Abstract
Considerable evidence suggests that the steroid hormone testosterone mediates major life-history trade-offs in vertebrates, promoting mating effort at the expense of parenting effort or survival. Observations from a range of wild primates support the “Challenge Hypothesis,” which posits that variation in male testosterone is more closely associated with aggressive mating competition than with reproductive physiology. In both seasonally and non-seasonally breeding species, males increase testosterone production primarily when competing for fecund females. In species where males compete to maintain long-term access to females, testosterone increases when males are threatened with losing access to females, rather than during mating periods. And when male status is linked to mating success, and dependent on aggression, high-ranking males normally maintain higher testosterone levels than subordinates, particularly when dominance hierarchies are unstable. Trade-offs between parenting effort and mating effort appear to be weak in most primates, because direct investment in the form of infant transport and provisioning is rare. Instead, infant protection is the primary form of paternal investment in the order. Testosterone does not inhibit this form of investment, which relies on male aggression. Testosterone has a wide range of effects in primates that plausibly function to support male competitive behavior. These include psychological effects related to dominance striving, analgesic effects, and effects on the development and maintenance of the armaments and adornments that males employ in mating competition.
Introduction
All organisms must manage fundamental trade-offs between growth, maintenance, and reproduction, in order to maximize fitness in changing environments. The endocrine system plays a key role in mediating life-history strategies, such as investment in current versus future reproduction, by coordinating morphological, physiological, and behavioral responses to environmental factors, including energy availability and social context (e.g. Ellison 2003, Stearns 1989, Wingfield et al. 2000, Zera & Harshman 2001). Substantial evidence suggests that the primary role of the steroid hormone testosterone is to promote reproductive effort, at the expense of long-term survival (Hau 2007; Ketterson & Nolan 1992, 1999). For example, testosterone drives the process of male genital development and supports sperm production (Dixson 2012, Weinbauer et al. 2004). It also spurs development of the secondary sexual characteristics that males use to compete with rivals and attract mates, including sexually dimorphic musculature (Bribiescas 2001, Kemnitz et al. 1988, Setchell et al. 2008). Finally, testosterone supports behavioral aspects of mating effort, by promoting both libido and aggression in reproductive contexts (Archer 2006, Dixson 2012, Isidori et al. 2005). Testosterone can adversely impact survival by increasing metabolic rate, exposure to predators, and risk of injury, and potentially by suppressing some aspects of immune function (Hilgarth & Wingfield 1997, Roberts et al. 2004, Wingfield et al. 2001).
Reproductive effort comprises both parenting effort, which is investment in offspring, and mating effort, which is investment in male-male competition and mate attraction. Elevated levels of testosterone predictably increase mating effort in vertebrates, and actively suppress parental activities in many species (Ketterson & Nolan 1999). Trade-offs between mating and parenting effort are widespread, as time and energy expended on male-male competition cannot normally be invested in offspring (Magrath & Komdeur 2003, Trivers 1972). As we shall see, however, not all forms of parenting are incompatible with mating effort (Stiver & Alonzo 2009). Silverback gorillas, for example, protect their offspring from infanticidal males, a form of paternal care that simultaneously functions as mating effort, since females abandon males who are ineffective defenders (Watts 1989). Testosterone is not expected to interfere with this type of investment.
The importance of testosterone in facilitating reproductive aggression is emphasized by the “challenge hypothesis,” which was originally formulated to explain both interspecific and individual differences in testosterone production by seasonally breeding birds (Wingfield et al. 1990). At the individual level, circulating testosterone in birds increases slightly during the breeding season, supporting spermatogenesis, sexual interest, and courtship behaviors. Following this initial rise, testosterone levels show transient surges to much higher concentrations during periods of social instability and heightened male aggression - “especially when establishing a territory, when being challenged by another male, or when mate guarding” (Wingfield et al. 2006:192). Under stable social conditions, and particularly when males are engaging in paternal care, testosterone levels are reduced back to baseline. Data from more than 80 bird species are consistent with these predictions (Hirschenhauser et al. 2003, Wingfield et al. 2000). Experimental manipulations in a range of birds have confirmed that high levels of testosterone often suppress parental behavior (De Ridder et al. 2000, Hegner & Wingfield 1987, Peters et al. 2002, Schoech et al. 1998, Stoehr & Hill 2000, Van Roo 2004), though not always (Lynn et al. 2002, 2005; Van Duyse et al. 2000).
At the species level, the challenge hypothesis predicts that the magnitude of the testosterone response to social instability is determined by mating system (specifically the form of male reproductive competition) and breeding strategy (specifically the form of male paternal care; Wingfield et al. 1990, 2000). Monogamous species in which males care for young are expected to show the largest increases in testosterone when challenges occur, because they normally maintain levels near the breeding baseline to avoid disrupting care. Polygynous species, by contrast, maintain elevated testosterone throughout the breeding season, because males provide less paternal care and face continual challenges. Their ability to elevate testosterone in response to acute challenges should thus be highly constrained. Again, these predictions are generally upheld in a wide range of birds (Hirschenhauser et al. 2003, Wingfield et al. 2000).
Such interspecific comparisons are complicated, however, by the difficulty of monitoring short-term changes in testosterone (Goymann et al. 2007, Goymann 2009). Acute responses are sometimes assessed experimentally, through simulated territorial intrusions employing decoys (Goymann et al. 2007, Landys et al. 2007). In most studies, though, the response is estimated as the difference between the breeding baseline and the maximum levels observed during the breeding season. When these experimental and observational measures are both available, they are not correlated, across species (Goymann 2009).
For primates in the wild, experimental data comparable to those from the bird literature are currently lacking. The ease of resampling from known individuals, however, means that longitudinal hormone data can often be collected in reproductive and non-reproductive contexts, as well as with changes in social status that influence reproductive opportunities. In this paper I review the relationship between testosterone and mating effort in primates, with specific emphasis on the ways in which testosterone promotes male-male competition. I also explore the effects of testosterone on sexual behavior and social signaling. The emphasis throughout is on wild studies, as captivity can have large but unpredictable effects on both behavior and hormone production (e.g. Muller & Wrangham 2005). Captive data are discussed, however, when relevant data from the wild are unavailable.
The challenge hypothesis and mating competition in primates
Unlike birds, many primates are not seasonal breeders, and most do not engage in direct paternal care. Consequently, the predictions of the challenge hypothesis must be adapted for novel mating and breeding systems (e.g. Muller & Wrangham 2001, 2004). The underlying logic is that testosterone supports competitive behavior while imposing a range of predictable costs that animals are better off avoiding. Thus, elevated levels of testosterone are only expected during periods of social instability and heightened aggression. As with birds, aggression in primates is expected to intensify when males are defending territories (or other resources critical for reproduction), being directly challenged by other males, or guarding mates. Mating itself is not expected to cause substantial elevations in testosterone, unless accompanied by an increased risk of aggression.
Taking primate social and mating systems into account, the challenge hypothesis suggests multiple predictions. (1) In seasonally breeding primates, males should increase testosterone production during the mating season. These increases should be associated with male mating competition, rather than mating itself. (2) In non-seasonal breeders, males should increase testosterone production when competing for fecund females, and more intense competition should result in larger increases. Again, the association should be with increased aggression, rather than mating. (3) When little aggression occurs in mating contexts, but males compete aggressively to maintain long-term access to females (e.g. in species living in one-male groups), testosterone should increase primarily when males are threatened with losing access to females, rather than during mating periods. (4) When male status is importantly associated with mating access, and dependent on aggression, high-ranking males should maintain higher testosterone levels than subordinates, particularly when dominance ranks are unstable, and being contested. (5) In territorial species, where territory size is importantly linked to reproduction, testosterone should increase during episodes of intergroup conflict. (6) From a life history perspective, males should show elevated testosterone during the parts of the lifespan in which they are competing most intensely for reproductive opportunities.
Prediction 1: Mating season increases in testosterone
Consistent with prediction 1, multiple studies of seasonally breeding primates report higher testosterone levels in the mating season than the non-mating season. These include three lemurs (Lemur catta: Cavigelli & Pereira 2000, Gould & Ziegler 2007; Propithecus verreauxi: Brockman et al. 2001; Eulemur fulvus: Ostner et al. 2002, 2008), golden lion tamarins (Leontopithecus rosalia: Bales et al. 2006), tufted capuchins (Cebus apella: Lynch et al. 2002), and four macaque species (Tibetan, Macaca thibetana: Xia et al. 2015; long-tailed, Macaca fascicularis: Girard-Buttoz et al. 2009, 2015; rhesus, Macaca mulatta: Mehlman et al. 1997, Higham et al. 2013a; Assamese, Macaca assamensis: Ostner et al. 2011). In two additional species - muriquis (Brachyteles arachnoides: Strier et al. 1999) and moustached tamarins (Saguinus mystax: Huck et al. 2005) – males did not increase testosterone during the mating season.
Are mating season rises in testosterone linked specifically to male mating competition? Unfortunately, some of the studies referenced above lack the behavioral data necessary to answer this question. For the remaining studies, results are mixed. Consistent with prediction 1, the two species that failed to show testosterone increases during the mating season, muriquis and moustached tamarins, also showed little or no aggression during that season (Huck et al. 2005, Strier et al. 1999). Further supporting prediction 1, ring-tailed lemurs, Assamese macaques, rhesus macaques, and long-tailed macaques all showed substantial increases in aggression during the mating season (Cavigelli & Pereira 2000, Gould & Ziegler 2007, Girard-Buttoz et al. 2015, Mehlman 1997, Ostner et al. 2011). For ring-tailed lemurs and Assamese macaques, individual rates of aggression and testosterone levels were correlated over the mating period (Cavigelli & Pereira 2000, Ostner et al. 2011). In long-tailed macaques, time spent mate-guarding, but not aggression, correlated positively with male androgen levels (Girard-Buttoz et al. 2015).
Contrary to prediction 1, three species – sifakas, redfronted lemurs, and tufted capuchins - showed elevated testosterone during the mating season without a corresponding increase in aggressive behavior (Brockman et al. 2001; Lynch et al. 2002, Ostner et al. 2002, 2008). Redfronted lemurs exhibited the most unusual pattern, with testosterone levels in the mating season higher than pre- and post-mating periods, but lower than levels in the birth season. The authors attribute this pattern to the need for males to defend their offspring from infanticide during the birth season, a possibility that is discussed further below (Ostner et al. 2002, 2008).
Across these studies, investigators considered a broad range of “non-mating” periods when making comparisons with the mating season. These included the birth season, the period of lactation, the pre-mating period, and the post-mating period. How such choices influence the overall pattern of findings is not clear.
Prediction 2: Testosterone and mating competition in non-seasonal breeders
In many primates, the availability of cycling females is unpredictable, and males likely maintain breeding levels of testosterone throughout the year (e.g. Muller & Wrangham 2004). In these species, testosterone levels are predicted to rise when males are competing over cycling females. More intense competition should produce greater increases. Because males compete most intensely over the most fecund females (i.e. those with the highest probability of successful conception), testosterone increases are expected to correlate positively with female fecundity.
Consistent with prediction 2, male gray-cheeked mangabeys (Lophocebus albigena: Arlet et al. 2011), white-faced capuchins (Cebus capucinus: Schoof et al. 2014), and chimpanzees (Pan troglodytes: Muller & Wrangham 2004, Sobolewski et al. 2013) all showed elevated testosterone levels in the presence of periovulatory females (i.e. those most likely to conceive). In the same context, male chimpanzees and gray-cheeked mangabeys (Arlet et al. 2009) showed increased rates of aggression. Comparable data are not available for white-faced capuchins, though direct aggression over mating opportunities by males within groups is characterized as rare in this species (Jack & Fedigan 2005, Schoof et al. 2014).
Female bonobos (Pan paniscus) sometimes cycle for years before successful conception. In the presence of “potentially fertile females” (defined as estrous females in the 6 months prior to conception), however, male bonobos showed elevated levels of urinary testosterone (Surbeck et al. 2012). During the same periods they also showed increased rates of male-male aggression. The males that showed the largest testosterone increases, however, also showed the lowest mating rates. Additionally, high testosterone males showed lower rates of grooming with females. The authors suggest that in bonobos male-female affiliation, rather than male-male competition, is paramount in male mating success, and that testosterone may interfere with such affiliation. If true, this raises an interesting question of why low-ranking males increase testosterone production in this context.
The evidence that elevated testosterone is associated with male competition, rather than simply mating, is compelling for chimpanzees. Although males in Kanyawara (Kibale National Park, Uganda) mated with mothers and nulliparas at similar rates, mothers were more attractive, and generated more intense mating competition (Muller et al. 2006). Accordingly, males showed testosterone increases in the presence of estrous mothers, but not with nulliparous females (Muller & Wrangham 2004). This finding was replicated in a second community, also in Kibale National Park, at Ngogo (Sobolewski et al. 2013).
Studies of male baboons have focused on testosterone production during consortships, defined as periods when males mate-guard estrous females, maintaining close proximity and exclusive mating access (Seyfarth 1978). Yellow baboon (Papio cynocephalus: Onyango et al. 2013) males had higher levels of fecal testosterone in months when they obtained consortships than those in which they did not. Chacma baboon males (Papio ursinus) similarly increased testosterone production during consortships (Beehner et al. 2006, Kalbitzer et al. 2015), but this was not true for Guinea baboons (Papio papio: Kalbitzer et al. 2015). Behavioral differences among these species are consistent with prediction 2, as consortships in Guinea baboons are not distinguished by the elevated levels of aggression that characterize Chacma and yellow baboon mate guarding (Bergman et al. 2005, Kalbitzer et al. 2015).
Prediction 3: Reproductive challenges outside of the mating context
In some primates, the most important reproductive challenges faced by males occur outside of the mating context. These include species living in one-male groups, as well as groups that have strong alpha males who monopolize reproduction when females are receptive. In such species, testosterone is expected to increase when the stability of established groups is threatened, especially by outside males who lack access to females.
Consistent with prediction 3, in multiple species, established males show elevated testosterone production, along with increased rates of aggression, when extragroup males attempt to join or take over a group. These include mantled howler monkeys (Alouatta palliata: Cristóbal-Azkarate et al. 2006), white-faced capuchins (Schoof & Jack 2013), ursine colobus monkeys (Colobus velerosus: Teichroeb & Sicotte 2008), gelada baboons (Theropithecus gelada: Pappano & Beehner 2014), and siamangs (Symphalangus syndactylus: Morino 2015). A similar pattern was documented experimentally in captive marmosets (Callithrix kuhlii: Ross et al. 2004). When resident male marmosets were exposed to unfamiliar intruders, their urinary testosterone levels increased in direct proportion to the amount of aggression directed at the intruder.
Black howler monkey males (Alouatta pigra), by contrast, showed no significant effect of either male immigration or encounters with extragroup males on testosterone production (Van Belle et al. 2009). Rangel-Negrín and colleagues (2011), however, reported that black howler males living in unimale groups had elevated testosterone levels, particularly after transitioning from multimale composition. They note that in black howlers, males in unimale groups are the most likely to be challenged and evicted by extragroup males. Thus, males living in multi-male groups are less likely to perceive encounters with extragroup males as salient challenges.
Prediction 4: Testosterone and male dominance rank
The logic of the challenge hypothesis suggests that positive associations between testosterone and male status should primarily be found when rank is (1) predictive of mating access, (2) maintained by aggression, and (3) unsettled (Muller & Wrangham 2004, Sapolsky 1993, Whitten 2000). When high-ranking males are not obliged to be more aggressive than others - either because the hierarchy is stable, or aggression does not influence mating success - then they should moderate testosterone production to avoid unnecessary costs.
In a number of primates, dominant males appear to consistently maintain higher testosterone levels than subordinates. These include sifakas (Brockman et al. 2001, Kraus et al. 1999), white-faced capuchins (Schoof et al. 2014, 2016), gray-cheeked mangabeys (Arelt et al. 2011), long-tailed macaques (Girard-Buttoz et al. 2015), mandrills (Mandrillus sphinx: Setchell et al. 2008, Wickings & Dixson 1992), mountain gorillas (Robbins & Czekala 1997), and orangutans (Marty et al. 2015). In these species, high-ranking males are generally able to monopolize estrous females, particularly during the periovulatory period. As a consequence, they are likely to face chronic challenges. By contrast, persistent differences in testosterone production between high and low ranking males were not detected in studies of redfronted lemurs (Ostner et al. 2002, 2008), Assamese macaques (Ostner et al. 2011), and tufted capuchins (Lynch et al. 2002). Consistent with prediction 4, in these species, high-ranking males were not able to monopolize females, and mating success was similar for high- and low-ranking males.
Multiple studies have found an effect of dominance instability on the relationship between testosterone and male rank. In rhesus macaques (Higham et al. 2013a), chacma baboons (Beehner et al. 2006), and olive baboons (Papio anubis: Sapolsky 1983, 1993), testosterone and rank were unrelated, except during periods of instability, when high-ranking individuals had the highest levels of testosterone. In sifakas (Brockman et al. 2001) and bearded capuchins (Sapajus libidinosus: Mendonca-Furtado et al. 2014), dominant males residing in unstable groups had significantly higher testosterone levels than those living in stable groups. And in golden lion tamarins, dominant males maintained elevated androgen levels in the presence of unrelated subordinate males, but not when subordinates were relatives (Bales et al. 2006). Consistent with prediction 4, males had less stable relationships with non-relatives. No correlation was found between testosterone and dominance rank in ursine colobus monkeys during a period of rank stability (Teichroeb & Sicotte 2008).
In wild chimpanzees, positive associations between dominance rank and testosterone production have been found in some studies (Muehlenbein et al. 2004, Muller & Wrangham 2004), but not others (Sobolewski et al. 2013). The same is true for bonobos (cf. Marshall & Hohmann 2005, Surbeck et al. 2012). Whether this variation is explained by rank stability is not yet known.
In chacma baboons, Beehner et al. (2006) found that males rising in rank had higher testosterone than males falling in rank, and that testosterone was predictive of future rank. This is consistent with the idea that testosterone facilitates aggressive behaviors that subsequently enhance status. Evidence that testosterone rises or falls in response to success or failure in competitive interactions is discussed below.
Prediction 5: Testosterone and territoriality
Testosterone supports male territorial behavior in a number of birds, lizards, and mammals, when acquisition or expansion of a territory increases reproductive opportunities (e.g. Bartsh et al. 1992, Sinervo et al. 2000, Wingfield 1994). In one study of wild chimpanzees, males showed elevated testosterone levels prior to conducting territorial boundary patrols, and levels remained high during and immediately after patrols compared to control days (Sobolewski et al. 2012). In chimpanzees, territorial expansion increases male reproductive opportunities by shortening female birth intervals (Williams et al. 2004). These findings are consistent with prediction 5, but relevant data from other primates are lacking.
Prediction 6: Testosterone and male life history
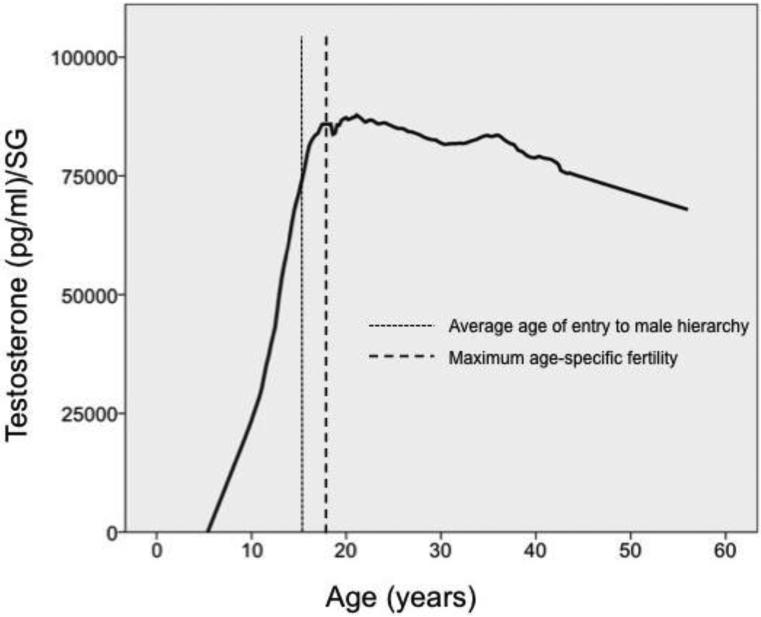
From a life history perspective, the challenge hypothesis suggests that males should maintain elevated testosterone during parts of the lifespan in which they are competing most intensely for reproductive opportunities, with reduced levels during periods of development and senescence. This prediction is supported by observations of chacma, yellow, and gelada baboons, which all showed peak levels of testosterone at the age of first sexual consortship, and diminished production later in life, as mating access also declined (Beehner et al. 2009). Testosterone senescence occurred at a later age in yellow baboons, consistent with the fact that older, low-ranking males in that species enjoyed greater mating access than equivalent chacma or gelada males. In chimpanzees, males attain adult levels of testosterone production around age 15-16, which coincides with the period when they first challenge adult males for status (Figure 1). Peak testosterone levels occur around the age of maximum reproductive output, 17-18 (Muller et al. 2016a,b). For chimpanzee males, a decline in testosterone production with advanced age is detectable, but both it and the age-specific decline in fertility are less steep than those in baboons (ibid.).
Figure 1.
Testosterone, age, and reproductive effort in wild chimpanzees (Kanyawara, Kibale National Park). The trend line is a LOESS curve through 138 yearly means from 20 unique males. Each mean included a minimum of 20 observations from the male at that age (n=6692 total urine samples). Specific gravity (SG) is used to correct for urinary dilution. Point of entry in the adult male hierarchy represents the age that a male was first seen to win an aggressive interaction with an adult male.
Predictions 1-6: Conclusions
Evidence from a broad range of primates thus supports the idea that testosterone is commonly elevated when males face reproductive challenges that require an aggressive response. The precise nature of such challenges is driven by the social and mating system. In species where male reproductive competition commonly occurs in mating contexts, testosterone rises during mating periods. In species where male-male aggression is less important in mating contexts, the relationship between testosterone and mating activity is less consistent.
Is some of the testosterone produced by males in mating contexts necessary to support spermatogenesis and sexual motivation? Although these are not well studied in the wild, captive data suggest that relatively low concentrations of circulating testosterone are generally adequate to maintain male reproductive function (Dixson 2012; for humans see Christiansen 2004, Weinbauer et al. 2004). Researchers consistently report, for example, that intact rhesus macaque males exposed to receptive females outside of the breeding season immediately exhibit normal sexual behavior; sustained increases in circulating testosterone occur subsequently (Bernstein et al. 1983, Michael & Zumpe 1993, Ruiz de Elvira et al. 1982). Castrated rhesus, by contrast, show a gradual decline in sexual behavior over time (reviewed in Dixson 2012). The evidence reviewed above is consistent with this kind of threshold effect being common. Muriquis, for example, exhibit extreme sperm competition, but fail to increase testosterone substantially during the mating season, during which they show little or no direct competition over access to females (Strier et al. 1999).
Testosterone and trade-offs between mating effort and paternal care
In a wide range of vertebrates, including birds, fish, anurans, and mammals (reviewed in Bales & Saltzman 2016; Lynn 2008, 2016; Marler et al. 2003; Wynne-Edwards 2001), associations between testosterone and paternal care are generally negative. In many species, experimental administration of testosterone actively suppresses paternal behavior (Clark & Galef 1999; Lynn 2008, 2016). There are exceptions to this pattern, however, and they occur in predictable contexts. First, in species where paternal care is critical for male reproductive success, males can become insensitive to testosterone during periods of caregiving (Lynn 2008). Second, in species that must exhibit aggressive behavior and paternal care more or less simultaneously, paternal behavior may not be adversely impacted by testosterone (Marler et al. 2003, Trainor & Marler 2001). In some cases paternal behavior may even be promoted by testosterone, after its conversion to estrogen (Trainor & Marler 2002).
The challenge hypothesis was originally formulated for seasonally breeding birds. In many of these species, males invest heavily in parenting effort, both by incubating eggs and provisioning offspring. These activities directly conflict with mating effort, as time and energy devoted to them cannot be used to support, for example, territorial or courtship displays. Trade-offs between mating and parenting effort are not universal, however (reviewed in Stiver & Alonzo 2009). Some forms of mating effort involve behaviors that are compatible with parenting, and when this is true, elevated testosterone production is not expected to suppress paternal behavior. Once again, predictions of the challenge hypothesis must be adapted to fit primate mating and breeding systems (e.g. Salzman & Ziegler 2014).
Only a few primates invest in costly direct paternal care, such as carrying and provisioning offspring (Fernandez-Duque et al. 2009, Muller & Emery Thompson 2012). Among those that do, hormone data from the wild are rare. A clear trade-off between mating effort and parenting effort has been identified in black tufted-ear marmoset fathers (Callithrix kuhlii), who both carry infants and share food with them (Bales & Jarcho 2013, Storey & Ziegler 2016). In captive animals, significant declines in testosterone were observed 3-4 weeks following parturition, coinciding with the peak rate of infant carrying by fathers (Nunes et al. 2000). This effect was most pronounced in experienced fathers. Furthermore, across fathers, testosterone was negatively correlated with infant carrying (Nunes et al. 2001). Direct contact with infants is thought to be responsible for this pattern, as captive common marmosets (Callithrix jacchus) showed a drop in serum testosterone levels within 20 minutes of exposure to the scent of their infants (Prudom et al. 2008). Such detailed data are not available for golden lion tamarins, but wild males in that species are highly solicitous of infants, and maintained lower levels of testosterone during the birth and infant care season than the mating season (Bales et al. 2006).
Although cotton-top tamarin (Saguinus oedipus) fathers also carry infants and share food with them (Bales & Jarcho 2013), in captivity they increased testosterone production just prior to the birth of their infants (Ziegler et al. 2004). A similar pattern was observed in a wild population of moustached tamarins (Huck et al. 2005). Because tamarin females show postpartum ovulation, this likely represents a situation where males must be prepared to engage in paternal care and mating competition simultaneously (Storey & Ziegler 2016). Evidence of a trade-off remains, however, as captive cotton-top tamarin males without infants increased testosterone production when exposed to the scent of a periovulatory female, whereas fathers did not (Ziegler et al. 2005).
In humans, considerable evidence supports a trade-off between mating and parenting effort, partly mediated by testosterone (reviewed in Gettler 2014, Gray & Anderson 2010, Roney & Gettler 2015, Storey & Ziegler 2016). For example, longitudinal studies reported that invested fathers decreased testosterone levels near parturition (Berg & Wynne-Edwards 2001, Storey et al. 2000). And a series of cross-sectional studies have variously shown that men in committed, long-term relationships, fathers, or both maintain lower levels of testosterone than unpaired men, or married men pursuing additional mates (e.g. Alvergne et al. 1999, Burnham et al. 2003, Gray & Campbell 2009, Gray et al. 2002, 2006, 2007ab; Hooper et al. 2011, Kuzawa et al. 2009, Muller et al. 2009). A longitudinal study from the Philippines demonstrated that this relationship likely results from men muting testosterone production in response to marriage and fatherhood, rather than low-testosterone men being more likely to marry and have children (Gettler et al. 2011). In various studies, men with elevated testosterone were found to be less responsive and less sympathetic to infant cries (Fleming et al. 2002), less interested in infants or infant stimuli (Roney et al. 2006, Storey et al. 2000, Weisman et al. 2014), and less involved with families and parenting (Alvergne et al. 2009, Mascaro et al. 2013). Further evidence for a link between androgens and men's mating effort comes from studies showing a positive association between testosterone and interest in extra-pair mating (Edelstein et al. 2011, McIntyre et al. 2006), incidence of extra-marital affairs (Booth & Dabbs 1993), and number of sexual partners (Bogaert & Fisher 1995, Peters et al. 2008, Pollet et al. 2011, van Anders et al. 2007). Finally, men who reported greater “interest in babies” had lower testosterone reactivity to cues of short-term mating (Zilioli et al. 2015).
In most primates the predominant form of direct paternal investment is not infant transport or provisioning, but physical protection – either from infanticide or predation (Muller & Emery Thompson 2012). Infanticide by male conspecifics is an important source of mortality in many species, particularly those living in one-male groups or groups with high reproductive skew (Palombit 2012). Because of their small body size, infants are also presumed to be at greater risk of predation than adults (Cheney & Wrangham 1987), though data are lacking for most species (for red colobus monkeys, Colobus badius, see Stanford 1998).
Male investment in infant defense is unlikely to conflict with mating effort for at least two reasons. First, there is some evidence that females in species at high risk for infanticide either abandon males who are unsuccessful at defending infants, or form preferential bonds with those who are effective defenders (Palombit 2009, 2012; Sterck & Korstjens 2000; Watts 1989). Consequently, male protection can simultaneously function as both mating and parenting effort (see Smuts & Gubernick 1992, van Schaik & Paul 1998). Second, substantial evidence supports the idea that testosterone facilitates aggressive male competition (reviewed below). Consequently, when paternal investment takes the form of aggression, testosterone should support that investment, rather than compromising it.
In several primates at high risk of infanticide, males show elevated testosterone in the period around parturition, when offspring are most vulnerable. These include redfronted lemurs (Ostner et al. 2002, 2008), sifakas (Brockman et al. 2001), and ursine colobus monkeys (Teichroeb & Sicotte 2008). Such increases have been argued to support aggression in the context of infant protection. A similar dynamic has been established experimentally in fish that exhibit paternal care, with androgens promoting male nest defense from brood predators (e.g. Desjardins et al. 2008, Dey et al. 2010).
Multiple experimental studies have found that men respond to the sound of infant cries by increasing testosterone production (Fleming et al. 2002; Storey et al. 2000; van Anders et al. 2012, 2014). By contrast, when men hear infant cries while engaging in nurturant behavior, their testosterone levels decline (Kuo et al. 2015, van Anders et al. 2014). One interpretation of these results is that when men hear infant cries, but are unable to respond to them, cries are perceived as evidence of a threat to the infant that necessitates an aggressive, protective response (van Anders et al. 2011). Alternatively, testosterone increases in this context could result from men perceiving infant cries as a kind of social provocation (Kuo et al. 2015).
Yellow baboons have comparatively low rates of infanticide, but males in the wild selectively support their offspring in fights with conspecifics (Alberts 2012, Buchan et al. 2003). This kind of paternal investment appears to be important for infants, resulting in earlier maturation (Charpentier et al. 2008). In Amboseli, proximity between males and their offspring was closer than that between males and unrelated infants, though it was not clear which party was responsible for maintaining that relationship. Additionally, males showed a positive correlation between testosterone production and the number of immature offspring they had in the group (Onyango et al. 2013). Paternal care in this case thus appears to involve tolerance and aggressive defense, rather than nurturance.
Silverback gorillas in Karisoke show a similar pattern, in that they were found in closer proximity to infants than were other males in the group (Rosenbaum 2012). In this case the relationship was clearly driven by the infants, however, who were attracted to the silverbacks (ibid.). Again tolerance and protection, rather than direct nurturance, appears to be the paternal contribution. Perhaps for this reason, no relationship was found between fecal testosterone levels and male-infant social relationships in this population (Rosenbaum et al. 2016).
In combination, the baboon and gorilla data support the idea that testosterone can have both positive and negative effects on paternal care, depending on the form that such care takes. When care involves infant defense, or requires males to compete aggressively with other males, testosterone might act as promoter rather than inhibitor. When care takes the form of nurturance, however, or is otherwise hindered by mating effort, then testosterone should impede care (van Anders et al. 2011). Because infant defense represents the dominant form of paternal investment in the order, trade-offs between mating and parenting effort are likely to be weaker in most primates than in birds. Men represent an exception, as they provide more investment than other primate males, including forms of direct nurturance that are likely compromised by testosterone.
What is testosterone doing?
Although studies of wild primates indicate that male testosterone is often elevated during reproductive competition, the proximate relationship between aggression and testosterone is not well understood. In some studies testosterone shows strong correlations with aggression, but in others it does not. One solution to this puzzle is that testosterone has both physiological and psychological effects that increase the probability of aggression between specific individuals, and in specific contexts, but without a direct causal link. Some of these effects have been studied in wild primates, but others are better documented in laboratory studies of humans and rodents. Here I briefly review testosterone's (1) psychological effects, (2) winner and loser effects, (3) analgesic effects, (4) effects on male armaments, (5) effects on male adornments, and (6) effects on male vocalizations. As we shall see, many of these effects plausibly provide an advantage in male mating competition.
Psychological effects
The psychological effects of testosterone have been best studied in humans, and are important to review here because of their relevance to status competition among primates generally (Eisenegger et al. 2011, Terburg & van Honk 2013). Numerous studies have found that testosterone is associated with the motivation to dominate others, and to avoid being dominated oneself (Grant & France 2001; Josephs et al. 2006; Mazur & Booth 1998; Schultheiss et al. 1999, 2003, 2005; Sellers et al. 2007; Stanton & Schultheiss 2009). Relatedly, testosterone appears to increase vigilance to signs of potential dominance behavior in others. For example, men with higher basal testosterone levels showed enhanced attention toward, and increased amygdala activation in response to, angry faces (Derntl et al. 2009, Stanton et al. 2009, van Honk et al. 1999, Wirth & Schultheiss 2007). Additionally, experimental testosterone administration decreased gaze aversion from, and increased both cardiac and amygdala reactivity to, angry faces (Hermans et al. 2008, Radke et al. 2015, Terburg et al. 2012, van Honk et al. 2001). Finally, among men who were chemically castrated, those receiving exogenous testosterone showed greater amygdala reactivity to angry faces than those receiving placebo (Goetz et al. 2014). Comparable data are not available from non-human primates. Captive rhesus macaques that were given exogenous testosterone significantly increased their time spent watching video clips of fights between unfamiliar conspecifics, however, suggesting equivalent effects (Kelly et al. 2014, Lacreuse et al. 2010).
Testosterone has the additional action, in a range of species, of reducing fear and anxiety in both social and non-social contexts. Animals treated with testosterone have shown reductions in fear-related behaviors in response to diverse stimuli, including isolation from conspecifics, human approach, unfamiliar environments, novel objects, and surprising events (rodents: Aikey et al. 2002, Fernandez-Guasti & Martinez-Mota 2005, Frye & Seliga 2001, Hodosy et al. 2012, Raynaud & Schradin 2014; cattle: Boissy & Bouissou 1994; sheep: Bouissou & Vandenheede 1996). Experimentally lowering testosterone levels has the opposite effect, increasing fear-related behaviors (King et al. 2005). Similar results have been documented in multiple human studies (e.g. Enter et al. 2014; Hermans et al. 2006, 2007; van Honk et al. 2005).
Data from non-human primates are again rare, but captive studies of rhesus macaques show mixed results. Consistent with the fear-reducing effects of testosterone in other species, monkeys who were gonadectomized spent less time in association with unfamiliar conspecifics than did intact animals, and took longer to retrieve food placed under the image of a threatening monkey face (Richards et al. 2009). Testosterone administration caused older, but not younger, monkeys to spend more time in proximity to unfamiliar objects (Kelly et al. 2014). By contrast, monkeys whose testosterone levels were lowered through pharmacological castration did not show increased anxiety (Lacreuse et al 2010, 2012), or even showed reduced anxiety (Suarez-Jimenez et al. 2013). This discrepancy may be explained by the fact that the GnRH agonist used to shut down testosterone production in these studies itself has fear-reducing properties (Suarez-Jimenez et al. 2013).
In a range of species, then, testosterone appears to increase the desire for high status, enhance sensitivity to status threats, and decrease fear in the face of such threats. All of these effects presumably increase the odds of successful aggression in contexts where status is threatened (reviewed in Eisenegger et al. 2011, Montoya et al. 2012, Oliveira & Oliveira 2014, Terburg & van Honk 2013).
Winner and loser effects
Experience in aggressive contests influences both the likelihood of engaging in and the outcome of future contests, with recent winners more likely to compete and win, and recent losers more likely to withdraw or lose (reviewed in Hsu et al. 2006). One prominent hypothesis for these effects involves self-assessment. If an individual is uncertain of its relative fighting ability, then the best strategy is to modulate aggression in response to the outcome of recent contests (Fawcett & Johnstone 2010, Rutte et al. 2006). Winning fights should, all else being equal, lead to increased aggression, and losing fights should lead to avoidance of further competition.
Evidence from a range of species suggests that testosterone is often involved in modulating competitive responses to winning and losing (Gleason et al. 2009). Winner effects are particularly well studied in California mice (Peromyscus californicus). Males in this species show acute increases in testosterone after winning aggressive encounters with intruder males. This testosterone response produces a shorter attack latency and increased likelihood of winning in future interactions, effects that disappear when the response is experimentally blocked (Fuxjager et al. 2011a, Oyegbile & Marler 2005, Trainor & Marler 2001, Trainor et al. 2004). In a closely related species, the white-footed mouse (Peromyscus leucopus), winning produces neither a rise in testosterone, nor an increase in the probability of winning future fights. When an exogenous testosterone surge is administered in response to victory, however, this species shows a subsequent winner effect (Fuxjager et al. 2011b). Similar dynamics have been documented in cichlid fish (Oliveira et al. 2009).
Loser effects, in which losing a conflict leads to an acute decline in testosterone, and a corresponding decrease in the probability of engaging in future aggression, are less well studied. In some species loser effects appear to be independent of androgen production. In the Mozambique tilapia (Oreochromis mossambicus), for example, loser effects were apparent even when losing was accompanied by an experimental testosterone increase (Oliveira et al. 2009). By contrast, administering testosterone to losers reversed the effect in Japanese quail (Hirschenhauser et al. 2013). And in mangrove killifish (Kryptolebias marmoratus), males with lower testosterone levels showed a stronger and longer lasting effect of losing than males with higher levels (Earley et al. 2013).
Humans show evidence of both winner and loser effects that are influenced by testosterone (Carré & Olmstead 2015). In multiple competitive contexts it is common for winners to show increases in testosterone, losers to show decreases, or both (reviewed in Archer 2006, Carré & Olmstead 2015, Oliveira & Oliveira 2014). Not all studies find these effects; important moderating factors include whether outcomes are (1) decisive, (2) attributed to individual effort (rather than chance or the effort of others), and (3) interpreted as salient to an individual's status (Carré 2009, Edwards et al. 2006, Gonzalez-Bono et al. 2000, Mehta et al. 2015, Casto & Edwards 2016). In one experimental study, losing combined with a decrease in testosterone made participants less likely to engage in future competition (Mehta & Josephs 2006). In additional studies, testosterone increases in response to winning predicted performance in, or the decision to participate in, later contests (Carré & McCormick 2008, Zilioli & Watson 2014). Acute rises in testosterone in response to winning also produced increases in subsequent aggressive behavior (Carré et al. 2011, 2013).
Captive data suggest that rhesus macaques living in unstable social groups show testosterone increases after winning aggressive encounters, and declines after losing (Bernstein et al. 1974; Rose et al. 1972, 1975). Winner and loser effects are generally understudied in non-human primates, however, particularly in the wild. One significant impediment to such research involves the ephemeral nature of potentially important endocrine responses. For example, in mouse studies, an acute rise in testosterone that lasts for only 40 minutes can affect subsequent competitive behavior over a period of 2-6 days (Gleason et al. 2009). Frequent urine sampling might enable detection of acute hormone shifts in wild primates that could be correlated with winning and losing, but most experimental approaches are prohibitively invasive.
Analgesic effects
If testosterone increases the probability of aggressive behavior, then it also increases the potential for injury. The perception of pain, in turn, is likely to interfere with effective aggression (Hau et al. 2004). Consequently, one of testosterone's effects is to decrease pain perception. Experimental studies in birds, rats, and mice have shown that blocking the action of testosterone increases sensitivity to painful stimuli, while testosterone administration decreases it (Craft et al. 2004, Hau et al. 2004).
In humans, substantial evidence suggests that women are more sensitive than men to multiple forms of pain, and sex differences in testosterone have been invoked to explain this difference (Fillingim et al. 2009, Mogil 2012, Racine et al. 2012). Consistent with this hypothesis, sensitivity to painful stimuli was negatively correlated with salivary testosterone in a sample of U.S. women (Bartley et al. 2015). Additionally, men with androgen deficiency exhibited greater tolerance to multiple forms of induced pain after treatment with exogenous testosterone (Basaria et al. 2015). Data from non-human primates are again lacking.
Effects on male armaments: sexually dimorphic musculature
Testosterone frequently supports the secondary sexual characteristics that males use as armaments in reproductive competition (Hau 2007). In red deer (Cervus elaphus), for example, androgens control the seasonal growth and development of male antlers, together with the sexually dimorphic neck musculature that powers them (Fletcher 1978, Li et al. 2003, Lincoln et al. 1972). For most primates, sexually dimorphic canines and musculature are the main weapons in male-male competition (Mitani et al. 1996, Plavcan 2004). Little is known about the relationship between testosterone and canine growth, but in captive rhesus macaques, experimental prenatal testosterone exposure increased both the developmental rate and adult size of canine teeth (Hurme & van Wagenen 1956, Zingeser & Phoenix 1978). By contrast, the effects of testosterone on sexually dimorphic musculature are well studied (e.g. Bhasin et al. 2004).
Both acute and chronic effects of testosterone on muscle tissue have been investigated. Sapolsky (1993) hypothesized that the acute, transient elevations in testosterone that accompany competition might have immediate metabolic effects on muscle that would provide an advantage in fights or chases (see also Crewther et al. 2011). This idea is ostensibly supported by in vitro studies showing that testosterone causes rapid increases in glucose uptake and calcium release in muscle cells (Estrada et al. 2000, 2003, Tsai & Sapolsky 1996). In vivo support has proven elusive, however. Androgen treatments in vivo had no immediate effect on the maximal force, power or fatigue resistance of muscles in mice, nor on their evoked calcium transient (Fraysse et al. 2014). And in humans, a series of careful studies have found no effect of acute, exercise-induced increases in men's testosterone on muscular performance, growth, or strength, either during or postexercise (West et al. 2009, 2010, 2012; West & Phillips 2012). More studies are needed, however, and surprises are possible.
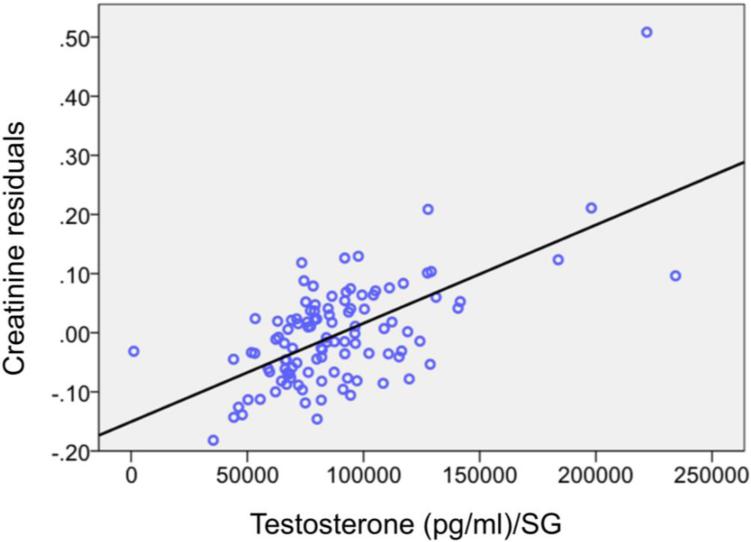
Chronic effects of testosterone on muscle development in primates are better established. For example, experimental administration of testosterone increased muscle mass in captive long-tailed macaques (Weinbauer et al. 2003). And in captive studies of male baboons, pig-tailed macaques, and rhesus macaques, serum testosterone levels correlated positively with upper-arm circumference, a proxy for muscle mass, after controlling for age (Muehlenbein et al. 2001, 2002). The only comparable wild data come from long-term studies of chimpanzees in which urinary creatinine served as a proxy for musculature (Emery Thompson et al. 2012, Muller et al. 2016a). Creatinine is produced at a relatively constant rate by skeletal muscle, and in humans correlates with measures of lean mass (e.g. Baxmann et al. 2008, Jassal et al. 2015). Male chimpanzees in Kanyawara, Kibale National Park, showed positive correlations between concentrations of urinary creatinine and testosterone (Figure 2; Emery Thompson et al. 2012, Muller et al. 2016a).
Figure 2.
Urinary testosterone levels correlated positively (r=0.63, p<0.001) with urinary creatinine, a proxy for skeletal muscle mass, in wild chimpanzees (Kanyawara, Kibale National Park). Each point (n=112) represents observations averaged over one year from one adult male (n=16 unique males). The x-axis shows mean yearly testosterone, corrected for specific gravity. The y-axis shows the unstandardized residuals of the regression of creatinine on specific gravity. Specific gravity (SG) is used to correct for urinary dilution. In humans, the relationship between creatinine and testosterone appears to be weaker than that observed in chimpanzees (see Emery Thompson et al. 2012, Muller et al. 2016a). The Kanyawara data include 6725 urine samples. Each point comprises a minimum of 20, and a mean of 60 samples.
In humans, administering supraphysiological doses of testosterone produces increases in muscle size and strength, as does providing testosterone supplementation to hypogonadal men (Bhasin et al. 2004). Modulating testosterone levels within the broad normal range, however, does not have clear effects on lean mass (Bhasin et al. 1998, 2004). Consequently, direct correlations between testosterone and measures of musculature in adult men are generally modest or nonexistent (reviewed in Alvarado et al. 2015).
Humans differ from most primates, though, in that sexually dimorphic musculature is important not only for male-male competition, but for parenting effort. In foraging and other small-scale societies, men often provision their families through work that requires episodic bursts of upper-body strength (Murdock & Provost 1973). Workload increases with the number of dependents (e.g. Hooper 2011, Wood & Marlowe 2013). As previously discussed, invested human fathers typically curtail testosterone production, probably to avoid trade-offs with increased mating effort. Consequently, a tight link between testosterone and muscle maintenance in men would not be adaptive, because falling testosterone during fatherhood would compromise men's physical abilities when they are most important for family provisioning (Alvarado et al. 2015).
Alvarado and colleagues (2015) hypothesize that strength and musculature in men should be more sensitive to physical demand, and less sensitive to changes in circulating testosterone, than in other primates. This prediction is supported by data from rural Poland, where fathers maintained lower levels of salivary testosterone than non-fathers, but greater labor demands, strength, and musculature (Alvarado et al. 2015). Within individuals, testosterone levels were lowest during the harvest season, when men performed heavy labor and showed increased muscle mass, and highest during the winter, when workloads were low and muscle mass decreased (Alvarado et al. 2016). Further support comes from direct comparisons of the relationship between testosterone and creatinine production in chimpanzees and Tsimane forager-horticulturalists. Although there is a weak, positive relationship between testosterone and creatinine in Tsimane men, testosterone accounts for half as much variance in urinary creatinine in that population as it does in wild chimpanzees (Muller et al. 2016a).
Effects on male adornments
Across primates, the presence of striking secondary sexual adornments, such as beards, capes, crests, cheek pads, and color patches, is strongly associated with a polygynous mating system (Dixson 2005). Many of these traits appear to function as badges of status, signaling the competitive abilities of the bearer (Dixson 2012). Badges are distinct from armaments, such as canines and musculature, in that they confer no direct advantage in fighting. Rather, in species with intense reproductive competition, where males risk injury in fights, such signals help to settle potentially dangerous encounters before they escalate (Senar 2006, Whiting et al. 2003). Sexually dimorphic adornments are most common in primate species maintaining large groups and multilevel social organization (Grueter et al. 2015). Status badges are likely to be beneficial in such complex societies, because competition is common, and animals have increased difficulty identifying individuals and tracking their competitive abilities (ibid.).
Although status badges generally evolve in the context of male-male competition, females in some species subsequently use them as indicators of male quality (Berglund et al. 1996). Consequently, male-male competition and female choice can both contribute as selective factors, but male-male competition is usually the initiating process. Experimental studies in both birds (e.g. Buchanan et al. 2003, Evans et al. 2000, Senar 2006), and lizards (Cooper et al. 1987; Cox et al. 2005, 2008; Salvador et al. 1996) indicate that testosterone often plays a direct physiological role in the development and maintenance of status badges. This relationship makes sense, because badges only function if they reliably signal an animal's fighting ability or aggressive motivation, and testosterone is closely associated with these (McGlothlin et al. 2008). The numerous costs imposed by increased testosterone production help to ensure that any testosterone-driven signal is honest.
Captive work with primates indicates that a threshold level of testosterone is necessary for the development of many male secondary sexual traits. Castration of male hamadryas baboons, for example, results in the loss of the long white shoulder cape, whereas exogenous testosterone administration restores it (Zuckerman & Parkes 1939). Castration similarly causes red coloration to fade in the sexual skins of male rhesus monkeys, hamadryas baboons, and patas monkeys (Erythrocebus patas) (Dixson 2012, Vandenbergh 1965, Zuckerman & Parkes 1939). Evidence for a direct, dose-dependent relationship between testosterone and the expression of such traits, however, is harder to come by.
The clearest primate evidence linking testosterone with changes in both color badges and competitive abilities comes from mandrills (reviewed in Setchell 2016). Male mandrills maintain red patches on the face and genitals that are sensitive to social status. For example, in a semi-free-ranging colony, males who fell in rank subsequently lost color, and those who rose in rank gained it (Setchell & Dixson 2001). Males became redder over periods of dominance instability, and during competition for estrous females (Setchell et al. 2008). Similarly-colored males were the most likely to threaten or fight each other, whereas pale males submitted to those with darker coloration (Setchell & Wickings 2005). Finally, in all of these cases, red coloration was correlated with fecal androgen levels, which changed in concert with color (Setchell et al. 2008).
In a study of wild gelada baboons, dominance status was similarly communicated by the redness of male chest patches (Bergman et al. 2009). Leader males had the reddest chests, and among leader males, those whose groups contained more females had redder chests still. Following group takeovers, males who lost females lost redness, and those who gained females grew redder (ibid.). Although the relationship between testosterone and red coloration in geladas has not been directly examined, coloration in bachelor males does not change until after a successful challenge, despite the fact that males show clear rises in testosterone prior to making a challenge (Pappano & Beehner 2014). Thus, it seems unlikely that the relationship between testosterone and coloration in geladas is as straightforward as in mandrills (see discussion in Higham et al. 2013b). In semi-free-ranging drills (Mandrillus leucophaeus), male coloration was found to signal status in a manner equivalent to that described for mandrills (Marty et al. 2009), but testosterone measures are again lacking.
Male rhesus macaques express red coloration on their faces, rumps, and genitals during the mating season (Baulu 1976), and in captive studies, females attend more to red-faced than pale-faced males (Waitt et al. 2003). As previously noted, castration led to declines in male sex-skin coloration (Vandenbergh 1965). Low doses of exogenous testosterone restored the coloration seen in nonbreeding males, and high doses produced the coloration of breeding males (Vandenbergh 1965; see also Rhodes et al. 1997). Studies with free-ranging, provisioned monkeys on Cayo Santiago, however, found no correlation between color, and either rank or testosterone production during the breeding season (Higham et al. 2013b). Females in this population showed clear preferences for dark red males over pale pink ones, however, suggesting that red coloration in rhesus males may currently be supported by female choice rather than male-male competition (Dubuc et al. 2014).
Less is known about the relationship between testosterone and non-color adornments in male primates. Adult male orangutans exhibit two morphs, only one of which possesses the suite of secondary sexual characteristics that includes projecting cheek pads, a throat pouch, long hair, and a musky odor (Knott & Kahlenbeg 2007). These dominant males exhibit higher androgen levels than undeveloped males, but further details are not available (Marty et al. 2015). Sifaka males also exhibit two morphs – one with a dark chest stain that is associated with sternal gland activity and scent marking, and one with a clean chest. Males with stained chests are the dominant males in multimale social groups, or the only adult males in single-male groups (Lewis & van Schaik 2007). Preliminary evidence shows that stained males have higher testosterone levels than clean males (Lewis 2009). In most species, though, relationships between testosterone, competition, and male adornments have yet to be examined in detail.
Effects on male vocalizations
In a number of anurans, birds, and rodents, testosterone regulates the production of vocalizations that males use to attract mates or repel sexual rivals (anurans: Marler & Ryan 1996, Solís & Penna 1997, Townsend & Moger 1987, Wilczynski et al. 2005; birds: Boseret et al. 2006, Harding 1991, Ketterson et al. 1992, McDonald et al. 2001, Nowicki & Ball 1989; rodents: Floody et al. 1979, Floody 1981, Kapusta & Pochroń 2011, Pasch et al. 2011). In some species testosterone has short-term, activational effects on the motivation to vocalize. In others, testosterone alters the acoustic structure of vocalizations through its long-term effects on the organs of vocal production. As with visual badges of status, vocalizations can provide valuable information to both rivals and mating partners. Among primates, loud calls in particular incur energetic costs that potentially make them honest signals of male quality, and provide cues to male condition (reviewed in Delgado 2006).
The relationship between testosterone and rates of loud calling has been examined in several primates, with mixed results. In a study of captive mouse lemurs (Microcebus murinus), castration reduced calling, but rates were not influenced by natural variation in circulating testosterone (Zimmermann 1996). Similarly, the rate of long-distance calling in wild male black howler monkeys was not affected by testosterone production (Rangel-Negrín et al. 2011). In male chimpanzees, however, mean monthly testosterone levels correlated with mean monthly rates of pant-hooting, and diurnal patterns of pant-hooting paralleled those of testosterone production (Fedurek et al. 2016). Comparable data are not available from orangutans, but in that species loud calls are only produced by dominant, flanged males (Mitani 1985), who maintain higher androgen levels than unflanged males (Marty et al. 2015). Harem-holding gelada baboon males give loud calls during competitive displays against bachelor males. In one study, high-testosterone gelada males gave more loud calls than low-testosterone males, and calling rates were elevated during months when testosterone levels were the highest (Benitez et al. 2015). High-ranking yellow baboon and crested macaque males (Macaca nigra) also produce loud calls at higher rates than low-ranking males (Kitchen 2003, Neumann et al. 2010), but a direct link with testosterone has not been investigated.
The evidence that testosterone affects the acoustic structure of primate calls is similarly mixed. In a study of Thomas langurs (Presbytis thomasi), several aspects of loud calls differed between life phases, but these did not correspond with changes in testosterone production (Wich et al. 2003). In white handed gibbons (Hylobates lar), by contrast, males with higher androgen levels produced songs of higher pitch (Barelli et al. 2013). In chimpanzees, males with higher testosterone levels produced pant-hoots with higher peak frequencies at the beginning of the climax (Fedurek et al. 2016). And in rhesus macaques, preliminary data suggested a positive association between androgen levels and acoustic frequency of male barks (Higham et al. 2013b). Higher frequency calls were also recorded in dominant yellow baboon males, but the relationship with testosterone was not examined (Fischer et al. 2004).
The association between high frequency calls and testosterone seems unusual, given that large body size is associated with low vocal tract resonant frequencies, and males in some species decrease these frequencies to exaggerate their formidability (Fitch & Reby 2001). In human men, for example, testosterone lengthens and thickens the vocal cords, causing lower voice pitch, which increases perceived social dominance (Apicella & Feinberg 2009, Archer 2006, Dabbs & Mallinger 1999, Hodges-Simeon et al. 2014, Puts et al. 2014). However, in some mammals, loud calls with high fundamental frequencies signal fitness and fighting ability (Garcia et al. 2013, Reby & McComb 2003, Reby et al. 2010). This is likely because producing loud, high-frequency calls requires intense lung pressure and large muscular effort that individuals low in strength or endurance cannot generate (Titze & Riede 2010). As was the case with male adornments, the relationship between loud calling and testosterone is one that needs further development in wild primates.
Future directions and conclusions
Much of the published research on testosterone and primate behavior employs either cross-sectional data, or longitudinal data that are limited to a single field season. Moving forward, an important goal is to track hormonal variation within individuals over longer periods. Primate research has a clear advantage here over bird studies, in that researchers sometimes observe known individuals over years or even decades. Such data are currently being collected in many species, and should help to answer questions raised in the preceding review. For example, how strong is the association between testosterone and the expression of ornaments (such as chest patches) or armaments (such as sexually dimorphic musculature) within individuals, and what factors affect the strength of that relationship? Why do mandrills show a robust relationship between red coloration and testosterone, whereas rhesus macaques do not (see Higham et al. 2013b for discussion)? Do levels of testosterone during adolescence predict aspects of morphology or behavior in adulthood? How does victory or defeat in an aggressive encounter affect subsequent testosterone production and dominance striving? How does testosterone change when males leave their natal groups, enter new groups, or attempt to take over new groups? And how do reproductive tactics and testosterone profiles change during senescence? Detailed, longitudinal data are necessary to answer such questions.
Another valuable endeavor will be to collect similar longitudinal data from human populations. Even in anthropology, human studies have mostly followed an epidemiological strategy of targeting large numbers of subjects, but with limited hormone measures on each. Hormones are generally sampled cross-sectionally or, if longitudinally, once every few years. Having more detailed data on smaller samples of humans, together with matching behavioral and demographic data, will provide a valuable point of comparison with non-human primates, for whom truly large sample sizes will never be available.
Most of the work on testosterone and aggression in primates looks at reproductive competition among males. Much less is known about the relationship between testosterone and intersexual competition. In recent years the literature on sexual coercion and male aggression against females in primates has grown considerably (Muller & Wrangham 2009), and an important next step will be to look at hormonal aspects of these relationships. Although a few studies have examined the relationship between testosterone and partner abuse in humans (e.g. Soler et al. 2000), comparable data from non-human primates are lacking.
Similarly, work on the relationship between testosterone and intergroup competition in primates is mostly absent. Recent human data suggest that testosterone might simultaneously promote in-group bonds while increasing between-group hostility (Diekhof et al. 2014). Species exhibiting cooperative territorial behavior should provide opportunities for investigating this dynamic.
The relationship between testosterone and immune function in primates is another important topic for future research. Although testosterone is often characterized as immunosuppressive, the evidence for such an effect is mixed. Some studies actually show positive associations between testosterone and measures of immune function (e.g. Gettler et al. 2014). Additionally, in studies that report a negative relationship, it is not usually clear whether testosterone is suppressing the immune system directly or indirectly (for example, by channeling energy into mating effort at the expense of immune function; Muehlenbein & Bribiescas 2005). Still other studies indicate that testosterone is not universally immunosuppressive, but has positive or negative effects on different aspects of the immune system (Ezenwa et al. 2012).
Progress in this area will require better non-invasive methods to assay immune function in the wild (e.g. Higham et al. 2015, Heistermann & Higham 2015). Most studies employ a proxy for immune function, such as parasite load, to examine this relationship. Wild gray-cheeked mangabeys, for example, showed a positive relationship between nematode infection and fecal testosterone levels (Arlet et al. 2015), and high-ranking male chimpanzees exhibited both higher parasite richness (specifically the number of unique helminth species) and fecal testosterone levels (Muehlenbein & Watts 2010). It is not clear from such studies, however, whether this relationship is driven by variation in immune function, or by other potential factors, such as exposure. In support of the latter hypothesis, social network analyses in one wild chimpanzee population showed that high-ranking males were more central, and thus more likely to be exposed to diverse pathogens (Rushmore et al. 2013, 2014). Prall and Muehlenbein (2014) review major issues and methodological concerns in this area.
The preceding review suggests that if testosterone does have direct, adaptive effects on immune function, then they are likely to be associated in some way with aggressive competition. This logic underlies the “immuneoredistribution hypothesis,” which proposes that high levels of testosterone temporarily shift immune cells to lymph nodes, skin, and other tissues “well positioned to combat challenges from new trauma” associated with aggression (Braude et al. 1999: 347). Regardless of whether this particular hypothesis is ultimately refuted, it represents a useful theoretical approach in considering specific aspects of immune function that should be positively or negatively impacted by testosterone.
Little has been said here about the technical issues surrounding hormone measurement, but it is possible that some of the conflicting findings discussed above result from differences in methodology. For example, it is clear that different species metabolize hormones differently, ultimately excreting testosterone as a range of metabolites, and at varying amounts in urine versus feces (Möhle et al. 2002). Consequently, the percentage of circulating testosterone recovered by an assay will depend on both the assay medium, and how closely the dominant metabolites are targeted by a specific antibody. Further, antibodies to testosterone generally show some cross-reactivity with other androgens. Thus, some studies purporting to measure testosterone may be primarily assaying related steroid molecules that show a different affinity for the androgen receptor. The choice of blood, urine, saliva, or feces as a medium will also affect the duration of hormone release over which one is sampling. Higham (2016) and Gray and colleagues (2016) provide current reviews of these and other methodological issues in hormone measurement. A clear message from these is that researchers should do more to ensure that they are measuring the hormones they claim to be measuring, and in ways that are comparable across studies.
Although less frequently discussed, similar problems are evident in the measurement of aggression. What counts as an aggressive behavior, and how rates of aggression are calculated vary substantially across studies and study species. Many researchers provide only cursory descriptions of their behavioral measures, however, so the scale of the problem is difficult to assess. Increased attention to this issue would undoubtedly facilitate more accurate comparisons across species and study sites.
In summary, the evidence reviewed above supports the role of testosterone as a mediator of life-history trade-offs in primates. Testosterone generally promotes mating effort at the expense of parenting effort or survival, especially when mating involves aggressive competition. Testosterone supports male competition through its psychological effects on status striving and competitive motivation, its analgesic effects, and its effects on the development and maintenance of male ornaments and armaments. When parenting effort takes the form of physical protection, however, rather than nurturant behaviors, then a stark trade-off between mating and parenting effort may not exist. This appears to be the case for most primates, with humans a prominent exception.
Highlights.
Testosterone promotes mating effort in primates, at the expense of survival.
In primates testosterone primarily facilitates aggression in reproductive contexts.
Trade-offs between parenting effort and mating effort are weak in most primates.
Testosterone's psychological and physiological effects promote male competition.
Acknowledgments
I thank James Higham and Jim Roney for inviting me to contribute this review, and James Higham and two anonymous reviewers for comments on the manuscript. Long-term research at Kanyawara is supported by the Leakey Foundation, the Wenner-Gren Foundation, the National Science Foundation (Grants 1355014 and 0849380), and the National Institute on Aging of the National Institutes of Health (Award R01AG049395).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Alberts SC. Magnitude and sources of variation in male reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 412–431. [Google Scholar]
- Alvarado LC, Muller MN, Emery Thompson M, Klimek M, Nenko I, Jasienska G. The Paternal Provisioning Hypothesis: Effects of workload and testosterone production on men's musculature. Am. J. Phys. Anthropol. 2015;158:19–35. doi: 10.1002/ajpa.22771. [DOI] [PubMed] [Google Scholar]
- Alvarado LC, Muller MN, Emery Thompson M, Klimek M, Nenko I, Jasienska G. Men's reproductive ecology and diminished hormonal regulation of skeletal muscle phenotype: An analysis of between- and within-individual variation among rural Polish men. Am. J. Phys. Anthropol. 2016;159(S62):78. [Google Scholar]
- Alvergne A, Faurie C, Raymond M. Variation in testosterone levels and male reproductive effort: Insight from a polygynous human population. Horm. Behav. 2009;56:491–497. doi: 10.1016/j.yhbeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Apicella CL, Feinberg DR, Marlowe FW. Voice pitch predicts reproductive success in male hunter-gatherers. Biol. Lett. 2007;3:682–684. doi: 10.1098/rsbl.2007.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Arlet ME, Kaasik A, Molleman F, Isbell L, Carey JR, Mänd R. Social factors increase fecal testosterone levels in wild male gray-cheeked mangabeys (Lophocebus albigena). Horm. Behav. 2011;59:605–611. doi: 10.1016/j.yhbeh.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Arlet ME, Chapman CA, Isbell L, Molleman F, Mänd R, Hõrak P, Carey JR. Social and ecological correlates of parasitic infections in adult male gray-cheeked mangabeys (Lophocebus albigena). Int. J. Primatol. 2011;36:967–986. [Google Scholar]
- Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia). Horm. Behav. 2006;49:88–95. doi: 10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Bales KL, Jarcho MR. Fathering in non-human primates. In: Cabrera NJ, Tamis-LeMonda CS, editors. Handbook of Father Involvement: Multidisciplinary Perspectives. Routledge; New York: 2013. pp. 37–54. [Google Scholar]
- Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm. Behav. 2016;77:249–259. doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelli C, Mundry R, Heistermann M, Hammerschmidt K. Cues to androgens and quality in male gibbon songs. PloS one. 2013;8:e82748. doi: 10.1371/journal.pone.0082748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, Palit S, Kuhn BL, Kerr KL, Terry EL, DelVentura JL, Rhudy JL. Natural variation in testosterone is associated with hypoalgesia in healthy women. Clin. J. Pain. 2015;31:730–739. doi: 10.1097/AJP.0000000000000153. [DOI] [PubMed] [Google Scholar]
- Bartsh SS, Johnston SD, Siniff DB. Territorial behavior and breeding frequency of male Weddell seals (Leptonychotes weddelli) in relation to age, size, and concentrations of serum testosterone and cortisol. Can. J. Zool. 1992;70:680–692. [Google Scholar]
- Basaria S, Travison TG, Alford D, Knapp PE, Teeter K, Cahalan C, Eder R, Lakshman K, Bachman E, Mensing G. Effects of testosterone replacement in men with opioid-induced androgen deficiency: A randomized controlled trial. Pain. 2015;156:280–288. doi: 10.1097/01.j.pain.0000460308.86819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulu J. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm. Behav. 1976;7:481–494. doi: 10.1016/0018-506x(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav. Ecol. Sociobiol. 2006;59:469–479. [Google Scholar]
- Beehner JC, Gesquiere L, Seyfarth RM, Cheney DL, Alberts SC, Altmann J. Testosterone related to age and life-history stages in male baboons and geladas. Horm. Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez ME, Bergman TJ, Beehner JC. Testosterone mediates loud call production in gelada males. Am. J. Phys. Anth. 2015;156(S83):60–61. [Google Scholar]
- Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin. Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- Berglund A, Bisazza A, Pilastro A. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linnean Soc. 1996;58:385–399. [Google Scholar]
- Bergman TJ, Ho L, Beehner JC. Chest color and social status in male geladas (Theropithecus gelada). Int. J. Primatol. 2009;30:791–806. [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. The interaction of hormones, behavior, and social context in nonhuman primates. In: Svare BB, editor. Hormones and Aggressive Behavior. Plenum Press; New York: 1983. pp. 535–561. [Google Scholar]
- Bhasin S, Bross R, Storer TW, Casaburi R. Androgens and muscles. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. Springer; New York: 1998. pp. 209–228. [Google Scholar]
- Bhasin S, Storer TW, Singh AB, Woodhouse L, Singh R, Artaza J, Taylor WE, Sinha-Hikim I, Jasuja R, Gonzalez-Cadavid N. Testosterone effects on the skeletal muscle. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. Cambridge University Press; Cambridge: 2004. pp. 255–282. [Google Scholar]
- Bogaert AF, Fisher WA. Predictors of university men's number of sexual partners. J. Sex Res. 1995;32:119–130. [Google Scholar]
- Boissy A, Bouissou MF. Effects of androgen treatment on behavioral and physiological responses of heifers to fear-eliciting situations. Horm. Behav. 1994;28:66–83. doi: 10.1006/hbeh.1994.1006. [DOI] [PubMed] [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men's marriages. Soc. Forces. 1993;72:463–477. [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria). J. Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bouissou M-F, Vandenheede M. Long-term effects of androgen treatment on fear reactions in ewes. Horm. Behav. 1996;30:93–99. doi: 10.1006/hbeh.1996.0013. [DOI] [PubMed] [Google Scholar]
- Braude S, Tang-Martinez Z, Taylor GT. Stress, testosterone, and the immunoredistribution hypothesis. Behav. Ecol. 1999;10:345–350. [Google Scholar]
- Bribiescas RG. Reproductive ecology and life history of the human male. Yearb. Phys. Anthropol. 2001;44:148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- Brockman DK, Whitten PL, Richard AF, Benander B. Birth season testosterone levels in male Verreaux's sifaka, Propithecus verreauxi: Insights into socio-demographic factors mediating seasonal testicular function. Behav. Ecol. Sociobiol. 2001;49:117–127. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Evans MR, Goldsmith AR. Testosterone, dominance signalling and immunosuppression in the house sparrow, Passer domesticus. Behav. Ecol. Sociobiol. 2003;55:50–59. [Google Scholar]
- Burnham TC, Chapman JF, Gray PB, McIntyre MH, Lipson SF, Ellison PT. Men in committed, romantic relationships have lower testosterone. Horm. Behav. 2003;44:119–122. doi: 10.1016/s0018-506x(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Carré JM. No place like home: testosterone responses to victory depend on game location. Am. J. Hum. Biol. 2009;21:392–394. doi: 10.1002/ajhb.20867. [DOI] [PubMed] [Google Scholar]
- Carré JM, Campbell JA, Lozoya E, Goetz SMM, Welker KM. Changes in testosterone mediate the effect of winning on subsequent aggressive behaviour. Psychoneuroendocrinol. 2013;38:2034–2041. doi: 10.1016/j.psyneuen.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM. Aggressive behavior and change in salivary testosterone concentrations predict willingness to engage in a competitive task. Horm. Behav. 2008;54:403–409. doi: 10.1016/j.yhbeh.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM, Hariri AR. The social neuroendocrinology of human aggression. Psychoneuroendocrinol. 2011;36:935–944. doi: 10.1016/j.psyneuen.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Carré JM, Olmstead NA. Social neuroendocrinology of human aggression: Examining the role of competition-induced testosterone dynamics. Neurosci. 2015;286:171–186. doi: 10.1016/j.neuroscience.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Carré JM, Putnam SK, McCormick CM. Testosterone responses to competition predict future aggressive behaviour at a cost to reward in men. Psychoneuroendocrinol. 2009;34:561–570. doi: 10.1016/j.psyneuen.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Casto KV, Edwards DA. Testosterone, cortisol, and human competition. Horm. Behav. 2016;82:21–37. doi: 10.1016/j.yhbeh.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Pereira ME. Mating season aggression and fecal testosterone levels in male ring-tailed lemurs (Lemur catta). Horm. Behav. 2000;37:246–255. doi: 10.1006/hbeh.2000.1585. [DOI] [PubMed] [Google Scholar]
- Charpentier MJE, Van Horn RC, Altmann J, Alberts SC. Paternal effects on offspring fitness in a multimale primate society. Proc. Natl. Acad. Sci. USA. 2008;105:1988–1992. doi: 10.1073/pnas.0711219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, Wrangham RW. Predation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 227–239. [Google Scholar]
- Christiansen K. Behavioural correlates of testosterone. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. Cambridge University Press; Cambridge: 2004. pp. 125–172. [Google Scholar]
- Clark MM, Galef BG., Jr A testosterone-mediated trade-off between parental and sexual effort in male mongolian gerbils (Meriones unguiculatus). J. Comp. Psychol. 1999;113:388–395. doi: 10.1037/0735-7036.113.4.388. [DOI] [PubMed] [Google Scholar]
- Cooper WE, Jr, Mendonca MT, Vitt LJ. Induction of orange head coloration and activation of courtship and aggression by testosterone in the male broad-headed skink (Eumeces laticeps). J. Herpetol. 1987;21:96–101. [Google Scholar]
- Cox RM, Skelly SL, Leo A, John-Alder HB, Beaupre SJ. Testosterone regulates sexually dimorphic coloration in the eastern fence lizard, Sceloporus undulatus. Copeia. 2005;2005:597–608. [Google Scholar]
- Cox RM, Zilberman V, John-Alder HB. Testosterone stimulates the expression of a social color signal in Yarrow's spiny lizard, Sceloporus jarrovii. J. Exp. Zool. Part A. 2008;309:505–514. doi: 10.1002/jez.481. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: The role of gonadal hormones. Europ. J. Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Crewther BT, Cook C, Cardinale M, Weatherby RP, Lowe T. Two emerging concepts for elite athletes. Sports Med. 2011;41:103–123. doi: 10.2165/11539170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cristóbal-Azkarate J, Chavira R, Boeck L, Rodríguez-Luna E, Veàl JJ. Testosterone levels of free-ranging resident mantled howler monkey males in relation to the number and density of solitary males: A test of the challenge hypothesis. Horm. Behav. 2006;49:261–267. doi: 10.1016/j.yhbeh.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Mallinger A. High testosterone levels predict low voice pitch among men. Pers. Indiv. Differ. 1999;27:801–804. [Google Scholar]
- De Ridder E, Pinxten R, Eens M. Experimental evidence of a testosterone-induced shift from paternal to mating behaviour in a facultatively polygynous songbird. Behav. Ecol. Sociobiol. 2000;49:24–30. [Google Scholar]
- Delgado RA. Sexual selection in the loud calls of male primates: signal content and function. Int. J. Primatol. 2006;27:5–25. [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinol. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Desjardins JK, Stiver KA, Fitzpatrick JL, Milligan N, Van Der Kraak GJ, Balshine S. Sex and status in a cooperative breeding fish: Behavior and androgens. Behav. Ecol. Sociobiol. 2008;62:785–794. [Google Scholar]
- Dey CJ, O'Connor CM, Gilmour KM, Van Der Kraak G, Cooke SJ. Behavioral and physiological responses of a wild teleost fish to cortisol and androgen manipulation during parental care. Horm. Behav. 2010;58:599–605. doi: 10.1016/j.yhbeh.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Wittmer S, Reimers L. Does competition really bring out the worst? Testosterone, social distance and inter-male competition shape parochial altruism in human males. PLoS ONE. 2014;9:e98977. doi: 10.1371/journal.pone.0098977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson A. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Humans. Oxford University Press; Oxford: 2012. [Google Scholar]
- Dixson A, Dixson B, Anderson M. Sexual selection and the evolution of visually conspicuous sexually dimorphic traits in male monkeys, apes, and human beings. Ann. Rev. Sex Res. 2005;16:1–19. [PubMed] [Google Scholar]
- Dubuc C, Allen WL, Maestripieri D, Higham JP. Is male rhesus macaque red color ornamentation attractive to females? Behav. Ecol. Sociobiol. 2014;68:1215–1224. doi: 10.1007/s00265-014-1732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley RL, Lu C-K, Lee IH, Wong SC, Hsu Y. Winner and loser effects are modulated by hormonal states. Front. Zool. 2013;10:6. doi: 10.1186/1742-9994-10-6. doi:10.1186/1742-9994-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA. Competition and testosterone. Horm. Behav. 2006;50:681–683. doi: 10.1016/j.yhbeh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends Cogn. Sci. 2011;15:263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Energetics and reproductive effort. Am. J. Hum. Biol. 2003;15:342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW. Technical note: variation in muscle mass in wild chimpanzees: Application of a modified urinary creatinine method. Am. J. Phys. Anth. 2012;149:622–627. doi: 10.1002/ajpa.22157. [DOI] [PubMed] [Google Scholar]
- Enter D, Spinhoven P, Roelofs K. Alleviating social avoidance: Effects of single dose testosterone administration on approach–avoidance action. Horm. Behav. 2014;65:351–354. doi: 10.1016/j.yhbeh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinol. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- Estrada M, Liberona JL, Miranda M, Jaimovich E. Aldosterone-and testosterone-mediated intracellular calcium response in skeletal muscle cell cultures. Am. J. Physiol. Endocrinol. Metab. 2000;279:E132–E139. doi: 10.1152/ajpendo.2000.279.1.E132. [DOI] [PubMed] [Google Scholar]
- Evans MR, Goldsmith AR, Norris SRA. The effects of testosterone on antibody production and plumage coloration in male house sparrows (Passer domesticus). Behav. Ecol. Sociobiol. 2000;47:156–163. [Google Scholar]
- Ezenwa VO, Ekernas LS, Creel S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012;26:123–133. [Google Scholar]
- Fawcett TW, Johnstone RA. Learning your own strength: Winner and loser effects should change with age and experience. Proc. Roy. Soc. B. 2010;277:1427–1434. doi: 10.1098/rspb.2009.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedurek P, Slocombe KE, Enigk DK, Thompson ME, Wrangham RW, Muller MN. The relationship between testosterone and long-distance calling in wild male chimpanzees. Behav. Ecol. Sociobiol. 2016 doi: 10.1007/s00265-016-2087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mendoza SP. The biology of paternal care in human and nonhuman primates. Ann. Rev. Anthropol. 2009;38:115–130. [Google Scholar]
- Fernández-Guasti A, Martínez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinol. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Kitchen DM, Seyfarth RM, Cheney DL. Baboon loud calls advertise male quality: Acoustic features and their relation to rank, age, and exhaustion. Behav. Ecol. Sociobiol. 2004;56:140–148. [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm. Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Fletcher TJ. The induction of male sexual behavior in red deer (Cervus elaphus) by the administration of testosterone to hinds and estradiol-17β to stags. Horm. Behav. 1978;11:74–88. doi: 10.1016/0018-506x(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Floody OR. The hormonal control of ultrasonic communication in rodents. Am. Zool. 1981;21:129–142. [Google Scholar]
- Floody OR, Walsh C, Flanagan MT. Testosterone stimulates ultrasound production by male hamsters. Horm. Behav. 1979;12:164–171. doi: 10.1016/0018-506x(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Vignaud A, Fane B, Schuh M, Butler-Browne G, Metzger D, Ferry A. Acute effect of androgens on maximal force-generating capacity and electrically evoked calcium transient in mouse skeletal muscles. Steroids. 2014;87:6–11. doi: 10.1016/j.steroids.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affect. Behav. Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Oyegbile TO, Marler CA. Independent and additive contributions of postvictory testosterone and social experience to the development of the winner effect. Endocrinol. 2011a;152:3422–3429. doi: 10.1210/en.2011-1099. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Montgomery JL, Marler CA. Species differences in the winner effect disappear in response to post-victory testosterone manipulations. Proc. Roy. Soc. B. 2011b;278:3497–3503. doi: 10.1098/rspb.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Charlton BD, Wyman MT, Fitch WT, Reby D. Do red deer stags Cervus elaphus use roar fundamental frequency (F0) to assess rivals? PLoS ONE. 2013;8:e83946. doi: 10.1371/journal.pone.0083946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT. Applying socioendocrinology to evolutionary models: Fatherhood and physiology. Evol. Anthropol. 2014;23:146–160. doi: 10.1002/evan.21412. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Agustin SS, Feranil AB, Kuzawa CW. Testosterone, immune function, and life history transitions in Filipino males (Homo sapiens). Int. J. Primatol. 2014;35:787–804. [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc. Natl. Acad. Sci. USA. 2011;108:16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Krummel S, Engelhardt A. Seasonal and social influences on fecal androgen and glucocorticoid excretion in wild male long-tailed macaques (Macaca fascicularis). Physiol. Behav. 2009;98:168–175. doi: 10.1016/j.physbeh.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Rahmi E, Agil M, Fauzan PA, Engelhardt A. Androgen correlates of male reproductive effort in wild male long-tailed macaques (Macaca fascicularis): A multi-level test of the challenge hypothesis. Physiol. Behav. 2015;141:143–153. doi: 10.1016/j.physbeh.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: When it occurs and why. Front. Neuroendocrinol. 2009;30:460–469. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Goetz SMM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carré JM. Testosterone rapidly increases neural reactivity to threat in healthy men: A novel two-step pharmacological challenge paradigm. Biol. Psychiatry. 2014;76:324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bono E, Salvador A, Ricarte J, Serrano MA, Arnedo M. Testosterone and attribution of successful competition. Aggress. Behav. 2000;26:235–240. [Google Scholar]
- Gould L, Ziegler TE. Variation in fecal testosterone levels, inter-male aggression, dominance rank and age during mating and post-mating periods in wild adult male ring-tailed lemurs (Lemur catta). Am. J. Primatol. 2007;69:1325–1339. doi: 10.1002/ajp.20438. [DOI] [PubMed] [Google Scholar]
- Goymann W. Social modulation of androgens in male birds. Gen. Comp. Endocrinol. 2009;163:149–157. doi: 10.1016/j.ygcen.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male–male androgen responsiveness - revisiting the challenge hypothesis. Horm. Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Grant VJ, France JT. Dominance and testosterone in women. Biol. Psychol. 2001;58:41–47. doi: 10.1016/s0301-0511(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Gray PB, McHale TS, Carré JM. A review of human male field studies of hormones and behavioral reproductive effort. Horm. Behav. 2016 doi: 10.1016/j.yhbeh.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Gray PB, Ellison PT, Campbell BC. Testosterone and marriage among Ariaal men of northern Kenya. Curr. Anthropol. 2007;48:750–755. [Google Scholar]
- Gray PB, Anderson KG. Fatherhood: Evolution and Human Paternal Behavior. Harvard University Press; Cambridge MA.: 2010. [Google Scholar]
- Gray PB, Campbell BC. Human male testosterone, pair bonding and fatherhood. In: Gray PB, Ellison PT, editors. Endocrinology of Social Relationships. Harvard University Press; Cambridge MA: 2009. pp. 270–293. [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evol. Hum. Behav. 2002;23:193–201. [Google Scholar]
- Gray PB, Parkin JC, Samms-Vaughan ME. Hormonal correlates of human paternal interactions: A hospital-based investigation in urban Jamaica. Horm. Behav. 2007;52:499–507. doi: 10.1016/j.yhbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Gray PB, Yang C-FJ, Pope HG. Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proc. Roy. Soc. B. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter CC, Isler K, Dixson BJ. Are badges of status adaptive in large complex primate groups? Evol. Hum. Behav. 2015;36:398–406. [Google Scholar]
- Harding CF. Neuroendocrine integration of social behaviour in male songbirds. In: Archer T, Hansen S, editors. Behavioral Biology: Neuroendocrine Axis. Lawrence Erlbaum Associates; Hillsdale, New York: 1991. pp. 53–66. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: Implications for the evolution of vertebrate life histories. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hau M, Dominguez OA, Evrard HC. Testosterone reduces responsiveness to nociceptive stimuli in a wild bird. Horm. Behav. 2004;46:165–170. doi: 10.1016/j.yhbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Higham JP. Urinary neopterin, a non-invasive marker of mammalian cellular immune activation, is highly stable under field conditions. Sci. Rep. 2015;5:16308. doi: 10.1038/srep16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinol. 2007;32:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J. A single administration of testosterone reduces fear-potentiated startle in humans. Biol. Psychiatry. 2006;59:872–874. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol. Psychiatry. 2008;63:263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Higham JP. Field endocrinology of nonhuman primates: Past, present, and future. Horm. Behav. 2016 doi: 10.1016/j.yhbeh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Higham JP, Kraus C, Stahl-Hennig C, Engelhardt A, Fuchs D, Heistermann M. Evaluating non-invasive markers of non-human primate immune activation and inflammation. Am. J. Phys. Anthropol. 2015;158:673–684. doi: 10.1002/ajpa.22821. [DOI] [PubMed] [Google Scholar]
- Higham JP, Heistermann M, Maestripieri D. The endocrinology of male rhesus macaque social and reproductive status: A test of the challenge and social stress hypotheses. Behav. Ecol. Sociobiol. 2013a;67:19–30. doi: 10.1007/s00265-012-1420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Pfefferle D, Heistermann M, Maestripieri D, Stevens M. Signaling in multiple modalities in male rhesus macaques: Sex skin coloration and barks in relation to androgen levels, social status, and mating behavior. Behav. Ecol. Sociobiol. 2013b;67:1457–1469. doi: 10.1007/s00265-013-1521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillgarth N, Wingfield JC. Beckage NE, editor. Testosterone and immunosuppression in vertebrates: implications for parasite-mediated sexual selection. Parasites and Pathogens. Springer. 1997:143–155. [Google Scholar]
- Hirschenhauser K, Gahr M, Goymann W. Winning and losing in public: Audiences direct future success in Japanese quail. Horm. Behav. 2013;63:625–633. doi: 10.1016/j.yhbeh.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Winkler H, Oliveira RF. Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm. Behav. 2003;43:508–519. doi: 10.1016/s0018-506x(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Hodges-Simeon CR, Gurven M, Puts DA, Gaulin SJC. Vocal fundamental and formant frequencies are honest signals of threat potential in peripubertal males. Behav. Ecol. 2014;25:984–988. doi: 10.1093/beheco/aru081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodosy J, Zelmanová D, Majzúnová M, Filová B, Malinová M, Ostatníková D, Celec P. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol. Biochem. Behav. 2012;102:191–195. doi: 10.1016/j.pbb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Hooper AEC, Gangestad SW, Thompson ME, Bryan AD. Testosterone and romance: the association of testosterone with relationship commitment and satisfaction in heterosexual men and women. Am. J. Hum. Biol. 2011;23:553–555. doi: 10.1002/ajhb.21188. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol. Rev. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Huck M, Löttker P, Heymann EW, Heistermann M. Characterization and social correlates of fecal testosterone and cortisol excretion in wild male Saguinus mystax. Int. J. Primatol. 2005;26:159–179. [Google Scholar]
- Hurme VO, VanWagenen G. Emergence of permanent first molars in the monkey (Macaca mulatta). Association with other growth phenomena. Yale J. Biol. Med. 1956;28:538. [PMC free article] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, Isidori A, Fabbri A, Lenzi A. Effects of testosterone on sexual function in men: Results of a meta-analysis. Clin. Endocrinol. 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- Jack KM, Fedigan LM. Why be alpha male? Dominance and reproductive success in wild white-faced capuchins (Cebus capucinus) In: Estrada A, Garber PA, Pavelka MSM, Luecke L, editors. New Perspectives in the Study of Mesoamerican Primates: Distribution, Ecology, Behavior, and Conservation. Springer; New York: 2006. pp. 367–386. [Google Scholar]
- Jassal SK, Wassel CL, Laughlin GA, Barrett-Connor E, Rifkin DE, Ix JH. Urine creatinine-based estimates of fat-free mass in community-dwelling older persons: The Rancho Bernardo study. J. Ren. Nutr. 2015;25:97–102. doi: 10.1053/j.jrn.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs RA, Sellers JG, Newman ML, Mehta PH. The mismatch effect: when testosterone and status are at odds. J. Pers. Soc. Psychol. 2006;90:999–1013. doi: 10.1037/0022-3514.90.6.999. [DOI] [PubMed] [Google Scholar]
- Kalbitzer U, Heistermann M, Cheney D, Seyfarth R, Fischer J. Social behavior and patterns of testosterone and glucocorticoid levels differ between male chacma and Guinea baboons. Horm. Behav. 2015;75:100–110. doi: 10.1016/j.yhbeh.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Kapusta J, Pochrón E. Effect of gonadal hormones and sexual experience on vocalizations and behavior of male bank voles (Myodes glareolus). Can. J. Zool. 2011;89:1117–1127. [Google Scholar]
- Kelly B, Maguire-Herring V, Rose CM, Gore HE, Ferrigno S, Novak MA, Lacreuse A. Short-term testosterone manipulations do not affect cognition or motor function but differentially modulate emotions in young and older male rhesus monkeys. Horm. Behav. 2014;66:731–742. doi: 10.1016/j.yhbeh.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Sladky KK, Flitsch TJ, Pomerantz SM, Goy RW. Androgenic influences on body size and composition of adult rhesus monkeys. Am. J. Physiol. 1988;255:E857–E864. doi: 10.1152/ajpendo.1988.255.6.E857. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Jr, Wolf L, Ziegenfus C. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat. 1992;140:980–999. [Google Scholar]
- Ketterson ED, Nolan V., Jr. Adaptation, exaptation, and constraint: A hormonal perspective. Am. Nat. 1999;153:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- King HM, Kurdziel LB, Meyer JS, Lacreuse A. Effects of testosterone on attention and memory for emotional stimuli in male rhesus monkeys. Psychoneuroendocrinol. 2012;37:396–409. doi: 10.1016/j.psyneuen.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Kitchen DM, Seyfarth RM, Fischer J, Cheney DL. Loud calls as indicators of dominance in male baboons (Papio cynocephalus ursinus). Behav. Ecol. Sociobiol. 2003;53:374–384. [Google Scholar]
- Knott CD, Kahlenberg SM. Orangutans in perspective: forced copulations and female mating resistance. In: Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. Oxford University Press; New York: 2007. pp. 290–304. [Google Scholar]
- Kraus C, Heistermann M, Kappeler PM. Physiological suppression of sexual function of subordinate males: a subtle form of intrasexual competition among male sifakas (Propithecus verreauxi)? Physiol. Behav. 1999;66:855–861. doi: 10.1016/s0031-9384(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Kuo PX, Saini EK, Thomason E, Schultheiss OC, Gonzalez R, Volling BL. Individual variation in fathers' testosterone reactivity to infant distress predicts parenting behaviors with their 1-year-old infants. Dev. Psychobiol. 2015;58:303–314. doi: 10.1002/dev.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Gettler LT, Muller MN, McDade TW, Feranil AB. Fatherhood, pairbonding and testosterone in the Philippines. Horm. Behav. 2009;56:429–435. doi: 10.1016/j.yhbeh.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Gore HE, Chang J, Kaplan ER. Short-term testosterone manipulations modulate visual recognition memory and some aspects of emotional reactivity in male rhesus monkeys. Physiol. Behav. 2012;106:229–237. doi: 10.1016/j.physbeh.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, King HM, Kurdziel LB, Partan SR, Caldwell KM, Chiavetta MR, Millette MM, Meyer JS, Grow DR. Testosterone may increase selective attention to threat in young male macaques. Horm. Behav. 2010;58:854–863. doi: 10.1016/j.yhbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Landys MM, Goymann W, Raess M, Slagsvold T. Hormonal responses to male-male social challenge in the blue tit Cyanistes caeruleus: Single-broodedness as an explanatory variable. Physiol. Biochem. Zool. 2007;80:228–240. doi: 10.1086/510564. [DOI] [PubMed] [Google Scholar]
- Lewis RJ. Chest staining variation as a signal of testosterone levels in male Verreaux's Sifaka. Physiol. Behav. 2009;96:586–592. doi: 10.1016/j.physbeh.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, van Schaik CP. Bimorphism in male Verreaux's sifaka in the Kirindy Forest of Madagascar. Int. J. Primatol. 2007;28:159–182. [Google Scholar]
- Li C, Littlejohn RP, Corson ID, Suttie JM. Effects of testosterone on pedicle formation and its transformation to antler in castrated male, freemartin and normal female red deer (Cervus elaphus). Gen. Comp. Endocrinol. 2003;131:21–31. doi: 10.1016/s0016-6480(02)00625-1. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Guinness F, Short RV. The way in which testosterone controls the social and sexual behavior of the red deer stag (Cervus elaphus). Horm. Behav. 1972;3:375–396. [Google Scholar]
- Lynch JW, Ziegler TE, Strier KB. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm. Behav. 2002;41:275–287. doi: 10.1006/hbeh.2002.1772. [DOI] [PubMed] [Google Scholar]
- Lynn SE. Behavioral insensitivity to testosterone: why and how does testosterone alter paternal and aggressive behavior in some avian species but not others? Gen. Comp. Endocrinol. 2008;157:233–240. doi: 10.1016/j.ygcen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Lynn SE. Endocrine and neuroendocrine regulation of fathering behavior in birds. Horm. Behav. 2016;77:237–248. doi: 10.1016/j.yhbeh.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Lynn SE, Hayward LS, Benowitz-Fredericks ZM, Wingfield JC. Behavioural insensitivity to supplementary testosterone during the parental phase in the chestnut-collared longspur, Calcarius ornatus. Anim. Behav. 2002;63:795–803. [Google Scholar]
- Lynn SE, Walker BG, Wingfield JC. A phylogenetically controlled test of hypotheses for behavioral insensitivity to testosterone in birds. Horm. Behav. 2005;47:170–177. doi: 10.1016/j.yhbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Magrath MJL, Komdeur J. Is male care compromised by additional mating opportunity? Trends Ecol. Evol. 2003;18:424–430. [Google Scholar]
- Marler CA, Bester-Meredith JK, Trainor BC. Paternal behavior and aggression: Endocrine mechanisms and nongenomic transmission of behavior. Adv. Study Behav. 2003;32:263–328. [Google Scholar]
- Marler CA, Ryan MJ. Energetic constraints and steroid hormone correlates of male calling behaviour in the túngara frog. J. Zool. 1996;240:397–410. [Google Scholar]
- Marshall AJ, Hohmann G. Urinary testosterone levels of wild male bonobos (Pan paniscus) in the Lomako Forest, Democratic Republic of Congo. Am. Nat. 1990;136:829–846. doi: 10.1002/ajp.20099. [DOI] [PubMed] [Google Scholar]
- Marty JS, Higham JP, Gadsby EL, Ross C. Dominance, coloration, and social and sexual behavior in male drills Mandrillus leucophaeus. Int. J. Primatol. 2009;30:807–823. [Google Scholar]
- Marty PR, Van Noordwijk MA, Heistermann M, Willems EP, Dunkel LP, Cadilek M, Agil M, Weingrill T. Endocrinological correlates of male bimaturism in wild Bornean orangutans. Am. J. Primatol. 2015;77:1170–1178. doi: 10.1002/ajp.22453. [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Rilling JK. Testicular volume is inversely correlated with nurturing-related brain activity in human fathers. Proc. Natl. Acad. Sci. USA. 2013;110:15746–15751. doi: 10.1073/pnas.1305579110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behav. Brain Sci. 1998;21:353–363. [PubMed] [Google Scholar]
- McDonald PG, Buttemer WA, Astheimer LB. The influence of testosterone on territorial defence and parental behavior in male free-living rufous whistlers, Pachycephala rufiventris. Horm. Behav. 2001;39:185–194. doi: 10.1006/hbeh.2001.1644. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J. Evol. Biol. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- McIntyre M, Gangestad SW, Gray PB, Chapman JF, Burnham TC, O'Rourke MT, Thornhill R. Romantic involvement often reduces men's testosterone levels - but not always: the moderating role of extrapair sexual interest. J. Pers. Soc. Psychol. 2006;91:642. doi: 10.1037/0022-3514.91.4.642. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M. CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry Res. 1997;72:89–102. doi: 10.1016/s0165-1781(97)00084-x. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone change after losing predicts the decision to compete again. Horm. Behav. 2006;50:684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Welker KM, Zilioli S, Carré JM. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinol. 2015;56:88–99. doi: 10.1016/j.psyneuen.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Mendonça-Furtado O, Edaes M, Palme R, Rodrigues A, Siqueira J, Izar P. Does hierarchy stability influence testosterone and cortisol levels of bearded capuchin monkeys (Sapajus libidinosus) adult males? A comparison between two wild groups. Behav. Process. 2014;109:79–88. doi: 10.1016/j.beproc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macaques. Am. J. Primatol. 1993;30:213–241. doi: 10.1002/ajp.1350300306. [DOI] [PubMed] [Google Scholar]
- Mitani JC. Sexual selection and adult male orangutan long calls. Anim. Behav. 1985;33:272–283. [Google Scholar]
- Mitani JC, Gros-Louis J, Richards AF. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am. Nat. 1996;147:966–980. [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nature Rev. Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Möhle U, Heistermann M, Palme R, Hodges JK. Characterization of urinary and fecal metabolites of testosterone and their measurement for assessing gonadal endocrine function in male nonhuman primates. Gen. Comp. Endocrinol. 2002;129:135–145. doi: 10.1016/s0016-6480(02)00525-7. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, Van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv. Emotion. 2012;36:65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino L. Social correlates of androgen levels in a facultatively monogamous ape (Symphalangus syndactylus): A test of the challenge hypothesis. Behav. Ecol. Sociobiol. 2015;69:243–251. [Google Scholar]
- Muehlenbein MP, Campbell BC, Murchison MA, Phillippi KM. Morphological and hormonal parameters in two species of macaques: Impact of seasonal breeding. Am. J. Phys. Anth. 2002;117:218–227. doi: 10.1002/ajpa.10030. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Campbell BC, Phillippi KM, Murchison MA, Richards RJ, Svec F, Myers L. Reproductive maturation in a sample of captive male baboons. J. Med. Primatol. 2001;30:273–282. doi: 10.1034/j.1600-0684.2001.d01-60.x. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Watts DP. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosoc. Med. 2010;4:21. doi: 10.1186/1751-0759-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Emery Thompson M. Mating, parenting, and male reproductive strategies. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 387–411. [Google Scholar]
- Muller MN, Emery Thompson M, Beheim BA, Enigk DK, Gurven M, Sabbi K, Trumble BC, Wrangham RW, Kaplan HS. Testosterone, musculature, and development in Kanyawara chimpanzees and Tsimane forager-horticulturalists. Am. J. Phys. Anthropol. 2016a;159(S62):236. [Google Scholar]
- Muller MN, Beheim BA, Hahn BH, Langergraber KE, Scully EJ, Vigilant L, Wrangham RW, Wroblewski EE, Pusey AE. Age specific fertility rates in male chimpanzees (Pan troglodytes).. American Society of Primatologists/International Primatological Society joint meeting; Chicago. 2016b. [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proc. Roy. Soc. B. 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Thompson ME, Wrangham RW. Male chimpanzees prefer mating with old females. Curr. Biol. 2006;16:2234–2238. doi: 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. The reproductive ecology of male hominoids. In: Ellison PT, editor. Reproductive Ecology and Human Evolution. Aldine; Chicago: 2001. pp. 397–427. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: A test of the „challenge hypothesis'. Anim. Behav. 2004;67:113–123. [Google Scholar]
- Muller MN, Wrangham RW. Testosterone and energetics in wild chimpanzees (Pan troglodytes schweinfurthii). Am. J. Primatol. 2005;66:119–130. doi: 10.1002/ajp.20132. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. Sexual Coercion in Primates and Humans. Harvard University Press; Cambridge: 2009. [Google Scholar]
- Murdock GP, Provost C. Factors in the division of labor by sex: A cross-cultural analysis. Ethnology. 1973;12:203–225. [Google Scholar]
- Neumann C, Assahad G, Hammerschmidt K, Perwitasari-Farajallah D, Engelhardt A. Loud calls in male crested macaques, Macaca nigra: a signal of dominance in a tolerant species. Anim. Behav. 2010;79:187–193. [Google Scholar]
- Nowicki S, Ball GF. Testosterone induction of song in photosensitive and photorefractory male sparrows. Horm. Behav. 1989;23:514–525. doi: 10.1016/0018-506x(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii). Anim. Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera K, French JA. Endocrine variation associated with the phases of paternal behavior in black tufted-ear marmosets (Callithrix kuhlii). Horm. Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Oliveira GA, Oliveira RF. Androgen responsiveness to competition in humans: the role of cognitive variables. Neurosci. Neuroecon. 2014;3:19–32. [Google Scholar]
- Oliveira RF, Silva A, Canário AVM. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc. Roy. Soc. B. 2009;276:2249–2256. doi: 10.1098/rspb.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango PO, Gesquiere LR, Altmann J, Alberts SC. Testosterone positively associated with both male mating effort and paternal behavior in savanna baboons (Papio cynocephalus). Horm. Behav. 2013;63:430–436. doi: 10.1016/j.yhbeh.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Kappeler PM, Heistermann M. Androgen and glucocorticoid levels reflect seasonally occurring social challenges in male redfronted lemurs (Eulemur fulvus rufus). Behav. Ecol. Sociobiol. 2008;62:627–638. doi: 10.1007/s00265-007-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Heistermann M, Schülke O. Male competition and its hormonal correlates in Assamese macaques (Macaca assamensis). Horm. Behav. 2011;59:105–113. doi: 10.1016/j.yhbeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Ostner J, Kappeler PM, Heistermann M. Seasonal variation and social correlates of androgen excretion in male redfronted lemurs (Eulemur fulvus rufus). Behav. Ecol. Sociobiol. 2002;52:485–495. doi: 10.1007/s00265-007-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 2005;48:259–267. doi: 10.1016/j.yhbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Palombit R. “Friendship” with males: A female counterstrategy to infanticide in chacma baboons of the Okavango Delta. In: Muller MN, Wrangham RW, editors. Sexual Coercion in Primates and Humans: An Evolutionary Perspective on Male Aggression Against Females. Harvard University Press; Cambridge MA: 2009. pp. 377–409. [Google Scholar]
- Palombit RA. Infanticide and the evolution of male-female bonds in animals. In: van Schaik CP, Janson CH, editors. Infanticide by Males and its Implications. Cambridge University Press; Cambridge: 2000. pp. 239–268. [Google Scholar]
- Palombit RA. Infanticide: male strategies and female counterstrategies. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. The Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 432–468. [Google Scholar]
- Pappano DJ, Beehner JC. Harem-holding males do not rise to the challenge: androgens respond to social but not to seasonal challenges in wild geladas. R. Soc. Open Sci. 2014;1:140081. doi: 10.1098/rsos.140081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch B, George AS, Hamlin HJ, Guillette LJ, Phelps SM. Androgens modulate song effort and aggression in Neotropical singing mice. Horm. Behav. 2011;59:90–97. doi: 10.1016/j.yhbeh.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Peters A, Cockburn A, Cunningham R. Testosterone treatment suppresses paternal care in superb fairy-wrens, Malurus cyaneus, despite their concurrent investment in courtship. Behav. Ecol. Sociobiol. 2002;51:538–547. [Google Scholar]
- Peters M, Simmons LW, Rhodes G. Testosterone is associated with mating success but not attractiveness or masculinity in human males. Anim. Behav. 2008;76:297–303. [Google Scholar]
- Phillips SM. Strength and hypertrophy with resistance training: chasing a hormonal ghost. Eur. J. Appl. Physiol. 2012;112:1981–1983. doi: 10.1007/s00421-011-2148-0. [DOI] [PubMed] [Google Scholar]
- Plavcan JM. Sexual selection, measures of sexual selection, and sexual dimorphism in primates. In: Kappeler PM, van Schaik C, editors. Sexual Selection in Primates: New and Comparative Perspectives. Cambridge University Press; Cambridge: 2004. pp. 230–252. [Google Scholar]
- Pollet TV, van der Meij L, Cobey KD, Buunk AP. Testosterone levels and their associations with lifetime number of opposite sex partners and remarriage in a large sample of American elderly men and women. Horm. Behav. 2011;60:72–77. doi: 10.1016/j.yhbeh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Prall SP, Muelenbein MP. Testosterone and immune function in primates: A brief summary with methodological considerations. 2015;35:805–824. [Google Scholar]
- Prudom SL, Broz CA, Schultz-Darken N, Ferris CT, Snowdon C, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus). Biol. Lett. 2008;4:603–605. doi: 10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts DA, Doll LM, Hill AK. Sexual selection on human voices. In: Shackelford TK, Weekes-Shackelford VA, editors. Evolutionary Perspectives on Human Sexual Psychology and Behavior. Springer; New York: 2014. pp. 69–86. [Google Scholar]
- Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - Part 1: Are there really differences between women and men? Pain. 2012;153:602–618. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Radke S, Volman I, Mehta P, van Son V, Enter D, Sanfey A, Toni I, de Bruijn ERA, Roelofs K. Testosterone biases the amygdala toward social threat approach. Sci. Adv. 2015;1:e1400074. doi: 10.1126/sciadv.1400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Negrín A, Dias PAD, Chavira R, Canales-Espinosa D. Social modulation of testosterone levels in male black howlers (Alouatta pigra). Horm. Behav. 2011;59:159–166. doi: 10.1016/j.yhbeh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Raynaud J, Schradin C. Experimental increase of testosterone increases boldness and decreases anxiety in male African striped mouse helpers. Physiol. Behav. 2014;129:57–63. doi: 10.1016/j.physbeh.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Reby D, Charlton BD, Locatelli Y, McComb K. Oestrous red deer hinds prefer male roars with higher fundamental frequencies. Proc. Roy. Soc. B. 2010;277:2747–2753. doi: 10.1098/rspb.2010.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: Acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 2003;65:519–530. [Google Scholar]
- Rhodes L, Argersinger ME, Gantert LT, Friscino BH, Hom G, Pikounis B, Hess DL, Rhodes WL. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, an aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J. Reprod. Fertil. 1997;111:51–57. doi: 10.1530/jrf.0.1110051. [DOI] [PubMed] [Google Scholar]
- Robbins MM, Czekala NM. A preliminary investigation of urinary testosterone and cortisol levels in wild male mountain gorillas. Am. J. Primatol. 1997;43:51–64. doi: 10.1002/(SICI)1098-2345(1997)43:1<51::AID-AJP4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: A review of the evidence. Anim. Behav. 2004;68:227–239. [Google Scholar]
- Roney JR, Gettler LT. The role of testosterone in human romantic relationships. Curr. Opin. Psychol. 2015;1:81–86. [Google Scholar]
- Roney JR, Hanson KN, Durante KM, Maestripieri D. Reading men's faces: women's mate attractiveness judgments track men's testosterone and interest in infants. Proc. Roy. Soc. B. 2006;273:2169–2175. doi: 10.1098/rspb.2006.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum S, Silk J, Stoinski TS. Male–immature relationships in multi-male groups of mountain gorillas (Gorilla beringei beringei). Am. J. Primatol. 2011;73:356–365. doi: 10.1002/ajp.20905. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Hirwa JP, Vigilant L, Santymire R, Stoinski TS. Dads and cads? Male reproductive success, androgen profiles, and male-infant social bonds in wild mountain gorillas (Gorilla beringei beringei). Am. J. Phys. Anth. 2016;159(S62):272–273. [Google Scholar]
- Ross CN, French JA, Patera KJ. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiol. Behav. 2004;83:437–445. doi: 10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Ruiz de Elvira M, Herndon J, Collins D. Effect of estradiol-treated females on all female groups of rhesus monkeys during the transition between the nonbreeding and breeding season. Folia Primatol. 1983;41:191–203. doi: 10.1159/000156131. [DOI] [PubMed] [Google Scholar]
- Rushmore J, Caillaud D, Matamba L, Stumpf RM, Borgatti SP, Altizer S. Social network analysis of wild chimpanzees with insights for disease transmission. J. Anim. Ecol. 2013;82:976–986. doi: 10.1111/1365-2656.12088. [DOI] [PubMed] [Google Scholar]
- Rushmore J, Caillaud D, Hall RJ, Stumpf RM, Meyers LA, Altizer S. Network-based vaccination improves prospects for disease control in wild chimpanzees. J. R. Soc. Interface. 2014;11:20140349. doi: 10.1098/rsif.2014.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, Brinkhof MWG. What sets the odds of winning and losing? Trends Ecol. Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Salvador A, Veiga JP, Martin J, Lopez P, Abelenda M, Puertac M. The cost of producing a sexual signal: testosterone increases the susceptibility of male lizards to ectoparasitic infestation. Behav. Ecol. 1996;7:145–150. [Google Scholar]
- Salzman W, Ziegler TE. Functional significance of hormonal changes in mammalian fathers. J. Neuroendocrinol. 2014;26:685–696. doi: 10.1111/jne.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology alfresco: Psychoendocrine studies of wild baboons. Recent Prog. Horm. Res. 1993;48:437–468. doi: 10.1016/b978-0-12-571148-7.50020-8. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Ketterson ED, Nolan V, Sharp PJ, Buntin JD. The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding sites in dark-eyed juncos. Horm. Behav. 1998;34:1–10. doi: 10.1006/hbeh.1998.1455. [DOI] [PubMed] [Google Scholar]
- Schoof VAM, Jack KM. The association of intergroup encounters, dominance status, and fecal androgen and glucocorticoid profiles in wild male white-faced capuchins (Cebus capucinus). Am. J. Primatol. 2013;75:107–115. doi: 10.1002/ajp.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof VAM, Jack KM, Ziegler TE. Male response to female ovulation in white-faced capuchins (Cebus capucinus): Variation in fecal testosterone, dihydrotestosterone, and glucocorticoids. Int. J. Primatol. 2014;35:643–660. [Google Scholar]
- Schoof VAM, Bonnell TR, Jack KM, Ziegler TE, Melin AD, Fedigan LM. Male endocrine response to seasonally varying environmental and social factors in a neotropical primate, Cebus capucinus. Am. J. Phys. Anth. 2016;159:671–682. doi: 10.1002/ajpa.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss OC, Campbell KL, McClelland DC. Implicit power motivation moderates men's testosterone responses to imagined and real dominance success. Horm. Behav. 1999;36:234–241. doi: 10.1006/hbeh.1999.1542. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Rohde W. Implicit power motivation predicts men's testosterone changes and implicit learning in a contest situation. Horm. Behav. 2002;41:195–202. doi: 10.1006/hbeh.2001.1745. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Stanton SJ. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Horm. Behav. 2004;46:592–599. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men's and women's implicit learning and testosterone changes after social victory or defeat. J. Pers. Soc. Psychol. 2005;88:174–188. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- Sellers JG, Mehl MR, Josephs RA. Hormones and personality: Testosterone as a marker of individual differences. J. Res. Pers. 2007;41:126–138. [Google Scholar]
- Senar JC. Color displays as intrasexual signals of aggression and dominance. In: Hill GE, McGraw KJ, editors. Bird Coloration: Function and Evolution. Harvard University Press; Cambridge MA: 2006. pp. 87–136. [Google Scholar]
- Setchell JM. Color in competition contexts in non-human animals. In: Elliot AJ, Fairchild MD, Franklin A, editors. Handbook of Color Psychology. Cambridge University Press; Cambridge: 2016. pp. 546–567. [Google Scholar]
- Setchell JM, Dixson AF. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Horm. Behav. 2001;39:177–184. doi: 10.1006/hbeh.2000.1628. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Jean Wickings E. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology. 2005;111:25–50. [Google Scholar]
- Setchell JM, Smith T, Wickings EJ, Knapp LA. Social correlates of testosterone and ornamentation in male mandrills. Horm. Behav. 2008;54:365–372. doi: 10.1016/j.yhbeh.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. Social relationships among adult male and female baboons. I. Behaviour during sexual consortship. Behav. 1978;64:204–226. [Google Scholar]
- Sinervo B, Miles DB, Frankino WA, Klukowski M, DeNardo DF. Testosterone, endurance, and Darwinian fitness: Natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Horm. Behav. 2000;38:222–233. doi: 10.1006/hbeh.2000.1622. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Gubernick DJ. Male-infant relationships in nonhuman primates: Paternal investment or mating effort. In: Hewlett BS, editor. Father-Child Relations: Cultural and Biosocial Contexts. Aldine Transaction; New Brunswick NJ: 1992. pp. 1–30. [Google Scholar]
- Sobolewski ME, Brown JL, Mitani JC. Territoriality, tolerance and testosterone in wild chimpanzees. Anim. Behav. 2012;84:1469–1474. [Google Scholar]
- Sobolewski ME, Brown JL, Mitani JC. Female parity, male aggression, and the challenge hypothesis in wild chimpanzees. Primates. 2013;54:81–88. doi: 10.1007/s10329-012-0332-4. [DOI] [PubMed] [Google Scholar]
- Soler H, Vinayak P, Quadagno D. Biosocial aspects of domestic violence. Psychoneuroendocrinol. 2000;25:721–739. doi: 10.1016/s0306-4530(00)00022-6. [DOI] [PubMed] [Google Scholar]
- Solis R, Penna M. Testosterone levels and evoked vocal responses in a natural population of the frog Batrachyla taeniata. Horm. Behav. 1997;31:101–109. doi: 10.1006/hbeh.1997.1366. [DOI] [PubMed] [Google Scholar]
- Stanford CB. Chimpanzee and Red Colobus: The Ecology of Predator and Prey. Harvard University Press; Cambridge MA.: 1998. [Google Scholar]
- Stanton SJ, Schultheiss OC. The hormonal correlates of implicit power motivation. J. Res. Pers. 2009;43:942–949. doi: 10.1016/j.jrp.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Wirth MM, Waugh CE, Schultheiss OC. Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biol. Psychol. 2009;81:118–122. doi: 10.1016/j.biopsycho.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. Trade-offs in life-history evolution. Funct. Ecol. 1989;3:259–268. [Google Scholar]
- Sterck EHM, Korstjens AH. Female dispersal and infanticide avoidance in primates. In: van Schaik CP, Janson CH, editors. Infanticide by Males and its Implications. Cambridge University Press; Cambridge: 2000. pp. 293–321. [Google Scholar]
- Stiver KA, Alonzo SH. Parental and mating effort: Is there necessarily a trade-off? Ethology. 2009;115:1101–1126. [Google Scholar]
- Stoehr AM, Hill GE. Testosterone and the allocation of reproductive effort in male house finches (Carpodacus mexicanus). Behav. Ecol. Sociobiol. 2000;48:407–411. [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol. Hum. Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Storey AE, Ziegler TE. Primate paternal care: Interactions between biology and social experience. Horm. Behav. 2016;77:260–271. doi: 10.1016/j.yhbeh.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strier KB, Ziegler TE, Wittwer DJ. Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Horm. Behav. 1999;35:125–134. doi: 10.1006/hbeh.1998.1505. [DOI] [PubMed] [Google Scholar]
- Suarez-Jimenez B, Gore HE, Hachey J, King HM, Lacreuse A. Testosterone modulation of anxiety in gonadally-suppressed male rhesus monkeys: A role for gonadotropins? Pharmacol. Biochem. Behav. 2013;104:97–104. doi: 10.1016/j.pbb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Surbeck M, Deschner T, Schubert G, Weltring A, Hohmann G. Mate competition, testosterone and intersexual relationships in bonobos, Pan paniscus. Anim. Behav. 2012;83:659–669. [Google Scholar]
- Teichroeb JA, Sicotte P. Social correlates of fecal testosterone in male ursine colobus monkeys (Colobus vellerosus): The effect of male reproductive competition in aseasonal breeders. Horm. Behav. 2008;54:417–423. doi: 10.1016/j.yhbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Terburg D, Aarts H, van Honk J. Testosterone affects gaze aversion from angry faces outside of conscious awareness. Psychol. Sci. 2012;23:459–463. doi: 10.1177/0956797611433336. [DOI] [PubMed] [Google Scholar]
- Terburg D, van Honk J. Approach–avoidance versus dominance - submissiveness: A multilevel neural framework on how testosterone promotes social status. Emot. Rev. 2013;5:296–302. [Google Scholar]
- Titze IR, Riede T. A cervid vocal fold model suggests greater glottal efficiency in calling at high frequencies. PLoS Comput. Biol. 2010;6:e1000897. doi: 10.1371/journal.pcbi.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DS, Moger WH. Plasma androgen levels during male parental care in a tropical frog (Eleutherodactylus). Horm. Behav. 1987;21:93–99. doi: 10.1016/0018-506x(87)90034-1. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Marler CA. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 2004;45:115–121. doi: 10.1016/j.yhbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus). Horm. Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. Roy. Soc. B. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man, 1871-1971. Aldine; Chicago: 1972. pp. 136–179. [Google Scholar]
- Tsai LW, Sapolsky RM. Rapid stimulatory effects of testosterone upon myotubule metabolism and sugar transport, as assessed by silicon microphysiometry. Aggress. Behav. 1996;22:357–364. [Google Scholar]
- van Anders SM, Goldey KL, Kuo PX. The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinol. 2011;36:1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Watson NV. Multiple partners are associated with higher testosterone in North American men and women. Horm. Behav. 2007;51:454–459. doi: 10.1016/j.yhbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Tolman RM, Jainagaraj G. Examining how infant interactions influence men's hormones, affect, and aggression using the Michigan infant nurturance simulation paradigm. Fathering. 2014;12:143–160. [Google Scholar]
- van Anders SM, Tolman RM, Volling BL. Baby cries and nurturance affect testosterone in men. Horm. Behav. 2012;61:31–36. doi: 10.1016/j.yhbeh.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Van Belle S, Estrada A, Ziegler TE, Strier KB. Social and hormonal mechanisms underlying male reproductive strategies in black howler monkeys (Alouatta pigra). Horm. Behav. 2009;56:355–363. doi: 10.1016/j.yhbeh.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Van Duyse E, Pinxten R, Eens M. Effects of testosterone on song, aggression, and nestling feeding behavior in male great tits, Parus major. Horm. Behav. 2002;41:178–186. doi: 10.1006/hbeh.2001.1747. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJLG. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol. Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Hermans E, Putnam P, Koppeschaar H, Thijssen J, Verbaten R, van Doornen L. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behav. Neurosci. 2001;115:238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Verbaten R, van den Hout M, Koppeschaar H, Thijssen J, de Haan E. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm. Behav. 1999;36:17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- Van Roo BL. Exogenous testosterone inhibits several forms of male parental behavior and stimulates song in a monogamous songbird: the blue-headed vireo (Vireo solitarius). Horm. Behav. 2004;46:678–683. doi: 10.1016/j.yhbeh.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Van Schaik CP, Paul A. Male care in primates: Does it ever reflect paternity? Evol. Anthropol. 1996;5:152–156. [Google Scholar]
- Vandenbergh JG. Hormonal basis of sex skin in male rhesus monkeys. Gen. Comp. Endocrinol. 1965;5:31–34. doi: 10.1016/0016-6480(65)90065-1. [DOI] [PubMed] [Google Scholar]
- Waitt C, Little AC, Wolfensohn S, Honess P, Brown AP, Buchanan-Smith HM, Perrett DI. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proc. Roy. Soc. B. 2003;270:S144–S146. doi: 10.1098/rsbl.2003.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DP. Infanticide in mountain gorillas: New cases and a reconsideration of the evidence. Ethology. 1989;81:1–18. [Google Scholar]
- Weinbauer GF, Niehaus M, Nieschlag E. The role of testosterone in spermatogenesis. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. Cambridge University Press; Cambridge: 2004. pp. 173–206. [Google Scholar]
- Weinbauer GF, Partsch CJ, Zitzmann M, Schlatt S, Nieschlag E. Pharmacokinetics and degree of aromatization rather than total dose of different preparations determine the effects of testosterone: a nonhuman primate study in Macaca fascicularis. J. Androl. 2003;24:765–774. doi: 10.1002/j.1939-4640.2003.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration, salivary testosterone, and father-infant social behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;49:47–52. doi: 10.1016/j.pnpbp.2013.11.006. [DOI] [PubMed] [Google Scholar]
- West DWD, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. Journal of Applied Physiology. 2010;108:60–67. doi: 10.1152/japplphysiol.01147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DWD, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J. Physiol. 2009;587:5239–5247. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DWD, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur. J. Appl. Physiol. 2012;112:2693–2702. doi: 10.1007/s00421-011-2246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting MJ, Nagy KA, Bateman PW. Evolution and maintenance of social status-signaling badges. In: Fox SF, McCoy JK, Baird TA, editors. Lizard Social Behaviour. John Hopkins University Press; Baltimore MD: 2003. pp. 47–82. [Google Scholar]
- Whitten PL. Evolutionary endocrinology of the cercopithecoids. In: Whitehead PF, Jolly CJ, editors. Old World Monkeys. Cambridge University Press; Cambridge: 2000. pp. 269–297. [Google Scholar]
- Wich SA, Van der Post DJ, Heistermann M, Möhle U, Van Hooff J, Sterck EHM. Life-phase related changes in male loud call characteristics and testosterone levels in wild Thomas langurs. Int. J. Primatol. 2003;24:1251–1265. [Google Scholar]
- Wickings EJ, Dixson AF. Testicular function, secondary sexual development, and social status in male mandrills (Mandrillus sphinx). Physiol. Behav. 1992;52:909–916. doi: 10.1016/0031-9384(92)90370-h. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS, O'Bryant EL. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm. Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Oehlert GW, Carlis JV, Pusey AE. Why do male chimpanzees defend a group range? Anim. Behav. 2004;68:523–532. [Google Scholar]
- Wingfield JC. Control of territorial aggression in a changing environment. Psychoneuroendocrinol. 1994;19:709–721. doi: 10.1016/0306-4530(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136:829–846. [Google Scholar]
- Wingfield JC, Jacobs JD, Tramontin AD, Perfito N, Meddle S, Maney DL, Soma K. Toward an ecological basis of hormone-behavior interactions in reproduction of birds. In: Wallen K, Schneider JE, editors. Reproduction in Context. MIT Press; Cambridge MA: 2000. pp. 85–128. [Google Scholar]
- Wingfield JC, Lynn S, Soma KK. Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav. Evol. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Moore IT, Goymann W, Wacker DW, Sperry T. Contexts and ethology of vertebrate aggression: Implications for the evolution of hormone-behavior interactions. In: Nelson RJ, editor. Biology of Aggression. Oxford University Press; Oxford: 2006. pp. 179–210. [Google Scholar]
- Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiol. Behav. 2007;90:496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Wood BM, Marlowe FW. Household and kin provisioning by Hadza men. Hum. Nat. 2013;24:280–317. doi: 10.1007/s12110-013-9173-0. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm. Behav. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- Xia D-P, Li J-H, Sun B-H, Weed JL, Kyes RC. Evaluation of fecal testosterone, rank and copulatory behavior in wild male Macaca thibetana at Huangshan. China. Pakistan J. Zool. 2015;47:1445–1454. [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Ann. Rev. Ecol. Syst. 2001;32:95–126. [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate's pregnancy. Horm. Behav. 2004;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Zilioli S, Ponzi D, Henry A, Kubicki K, Nickels N, Wilson MC, Maestripieri D. Interest in babies negatively predicts testosterone responses to sexual visual stimuli among heterosexual young men. Psychol. Sci. 2015;27:114–118. doi: 10.1177/0956797615615868. [DOI] [PubMed] [Google Scholar]
- Zilioli S, Watson NV. Testosterone across successive competitions: Evidence for a ‘winner effect’ in humans? Psychoneuroendocrinol. 2014;47:1–9. doi: 10.1016/j.psyneuen.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Zimmermann E. Castration affects the emission of an ultrasonic vocalization in a nocturnal primate, the grey mouse lemur (Microcebus murinus). Physiol. Behav. 1996;60:693–697. doi: 10.1016/0031-9384(96)81674-x. [DOI] [PubMed] [Google Scholar]
- Zingeser MR, Phoenix CH. Metric characteristics of the canine dental complex in prenatally androgenized female rhesus monkeys (Macaca mulatta). Am. J. Phys. Anth. 1978;49:187–192. doi: 10.1002/ajpa.1330490206. [DOI] [PubMed] [Google Scholar]
- Zuckerman S, Pakes AS. Observations on the secondary sexual characters in monkeys. J. Endocrin. 1939;1:430–439. [Google Scholar]