Abstract
Intracellular calcium (Ca2+) oscillation is an initial event in digestive enzyme secretion of pancreatic acinar cells. Reactive oxygen species are known to be associated with a variety of oxidative stress-induced cellular disorders including pancreatitis. In this study, we investigated the effect of hydrogen peroxide (H2O2) on intracellular Ca2+ accumulation in mouse pancreatic acinar cells. Perfusion of H2O2 at 300 µM resulted in additional elevation of intracellular Ca2+ levels and termination of oscillatory Ca2+ signals induced by carbamylcholine (CCh) in the presence of normal extracellular Ca2+. Antioxidants, catalase or DTT, completely prevented H2O2-induced additional Ca2+ increase and termination of Ca2+ oscillation. In Ca2+-free medium, H2O2 still enhanced CCh-induced intracellular Ca2+ levels and thapsigargin (TG) mimicked H2O2-induced cytosolic Ca2+ increase. Furthermore, H2O2-induced elevation of intracellular Ca2+ levels was abolished under sarco/endoplasmic reticulum Ca2+ ATPase-inactivated condition by TG pretreatment with CCh. H2O2 at 300 µM failed to affect store-operated Ca2+ entry or Ca2+ extrusion through plasma membrane. Additionally, ruthenium red, a mitochondrial Ca2+ uniporter blocker, failed to attenuate H2O2-induced intracellular Ca2+ elevation. These results provide evidence that excessive generation of H2O2 in pathological conditions could accumulate intracellular Ca2+ by attenuating refilling of internal Ca2+ stores rather than by inhibiting Ca2+ extrusion to extracellular fluid or enhancing Ca2+ mobilization from extracellular medium in mouse pancreatic acinar cells.
Keywords: Hydrogen peroxide, Intracellular Ca2+ stores, Pancreatic acinar cells, Reactive oxygen species, Sarcoplasmic reticulum Ca2+ ATPase
INTRODUCTION
Reactive oxygen species (ROS) are formed as a result of partial reduction of oxygen during aerobic respiration [1]. They cause oxidative damage to various biological molecules including DNA, lipids, and proteins, thereby disrupting normal cellular function [2,3,4]. Under physiological conditions, ROS are controlled by intracellular free radical scavengers and antioxidant enzymes to protect cells from injuries [5]. However, imbalance between ROS generating and scavenging systems can lead to oxidative stress which can morphologically and functionally damage cells [6]. It is well-known that hydrogen peroxide (H2O2), one type of ROS, can disrupt normal functions in various cell types [2,3]. It is correlated with overloaded intracellular Ca2+ [7,8,9]. However, the mechanism of H2O2-induced Ca2+ accumulation has known to be complicated due to cell-to-cell difference in expression and participation of Ca2+ modulating transporters. It has been reported that H2O2 can enhance Ca2+ release from intracellular store [10,11,12], stimulate Ca2+ entry from extracellular medium [13,14,15,16], and attenuate Ca2+ extrusion by plasma membrane Ca2+ ATPase (PMCA) or sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inactivation [17,18] in various cell types.
Pancreatic acinar cells synthesize and secrete a variety of digestive enzyme, tightly regulated by intracellular repetitive Ca2+ oscillation [19,20]. A physiological concentration of carbamylcholine (CCh) could generate Ca2+ oscillation known to be initiated by inositol 1,4,5-trisphospate receptors-mediated Ca2+ release from the intracellular store followed by activation of Ca2+ entry from extracellular medium [21,22]. The loaded Ca2+ is rapidly cleared to the internal store through SERCA or to the extracellular space through PMCA [23]. Overloaded Ca2+ can cause premature intracellular digestive enzyme activation and cellular injury, one of characteristics of pancreatitis [24,25].
Although the pathophysiology of pancreatitis remains unclear at the present time, it has been proposed that oxidative stress due to excess generation of ROS is involved in acute pancreatitis [26]. A prominent feature of acute pancreatitis is disruption of Ca2+ homeostasis within pancreatic acinar cells, and cytosolic Ca2+ accumulation has been shown to cause elevation of ROS in acinar cells that promote cell death [27]. Moreover, there are evidences showing that antioxidants can provide benefits to pancreatitis patients with pancreatic cell injury [28]. However, how ROS accumulates intracellular Ca2+ in pancreatic acinar cell is unclear at the present time. The objective of this study was to characterize the effect of H2O2 on CCh-induced intracellular Ca2+ signals and the underlying mechanism involved in Ca2+ accumulation in mouse pancreatic acinar cells. Here we report that H2O2 could accumulate intracellular Ca2+ by reducing refilling of intracellular Ca2+ stores, rather than by inhibiting Ca2+ extrusion to extracellular fluid or enhancing Ca2+ mobilization from extracellular medium in mouse pancreatic acinar cells.
METHODS
Animals
Male BALB/c mice at 8~10 weeks old were humanely handled and housed under specific pathogen-free conditions in clean polypropylene cages. They were maintained in air conditioned room at 20~22℃ with a constant photoperiod of 12 hours light/dark cycle. Mice were provided free access to pallet diet and drinking water ad libitum. All animal experiments were performed in accordance with the Guideline for the Care and Use of Laboratory Animal provided by NIH. All experiments adhered to Konyang University policies regarding the care and use of animals.
Materials
Type II collagenase was purchased from Roche Diagnostics GmbH (Mannheim, Germany). Fura-2/acetoxymethyl ester (fura-2/AM) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Thapsigargin (TG) was purchased from Tocris (Avonmouth, BS, UK). All other materials were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of pancreatic acinar cells
Small clusters of pancreatic acinar cells (10~15 cells per experiment) were freshly isolated using collagenase digestion method as described previously [29,30]. Briefly, the pancreas was removed from mice after CO2 asphyxiation and cervical dislocation. The dissected tissue was enzymatically digested with type II collagenase in HEPES-buffered physiological saline containing 0.01% trypsin inhibitor (soybean) and 0.1% bovine serum albumin (BSA) for 30 minutes followed by mechanical dissociation of cells by gentle agitation. Cells were then filtered through 100 mm nylon mesh and centrifuged at 75 g with 1% BSA. After isolation, cells were resuspended in HEPES-buffered physiological saline containing 137 mM NaCl, 4.7 mM KCl, 0.56 mM MgCl2, 1 mM Na2HPO4, 10 mM HEPES, 1.28 mM CaCl2 and 5.5 mM glucose (pH 7.4 adjusted with NaOH) until use. For Ca2+-free condition, HEPES-buffered physiological saline without adding Ca2+ was supplemented with 5 mM ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA).
Intracellular Ca2+ measurements
To measure intracellular Ca2+, the isolated acinar cells were loaded with 5 µM Fura-2/AM and incubated at room temperature in dark condition for 40 minutes. Fura-2/AM loaded cells were mounted onto a cover-glass at the bottom of perfusion chambers. Cells were continuously perfused with HEPES-buffered physiological saline using an electronically controlled perfusion system (Warner Instrument, Hamden, CT, USA). Cells were excited alternately with light at 340 nm and 380 nm using a Polychrome V monochrometer (TILL Photonics, Pleasanton, CA, USA). Fluorescence emission at 505 nm was detected with a Cool-SNAP HQ2 camera (Photometrics, Tucson, AZ, USA) attached to an inverted microscope. The fluorescence ratio of 340/380 was measured using Till-Photonics imaging system. Stimuli were dissolved in HEPES-buffered physiological saline and used to continuously perfuse cells in the perfusion chamber at a flow rate of 1 ml/minute using an electronic controlled perfusion system (Waner Instrument, Hamden, CT, USA).
Data analysis
Values are expressed as mean±SEM. Student t test were used for data analysis. Differences were considered as statistically significant when the p value was less than 0.05. Ca2+ entry rates and extrusion rates were estimated by fitting the increasing and decreasing fluorescence to a single exponential function using Origin program.
RESULTS
Effects of hydrogen peroxide (H2O2) on CCh-induced intracellular Ca2+ oscillation
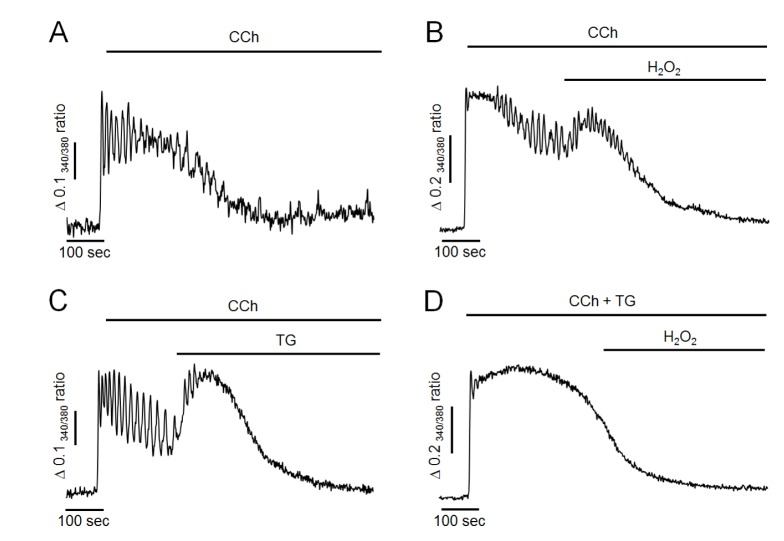
First, the effects of H2O2 on intracellular Ca2+ oscillation were performed in pancreatic acinar cells. Intracellular Ca2+ oscillation was evoked by 500 nM of CCh perfusion in the presence of extracellular Ca2+ at 1.28 mM in intact cells. As shown in Fig. 1A, CCh at 500 nM generated repetitive and sustained Ca2+ oscillation. After the steady state, perfusion of H2O2 at 300 µM resulted in additional elevation of intracellular Ca2+ levels and termination of Ca2+ oscillation in 97±4% cells (n=7, 98 cells). These effects were irreversible even when H2O2 was washed out. Since, in preliminary study, only small proportion of cells were response to H2O2 at 100 µM (34±3%, n=5, 73 cells), we used H2O2 at a concentration of 300 µM in the following studies. Additionally, pretreatment of antioxidants such as catalase at 30µg/ml or 1,4-dithiothreitol (DTT) at 2 mM with CCh completely prevented the effects of H2O2 (i.e., the additional elevation of intracellular Ca2+ levels and the termination of Ca2+ oscillation) (Fig. 1B, C). These results suggest that H2O2 could accumulate intracellular Ca2+ and disrupt normal oscillatory Ca2+ signals in mouse pancreatic acinar cells.
Fig. 1. Effects of hydrogen peroxide (H2O2) and antioxidants on CCh-induced intracellular Ca2+ oscillation in intact pancreatic acinar cells.
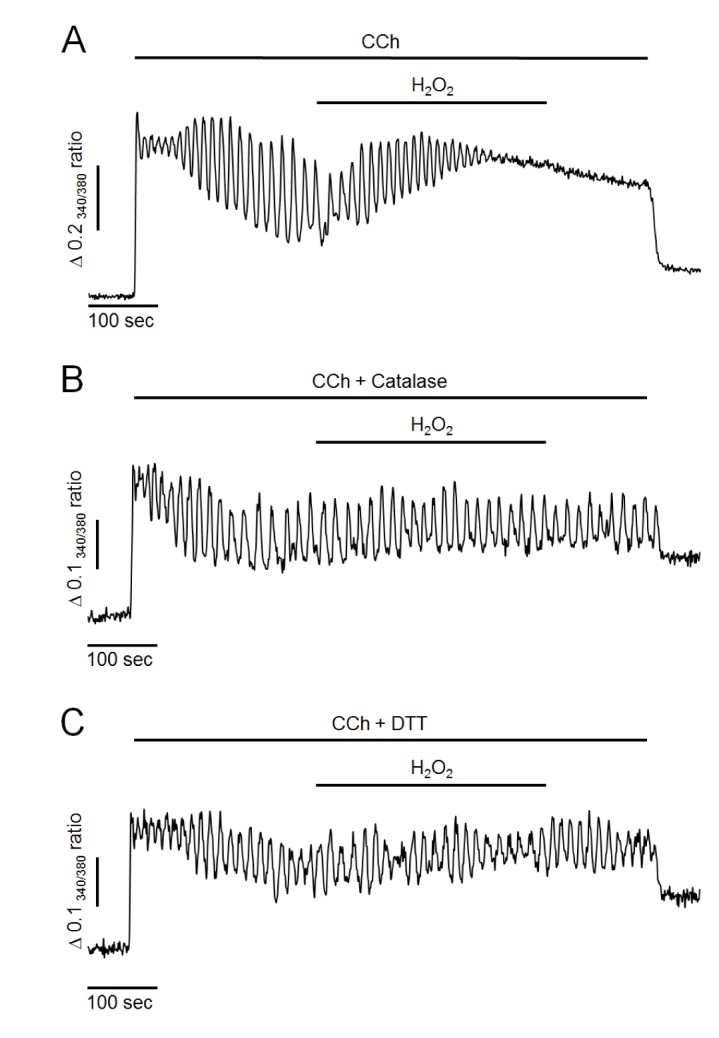
(A) Representative trace showing the effect of H2O2 on CCh-induced Ca2+ oscillation. (B, C) Representative traces showing the effects of antioxidants (30 µg/ml of catalase and 2 mM of DTT) on H2O2-induced intracellular Ca2+ changes. Oscillatory Ca2+ signals were induced by perfusion with 500 nM of CCh in HEPES buffer containing normal extracellular Ca2+. H2O2 at 300 µM was perfused for 5 minutes. All data were obtained from at least five separate experiments (71~98 cells) and expressed as changes of 340/380 ratio. The perfusion of H2O2 resulted in an elevation of intracellular Ca2+ concentration and a termination of Ca2+ oscillation. Antioxidants completely prevent H2O2-induced Ca2+ accumulation.
H2O2 does not affect Ca2+entry or Ca2+extrusion through plasma membrane
Next, we determined whether H2O2-induced Ca2+ accumulation was caused by facilitating Ca2+ entry from extracellular medium or reducing Ca2+ extrusion to extracellular medium through plasma membrane. As shown in Fig. 2A, Ca2+ store was initially depleted with 1 µM of TG in Ca2+-free medium. Store-operated Ca2+ entry was then stimulated by adding extracellular Ca2+ at 1.28 mM. Ca2+ extrusion through plasma membrane was then stimulated by changing to Ca2+-free medium in intact cells. In the control experiment, the adding of extracellular Ca2+ remarkably stimulated Ca2+ entry from extracellular fluid with a Ca2+ entry rate of 0.053±0.009 S−1. H2O2-induced Ca2+ entry rate was 0.056±0.007 S−1, which was not significantly different from the control value (Fig. 2B, C). In H2O2-treated cells, the removing of extracellular Ca2+ clearly extruded intracellular Ca2+ to external space (Fig. 2A). The Ca2+ extrusion rate was 0.033±0.005 S−1, which was not significantly different from its control value at 0.030±0.003 S−1 (Fig. 2D, E). Thus, neither Ca2+ entry from extracellular medium nor Ca2+ extrusion to extracellular medium was modified by H2O2 treatment. Therefore, H2O2-induced Ca2+ accumulation was not due to facilitating Ca2+ entry from extracellular medium or reducing Ca2+ extrusion to extracellular medium through plasma membrane in pancreatic acinar cells.
Fig. 2. H2O2 does not affect Ca2+ entry or Ca2+ extrusion in TG-treated pancreatic acinar cells.
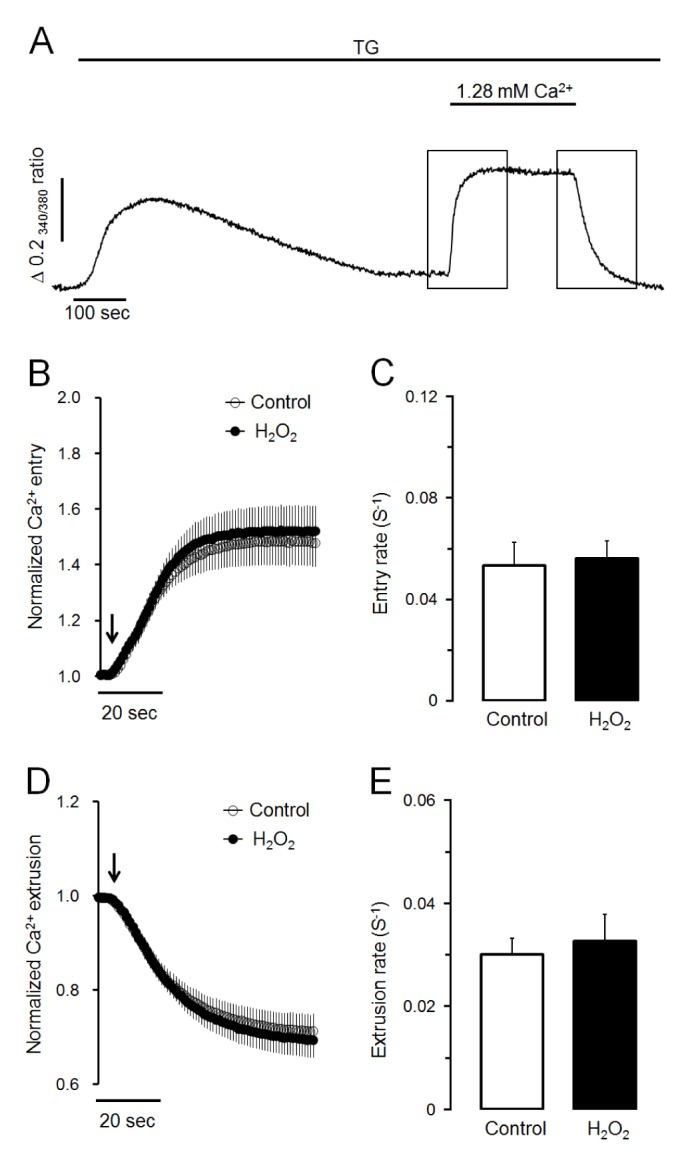
(A) Representative trace showing the effect of SERCA inactivation using TG on Ca2+ entry from extracellular medium and Ca2+ extrusion to extracellular medium. To deplete intracellular Ca2+ stores, TG at 1 µM was treated in Ca2+-free medium. After depletion of intracellular Ca2+ stores, 1.28 mM of Ca2+ was added and removed to activate Ca2+ entry and Ca2+ extrusion, respectively. (B, C) Effects of H2O2 on normalized Ca2+ entry and Ca2+ entry rate in TGtreated pancreatic acinar cells. Values are expressed as means±SEM obtained from six separate experiments (76 cells). (D, E) Effects of H2O2 on normalized Ca2+ extrusion and Ca2+ extrusion rate in TG-treated cells. H2O2 at 300 µM did not modify Ca2+ entry or Ca2+ extrusion through plasma membrane in TG-treated pancreatic acinar cells.
TG mimicsH2O2-induced Ca2+responses and pretreatment of TG completely abolishesH2O2-induced Ca2+responses in Ca2+-free medium
Next, we evaluated whether H2O2 could elevate intracellular Ca2+ levels in Ca2+-free medium because H2O2 did not facilitate Ca2+ entry or reduce Ca2+ extrusion through plasma membrane. As shown in Fig. 3A, in Ca2+-free medium, 500 nM of CCh resulted in Ca2+ oscillation in the initial state, indicating that CCh initially mobilized Ca2+ from intracellular stores. However, oscillatory signals were ceased and returned to baseline levels after 300~500 sec of CCh perfusion in Ca2+-free medium due to discontinued Ca2+ supply from extracellular fluid. After 200 sec of CCh perfusion, treatment with 300 µM H2O2 still resulted in an additional elevation of intracellular Ca2+ levels even when extracellular Ca2+ was eliminated (Fig. 3B). Furthermore, additional elevation of intracellular Ca2+ concentration was mimicked by TG treatment in Ca2+-free medium (Fig. 3C). However, the H2O2-induced additional increase of Ca2+ was completely abolished under SERCA-inactivated condition by TG pretreatment with CCh. Since, in this condition, intracellular TG-sensitive Ca2+ stores were already depleted, TG-insensitive other Ca2+ pools may not participate on H2O2-induced additional elevation of intracellular Ca2+ concentration. These results suggested that H2O2 could accumulate intracellular Ca2+ through inhibiting Ca2+ refilling to intracellular store by inactivation of SERCA, similar to the effect of TG.
Fig. 3. Effects of H2O2 and TG on CCh-induced intracellular Ca2+ response in Ca2+-free medium.
(A) Representative trace showing CCh-induced intracellular Ca2+ response in Ca2+-free medium. (B) H2O2 -induced additional elevation of intracellular Ca2+ levels. (C) TG mimicked the additional elevation of intracellular Ca2+ levels. (D) Pretreatment of TG with CCh completely abolished H2O2-induced additional elevation of intracellular Ca2+ levels. All data were obtained from at least five separate experiments (74~103 cells). Perfusion of H2O2 at 300 µM resulted in additional elevation of intracellular Ca2+ levels, which was mimicked by 1 µM of TG perfusion in Ca2+-free medium. H2O2-induced additional increase of Ca2+ was completely abolished by inactivation of SERCA with TG pretreatment.
Ruthenium red does not attenuate H2O2-induced Ca2+responses in Ca2+-free medium
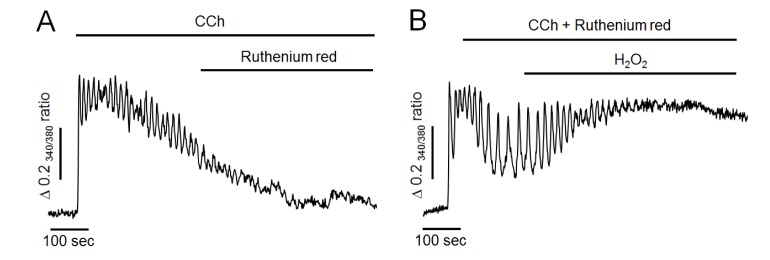
To further determine whether H2O2 could reduce mitochondrial Ca2+ buffering effect, ruthenium red at 50 µM, a mitochondrial Ca2+ uniporter blocker, was used in the following experiment. As shown in Fig. 4A, ruthenium red has no any effect to compare the control experiment (Fig. 3A), and failed to mimic H2O2-induced additional elevation of intracellular Ca2+ levels in Ca2+-free medium. Thus, it is unlikely that mitochondria remarkable participate on Ca2+ elimination after CCh stimulation. Moreover, H2O2 still elevated intracellular Ca2+ levels even when mitochondrial uniporter was blocked by pretreatment of ruthenium red with CCh (Fig. 4B). This result indicated that Ca2+ accumulation induced by H2O2 might not be by reducing Ca2+-buffering capacity of mitochondria in pancreatic acinar cells.
Fig. 4. Effect of ruthenium red on H2O2-induced intracellular Ca2+ response in Ca2+-free medium.
(A) Representative trace showing the effect of ruthenium red on CCh-induced intracellular Ca2+ response in Ca2+-free medium. (B) Pretreatment of ruthenium red with CCh failed to attenuate H2O2-induced additional elevation of intracellular Ca2+ levels. All data were obtained from six and seven separate experiments (70 and 81 cells). Perfusion of ruthenium red at 50 µM, a mitochondrial Ca2+ uniporter inhibitor, did not mimic H2O2-induced additional elevation of intracellular Ca2+ levels. After pretreatment of ruthenium red with CCh, H2O2 still elevated intracellular Ca2+ concentration.
DISCUSSION
The present study clearly provided evidence that H2O2, a reactive oxygen species, could accumulate cytosolic Ca2+ through attenuating refilling of intracellular Ca2+ store in mouse pancreatic acinar cells. Cytosolic free Ca2+ plays a pivotal role in the stimulus-secretion coupling process in pancreatic acinar cells [19,20]. Ca2+ can be mobilized to elicit physiological responses from both the external fluid and the internal stores such as endoplasmic reticulum and acidic store. Acetylcholine and cholecystokinin (CCK), the major agonists in pancreatic exocrine gland, are known to generate repetitive and transient oscillatory Ca2+ signals [21,22]. The balance between Ca2+ mobilization and Ca2+ elimination is important to generate Ca2+ oscillation in exocrine cells. These processes are regulated by the action of a variety of channels, pumps, and exchangers for Ca2+ localized both in the plasma membrane and the ER membrane [19,20]. Since the accumulation of intracellular Ca2+ causes cellular damage associated with acute and chronic pancreatitis [24,25], basal intracellular Ca2+ concentrations have to be finely regulated to low resting values under normal condition.
Although oxidant-induced intracellular Ca2+ overload has been revealed in various cell types, the underlying mechanisms of Ca2+ mobilization and elimination are complicated [10,11,12,13,14,15,16,17,18]. It has been known that the involvement of oxidants in Ca2+ homeostasis is mediated by the modification of disulfide bonds between cysteine residues of Ca2+ regulating proteins including SERCA, PMCA and Na+/Ca2+ exchanger (NCX) [31,32]. These molecules have different isoforms with different expression characteristics and regulation properties, thus giving versatility of Ca2+ signaling [33]. The present study was designed to investigate the exact mechanism of how H2O2 could cause intracellular Ca2+ accumulation in pancreatic acinar cells. When acinar cells were exposed to 300 µM of H2O2 in normal buffer, there was additional elevation of cytosolic Ca2+ and termination of oscillatory Ca2+ signals. These effects of H2O2 on Ca2+ signals were completely prevented by pretreatment with catalase (an enzyme that can degrade hydrogen peroxide) and DTT (a sulfhydryl reducing agent). Although cytosolic H2O2 concentrations produced by oxidative stress in pancreatic acinar cells is not known, only small proportion of cells (34%) were response to 100 µM of H2O2 and most cells (97%) were response to 300 µM of H2O2 in the present study. In general, H2O2 at concentrations from 10 µM to 1 mM caused intracellular Ca2+ accumulation in various cell types [10,11,12,13,14,15,16,17]. These results suggest that excess generation of oxidants in pathologic conditions could disturb Ca2+ homeostasis mediated by sulfhydryl group oxidation in pancreatic acinar cells.
Next, we investigated whether H2O2 actually induced Ca2+ entry from extracellular fluid through plasma membrane. In ventricular myocyte, ROS can enhance Ca2+ entry through modulating the function of voltage-gated L-type Ca2+ channels in plasma membrane [34]. It has been reported that ROS play physiological roles in platelet aggregation by activating SOC-mediated Ca2+ entry in human platelets [35]. Transient receptor potential (TRP) channels, such as TRPC3, TRPM2, TRPM7, and TRPPA1 are also known to sensitive to ROS [36]. They participate in neurodegeneration process of neuronal cells. However, H2O2-induced Ca2+ accumulation still occurred in Ca2+-free medium in this study after extracellular Ca2+ sources were eliminated. Furthermore, H2O2 failed to attenuate SOC-mediated Ca2+ entry by adding extracellular Ca2+ at 1.28 mM after ER Ca2+ stores were depleted in Ca2+-free medium by pretreatment of TG. In pancreatic acinar cells, evidence of the existence or the role of voltage-gated Ca2+ channels or NCX has not been presented. The role of TRP channels and their sensitivities to H2O2 have also not been fully elucidated at the present time. Our results suggested that Ca2+ entry channels in plasma membrane might not be the primary targets of H2O2-induced Ca2+ accumulation in mouse pancreatic acinar cells.
In this study, H2O2-induced additional elevation of intracellular Ca2+ concentration was mimicked by TG treatment in Ca2+-free medium. Moreover, H2O2-induced additional increase of Ca2+ was completely abolished in SERCA-inactivated condition by TG pretreatment with CCh. These results strongly suggest that H2O2 could accumulate intracellular Ca2+ through inhibiting refilling to intracellular Ca2+ store, similar to the effect of TG by inactivating SERCA. Since SERCA contains 20~28 cysteine residues, its activity can be effectively modulated by oxidants. It has been reported that ROS could attenuate the activity of this pump by modifying sulfhydryl groups [37]. Distinct SERCA isoforms are known to show different susceptibilities to ROS due to different location of cysteine residues [38]. In rat pancreatic acinar cell, there was no expression of SERCA1 mRNA and SERCA2 mRNA expression was down-regulated in acute pancreatitis [39]. The different sensitivity to H2O2 between SERCA subtypes is not known at the present time. Thus further studies are needed to elucidate the mechanism of H2O2 on calcium accumulation and cell damage. PMCA also could contribute to ROS-induced cytosolic Ca2+ accumulation because this pump has abundant cysteine residues [37]. In this study, 300 µM of H2O2 failed to attenuate Ca2+ extrusion through plasma membrane in TG-treated experiment. However 10 folds higher concentration of H2O2 partially inhibited Ca2+ extrusion to extracellular fluid under similar conditions (data not shown). These findings strongly suggest that the primary target for H2O2-induced Ca2+ accumulation might be SERCA rather than PMCA in mouse pancreatic acinar cells.
The overloaded Ca2+ also could be eliminated by buffering action of mitochondria. CCK can evoke oscillatory Ca2+ signals and substantial mitochondrial Ca2+ uptake in pancreatic acinar cells [40,41]. H2O2 can cause mitochondrial Ca2+ release abolished by pretreatment of FCCP or CCCP, a mitochondrial uncoupler [40,41]. However, in another study, mitochondrial Ca2+ uptake did not occur in unstimulated resting cells [18]. In addition, H2O2-induced mitochondrial Ca2+ uptake was very slow at low capacity even cells were stimulated by CCK [18]. In the present study, ruthenium red alone has no effect on CCh-induced Ca2+ response in Ca2+ free medium, and H2O2 still elevated intracellular Ca2+ levels even when mitochondrial uniporter was blocked by pretreatment of ruthenium red with CCh. Thus, it is unlikely that mitochondria are the major source of H2O2-induced elevation of cytosolic Ca2+.
Based on the above results, we conclude that the primary target molecule for excessively generated H2O2 in pathological conditions is likely to be the sulfhydryl group of SERCA. We also conclude that H2O2 can accumulate intracellular Ca2+ by attenuating the refilling of intracellular Ca2+ stores through ER membrane rather than by Ca2+ entry or Ca2+ extrusion through plasma membrane in mouse pancreatic acinar cells.
ACKNOWLEDGEMENTS
This work was supported by a grant (NRF-2016R1D1A1B03935363) of the National Research Foundation (NRF) funded by the Korea Government.
Footnotes
Author contributions: M.N.Y. performed Ca2+ measurement and analyzed the data. D.K.K. and S.H.K. conceived the idea and disigned the study. H.S.P. supervised and coordinated the study and reviewed the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, Lichtenstein A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22:73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 5.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 7.Galan C, Jardin I, Dionisio N, Salido G, Rosado JA. Role of oxidant scavengers in the prevention of Ca2+ homeostasis disorders. Molecules. 2010;15:7167–7187. doi: 10.3390/molecules15107167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee R, Criddle DN, Gukovskaya A, Pandol S, Petersen OH, Sutton R. Mitochondrial injury in pancreatitis. Cell Calcium. 2008;44:14–23. doi: 10.1016/j.ceca.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiselyov K, Muallem S. ROS and intracellular ion channels. Cell Calcium. 2016;60:108–114. doi: 10.1016/j.ceca.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Shen X. H2O2 directly activates inositol 1,4,5-trisphosphate receptors in endothelial cells. Redox Rep. 2005;10:29–36. doi: 10.1179/135100005X21660. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Kaneko YK, Sawatani T, Noguchi A, Ishikawa T. Obligatory role of early Ca2+ responses in H2O2-induced β-cell apoptosis. Biol Pharm Bull. 2015;38:1599–1605. doi: 10.1248/bpb.b15-00396. [DOI] [PubMed] [Google Scholar]
- 12.Altinkilic S, Naziroğlu M, Uğuz AC, Ozcankaya R. Fish oil and antipsychotic drug risperidone modulate oxidative stress in PC12 cell membranes through regulation of cytosolic calcium ion release and antioxidant system. J Membr Biol. 2010;235:211–218. doi: 10.1007/s00232-010-9267-0. [DOI] [PubMed] [Google Scholar]
- 13.Grupe M, Myers G, Penner R, Fleig A. Activation of store-operated ICRAC by hydrogen peroxide. Cell Calcium. 2010;48:1–9. doi: 10.1016/j.ceca.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bari MR, Akbar S, Eweida M, Kuhn FJ, Gustafsson AJ, Luckhoff A, Islam MS. H2O2-induced Ca2+ influx and its inhibition by N-(p-amylcinnamoyl) anthranilic acid in the beta-cells: involvement of TRPM2 channels. J Cell Mol Med. 2009;13:3260–3267. doi: 10.1111/j.1582-4934.2009.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giambelluca MS, Gende OA. Hydrogen peroxide activates calcium influx in human neutrophils. Mol Cell Biochem. 2008;309:151–156. doi: 10.1007/s11010-007-9653-9. [DOI] [PubMed] [Google Scholar]
- 16.Redondo PC, Jardin I, Hernandez-Cruz JM, Pariente JA, Salido GM, Rosado JA. Hydrogen peroxide and peroxynitrite enhance Ca2+ mobilization and aggregation in platelets from type 2 diabetic patients. Biochem Biophys Res Commun. 2005;333:794–802. doi: 10.1016/j.bbrc.2005.05.178. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca2+-ATPase. Free Radic Biol Med. 1999;27:810–821. doi: 10.1016/s0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]
- 18.Bruce JI, Elliott AC. Oxidant-impaired intracellular Ca2+ signaling in pancreatic acinar cells: role of the plasma membrane Ca2+-ATPase. Am J Physiol Cell Physiol. 2007;293:C938–C950. doi: 10.1152/ajpcell.00582.2006. [DOI] [PubMed] [Google Scholar]
- 19.Petersen OH. Calcium signalling and secretory epithelia. Cell Calcium. 2014;55:282–289. doi: 10.1016/j.ceca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Williams JA. Regulation of acinar cell function in the pancreas. Curr Opin Gastroenterol. 2010;26:478–483. doi: 10.1097/MOG.0b013e32833d11c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yule DI, Straub SV, Bruce JI. Modulation of Ca2+ oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans. 2003;31:954–957. doi: 10.1042/bst0310954. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci DR, Groblewski GE, Sneyd J, Yule DI. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J Biol Chem. 2000;275:33704–33711. doi: 10.1074/jbc.M004278200. [DOI] [PubMed] [Google Scholar]
- 23.Putney JW. The physiological function of store-operated calcium entry. Neurochem Res. 2011;36:1157–1165. doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerasimenko JV, Gerasimenko OV, Petersen OH. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol. 2014;592:269–280. doi: 10.1113/jphysiol.2013.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton R, Criddle D, Raraty MG, Tepikin A, Neoptolemos JP, Petersen OH. Signal transduction, calcium and acute pancreatitis. Pancreatology. 2003;3:497–505. doi: 10.1159/000075581. [DOI] [PubMed] [Google Scholar]
- 26.Perez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol. 2015;5:1–14. doi: 10.1016/j.redox.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward JB, Sutton R, Jenkins SA, Petersen OH. Progressive disruption of acinar cell calcium signaling is an early feature of cerulein-induced pancreatitis in mice. Gastroenterology. 1996;111:481–491. doi: 10.1053/gast.1996.v111.pm8690215. [DOI] [PubMed] [Google Scholar]
- 28.Criddle DN. Reactive oxygen species, Ca2+ stores and acute pancreatitis; a step closer to therapy? Cell Calcium. 2016;60:180–189. doi: 10.1016/j.ceca.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Park HS, Betzenhauser MJ, Zhang Y, Yule DI. Regulation of Ca2+ release through inositol 1,4,5-trisphosphate receptors by adenine nucleotides in parotid acinar cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G97–G104. doi: 10.1152/ajpgi.00328.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park HS, Betzenhauser MJ, Won JH, Chen J, Yule DI. The type 2 inositol (1,4,5)-trisphosphate (InsP3) receptor determines the sensitivity of InsP3-induced Ca2+ release to ATP in pancreatic acinar cells. J Biol Chem. 2008;283:26081–26088. doi: 10.1074/jbc.M804184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaidi A, Barŕon L, Sharov VS, Schoneich C, Michaelis EK, Michaelis ML. Oxidative inactivation of purified plasma membrane Ca2+-ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry. 2003;42:12001–12010. doi: 10.1021/bi034565u. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 33.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 34.Viola HM, Arthur PG, Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res. 2007;100:1036–1044. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 35.Redondo PC, Salido GM, Pariente JA, Rosado JA. Dual effect of hydrogen peroxide on store-mediated calcium entry in human platelets. Biochem Pharmacol. 2004;67:1065–1076. doi: 10.1016/j.bcp.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Arch. 2005;451:243–249. doi: 10.1007/s00424-005-1439-x. [DOI] [PubMed] [Google Scholar]
- 37.Vats YA, Fedirko NV, Klevets MY, Voitenko NV. Role of SH groups in the functioning of Ca2+-transporting ATPases regulating Ca2+ homeostasis and exocytosis. Neurophysiology. 2002;34:5–12. [Google Scholar]
- 38.Burk SE, Lytton J, MacLennan DH, Shull GE. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989;264:18561–18568. [PubMed] [Google Scholar]
- 39.Xue P, Deng LH, Zhang ZD, Yang XN, Xia Q, Xiang DK, Huang L, Wan MH. Effect of Chaiqinchengqi decoction on sarco/endoplasmic reticulum Ca2+-ATPase mRNA expression of pancreatic tissues in acute pancreatitis rats. World J Gastroenterol. 2008;14:2343–2348. doi: 10.3748/wjg.14.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pariente JA, Camello C, Camello PJ, Salido GM. Release of calcium from mitochondrial and nonmitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J Membr Biol. 2001;179:27–35. doi: 10.1007/s002320010034. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez A, Granados MP, Salido GM, Pariente JA. H2O2-induced changes in mitochondrial activity in isolated mouse pancreatic acinar cells. Mol Cell Biochem. 2005;269:165–173. doi: 10.1007/s11010-005-3457-6. [DOI] [PubMed] [Google Scholar]