Abstract
Plasma membrane hyperpolarization associated with activation of Ca2+-activated K+ channels plays an important role in sperm capacitation during fertilization. Although Slo3 (slowpoke homologue 3), together with the auxiliary γ2-subunit, LRRC52 (leucine-rich-repeat–containing 52), is known to mediate the pH-sensitive, sperm-specific K+ current KSper in mice, the molecular identity of this channel in human sperm remains controversial. In this study, we tested the classical BKCa activators, NS1619 and LDD175, on human Slo3, heterologously expressed in HEK293 cells together with its functional interacting γ2 subunit, hLRRC52. As previously reported, Slo3 K+ current was unaffected by iberiotoxin or 4-aminopyridine, but was inhibited by ~50% by 20 mM TEA. Extracellular alkalinization potentiated hSlo3 K+ current, and internal alkalinization and Ca2+ elevation induced a leftward shift its activation voltage. NS1619, which acts intracellularly to modulate hSlo1 gating, attenuated hSlo3 K+ currents, whereas LDD175 increased this current and induced membrane potential hyperpolarization. LDD175-induced potentiation was not associated with a change in the half-activation voltage at different intracellular pHs (pH 7.3 and pH 8.0) in the absence of intracellular Ca2+. In contrast, elevation of intracellular Ca2+ dramatically enhanced the LDD175-induced leftward shift in the half-activation potential of hSlo3. Therefore, the mechanism of action does not involve pH-dependent modulation of hSlo3 gating; instead, LDD175 may modulate Ca2+-dependent activation of hSlo3. Thus, LDD175 potentially activates native KSper and may induce membrane hyperpolarization-associated hyperactivation in human sperm.
Keywords: Ca2+-activated potassium channel, hLRRC52, hSlo3, KSper, LDD175, Sperm motility
INTRODUCTION
Within minutes of vaginal deposition, sperm begin to leave the seminal fluid and swim toward the ovum. The journey of sperm towards the oocyte is a guided event that is capacitated by changes in the local environment surrounding the sperm, including alkalinization and chemotactic hormonal and temperature gradients, among others. Ion channels in sperm are responsible for capacitation and capacitation-associated activities that mediate the sperm hyperactivation underlying male fertility.
Catsper (cation channel sperm-associated 1) and mSlo3 (mouse slowpoke homologue 3; also known as Kcnu1 [potassium channel subfamily U member 1] and Kcnma3 [potassium Ca2+-activated channel subfamily M alpha 1]) in mice, and HVCN1 (hydrogen voltage-gated channel 1) in humans, are ion channels localized to the principal piece of sperm flagellum that are involved in regulating the sperm hyperactivation process necessary for motility. Whereas the Ca2+ permeating channel Catsper can be activated directly by intracellular alkalinization and progesterone, mSlo3, which mediates a weakly outwardly rectifying K+ conductance in sperm known as KSper, can sense pH or Ca2+ and set the membrane potential in a way that favors Catsper-mediated Ca2+ influx. However, the characteristics of this K+ conductance in human sperm have not been fully elucidated.
Electrophysiological studies have characterized the pH-sensitive K+ conductance that sets the membrane potential in mouse sperm [1,2,3]. Subsequent studies have shown that this current is almost certainly mediated by a complex comprising the pore-forming mSlo3 α-subunit and the auxiliary γ2-subunit, LRRC52 (leucinerich-repeat–containing 52) [4]. However, the electrophysiological properties of human KSper differ from those of mouse KSper in that KSper can be inhibited by progesterone, exhibits a distinct pharmacological profile, and is more dependent on regulation by Ca2+ [5]. On the basis of these observations, Mannowetz et al. proposed that Slo1, also known as the BKCa (large-conductance Ca2+-activated potassium) channel (KCNMA1), rather than Slo3, is responsible for human KSper. Brenker et al. subsequently demonstrated that human KSper exhibits hallmarks of human Slo3 (hSlo3), including insensitivity to the BKCa channel inhibitors iberiotoxin (IbTx) and tetraethylammonium (TEA), Ca2+-dependent rather than pH-dependent activation, and inhibition by progesterone.
Collectively, these studies suggest that discrepancies in physiology between human and mouse spermatozoa may reflect the involvement of a different regulatory subunit or subfamily in the modulation of KSper, highlighting the importance of understanding the specific pharmacological profile of hSlo3.
The fact that mSlo3 is responsible for capacitation in murine sperm has motivated the search for strong pharmacological modulators of hSlo3 that may provide a biological tool for validating the role of hSlo3 in human sperm physiology and help to establish new therapeutic concepts for male infertility. Several recent studies have demonstrated that general BKCa channel blockers inhibit heterologously expressed Slo3 and human native KSper. However, the resulting modulators display limited preference for hSlo3 relative to hSlo1, and thus do not markedly discriminate between the two channels in vivo or in vitro [5,6]. Therefore, we undertook an alternative approach, applying well-known BKCa activators to heterologously expressed hSlo3 K+ channels and validating their electrophysiological properties. We found that the BKCa activators, LDD175 (which is recognized also as CTBIC) and NS1619, inversely regulated hSlo3, with NS1619 suppressing hSlo3 activity and LDD175 enhancing hSlo3 activity in a Ca2+-dependent manner, rather than an intracellular pH (pHi)-dependent manner.
METHODS
Cell culture and transfection
Human embryonic kidney 293 (HEK293) cells were cultured at 37℃ and 5% CO2 in Dulbecco's Modified Eagle Medium (GIBCO) supplemented with 1X antibiotic-antimycotic reagent (Life Technologies) and 10% fetal bovine serum. HEK293 cells were transiently transfected using Lipofectamine 2000 Transfection Reagent (Life Technologies) as recommended by the vendor. In brief, 1 µg DNA and 5 µl transfection reagent were separately and thoroughly mixed in 50 µl and 45 µl of Opti-MEM (31985-070; Life Technologies), respectively, and incubated at room temperature for 5 min. Thereafter, the two solutions were mixed and incubated at room temperature for 20 min before adding to 90% confluent HEK293 cells grown in a 12-well plate in antibiotic- and serum-free Opti-MEM media. hSlo3 function was investigated by co-expressing 0.45 µg of an hSlo3 (NP_114016.1) pCDNA3 expression plasmid, 0.45 µg of an hLRRC52 (NP_114016.1) pCDNA3 expression plasmid and 0.1 µg of a GFP-expressing vector. For rat Slo1 (rSlo1)-overexpression experiments, 0.5 µg rSlo1 (NM_001005214.3) in pCDNA3.1 vector was co expressed with 0.5 µg empty pCDNA3.1 vector. Cells were harvested 24 h after transfection, plated onto coverslips, and analyzed by electrophysiology using the whole-cell configuration of the patch-clamp technique.
Electrophysiology
Patch pipettes were pulled from thin-wall filament glass capillaries GC 150TF-7.5 (Harvard Apparatus) to a resistance of 3~4 MΩ using a vertical pipette puller (PC-10; Narishige Group Products). An inverted microscope (ECLIPSE Ti; Nikon) was used to identify transfected cells based on their green fluorescence upon illumination at 514 nm. Whole-cell voltage-clamp experiments were performed at room temperature using an Axopatch 200B patch-clamp amplifier (Axon Instruments) connected to a Digidata-1440A Digitizer (Axon Instruments). A total of 12 step-pulses from –100 mV to +140 mV were applied from a holding potential of –100 mV. Each test pulse was 400 ms long, and the gap between pulses was 600 ms. Linear voltage-ramp protocols from –100 to +140 mV (holding potential -100 mV) were used in tests of drug responses. Recorded currents were compensated for cell capacitance. All experiments were performed at room temperature (21~25℃). Gigaohm seals were formed in a standard extracellular solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES and 0.5 mM EGTA; pH adjusted to 7.4 using NaOH). Activation of hSlo3 by alkalinization was studied using the following pipette solution: 130 mM K-aspartate, 10 mM NaCl, 1 mM EGTA, 5 mM HEPES, 15 mM D-glucose, pH 6.2 or 7.3 (adjusted using KOH). Activation of hSlo3 by Ca2+ was studied using the following pipette solutions: divalent-free solution (130 mM K-aspartate, 10 mM NaCl, 1 mM EGTA, and 20 mM HEPES, adjusted to pH 7.3 using KOH), intracellular solutions with different free Ca2+ concentrations (130 mM K-aspartate, 10 mM NaCl, 5 mM EGTA, and 20 mM HEPES; adjusted to pH 7.3 using KOH). Different volumes of a calcium stock solution were added to this base solution in order to obtain final free calcium concentrations of 80, 200, and 1,000 µM. Free Ca2+ concentrations were calculated using the MaxChelator tool (maxchelator.stanford.edu/).
Solutions and chemicals
TEA (tetraethylammonium), NH4Cl, 4-AP (4-aminopyridine), and NS1619 (1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl) phenyl]-5-(trifluoromethyl)-2H-benzimidazole-2-one) were purchased from Sigma Aldrich (USA). Iberiotoxin (IbTX) was purchased from TOCRIS Biosciences (USA). LDD175 (4-chloro-7-trifluoromethyl-10H-benzo[4,5]furo[3,2-b]indole-1-carboxylic acid) was kindly provided by Professor Chul-Seung Park (GIST, Gwangju, Korea). TEA and NH4Cl were directly diluted in physiological perfusion solution prior to use. Stock solutions of 4-AP (1 M) and IbTX (100 µM) were prepared by dissolving the appropriate amount of each chemical in distilled water. Stock solutions of LDD175 (100 mM) and NS1619 (50 mM) were prepared in DMSO (Sigma Aldrich, USA) and 99% ethanol, respectively. All stock solutions were stored at –20℃ and used freshly reconstituted to appropriate concentrations in perfusion solutions prior to use.
Statistical analysis
Statistical significance of differences among three or more groups was calculated using a one-way analysis of variance (ANOVA) with Bonferroni correction using Origin Pro 8.1 software, and differences between two groups were calculated using unpaired or paired Student's t-test. All data are given as means±standard error. Gating conductance (G)–voltage (V) curves were calculated by fitting the slope of current (I)–voltage (V) curves to the linear relation, Y=mX+c. Independent Boltzmann fitting (y=A2+(A1–A2)/(1+exp((x–x0)/dx)) was used to calculate activation and inactivation kinetics. Maximum conductivity recorded for each cell was used as the maximum conductivity (Gmax) to normalize the conductivity of each cell. A standard exponential fit in Clampfit software 10.6.2.2 (Molecular Devices, USA) was used to calculate the time constant in step pulses.
RESULTS
Characterization of hSlo3
Prior to measurement of hSlo3 currents, we examined the response of endogenous potassium currents to the test protocols. A +116 mV voltage step elicited a 64.63±6.02 pA/pF (n=20) current in non-transfected HEK293 cells (Fig. 1A). Potassium-carrying currents were not significantly increased by overexpression of hSlo3 (p=0.99, n=8) or hLRRC52 (p=0.94, n=8) alone (data not shown). However, overexpression of hSlo3 and hLRRC52 at a 1:1 ratio resulted in a 147±14.56 pA/pF (n=30) current at +116 mV that displayed the characteristic step-current shape of hSlo3+hLRRC52 currents (Fig. 1A). Notably, the shape of voltage step-induced currents in HEK293 cells transfected with hSlo3+hLRRC52 was different from that of endogenous K+ currents recorded from non-transfected HEK293 cells.
Fig. 1. Pharmacological characterization of hSlo3 currents.
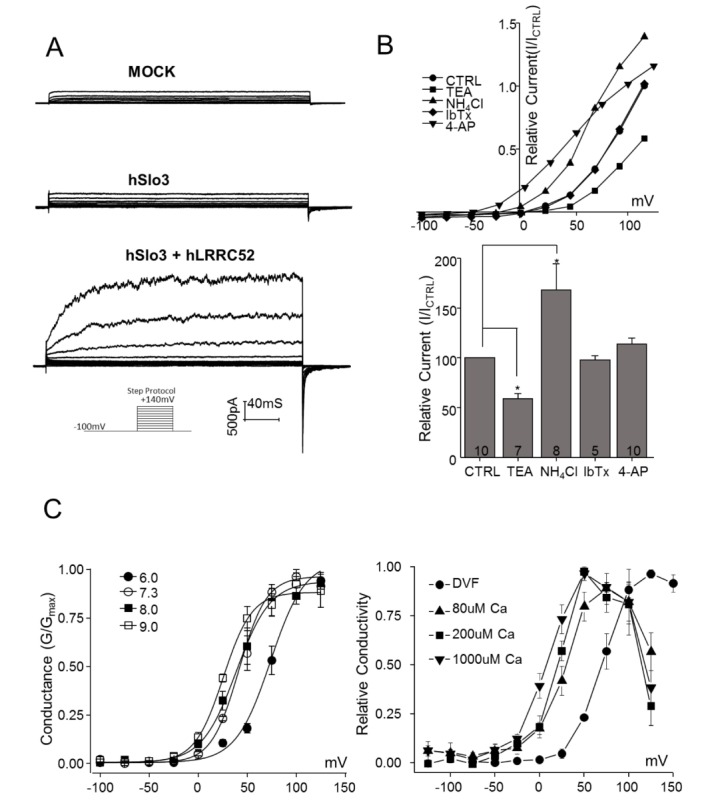
(A) Representative traces of endogenous currents in HEK293 cells (upper panel; MOCK), currents in cells transfected only with the hLRRC52 subunit (middle panel; hLRRC52), and currents in cells co-transfected with hSlo3 and the hLRRC52 subunit (lower panel; hSlo3+hLRRC52). Currents were recorded using a step-depolarizing pulse from –100 to +140 mV, with a holding potential of –100 mV. (B) Upper panel: Pharmacological experiments designed to verify hSlo3 K+ currents, recorded as in (A) in the presence of TEA (20 mM), IbTx (100 nM), NH4Cl (10 mM), or 4-AP (25 mM). Lower panel: Inhibition was calculated as the percentage of current remaining after treatment relative to that prior to treatment at 116 mV. (C) G~V curves shifted towards the left with increasing alkalinization and internal Ca2+. Left panel: hSlo3 G~V curves, tested at various pHi values: pH 6.0 (●), pH 7.3 (○), pH 8.0 (□), pH 9.0 (□). Right panel: hSlo3 G~V curves, tested at various [Ca2+]i: DVF (●), 80 µM Ca (▲), 200 µM Ca (■), 1000 µM Ca (▼). Data are presented as averages of results from five patches. Lines are fits to the Boltzmann equation. Vh values for each Boltzmann component are indicated for each condition. The number of cells is indicated in a bar graph. *p<0.05.
The endogenous current was inhibited by 45.42%±0.15% (n=4) by 20 mM TEA, but was unaffected by 10 mM NH4Cl (p=0.15, n=5) and 100 nM IbTX (p=0.96, n=5) (data not shown). In keeping with their classic characteristics, hSlo3+hLRRC52 currents measured in co-transfected HEK293 cells were inhibited by exposure to 20 mM TEA (41.0±5.19%; p=0.02, n=7) and were increased by exposure to 10 mM NH4Cl (68.04±8.60%; p=0.03, n=8). Furthermore, hSlo3+hLRRC52 currents were unaffected by both 100 nM IbTX (p=0.61, n=5) and 25 mM 4-AP (p=0.06, n=10) compared at peak current elicited by ramp command potential (Fig. 1B). Although 4-AP moderately potentiated the K+ current in a certain range of command potential (–50 mV to +80 mV), it did not show the inhibitory effect as previously reported.
Because Slo3 is suggested to be a pH-sensitive channel, we tested the response of hSlo3+hLRRC52 to changes in internal pH values. Conductance curves for hSlo3+hLRRC52 shifted towards the left with increasing internal alkalinization (pH 6.0~9.0), resulting in a decrease in the half-activation voltage (Fig. 1C). At pH 6.0, 7.3, 8.0 and 9.0, half-activation voltages of hSlo3+hLRRC52 currents were 73.48±8.56 (n=5), 41.39±4.05 (n=10), 38.50±3.10 (n=6) and 25.41±1.08 mV (n=5), respectively.
Furthermore, hSlo3 increases its activity in respond to stimuli that can increase intracellular calcium concentration. Therefore, we examined the effect of increasing intracellular calcium concentration to hSlo3+hLRRC52. The increase in intracellular calcium concentration not only increased the activity of Slo3 but shifted the GV curve to the left, and the half activation voltage was changed (Fig. 1D). At 0, 80, 200, 1000 µM of internal free calcium concentration, half-activation voltages of hSlo3+hLRRC52 currents were 36.93±4.74 (n=7), 25.40±2.04 (n=5), 19.03±4.01 (n=4) and 6.26±3.63 mV (n=4), respectively.
Effects of NS1619 on hSlo3 K+ currents
We next applied 50 µM NS1619, a Slo1 activator, to transfected and non-transfected HEK293 cells. Outward endogenous K+ currents in HEK293 cells trended slightly lower (–2.44%±6.29%) following external perfusion of NS1619, although this difference did not reach statistical significance (p=0.66, n=5; data not shown). In contrast, extracellular application of NS1619 to HEK293 cells transfected with hSlo3+hLRRC52 induced a rapid, concentration-dependent decrease in K+ currents, reducing these currents by 50.29%±7.92% (p<0.01, n=5) at a concentration of 50 µM (Fig. 2A). This latter concentration was close to the calculated half-maximal inhibitory concentration (IC50) value of 27.54±6.70 µM (n=5~10) (Fig. 2C). Furthermore, this NS1619-induced inhibition of hSlo3+hLRRC52 currents resulted in rapid depolarization of the cell membrane by an average of 11.38±1.02 mV (p=0.01, n=7) (Fig. 2B).
Fig. 2. Effects of the BKCa activator NS1619 on hSlo3 K+ currents.
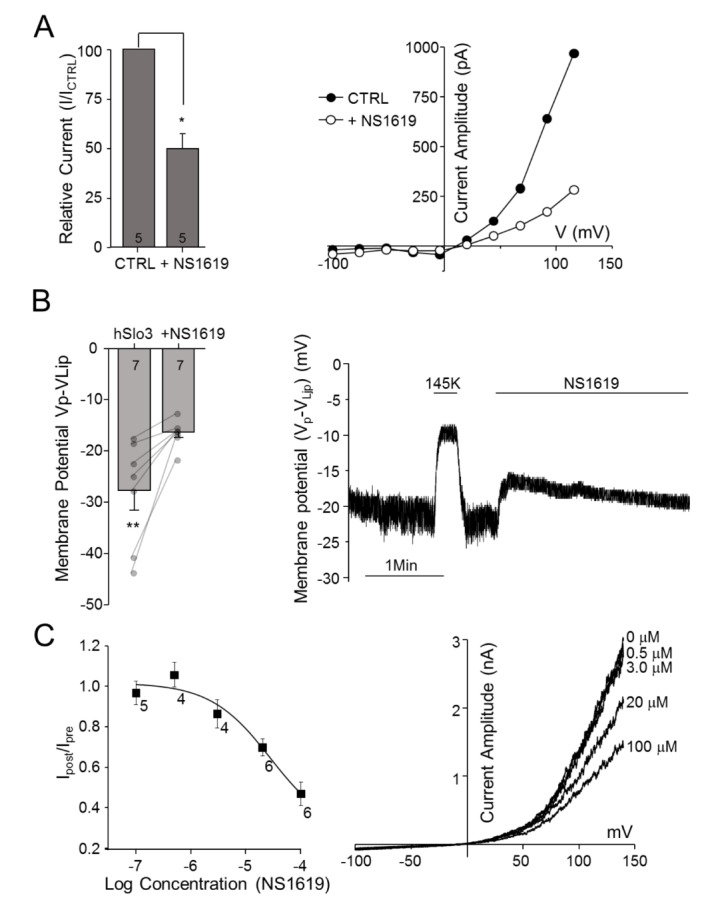
(A) Left panel: Extracellular application of 50 µM NS1619 inhibits hSLo3+hLRRC52 currents. Inhibition was calculated as the percentage of current remaining after treatment relative to that prior to treatment at 116 mV. Right panel: Representative traces showing I~V relationships before (●) and after (○) treatment. (B) Inhibition of hSlo3+hLRRC52 current by 50 µM NS1619, determined by measuring changes in membrane potential before and after treatment. Left panel: NS1619-induced membrane potential depolarization. Right panel: Representative continuous trace showing changes in membrane potential during the application of 50 µM NS1619. (C) Left panel: Concentration-response curve for NS1619 inhibition of hSlo3+hLRRC52. Right panel: Representative I~V traces showing changes in current with the application of NS1619. The number of cells is indicated in a bar graph. *p<0.05, **p<0.01.
Effects of LDD175 on hSlo3 K+ currents
We next tested the effects of the Slo1 activator LDD175 on non-transfected and transfected HEK293 cells. As shown in Fig. 3B, LDD175 induced a concentration-dependent increase in K+ current in hSlo3+hLRRC52-transfected cells, enhancing this current by 68.65%±13.80% at a concentration of 30 µM (p=0.00657, n=5) (Fig. 3A). A regression analysis of concentration-response curves yielded a half-maximal effective concentration (EC50) value of 2.34±0.53 µM (n=4~12). The increase in channel activity induced by perfusion of 30 µM LDD175 was associated with a small, but statistically insignificant (p=0.22496, n=5), shift in membrane potential towards more negative values (by 3.32±2.31 mV at I=0) (Fig. 3C).
Fig. 3. LDD175 activates hSLo3+hLRRC52 currents.
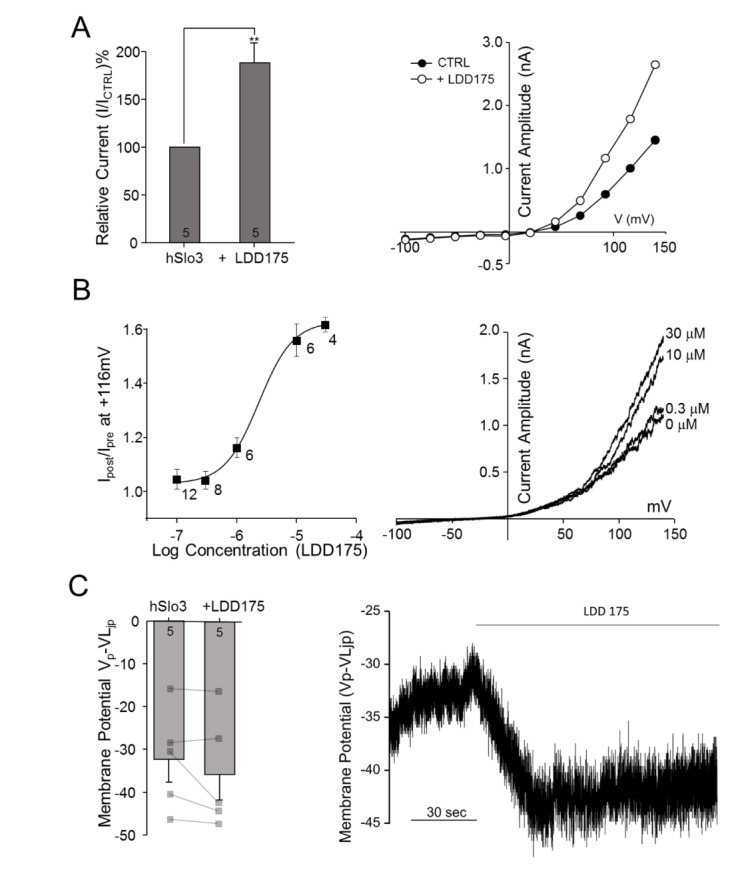
(A) Left panel: Extracellular application of 30 µM LDD175 enhances hSLo3+hLRRC52 currents. Activation was calculated as the percentage of current remaining after treatment relative to that prior to treatment at 116 mV. Right panel: Representative traces showing I~V relationships before (●) and after (○) treatment. (B) Left panel: Concentration-response curve for LDD175-induced activation of hSlo3+hLRRC52. Right panel: Representative I~V traces showing changes in current with the application of LDD175. (C) Activation of hSlo3+hLRRC52 current by 30 µM LDD175, as evidenced by changes in membrane potential after treatment. Left panel: Treatment with 30 µM LDD175 induces membrane potential hyperpolarization. Right panel: Representative continuous trace showing changes in membrane potential during the application of 30 µM LDD175. The number of cells is indicated in a bar graph. **p<0.01.
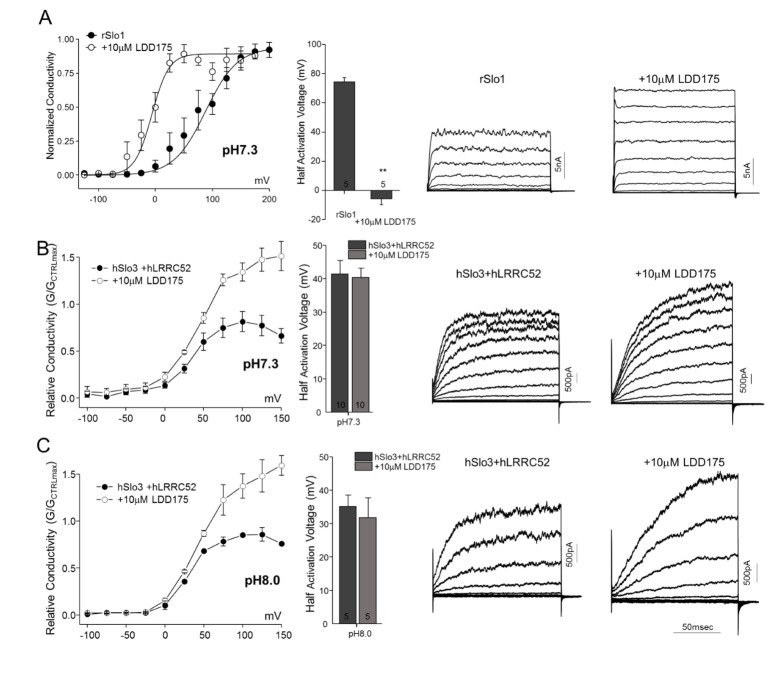
Effects of internal pH on LDD175-induced activation of rSlo1 and hSlo3 K+ currents
Application of 10 µM LDD175 induced a change in G~V curves for rSlo1 exogenously expressed in HEK293 cells, shifting the half-activation voltage from 74.45±2.87 mV (n=5~10) to –5.64±4.05 mV (n=5~10) (Fig. 4A). In contrast, 10 µM LDD175 had no significant effect on the half-activation voltage of hSlo3+hLRRC52 gating at any pH, despite modest, but insignificant, trends towards a decrease in the half-activation voltage of channel gating at pH 7.3 (41.38±4.05 to 40.32±2.80 mV; n=10, p=0.8349) and pH 8.0 (35.10±3.45 to 31.85±5.84 mV; n=5, p=0.6447). Thus, the absence of a decrease in the halfactivation voltage with alkalinization—a characteristic feature of Slo3—indicates that the Slo3 activation voltage does not respond to LDD175. However, under both internal pH conditions, the relative conductivity of hSlo3+hLRRC52 increased, indicating a possible impact on this parameter by LDD175 (Fig. 4B and C) that may be related to an increase in the total number of channels opened at a given time.
Fig. 4. LDD175 potentiates K+ currents in both rSlo1-transfected and hSlo3+hLRRC52–co-transfected cells.
(A) The application of 10 µM LDD175 causes a rightward shift in the rSlo1 steady-state G~V relationship (left panel), significantly decreases Vh values (middle panel), and increases the amplitude of step currents. (B, C) External application of 10 µM LDD175 significantly increases the relative conductivity of hSlo3+hLRRC52 at pH 7.3 (B, left panel) and pH 8.0 (C, left panel), and increases the amplitude and changes the shape of step currents (right panels). The number of cells is indicated in a bar graph. **p<0.01
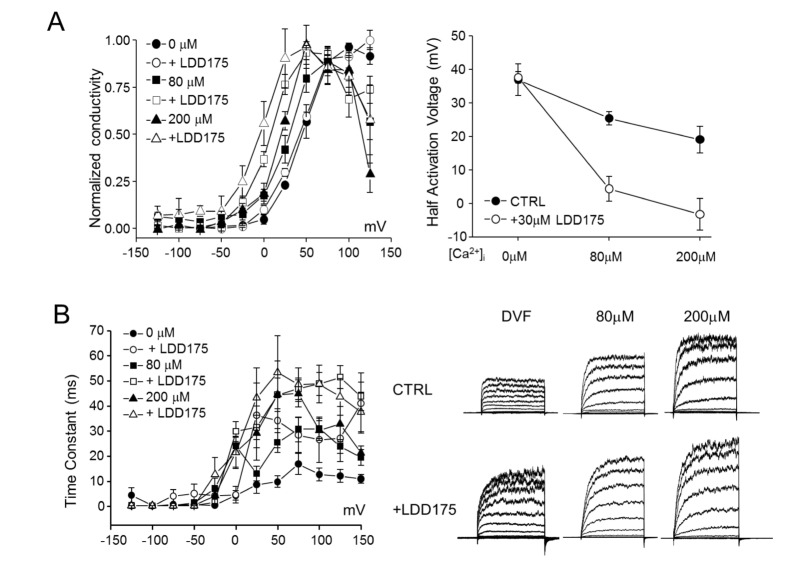
Effects of internal free Ca2+ on LDD175-induced activation of hSlo3 K+ currents
Increasing the internal free Ca2+ level resulted in an increase in total conductivity and a drop in activation voltage in hSlo3+hLRRC52 co-transfected HEK293 cells (Fig. 5A). Increasing the internal free Ca2+ potentiated the effect of LDD175 on activation voltage. Under internal divalent-free (DVF) conditions, half-activation voltage was largely unchanged by LDD175 application (36.97±4.74 to 37.60±1.65 mV). However, with increasing internal free Ca2+ levels, application of extracellular LDD175 decreased the half-activation voltage, reducing it from 25.40±2.04 to 4.31±3.75 mV at 80 µM internal Ca2+ and 19.04±4.01 to –3.28±4.68 mV at 200 µM internal Ca2+.
Fig. 5. Intracellular-Ca2+–dependent regulation of hSlo3+hLRRC52 currents by LDD175 in co-transfected HEK293 cells.
(A) Left panel: Steady-state G~V relationships for hSlo3+hLRRC52 at different [Ca2+]i, and their half-activation voltages. Right panel: Increasing internal free Ca2+ facilitates the effect of LDD175 (○) compared with controls (●). (B) Left panel: Activation time constants calculated in the presence or absence of LDD175. Right panel: Representative currents.
The negligible decrease in half-activation voltage and associated increase in total conductivity of hSlo3+hLRRC52 induced by LDD175 suggests that LDD175 might cause more channels to open at a given time. Therefore, we studied the activation time constant for hSlo3+hLRRC52 at different voltage steps. hSlo3+hLRRC52 step currents showed further development with increases in internal Ca2+ levels and application of LDD175, resulting in an increase in the time constant at 100-mV step pulses from 12.63±2.59 to 26.55±9.07 ms under DVF conditions, 30.95±4.02 to 48.72±4.63 ms at 80 µM intracellular Ca2+ and 30.23±3.94 to 48.72±7.57 ms at 200 µM intracellular Ca2+. The increase in time constants was most pronounced under DVF conditions and became smaller with increasing internal free Ca2+ concentration (Fig. 5B).
DISCUSSION
Sperm-specific ion channels contribute to the changes in membrane potential, intracellular Ca2+ and pHi that control not only the acrosomal reaction, but also sperm motility. However, the molecular identity of the membrane potential-regulating KSper in human sperm is still controversial. Thus, studying the pharmacological properties of this current is beneficial for understanding the molecular nature of the sperm K+ channel.
Here, we show for the first time that two general activators of BKCa channels differentially regulate sperm-specific hSlo3 channel, a molecular candidate for human KSper. Interestingly, the well-known BKCa channel opener, NS1619, inhibited the K+ current mediated by heterologously expressed hSlo3+hLRRC52 in a concentration-dependent manner. By contrast, LDD175, another novel BK channel activator, potentiated the hSlo3 K+ current in a concentration-dependent manner. Moreover, whereas LDD175 negatively shifted the G~V relationship of rSlo1, regulating it over a physiological range of membrane potentials, as is well known; an equivalent concentration of LDD175 did not alter the half-maximal voltage for activation of hSlo3 in the absence of intracellular Ca2+, regardless of pHi. However, LDD175-induced activation of hSlo3 was enhanced in the presence of intracellular Ca2+, which shifted its current-voltage relationship to more negative voltages.
Genetic ablation of Slo3 has revealed that mSlo3, a pH-sensitive, Ca2+-independent channel, is the principal K+ channel in murine sperm. Moreover, its γ2 axillary subunit, LRRC52, affects the activation voltage of mSlo3, causing the channel to gate at physiological membrane potentials [4,7,8]. Unexpectedly, the K+ current recorded from human sperm exhibited clear differences in pharmacological properties, including pHi-dependence and intracellular Ca2+ sensitivity. Specifically, whereas mouse KSper is only sensitive to internal alkalinization, human KSper activates in an intracellular Ca2+-dependent manner with minimal regulation by pHi [9]. These differences between mouse and human KSper inform the debate surrounding the molecular identity of human KSper.
It is known that stimuli including alkalinization and progesterone can cause intracellular Ca2+ to rise in human sperm, leading to hyperactivated motility, capacitation, and the acrosome reaction [10]. Although the precise mechanism is not completely understood, Ca2+ channels and K+ channels play a role in controlling sperm membrane potential and [Ca2+]i. In particular, pH-sensitive efflux of K+ is detected in sperm and is thought to aid in hyperpolarization of the membrane potential, which would maximize Ca2+ entry through CatSper channels. Therefore, understanding the properties of human KSper would provide important insight into the physiology of sperm.
It is known that a broad range of BKCa channel blockers, including quinidine, barium and TEA, exert inhibitory action on either native human KSper or hSlo3 currents in heterologous overexpression systems [11]. However, we found that IbTx, a selective BKCa blocker, and 4-AP, a voltage-gated K+ channel blocker, had minimal effects on hSlo3 currents in transfected HEK293 cells. We also found that 4-AP had a modest potentiation at the -50 mV to +80 mV while there is no significant difference on peak amplitude. 4-AP was known to inhibit mouse Slo3 intracellularly while it did not exhibit the inhibitory effect extracellularly [11]. Mansell et al. showed that sustained outward KSper current from human sperm was ineffective, instead, transient tail current of KSper was potentiated by 4-AP as well as progesterone [12]. Therefore, the potentiation of hSlo3 in the range of depolarized membrane potential is evidence of the functional role of Slo3 in KSper.
Mannowetz et al. reported that Slo1 is the principal channel underlying KSper in human sperm, based on their observation that the peptide toxins, charybdotoxin and IbTx, as well as progesterone, inhibited this current; by contrast, mouse KSper was found to be insensitive to these agents [5]. However, Brenker et al. subsequently refuted this idea, demonstrating a different pharmacological profile that human KSper was insensitive to IbTx and progesterone could not elicit Slo1 [6]. Our data support the conclusion that hSlo3 is insensitive to IbTx and 4-AP, and is minimally regulated by changes in pHi. However, these observations are contrast to the previously reported pHi-sensitivity of human KSper [6,12]. These discrepancies may reflect the possibility that other functional subunits of Ca2+-sensitive K+ channels, including β- or γ-subunits, modify access of toxins and blockers to their binding sites on pore-forming α-subunits. It is also possible that differences in extracellular Ca2+, which can inhibit monovalent CatSper currents (as Brenker et al. have argued) may contribute to reported differences in KSper. Taken together, our pharmacological data suggest that the human α-subunit, Slo3, and its axillary γ2-subunit, LRRC52, underlie KSper and play an important role in human sperm physiology.
Several activators of BKCa channels that act through a variety of mechanisms have been developed and used extensively for studying the function of BKCa channels in biological systems. Among them, NS1619, a synthetic benzimidazolone derivative, activates the BKCa channel either through direct interactions with the channel [13] or through the closely associated release of internal Ca2+ [14]. It has also been reported that NS1619 activates Slo1 by functionally interacting with the S6/RCK linker connecting the transmembrane S6 segment with the cytosolic RCK1 domain [15]. However, none of these studies evaluated changes in Slo3 activity caused by NS1619, which our data show caused a concentration-dependent inhibition of hSlo3 K+ current. This inhibitory action of NS1619 may interfere with the ability of α (Slo3) and γ2 subunits to potentiate channel activity; alternatively, the binding regions in the S6/RCK linker of Slo3 that promote the open state of the channel may differ from those in Slo1.
LDD175, a synthetic benzofuroindole analogue, activates the BKCa channel through specific effects on the α-subunit. Since Gormemis et al. first reported the potent activity of the BKCa channel expressed in Xenopus oocytes [16], LDD175 has demonstrated relaxation-inducing actions on the urinary bladder, antispasmodic actions on intestinal motility, and erectile responses in corporal smooth muscle [17,18,19]. Although the underlying mechanism of action of LDD175 on BKCa is not fully elucidated, it is known that LDD175 shifts the conductance-voltage relationship of the channel leftward without changing its voltage dependence [20]. It is also known that LDD175 acts extracellularly at the interface between the turret region and the P-region of the Slo1 α-subunit to potentiate BKCa channel activity [21]. In addition, the fact that the presence of β-subunits has only a minor effect on LDD175 actions implies that the interaction occurs only in the α-subunit.
Our data show that 10 µM LDD175 dramatically shifted the half-activation voltage of rSlo1 to a hyperpolarized potential, whereas this concentration of LDD175 had no significant effect on hSlo3 at pH 7.3 or pH 8.0 in the absence of Ca2+. However, LDD175 caused a shift in the half-activation voltage in the presence of intracellular Ca2+ similar to that of rSlo1.
A sequence alignment reveals four amino acids of hSlo3 that are positioned at sites homologous to those in rSlo1 proposed to be critical for LDD175 binding to the rSlo1 α-subunit [21]. Therefore, the mechanism of action of LDD175 on hSlo3 may be similar to that on rSlo1, where it interacts extracellularly at the interface between a-subunits.
Although our data demonstrate that the activity of hSlo3 is dependent on intracellular Ca2+, this isoform lacks negatively charged amino acids that account for intracellular Ca2+ binding in Slo1. Further investigations should seek to elucidate the mechanism underlying the Ca2+-dependent regulation of Slo3, which is important in sperm physiology.
In summary, the present results have defined distinct pharmacological differences between the closely related Slo1 and Slo3 K+ channels using two activators with different mechanisms of action. To date, hSlo3, a strong molecular candidate for KSper, exhibits LDD175-dependent activation, which may explain how KSper regulates membrane potential and consequentially regulates intracellular Ca2+ increases through CatSper in human sperm. Therefore, further investigation is clearly required to determine whether native KSper shares LDD175-activation and NS1619-inhibition features similar to those of heterologously expressed hSlo3.
ACKNOWLEDGEMENTS
We wish to thank Christopher J. Lingle (Washington University) for his generous gift of hSlo3 and hLRRC52 expression constructs. LDD175 was kindly provided by Professor Chul-Seung Park, GIST, Gwangju, Korea. This work was supported by a research fund of Chungnam National University (2012-1905).
Footnotes
Author contributions: T.D.W., J.H.K., D.K.Y., and K.P.L. designed the research; T.D.W. and J.H.K. performed the experiments; all authors contributed to the analysis of data and preparation of the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 2.Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A. 2011;108:5879–5884. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang C, Zeng XH, Zhou Y, Xia XM, Lingle CJ. LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated Slo3 channel. Proc Natl Acad Sci U S A. 2011;108:19419–19424. doi: 10.1073/pnas.1111104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannowetz N, Naidoo NM, Choo SA, Smith JF, Lishko PV. Slo1 is the principal potassium channel of human spermatozoa. Elife. 2013;2:e01009. doi: 10.7554/eLife.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenker C, Zhou Y, Müller A, Echeverry FA, Trötschel C, Poetsch A, Xia XM, Bönigk W, Lingle CJ, Kaupp UB, Strünker T. The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife. 2014;3:e01438. doi: 10.7554/eLife.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonetti MD, Yuan P, Hsiung Y, Mackinnon R. Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc Natl Acad Sci U S A. 2012;109:19274–19279. doi: 10.1073/pnas.1215078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng XH, Yang C, Xia XM, Liu M, Lingle CJ. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc Natl Acad Sci U S A. 2015;112:2599–2604. doi: 10.1073/pnas.1423869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapham DE. Sperm BerserKers. Elife. 2013;2:e01469. doi: 10.7554/eLife.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okabe M. The cell biology of mammalian fertilization. Development. 2013;140:4471–4479. doi: 10.1242/dev.090613. [DOI] [PubMed] [Google Scholar]
- 11.Tang QY, Zhang Z, Xia XM, Lingle CJ. Block of mouse Slo1 and Slo3 K+ channels by CTX, IbTX, TEA, 4-AP and quinidine. Channels (Austin) 2010;4:22–41. doi: 10.4161/chan.4.1.10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansell SA, Publicover SJ, Barratt CL, Wilson SM. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol Hum Reprod. 2014;20:392–408. doi: 10.1093/molehr/gau003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, Jr, Kozlowski ES, Romine JL, Trojnacki JT, Mckay MC, Zhong J, Dworetzky SI. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- 14.Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentzen BH, Nardi A, Calloe K, Madsen LS, Olesen SP, Grunnet M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol Pharmacol. 2007;72:1033–1044. doi: 10.1124/mol.107.038331. [DOI] [PubMed] [Google Scholar]
- 16.Gormemis AE, Ha TS, Im I, Jung KY, Lee JY, Park CS, Kim YC. Benzofuroindole analogues as potent BK(Ca) channel openers. Chembiochem. 2005;6:1745–1748. doi: 10.1002/cbic.200400448. [DOI] [PubMed] [Google Scholar]
- 17.dela Peña IC, Yoon SY, Kim SM, Lee GS, Ryu JH, Park CS, Kim YC, Cheong JH. Bladder-relaxant properties of the novel benzofuroindole analogue LDD175. Pharmacology. 2009;83:367–378. doi: 10.1159/000218739. [DOI] [PubMed] [Google Scholar]
- 18.Dela Peña IC, Yoon SY, Kim SM, Lee GS, Park CS, Kim YC, Cheong JH. Inhibition of intestinal motility by the putative BK(Ca) channel opener LDD175. Arch Pharm Res. 2009;32:413–420. doi: 10.1007/s12272-009-1315-x. [DOI] [PubMed] [Google Scholar]
- 19.Sung HH, Choo SH, Han DH, Chae MR, Kang SJ, Park CS, So I, Park JK, Lee SW. Effect of the novel BKCa channel opener LDD175 on the modulation of corporal smooth muscle tone. J Sex Med. 2015;12:29–38. doi: 10.1111/jsm.12744. [DOI] [PubMed] [Google Scholar]
- 20.Ha TS, Lim HH, Lee GE, Kim YC, Park CS. Electrophysiological characterization of benzofuroindole-induced potentiation of large-conductance Ca2+-activated K+ channels. Mol Pharmacol. 2006;69:1007–1014. doi: 10.1124/mol.105.016170. [DOI] [PubMed] [Google Scholar]
- 21.Lee BC, Lim HH, Kim S, Youn HS, Lee Y, Kim YC, Eom SH, Lee KW, Park CS. Localization of a site of action for benzofuroindole-induced potentiation of BKCa channels. Mol Pharmacol. 2012;82:143–155. doi: 10.1124/mol.112.078097. [DOI] [PubMed] [Google Scholar]