD (standardized mean difference = 0.21; 11 trials) (Sonuga-Barke et al., 2013). Further, omega-3 supplementation improved attention in both youth with ADHD and youth without ADHD in a recent RCT (Bos et al., 2015). Prior research offers evidence for the effects of omega-3 supplementation on depression and behavior problems with some (mixed) evidence of modest effects on ADHD symptoms specifically. As reported side effects have been mild (primarily gastrointestinal complaints), omega-3 supplementation may be both safe and efficacious in reducing depression symptoms and warrants further investigation as an intervention for disruptive behaviors among youth with primary depression. Effects of combined omega-3 and psychotherapy on behavioral symptoms are currently unknown.
The current study is a secondary analysis of a 2×2 pilot RCT of PEP vs. active monitoring and omega-3 supplementation vs. placebo among children and adolescents (ages 7 – 14) with depressive disorders. Prior analysis of this RCT, which focused on the a priori primary outcome (mood symptoms), indicated that family-level factors moderated treatment effects. Among youth with a history of maternal depression, PEP monotherapy demonstrated improvement in depression symptoms while placebo + active monitoring did not; when baseline family environmental stress was low, PEP and omega-3 alone and in combination each yielded a significant decline in depression severity while placebo did not (Fristad et al., in review). A parallel RCT including youth with bipolar disorder not otherwise specified or cyclothymic disorder indicated significant large effects of PEP vs. active monitoring (d = 1.24) and combined PEP + omega-3 vs. placebo (d = 1.70) on depression severity (Fristad et al., 2015). These primary findings support PEP and omega-3 supplementation, alone and in combination, as promising for treatment of youth depression. Although depression and disruptive behavior problems are commonly comorbid, little research has investigated the effects of depression interventions on comorbid disruptive behavior problems. Thus, the current analyses expand the prior findings by examining effects of PEP, omega-3 supplementation, and their combination on behavior problems among youth with primary depression. Consistent with previous PEP (Boylan et al., 2013) and omega-3 supplementation RCTs (Raine et al., 2015; Tammam et al., 2016), we expected that youth randomized to PEP, omega-3, and combined treatment would experience greater behavioral improvements than the placebo group.
Methods
Participants
Between July 2011 and May 2014, youth and their parents were recruited from community advertisements and clinician referrals in a Midwestern U.S. city to participate in an RCT of PEP vs. no PEP and omega-3 vs. placebo in a 2×2 design. Eligibility criteria included the diagnosis of DSM-IV-TR MDD, dysthymic disorder, or depressive disorder not otherwise specified (American Psychiatric Association, 2000), ages 7 – 14 years, and Children’s Depression Rating Scale-Revised score of ≥ 40. Participants were excluded if they exhibited impairing psychosis, active suicidal ideation, or evidence of intellectual disability (IQ<70 and impaired adaptive functioning); received mental health treatment (other than stable medication for ADHD or sleep problems) or omega-3 supplementation within 1 month prior to baseline; had a rating of marked or severe on mood items in the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) (Kaufman et al., 1997), or had a severe medical condition. The target sample size was 60, as determined by power analysis. Of 178 screened youth, 72 (40%) met criteria for and enrolled in the current study (Trial registration: www.ClinicalTrials.gov/ct2/show/NCT01341925); another 23 (13%) enrolled in a parallel trial for youth with bipolar disorder not otherwise specified (Trial registration: www.ClinicalTrials.gov/ct2/show/NCT01507753).
The two measures of behavioral problems, the Swanson, Nolan, and Pelham-IV (SNAP-IV) and Eyberg Child Behavior Inventory (ECBI), were added after the study commenced; thus, only a subset of the total had data on these measures. Forty-eight youth (66.7% of the total sample) completed the SNAP-IV at least twice during the trial (from baseline to 12-weeks, inclusively) and were included in the current analyses: 12 in combined treatment, 14 in PEP monotherapy, 11 in omega-3 monotherapy, 11 in placebo only; 38 youth (52.8%) had ECBI scores for at least two time points: nine in combined treatment, 12 in PEP monotherapy, eight in omega-3 monotherapy, nine in placebo only. Inclusion (vs. exclusion due to insufficient data) in the SNAP-IV and ECBI analyses did not significantly differ across treatment groups.
Table 1 depicts participant characteristics by whether or not they had sufficient data to be included in longitudinal analyses of either of the two outcome measures; Table 2 presents participant characteristics by treatment group among youth who were included in the longitudinal analyses. Both the SNAP-IV (n = 48) and ECBI (n = 38) samples respectively were primarily in the middle childhood age range (75.0% and 81.6% were ages 7 – 12). Just over half (52.1% and 57.9%) were male and just over one-third (35.4% and 36.8%) received public health insurance. Nearly half (45.8% and 42.1%) had MDD; comorbidity was common: 83.3% and 79.0% had an anxiety disorder; 54.2% and 63.2% had ADHD; and 16.7% and 18.4% had a disruptive behavior disorder (DBD; ODD, conduct disorder, or DBD not otherwise specified) in the SNAP-IV and ECBI samples.
Table 1.
Participant characteristics by status on outcome measures - n (%) unless otherwise indicated
| Characteristic | Total | SNAP-IV Completed | ECBI Completed | ||
|---|---|---|---|---|---|
| N=72 | Yes n=48 |
No n=24 |
Yes n=38 |
No n=34 |
|
| Child Age (M ± SD) | 11.65 ± 2.14 | 11.17 ± 2.21 | 12.61 ± 1.64 | 11.06 ± 2.06 | 12.31 ± 2.05 |
| 7 to 12.9 years old | 48 (66.7) | 36 (75.0) | 12 (50.0) | 31 (81.6) | 17 (50.0) |
| Child Sex: Male | 41 (56.9) | 25 (52.1) | 16 (66.7) | 22 (57.9) | 19 (55.9) |
| Child Race | |||||
| White | 41 (56.9) | 30 (62.5) | 11 (45.8) | 24 (63.2) | 17 (50.0) |
| Black/African-American | 22 (30.6) | 14 (29.2) | 8 (33.3) | 11 (29.0) | 11 (32.4) |
| Biracial | 9 (12.5) | 4 (8.3) | 5 (20.8) | 3 (7.9) | 6 (17.7) |
| Child Ethnicity: Hispanic | 7 (9.7) | 4 (8.3) | 3 (12.5) | 3 (7.9) | 4 (11.8) |
| Child Insurance: Medicaid | 23 (31.9) | 17 (35.4) | 6 (25.0) | 14 (36.8) | 9 (26.5) |
| Depressive Disorder | |||||
| Major Depression | 37 (51.4) | 22 (45.8) | 15 (62.5) | 16 (42.1) | 21 (61.8) |
| Dysthymia | 5 (6.9) | 3 (6.3) | 2 (8.3) | 2 (5.3) | 3 (8.8) |
| Depression NOS | 30 (41.7) | 23 (47.9) | 7 (29.2) | 20 (52.6) | 10 (29.4) |
| Comorbid Disorders | |||||
| Anxiety Disorder | 56 (77.8) | 40 (83.3) | 16 (66.7) | 30 (79.0) | 26 (76.5) |
| ADHD | 41 (56.9) | 26 (54.2) | 15 (62.5) | 24 (63.2) | 17 (50.0) |
| DBD | 22 (30.6) | 8 (16.7) | 14 (58.3) | 7 (18.4) | 15 (44.1) |
| Parent Age (M ± SD) | 41.82 ± 8.07 | 41.02 ± 7.87 | 43.41 ± 8.40 | 41.59 ± 7.74 | 42.07 ± 8.54 |
| Household Income | |||||
| <$20,000 | 13 (18.1) | 9 (18.8) | 4 (16.7) | 7 (18.4) | 6 (17.7) |
| $20,000 – 40,000 | 19 (26.4) | 15 (31.3) | 4 (16.7) | 11 (29.0) | 8 (23.5) |
| $40,000 – 60,000 | 13 (18.1) | 7 (14.6) | 6 (25.0) | 7 (18.4) | 6 (17.7) |
| $60,000 – 80,000 | 8 (11.1) | 4 (8.3) | 4 (16.7) | 3 (7.9) | 5 (14.7) |
| $80,000 – 100,000 | 3 (4.2) | 2 (4.2) | 1 (4.2) | 2 (5.3) | 1 (2.9) |
| >$100,000 | 15 (20.8) | 10 (20.8) | 5 (20.8) | 7 (18.4) | 8 (23.5) |
Note. One parent did not report household income. Participants whose parents completed the SNAP-IV or ECBI at two or more time points were younger than those whose parents did not, t(59.5) = 3.12, p=.003 and t(70) = 2.56, p=.013, respectively. Youth whose parents completed the SNAP-IV or the ECBI at two or more time points were also less likely to have a DBD diagnosis than those whose parents did not, X2=13.09, p<.001 and X2=5.58, p=.018, respectively.
ADHD=Attention-deficit/Hyperactivity Disorder; DBD=Disruptive Behavior Disorder (Oppositional Defiant Disorder, Conduct Disorder, or Disruptive Behavior Disorder Not Otherwise Specified); ECBI=Eyberg Child Behavior Inventory; NOS=Not Otherwise Specified; SNAP-IV=Swanson, Nolan, and Pelham Questionnaire-IV.
Table 2.
Characteristics of participants included in SNAP-IV and ECBI analyses by randomization group - n (%) unless otherwise indicated
| Characteristic | SNAP-IV Completers | ECBI Completers | ||||||
|---|---|---|---|---|---|---|---|---|
| Combined | PEP | Omega-3 | Placebo | Combined | PEP | Omega-3 | Placebo | |
| n=12 | n=14 | n=11 | n=11 | n=9 | n=12 | n=8 | n=9 | |
| Child Age (M ± SD) | 10.85 ± 2.35 | 11.61 ± 2.26 | 12.13 ± 1.85 | 10.01 ± 1.95 | 11.09 ± 2.01 | 11.73 ± 2.00 | 12.10 ± 2.04 | 9.23 ± 1.00 |
| 7 to 12.9 years old | 10 (83.3) | 9 (64.3) | 8 (72.7) | 9 (81.8) | 8 (88.9) | 8 (66.7) | 6 (75.0) | 9 (100.0) |
| Child Sex: Male | 7 (58.3) | 6 (42.9) | 4 (36.4) | 8 (16.7) | 5 (55.6) | 5 (41.7) | 4 (50.0) | 8 (88.9) |
| Child Race | ||||||||
| White | 7 (58.3) | 9 (64.3) | 6 (54.5) | 8 (72.7) | 5 (55.6) | 8 (66.7) | 4 (50.0) | 7 (77.8) |
| Black/African-American | 4 (33.3) | 4 (28.6) | 4 (36.4) | 2 (18.2) | 3 (33.3) | 3 (25.0) | 3 (37.5) | 2 (22.2) |
| Biracial | 1 (8.3) | 1 (7.1) | 1 (9.1) | 1 (9.1) | 1 (11.1) | 1 (8.3) | 1 (12.5) | 0 |
| Child Ethnicity: Hispanic | 2 (16.7) | 2 (14.3) | 0 | 0 | 2 (22.2) | 1 (8.3) | 0 | 0 |
| Child Insurance: Medicaid | 2 (16.7) | 4 (28.6) | 6 (54.5) | 5 (45.5) | 2 (22.2) | 3 (25.0) | 4 (50.0) | 5 (55.6) |
| Depressive Disorder | ||||||||
| Major Depression | 3 (25.0) | 7 (50.0) | 7 (63.3) | 5 (45.5) | 1 (11.1) | 6 (50.0) | 5 (62.5) | 4 (44.4) |
| Dysthymia | 0 | 1 (7.1) | 0 | 2 (18.2) | 0 | 0 | 0 | 2 (22.2) |
| Depression NOS | 9 (75.0) | 6 (42.9) | 4 (36.4) | 4 (36.4) | 8 (88.9) | 6 (50.0) | 3 (37.5) | 3 (33.3) |
| Comorbid Disorders | ||||||||
| Anxiety Disorder | 9 (75.0) | 11 (78.6) | 10 (90.9) | 10 (90.9) | 6 (66.7) | 9 (75.0) | 7 (87.5) | 8 (88.9) |
| ADHD | 5 (41.7) | 10 (71.4) | 4 (36.4) | 7 (63.6) | 4 (44.4) | 9 (75.0) | 4 (50.0) | 7 (77.8) |
| DBD | 2 (16.7) | 4 (28.6) | 1 (9.1) | 1 (9.1) | 2 (22.2) | 3 (25.0) | 1 (12.5) | 1 (11.1) |
| Parent Age (M ± SD) | 40.42 ± 7.59 | 41.25 ± 7.20 | 40.54 ± 6.71 | 41.85 ± 10.68 | 42.54 ± 7.48 | 42.18 ± 7.10 | 41.72 ± 6.19 | 39.74 ± 10.61 |
| Household Income | ||||||||
| <$20,000 | 2 (16.7) | 1 (7.1) | 3 (27.3) | 3 (27.3) | 1 (11.1) | 1 (8.3) | 2 (25.0) | 3 (33.3) |
| $20,000 – 40,000 | 4 (33.3) | 4 (28.6) | 3 (27.3) | 4 (36.4) | 4 (44.4) | 2 (16.7) | 1 (12.5) | 4 (44.4) |
| $40,000 – 60,000 | 0 | 3 (21.4) | 2 (18.2) | 2 (18.2) | 0 | 3 (25.0) | 2 (25.0) | 2 (22.2) |
| $60,000 – 80,000 | 1 (8.3) | 3 (21.4) | 0 | 0 | 0 | 3 (25.0) | 0 | 0 |
| $80,000 – 100,000 | 1 (8.3) | 1 (7.1) | 0 | 0 | 1 (11.1) | 1 (8.3) | 0 | 0 |
| >$100,000 | 3 (25.0) | 2 (14.3) | 3 (27.3) | 2 (18.2) | 2 (22.2) | 2 (16.7) | 3 (37.5) | 0 |
Note. One parent whose did not report household income. Among ECBI completers, youth in the placebo group were significantly younger than youth in PEP monotherapy (M difference=−2.51, SE= 0.81, p =.019, d=−1.59) and youth in omega-3 monotherapy (M difference=−2.87, SE= 0.89, p =.014, d=−1.93). ADHD=Attention-deficit/Hyperactivity Disorder; DBD=Disruptive Behavior Disorder (Oppositional Defiant Disorder, Conduct Disorder, or Disruptive Behavior Disorder Not Otherwise Specified); ECBI=Eyberg Child Behavior Inventory; NOS=Not Otherwise Specified; PBO=placebo; PEP=Individual-Family Psychoeducational Psychotherapy; SNAP-IV=Swanson, Nolan, and Pelham Questionnaire-IV.
Participants who had sufficient SNAP-IV data and those with sufficient ECBI data were younger, t(59.5) = 3.12, p = .003, d = 0.71 and t(70) = 2.56, p = .013, d = 0.61, respectively, and less likely to have a DBD diagnosis, X2= 13.09, p < .001 and X2= 5.58, p = .018, respectively, than youth who did not complete SNAP-IV or ECBI forms (Table 1). There were no significant differences by randomization group for those included in SNAP-IV analyses; only age differed across randomization groups in the ECBI sample, F (3, 34) = 4.42, p = .010, partial-eta2 =.28, with the placebo group being younger than either PEP, p = .019, d = −1.59, or omega-3, p = .014, d = −1.93, monotherapy groups (see Table 2).
Procedure
Details regarding study design and participant flow can be found elsewhere (Fristad et al., in review; Fristad et al., 2015). The local Institutional Review Board approved all study procedures. Youth provided written informed assent and their parents provided written informed consent and HIPAA authorizations prior to completing the screening assessment. Those who met trial criteria and attended the baseline assessment were block-randomized to receive combined treatment (PEP + omega-3 supplementation), PEP monotherapy (plus placebo), omega-3 monotherapy, or placebo alone. All received a daily multivitamin/mineral to standardize micronutrients. Follow-up assessments occurred at 2, 4, 6, 9, and 12 weeks from the baseline assessment.
Interventions
Families randomized to PEP – a manualized, family-focused, CBT-based psychotherapy (Fristad et al., 2011) – were invited to weekly parent sessions and weekly child sessions (each lasting 45–50 minutes) throughout the 12-week trial, for a total of 24 possible sessions. Parents participated for 5–10 minutes at the beginning of each child session to check in on the child’s progress since the last session and at 5–10 minutes at the end of each child session to review new session material. Thus, every PEP session includes parent involvement. Among the families randomized to PEP, those who were included in the SNAP-IV analyses attended a median of 17 sessions (range: 2 – 24) and those who were included in the ECBI analyses attended a median of 18 sessions (range 6–24). The goals of PEP are to provide psychoeducation about mood disorders, facilitate collaboration with the school system, teach youth coping strategies and parents stress management strategies and teach both problem-solving and communication skills; as previously noted, parent sessions also include identifying and improving negative family cycles. Specific information about session content can be found elsewhere (Fristad et al., 2011). Study therapists were doctoral-level clinicians, supervised by MAF.
After randomization, study staff provided families a pill minder of placebo or omega-3 supplements, depending on the study condition, and asked the families to bring it to each follow-up assessment to be refilled. Twice daily, participants took either two 500 mg capsules of omega-3 resulting in a daily dose of 2000 mg (1400 mg eicosapentaenoic acid [EPA], 200 mg docosahexaenoic acid [DHA], 400 mg other omega-3 fatty acids) or two placebo capsules, matched to odor and appearance of omega-3 capsules. OmegaBrite (www.omegabrite.com; Las Vegas, NV) manufactured and supplied the omega-3 and placebo capsules.
Assessments
Parents and children participated in structured and semi-structured diagnostic interviews and completed youth self-report and parent-report questionnaires at each assessment. Interviewer training included didactics, mock interviews and rating of live and videotaped interviews. In consensus conferences after each interview, interviewers reviewed content with an expert clinician (MAF or LEA) to determine diagnoses, severity ratings, and, after screening, study eligibility. Families and all study staff other than those dispensing the capsules were masked to omega-3/placebo condition. Families, interviewers who completed the follow-up assessments, and the clinician (LEA) who completed consensus conferences for follow-up assessments were masked to participants’ PEP/active monitoring condition.
Parents provided information such as youths’ sex, age, and socioeconomic status on a demographics questionnaire at screen. The screening assessment also included the Depression (KDRS) and Mania Rating Scales (KMRS) of the KSADS, a semi-structured parent and child interview (Ambrosini, Metz, Prabucki, & Lee, 1989; Axelson et al., 2003; Chambers et al., 1985), assisted in determining mood disorder diagnoses. The KDRS has demonstrated excellent test-retest reliability in diagnosing depressive disorders (kappa = .90) (Kaufman et al., 1997); the KMRS has excellent inter-rater reliability (intraclass correlation coefficient [ICC] = .97) and convergent validity with the Clinical Global Impressions-Severity scale (r = .91) (Axelson et al., 2003). Inter-rater reliability for videotaped interviews in the current study was excellent for the KDRS (ICC = .89) and KMRS (ICC = .82). All diagnoses were verified in consensus conferences.
Parents reported youth behavior problems via two questionnaires. Only a subset of participating families have data on these measures, as they were added after the study had commenced. The Swanson, Nolan and Pelham Questionnaire-IV (SNAP-IV) assesses symptoms of DSM-IV ADHD and ODD on a 0–3 scale (Swanson, 1992); parents completed the 26-item, Multi-modal Treatment Study of ADHD (MTA) version of the SNAP-IV (Bussing et al., 2008). Scale scores are calculated as average rating per item (raw sum of ratings/number of items in the scale). Average rating per item on the SNAP-IV Total, ADHD-Inattention and Hyperactivity/Impulsivity subscales (each with nine items), and ODD subscale (eight items) were outcomes of interest in the current analyses. The measure’s developers suggest tentative cut scores for clinical significance for the Inattention, Hyperactive/Impulsive, and ODD subscales (but not for the total score). These cut scores (mean item ratings ≥ 1.78, 1.44, and 1.88 for Inattention, Hyperactivity/Impulsivity, and ODD subscales, respectively) were used to help characterize the prevalence of behavior problems in this sample. The SNAP-IV has demonstrated good internal consistency with α = .94, .90, .79, and .89 for total, inattention, hyperactivity/impulsivity, and ODD scales, respectively (Bussing et al., 2008). It has demonstrated good validity in discriminating youth with and without behavioral problems and differentially predicting ADHD subtypes, and is sensitive to treatment response (Swanson, 1992; Swanson et al., 2001). Parents also completed the Eyberg Child Behavior Inventory (ECBI), a 36-item parent-report of child behavior problems including those related to ADHD and ODD (Eyberg &Pincus, 1999). The ECBI includes an Intensity subscale (score range 36–252; clinical cut-off ≥ 131) assessing the frequency of behaviors and a Problem subscale (score range 0 – 36; clinical cut-off ≥ 15) assessing parents’ perceptions of how problematic the behaviors are for their child. The ECBI has demonstrated good discriminant validity (significantly differentiating youth with identified behavior problems from both healthy controls and a clinical sample without identified behavior problems) and reliability (internal consistency range: α = .93–.95; inter-rater reliability range: r = .61–.79) (Eyberg &Pincus, 1999; Eyberg &Ross, 1978). Screening, baseline and all follow-up assessments administered the SNAP-IV. The ECBI was administered at baseline and the 6- and 12-week assessments.
Data Analytic Plan
As only a subset of the full sample completed the SNAP-IV and the ECBI, chi-squares and t-tests examined demographic and clinical characteristics comparing youth who had valid SNAP-IV and ECBI scores and those who did not. Linear mixed effects models (LME) examined whether behavioral symptom trajectories (as measured by the SNAP-IV and the ECBI) differed between each of the active treatment groups and placebo across the 12-week trial, using restricted maximum likelihood estimation (REML). LME accommodates missing data for participants with ≥ two non-missing data points. Thus models fit to SNAP-IV and ECBI trajectories could use data from the subset of the intent-to-treat sample who had data on the outcome measure at least two time points during the 12-week trial (n s = 48 and 38, respectively). Intercepts and slopes were modeled as random effects; treatment group (dummy-coded relative to placebo), time (weeks since baseline), and treatment-group-by-time were modeled as fixed effects. Additional models examined planned contrasts of omega-3 (collapsing across the combined and omega-3 monotherapy groups) vs. placebo (PEP monotherapy and placebo only groups) and PEP (combined and PEP monotherapy groups) vs. no PEP (omega-3 monotherapy and placebo only groups). LME analyses were completed in IBM SPSS Statistics version 23. Effect sizes were calculated from the unstandardized coefficients associated with group-by-time interactions using methods described by Feingold (2009). Given the exploratory nature of this investigation, all analyses used α = .05 for statistical significance without correction for number of tests.
Results
Table 3 summarizes levels of behavior problems present in the sample throughout the 12-week trial. Neither screening nor baseline SNAP-IV or ECBI scores were significantly associated with trial attrition, study capsule adherence, or number of therapy sessions attended.
Table 3.
Behavior Problems Throughout the 12-Week Trial - M ± SD unless otherwise specified
| Measure | Overall | Combined | PEP | Omega-3 | Placebo | ||
|---|---|---|---|---|---|---|---|
| Baseline | SNAP-IV n | 49 | 12 | 14 | 12 | 11 | |
| Total Score | 1.40 ± 0.61 | 1.30 ± 0.44 | 1.45 ± 0.79 | 1.33 ± 0.56 | 1.51 ± 0.64 | ||
| Inattention Score | 1.61 ± 0.70 | 1.44 ± 0.62 | 1.70 ± 0.78 | 1.52 ± 0.68 | 1.80 ± 0.70 | ||
| % ≥ cut-off | 34.7% | 25.0% | 35.7% | 33.3% | 45.5% | ||
| H/I Score | 1.04 ± 0.78 | 1.00 ± 0.55 | 1.13 ± 0.91 | 0.85 ± 0.75 | 1.19 ± 0.89 | ||
| % ≥ cut-off | 30.6% | 25.0% | 35.7% | 25.0% | 36.4% | ||
| ODD Score | 1.55 ± 0.75 | 1.49 ± 0.55 | 1.52 ± 0.92 | 1.66 ± 0.82 | 1.56 ± 0.70 | ||
| % ≥ cut-off | 34.7% | 16.7% | 35.7% | 50.0% | 36.4% | ||
| ECBI n | 49 | 12 | 14 | 12 | 11 | ||
| Problem Score | 15.71 ± 9.08 | 13.00 ± 9.15 | 16.00 ± 10.74 | 14.83 ± 6.67 | 19.00 ± 9.19 | ||
| % ≥ cut-off | 56.3% | 25.0% | 64.3% | 50.0% | 72.7% | ||
| Intensity Score | 130.40 ± 35.87 | 125.25 ± 29.56 | 127.64 ± 46.06 | 135.53 ± 35.97 | 133.91 ± 30.77 | ||
| % ≥ cut-off | 55.1% | 58.3% | 50.0% | 58.3% | 54.5% | ||
| Week 6 | SNAP-IV n | 37 | 9 | 12 | 8 | 8 | |
| Total Score | 1.24 ± 0.70 | 1.01 ± 0.35 | 1.31 ± 0.78 | 1.07 ± 0.62 | 1.55 ± 0.86 | ||
| Inattention Score | 1.50 ± 0.75 | 1.32 ± 0.54 | 1.58 ± 0.79 | 1.29 ± 0.79 | 1.76 ± 0.90 | ||
| % ≥ cut-off | 37.8% | 22.2% | 50.0% | 37.5% | 37.5% | ||
| H/I Score | 0.88 ± 0.82 | 0.70 ± 0.41 | 1.00 ± 0.87 | 0.51 ± 0.59 | 1.28 ± 1.14 | ||
| % ≥ cut-off | 24.3% | 11.1% | 33.3% | 12.5% | 37.5% | ||
| ODD Score | 1.35 ± 0.80 | 1.01 ± 0.55 | 1.35 ± 0.84 | 1.44 ± 1.04 | 1.63 ± 0.71 | ||
| % ≥ cut-off | 21.6% | 0% | 16.7% | 50.0% | 25.0% | ||
| ECBI n | 37 | 9 | 12 | 8 | 8 | ||
| Problem Score | 13.18 ± 9.32 | 9.50 ± 6.35 | 14.83 ± 9.61 | 11.29 ± 9.62 | 16.43 ± 11.36 | ||
| % ≥ cut-off | 47.1% | 22.2% | 50.0% | 50.0% | 50.0% | ||
| Intensity Score | 117.90 ± 37.03 | 110.11 ± 15.26 | 118.58 ± 44.44 | 114.04 ± 44.34 | 129.50 ± 38.29 | ||
| % ≥ cut-off | 35.1% | 11.1% | 33.3% | 37.5% | 62.5% | ||
| Week 12 | SNAP-IV n | 39 | 8 | 12 | 9 | 10 | |
| Total Score | 1.18 ± 0.69 | 0.78 ± 0.58 | 1.19 ± 0.73 | 0.96 ± 0.52 | 1.68 ± 0.62 | ||
| Inattention Score | 1.39 ± 0.73 | 1.01 ± 0.67 | 1.53 ± 0.72 | 1.06 ± 0.48 | 1.82 ± 0.77 | ||
| % ≥ cut-off | 33.3% | 12.5% | 50.0% | 11.1% | 50.0% | ||
| H/I Score | 0.91 ± 0.88 | 0.49 ± 0.69 | 0.84 ± 0.86 | 0.63 ± 0.64 | 1.59 ± 0.92 | ||
| % ≥ cut-off | 28.2% | 12.5% | 25.0% | 11.1% | 60.0% | ||
| ODD Score | 1.25 ± 0.74 | 0.86 ± 0.62 | 1.21 ± 0.79 | 1.23 ± 0.82 | 1.61 ± 0.58 | ||
| % ≥ cut-off | 23.1% | 0% | 16.7% | 44.4% | 30.0% | ||
| ECBI n | 39 | 8 | 12 | 9 | 10 | ||
| Problem Score | 12.03 ± 8.88 | 6.86 ± 4.60 | 11.75 ± 9.65 | 9.11 ± 6.27 | 18.60 ± 9.16 | ||
| % ≥ cut-off | 39.5% | 0% | 41.7% | 33.3% | 70.0% | ||
| Intensity Score | 116.87 ± 36.96 | 101.13 ± 26.25 | 113.58 ± 46.29 | 108.76 ± 34.84 | 140.70 ± 24.50 | ||
| % ≥ cut-off | 28.2% | 0% | 33.3% | 11.1% | 60.0% | ||
Note. ECBI=Eyberg Child Behavior Inventory; H/I=Hyperactivity/Impulsivity subscale; ODD=Oppositional Defiant Disorder subscale; PEP=Individual-Family Psychoeducational Psychotherapy; SNAP-IV=Swanson, Nolan, and Pelham Questionnaire-IV.
Treatment Effects
Combined therapy and monotherapies relative to placebo
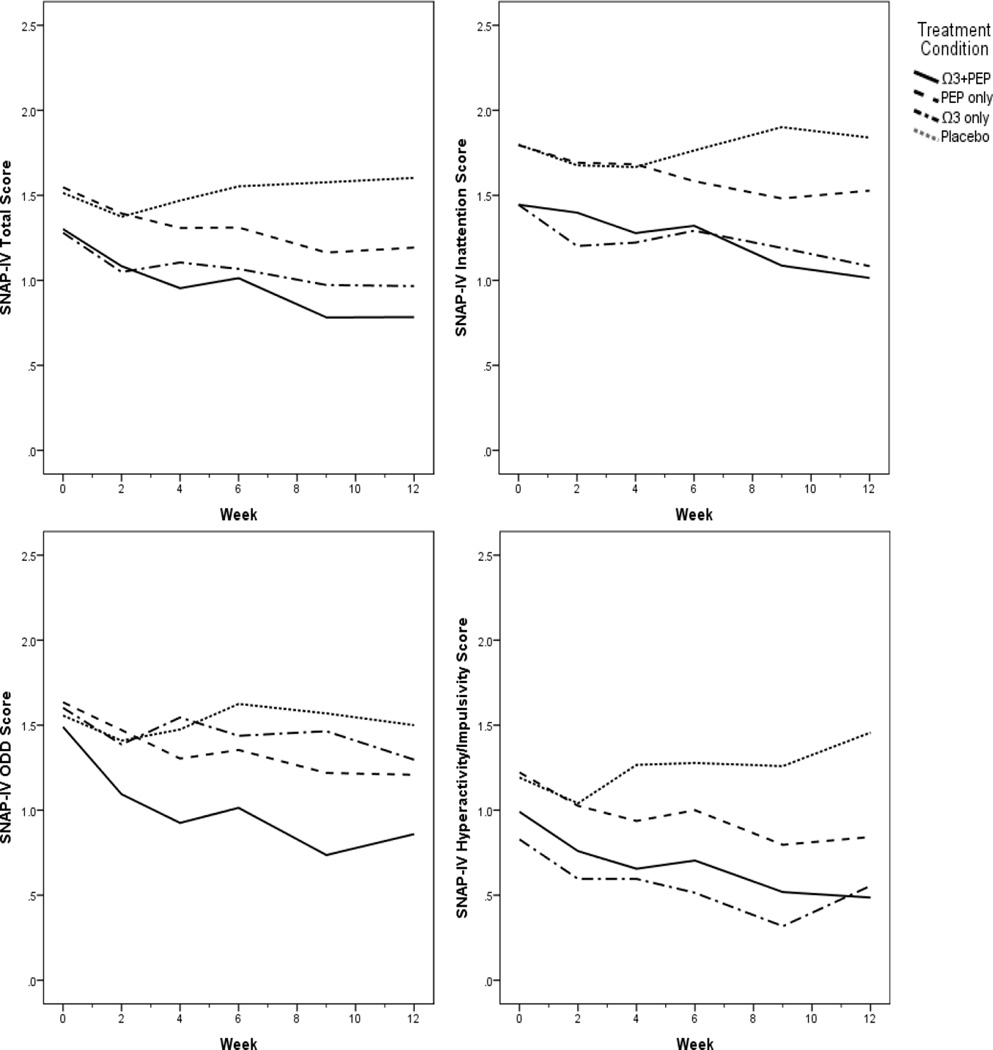
Table 4 and Figures 1 and 2 present results of the LME modeling trajectories in each of the three active treatment groups relative to placebo alone. Baseline SNAP-IV and ECBI scores did not significantly differ among treatment groups. LME models indicated a significant effect of combined IF-PEP + omega-3 on SNAP-IV Total score (p=.022, d=0.80) and Hyperactivity/Impulsivity subscale (p=.008, d=0.80) trajectories and a trend on ODD scores (p=.094, d=0.70). The effect of combined treatment on the Inattention subscale was nonsignificant but had a medium effect size (d=0.58). In each case, youth in the combined group saw greater improvement in behavioral symptoms than those receiving only placebo. Youth in omega-3 monotherapy experienced placebo-controlled improvement in Inattention (p=.038, d=0.80) and marginally better improvement in Hyperactivity/Impulsivity (p=.052, d=0.59) scores. Youth in IF-PEP monotherapy did not experience statistically significant placebo-controlled improvement, although small effect sizes were noted for Total (d=0.46), Inattention (d =0.35), Hyperactivity/Impulsivity (d=0.45) and ODD (d=0.38) scores.
Table 4.
Results of Linear Mixed-Effects Models of the Effect of PEP and Ω3 on Behavior Problems
| Measure | Treatment Group X Time Contrast | Estimate [95% CI] | df | t | p-value | d |
|---|---|---|---|---|---|---|
| SNAP-IV Total | Combined vs. Placebo only (PBO) | −0.04 [−0.07 – −0.01] | 38.5 | −2.4 | .022 | 0.80 |
| PEP monotherapy vs. PBO | −0.02 [−0.05 – 0.01] | 36.1 | −1.5 | .149 | 0.46 | |
| Ω3 monotherapy vs. PBO | −0.04 [−0.06 – 0.01] | 40.7 | −1.6 | .122 | 0.80 | |
| Ω3 (monotherapy/combined) vs. no Ω3 | −0.02 [−0.04 – 0.00] | 40.8 | −1.8 | .080 | 0.42 | |
| PEP (monotherapy/combined) vs. no PEP | −0.02 [−0.04 – 0.01] | 40.6 | −1.4 | .155 | 0.35 | |
| SNAP-IV Inattention | Combined vs. PBO | −0.03 [−0.07 – 0.01] | 39.0 | −1.6 | .113 | 0.58 |
| PEP monotherapy vs. PBO | −0.02 [−0.06 – 0.02] | 36.8 | −1.1 | .298 | 0.35 | |
| Ω3 monotherapy vs. PBO | −0.05 [−0.09 – −0.00] | 41.3 | −2.1 | .038 | 0.80 | |
| Ω3 (monotherapy/combined) vs. no Ω3 | −0.03 [−0.06 – 0.00] | 41.9 | −1.9 | .059 | 0.49 | |
| PEP (monotherapy/combined) vs. no PEP | −0.00 [−0.03 – 0.03] | 41.4 | −0.3 | .785 | 0.07 | |
| SNAP-IV H/I | Combined vs. PBO | −0.05 [−0.08 – −0.01] | 37.0 | −2.8 | .008 | 0.80 |
| PEP monotherapy vs. PBO | −0.03 [−0.06 – 0.00] | 33.4 | −1.7 | .091 | 0.45 | |
| Ω3 monotherapy vs. PBO | −0.03 [−0.07 – −0.00] | 39.3 | −2.0 | .052 | 0.59 | |
| Ω3 (monotherapy/combined) vs. no Ω3 | −0.03 [−0.05 – 0.00] | 38.2 | −2.2 | .034 | 0.44 | |
| PEP (monotherapy/combined) vs. no PEP | −0.02 [−0.04 – 0.01] | 37.8 | −1.6 | .125 | 0.33 | |
| SNAP-IV ODD | Combined vs. PBO | −0.04 [−0.09 – 0.01] | 38.9 | −1.7 | .094 | 0.70 |
| PEP monotherapy vs. PBO | −0.02 [−0.07 – 0.02] | 36.9 | −1.0 | .325 | 0.38 | |
| Ω3 monotherapy vs. PBO | 0.00 [−0.05 – 0.05] | 41.2 | 0.0 | .980 | −0.01 | |
| Ω3 (monotherapy/combined) vs. no Ω3 | −0.01 [−0.05 – 0.03] | 41.4 | −0.5 | .597 | 0.15 | |
| PEP (monotherapy/combined) vs. no PEP | −0.03 [−0.07 – 0.00] | 41.6 | −1.9 | .071 | 0.51 | |
| ECBI Problem | Combined vs. PBO | −0.36 [−0.80 – 0.09] | 64.2 | −1.6 | .115 | 0.49 |
| PEP monotherapy vs. PBO | −0.33 [−0.71 – 0.05] | 63.2 | −1.7 | .087 | 0.46 | |
| Ω3 monotherapy vs. PBO | −0.24 [−0.67 – 0.20] | 63.2 | −1.1 | .277 | 0.33 | |
| Ω3 (monotherapy/combined) vs. no Ω3 | −0.11 [−0.41 – 0.20] | 65.7 | −0.7 | .489 | 0.15 | |
| PEP (monotherapy/combined) vs. no PEP | −0.23 [−0.53 – 0.06] | 65.5 | −1.6 | .115 | 0.32 | |
| ECBI Intensity | Combined vs. PBO | −2.84 [−5.03 – −0.66] | 37.0 | −2.6 | .012 | 1.07 |
| PEP monotherapy vs. PBO | −1.80 [−3.80 – 0.20] | 34.6 | −1.8 | .077 | 0.68 | |
| Ω3 monotherapy vs. PBO | −1.73 [−4.00 – 0.53] | 37.6 | −1.5 | .130 | 0.65 | |
| Ω3 (monotherapy/combined) vs. no Ω3 | −1.28 [−2.86 – 0.29] | 38.0 | −1.7 | .106 | 0.48 | |
| PEP (monotherapy/combined) vs. no PEP | −1.46 [−3.01 – 0.11] | 38.9 | −1.9 | .066 | 0.55 | |
Note. Positive d values indicate an effect favoring the treatment group. ECBI Problem and ECBI Intensity analyses controlled for child age. Ω3=omega-3; CI=Confidence Interval; ECBI=Eyberg Child Behavior Inventory; H/I=Hyperactivity/Impulsivity subscale; PEP=Individual-Family Psychoeducational Psychotherapy; ODD=Oppositional Defiant Disorder subscale; SNAP-IV=Swanson, Nolan, and Pelham Questionnaire-IV.
Figure 1.
Plots of SNAP-IV score trajectories by treatment group. Ω3=omega-3; ODD=Oppositional Defiant Disorder; PBO=placebo; PEP=Individual-Family Psychoeducational Psychotherapy; SNAP-IV=Swanson, Nolan, and Pelham Questionnaire-IV.
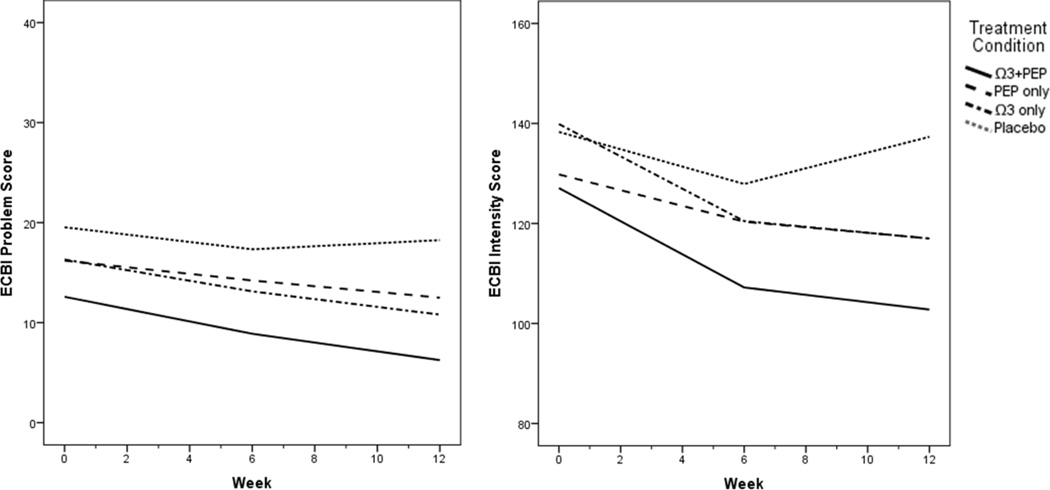
Figure 2.
Plots of ECBI score trajectories by treatment group. Ω3=omega-3; ECBI=Eyberg Child Behavior Inventory; PBO=placebo; PEP=Individual-Family Psychoeducational Psychotherapy.
LME analyses of ECBI scores controlled for child age as age significantly differed across treatment groups. Similar to the SNAP-IV outcomes, youth in combined treatment had more favorable ECBI Intensity trajectories (p=.012, d=1.07) than those in placebo alone. The effect of combined treatment on ECBI Problem scores was nonsignificant with a small-to-medium effect size (d=0.49). There were also marginal effects favoring IF-PEP monotherapy over placebo only on ECBI Problem (p=.087, d=0.46) and Intensity scales (p=.077, d=0.68).
Omega-3 vs. control
In analyses comparing all youth who received omega-3 (combined and omega-3 monotherapy pooled) to all who received placebo (IF-PEP monotherapy and placebo only pooled), omega-3 yielded more favorable trajectories than placebo on the SNAP-IV Hyperactivity/Impulsivity subscale (p=.034, d=0.44) and marginally more favorable on Total (p=.080, d=0.42), and Inattention scores (p=.059, d=0.49), but not on ODD scores. On the ECBI, there were no significant effects of pooled omega-3 versus pooled placebo groups.
IF-PEP vs. control
Youth who participated in IF-PEP (combined and IF-PEP monotherapy) compared to those who did not (omega-3 monotherapy and placebo) experienced marginally greater improvements in SNAP-IV ODD (p=.071, d=0.51) and ECBI Intensity scores (p=.066, d=0.55) but not on SNAP-IV Total scores or ADHD subscales nor on ECBI Problem scores.
Discussion
This study provides further preliminary support for the efficacy of PEP and omega-3 supplementation in treating child behavior problems among youth with a primary depression diagnosis. Specifically, there were large, significant effects of combined treatment relative to placebo on the SNAP-IV Total, Hyperactivity/Impulsivity, and ECBI Intensity scores, suggesting that combined treatment benefits severity of overall behavior problems and hyperactivity as well as the frequency of disruptive behaviors. Combined treatment also yielded a medium but nonsignificant effect on ODD symptoms. Further, youth who received omega-3 monotherapy experienced significant placebo-controlled improvement in inattention (a large effect). Although nonsignificant in the LME, IF-PEP monotherapy had small to medium effects on ECBI Intensity and Problem Scores relative to placebo.
Comparing youth who received omega-3 supplementation (omega-3 monotherapy and combined therapy groups pooled) to those who received placebo (IF-PEP monotherapy and placebo–only groups pooled), there was a significant small effect of omega-3 supplementation on hyperactivity/impulsivity as well as trending small to medium effects on inattention, overall behavior problems, and frequency of disruptive behaviors. Comparisons of both groups who received IF-PEP (monotherapy and combined groups pooled) to those who did not (omega-3 monotherapy and placebo groups pooled) indicated nonsignificant medium effects on ODD symptoms and frequency of disruptive behaviors.
Taken together, these results suggest that there are promising effects of combined PEP and omega-3 supplementation. Combination treatment yielded the most robust effects across behavioral outcomes, providing preliminary support for combining these interventions in treating the severity and frequency of behavior problems overall as well as hyperactivity/impulsivity among youth with depression. Omega-3 supplementation alone may have particular promise in alleviating inattentive symptoms among youth with depression. None of the treatments had a significant effect on SNAP-IV ODD scores or ECBI Problem scores relative to placebo, although trends were evident, with combined treatment yielding medium effects. Thus, this study offers only modest support for the efficacy of omega-3 supplementation and/or PEP in treating oppositional behaviors.
Only one previous study known to the authors has investigated combined psychosocial and biologic interventions in treating behavior problems among youth with depression. Results from TADS also found combined treatment (CBT + fluoxetine) to be superior to placebo in treating disruptive behavior in youth with depression (Jacobs et al., 2010). However, while TADS included only adolescents, the current study was comprised predominantly of children aged 12 or younger. Thus, this study added to the extant literature by providing preliminary support for combined biological and psychosocial treatment for behavior problems among a sample of depressed youth that included school-aged children and by showing this for omega-3 fatty acids as the biological treatment rather than a prescription drug.
Limitations and Future Directions
Because the outcome measures of interest were added mid-trial, only 67% and 53% of participants completed the SNAP-IV and ECBI, respectively, resulting in a small sample size that limited power to detect treatment effects. While ADHD was common among the subsample included in the current analyses, there was a lower rate of ODD, conduct disorder, and DBD not otherwise specified among youth who had behavior outcomes data compared to those who did not. Only 17% and 18% of youth included in SNAP-IV and ECBI analyses, respectively, had a pre-randomization diagnosis of ODD, conduct disorder, or DBD not otherwise specified. Thus, the low incidence of clinically impairing problems (other than ADHD) may have prevented detection of significant treatment effects of treatment in this exploratory pilot study with small sample sizes. Nevertheless, large and significant effects were detected for combined treatment. Another limitation is that participants’ depression symptoms were mild; thus, results may not be generalizable to youth with severe depression. However, results were consistent with previous research demonstrating that CBT (Boylan et al., 2013; Smeets et al., 2015; Sukhodolsky et al., 2004) and omega-3 supplementation (Bos et al., 2015; Raine et al., 2015; Sonuga-Barke et al., 2013; Tammam et al., 2016) are efficacious for behavior problems outside the context of a primary mood disorder, suggesting that the results presented here may generalize to youth without a mood disorder. Intervention lasted only 12 weeks; future studies should address whether benefits of combined treatment (or monotherapies) are maintained or increased over a longer treatment or post-treatment follow-up. The sample size did not allow for moderator analyses. Identification of moderators of treatment effects on disruptive behaviors in a larger study will allow for more individualized treatment planning. Additionally, future examination of the relative performance of IF-PEP and omega-3 monotherapies could provide further guidance to clinicians. The fact that all participants had a daily multi-vitamin/mineral supplement poses two limitations: one is that the results may not generalize to patients suffering deficient micronutrient intake; the other is that insofar as the vitamin/mineral supplement itself contributed to improvement in the placebo-alone group, the superiority of the active treatments may have been partly obscured. Lastly, as the only study known to the authors to investigate the combination of these interventions, replication in a larger study will provide further clarity regarding the clinical implications of these findings.
Conclusions
This study provides preliminary support for the efficacy of combined IF-PEP and omega-3 supplementation for behavior problems in children and adolescents with depression. A large multi-site RCT is warranted.
Acknowledgments
This research was supported by National Institute of Mental Health award# R34 MH85875; the content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors would like to thank staff who collected data and provided therapy, families who participated, and OmegaBrite, who provided study capsules.
Disclosures: Dr. Young has received research funding from Psychnostics, LLC. Dr. Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, Supernus, and YoungLiving (as well as NIH and Autism Speaks) and has consulted with or been on advisory boards for Arbor, Gowlings, Ironshore, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, Tris Pharma, and Waypoint. Dr. Fristad receives royalties from Guilford Press, American Psychiatric Press and CFPSI for treatment manuals, workbooks and a diagnostic instrument reported on in this study and honoraria from Physician’s Post-Graduate Press and the American Occupational Therapy Association.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent/assent was obtained from all individual participants included in the study.
References
- Ahn J, Ahn HS, Cheong JH, dela Pena I. Natural product-derived treatments for attention-deficit/hyperactivity disorder: Safety, efficacy, and therapeutic potential of combination therapy. Neural Plasticity, 2016. 2016 doi: 10.1155/2016/1320423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini PJ, Metz C, Prabucki K, Lee J-C. Videotape reliability of the third revised edition of the K-SADS. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th. Washington, DC: American Psychiatric Association; 2000. text revision ed. [Google Scholar]
- Angold A, Costello EJ. Depressive comorbidity in children and adolescents: Empirical, theoretical, and methodological issues. American Journal of Psychiatry. 1993;150:1779–1791. doi: 10.1176/ajp.150.12.1779. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Gordon M. Cognitive behavioral treatment outcomes in adolescent ADHD. Focus. 2012;10:334–345. doi: 10.1177/1087054712443155. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child and Adolescent Psychiatric Clinics of North America. 2002;11:619–637. doi: 10.1016/s1056-4993(02)00011-1. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent D the AACAPWork Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: Systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos DJ, Oranje B, Veerhoek ES, Van Diepen RM, Weusten JM, Demmelmair H, Durston S. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2015;40:2298–2306. doi: 10.1038/npp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan K, MacPherson HA, Fristad MA. Examination of disruptive behavior outcomes and moderation in a randomized psychotherapy trial for mood disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:699–708. doi: 10.1016/j.jaac.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Bussing R, Fernandez M, Harwood M, Hou W, Garvan CW, Eyberg SM, Swanson JM. Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms psychometric properties and normative ratings from a school district sample. Assessment. 2008;15:317–328. doi: 10.1177/1073191107313888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview: Test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive-behavioral treatment of adolescent depression: Efficacy of acute group treatment and booster sessions. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:272–279. doi: 10.1097/00004583-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. European Journal of Clinical Nutrition. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? Journal of Child Psychology and Psychiatry. 2006;47:1263–1271. doi: 10.1111/j.1469-7610.2006.01682.x. [DOI] [PubMed] [Google Scholar]
- Diamond GS, Reis BF, Diamond GM, Siqueland L, Isaacs L. Attachment-based family therapy for depressed adolescents: A treatment development study. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1190–1196. doi: 10.1097/00004583-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Eyberg SM, Pincus D. Eyberg Child Behavior Inventory & Sutter-Eyberg Student Behavior Inventory-Revised: Professional manual. Odessa, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- Eyberg SM, Ross AW. Assessment of child behavior problems: The validation of a new inventory. Journal of Clinical Child Psychology. 1978;7:113–116. [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad MA, Goldberg-Arnold JS, Leffler JM. Psychotherapy for children with bipolar and depressive disorders. New York, NY: Guilford Press; 2011. [Google Scholar]
- Fristad MA, Verducci JS, Walters K, Young ME. Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Archives of General Psychiatry. 2009;66:1013–1021. doi: 10.1001/archgenpsychiatry.2009.112. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Vesco AT, Young AS, Healy KZ, Nader ES, Gardner W, Arnold LE. Pilot RCT for children and adolescents with depression: Responders to omega-3 and Individual-Family Psychoeducational Psychotherapy. Manuscript submitted for review. in review doi: 10.1080/15374416.2016.1233500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad MA, Young AS, Vesco AT, Nader ES, Healy KZ, Gardner W, Arnold LE. A randomized controlled trial of Individual Family Psychoeducational Psychotherapy & omega-3 fatty acids in youth with subsyndromal bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2015;25:764–774. doi: 10.1089/cap.2015.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies D, Sinn J, Lad SS, Leach MJ, Ross MJ. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database of Systematic Reviews. 2012;7:CD007986. doi: 10.1002/14651858.CD007986.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Becker-Weidman EG, Reinecke MA, Jordan N, Silva SG, Rohde P, March JS. Treating depression and oppositional behavior in adolescents. Journal of Clinical Child and Adolescent Psychology. 2010;39:559–567. doi: 10.1080/15374416.2010.486318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knapp M, McCrone P, Fombonne E, Beecham J, Wostear G. The Maudsley long-term follow-up of child and adolescent depression 3. Impact of comorbid conduct disorder on service use and costs in adulthood. British Journal of Psychiatry. 2002;180:19–23. doi: 10.1192/bjp.180.1.19. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Presentation and course of major depressive disorder during childhood and later years of the life span. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:705–715. doi: 10.1097/00004583-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Hops H, Andrews J. Cognitive-behavioral treatment for depressed adolescents. Behavior Therapy. 1990;21:385–401. [Google Scholar]
- Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: A controlled, double-blind pilot study. American Journal of Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- Owen C, Rees A-M, Parker G. The role of fatty acids in the development and treatment of mood disorders. Current Opinion in Psychiatry. 2008;21:19–24. doi: 10.1097/YCO.0b013e3282f29841. [DOI] [PubMed] [Google Scholar]
- Pliszka S AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Raine A, Portnoy J, Liu J, Mahoomed T, Hibbeln JR. Reduction in behavior problems with omega-3 supplementation in children aged 8–16 years: A randomized, double-blind, placebo-controlled, stratified, parallel-group trial. Journal of Child Psychology and Psychiatry. 2015;56:509–520. doi: 10.1111/jcpp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AJ. The importance of omega-3 fatty acids for behaviour, cognition and mood. Scandinavian Journal of Nutrition. 2003;47:92–98. [Google Scholar]
- Rohde P, Clarke GN, Lewinsohn PM, Seeley JR, Kaufman NK. Impact of comorbidity on a cognitive-behavioral group treatment for adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:795–802. doi: 10.1097/00004583-200107000-00014. [DOI] [PubMed] [Google Scholar]
- Smeets KC, Leeijen AA, van Molender Molen MJ, Scheepers FE, Buitelaar JK, Rommelse NN. Treatment moderators of cognitive behavior therapy to reduce aggressive behavior: A meta-analysis. European Child & Adolescent Psychiatry. 2015;24:255–264. doi: 10.1007/s00787-014-0592-1. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M European ADHD: Guidelines Group. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. American Journal of Psychiatry. 2013;170:275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- Steiner H, Remsing L AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:126–141. doi: 10.1097/01.chi.0000246060.62706.af. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Kassinove H, Gorman BS. Cognitive-behavioral therapy for anger in children and adolescents: A meta-analysis. Aggression and Violent Behavior. 2004;9:247–269. [Google Scholar]
- Swanson JM. School-based assessments and interventions for ADD students. Irvine, CA: KC Publishing; 1992. [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, Wu M. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- TADS Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for adolescents with depression study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Tammam JD, Steinsaltz D, Bester DW, Semb-Andenaes T, Stein JF. A randomised double-blind placebo-controlled trial investigating the behavioural effects of vitamin, mineral and n-3 fatty acid supplementation in typically developing adolescent schoolchildren. British Journal of Nutrition. 2016;115:361–373. doi: 10.1017/S0007114515004390. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. FDA launches a multi-pronged strategy to strengthen safeguards for children treated with antidepressant medications. Silver Spring, MD: U.S. Food and Drug Administration; 2004. [Google Scholar]
- Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: A meta-analysis. Psychological Bulletin. 2006;132:132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]