Abstract
Virtual surgical planning (VSP) has recently been introduced in craniomaxillofacial surgery with the goal of improving efficiency and precision for complex surgical operations. Among many indications, VSP can also be applied for the treatment of congenital and acquired craniofacial defects, including orbital fractures. VSP permits the surgeon to visualize the complex anatomy of craniofacial region, showing the relationship between bone and neurovascular structures. It can be used to design and print using three-dimensional (3D) printing technology and customized surgical models. Additionally, intraoperative navigation may be useful as an aid in performing the surgery. Navigation is useful for both the surgical dissection as well as to confirm the placement of the implant. Navigation has been found to be especially useful for orbit and sinus surgery. The present paper reports a case describing the use of VSP and computerized navigation for the reconstruction of a large orbital floor defect with a custom implant.
Keywords: Neuronavigation, Orbital trauma, Orbital implants, Virtual surgical planning
Introduction
Reconstruction of the skull and facial regions represents a challenge for the maxillofacial surgeon due to the complex anatomy, the variety of techniques and materials, and the esthetical and psychological implications of the area. Recent innovations such as computer-assisted surgical planning and intraoperative navigation could potentially improve the efficacy, precision, and predictability of the surgical treatment. Multi-planar computed tomography (CT) scans, associated with 3D reconstruction software, show in detail the individual anatomical variability and help the surgeon to identify a specific bone area to resect or reconstruct.1 The software's reconstruction abilities could also be used to virtually display the patient's anatomy throughout the case, allowing stereotactic navigation.2 Computerized navigation consists of the virtual interface between the intraoperative positions of the surgical instruments with the reconstruction of patient anatomy, obtained by CT scans. During the surgery, the navigation system controls the position of the implants or the mobilized bone and verifies the final location. Intraoperative navigation enhances surgeons' ability to measure the extent of resection, to identify important anatomical landmarks and to confirm the orientation of bone grafts. It is possible to reduce human error, achieving greater adherence to the preoperative plan.3 Furthermore, intraoperative navigation could reduce the incidence of postsurgical complications due to a wrong positioning or orientation of bone grafts, plates, or fixation screws. More recently VSP has been combined with 3D printing technology, improving surgical efficiency and precision through the production of 3D surgical models, guides, and implants.4, 5 These devices increase indications for the surgeon to utilize VSP, offering additional effective tools in preoperative planning and intraoperative decision-making. 3D printer scan reads and analyzes CT scan data and creates customized surgical models. 3D printers produce layers of bioplastic under computer control. Both VSP and 3D models are used to precisely place the ideal amount, shape, and dimensions of autologous tissue or bioprosthetic material needed for reconstruction.6 They can also be utilized as templates in resective surgery to accurately identify the border of the resection, and/or to intraoperatively design and prepare the reconstructive phase more efficiently and precisely.7 Moreover, another advantage of using 3D models and guides is the reduction of the operative time and potential reduction of complications due to prolonged operative times.8 These advances improve efficiency, accuracy, and safety of the surgical management of orbital trauma. Volumetric analyses of anatomical structures could be applied for the design of standardized and custom patient-made anatomic implants for orbital reconstruction and midfacial defects as well.9
Various materials have been used for the purpose of reconstruction and replacing missing tissue. The process of 3D modeling and custom implants is continuously evolving with advancements in design and manufacturing processes. Implants are manufactured by machining a block of material (subtractive manufacturing) or by adding material layer by layer and fusion of the layers (additive manufacturing).
Polyetheretherketone (PEEK) custom implants have been used to correct cranial, frontal, malar and mandibular defects.10, 11, 12 PEEK is a very strong thermoplastic material, which retains its chemical and mechanical properties even at high temperatures. The material exhibits excellent biocompatibility and biostability and maintains its physical and chemical characteristics with long-term exposure to body fluids. The modulus of elasticity of PEEK is similar to that of cortical bone, preventing any stress shielding which is preferred over metallic implants owing to its high modulus of elasticity. PEEK is also radiolucent facilitating postoperative imaging procedures. Craniofacial implants can be designed to replace exact anatomy even in bulky regions as the material is very light. The material can be repeatedly sterilized by common methods as autoclave, gamma or ethylene oxide. PEEK lends itself to machining of complex organic shapes. PEEK implants can be fixated to the adjacent bone with standard screws and plates of surgeons' choice. PEEK implants are made from a block of extruded material using a computerized numerical control (CNC) machining. PEEK implants can be used in non-load bearing regions of the craniofacial skeleton.
The present paper reports a case of a secondary reconstruction of a large orbital floor defect using a computer-designed PEEK patient specific implant.
Case report
Presentation
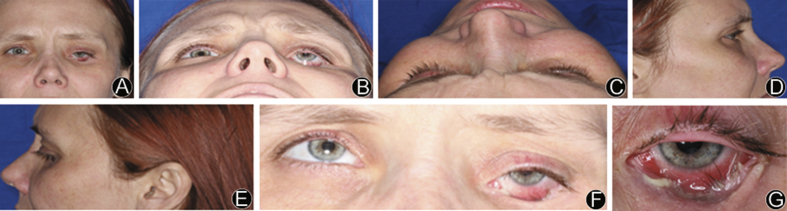
A 33-year old female patient presented with eye and sinus pain, bleeding, and an ongoing infection in her left orbit (Fig. 1). The patient reported that 6 years prior to her presentation she had been involved in a motor vehicle accident in which she sustained complex panfacial fractures. The patient underwent facial reconstruction at the time of initial trauma (Fig. 2a); however, the orbital plate had become malpositioned inferiorly and infected (Fig. 2b and c). On exam she presented with entropion, dystopia, enophthalmos of the left eye and purulent discharge at lower fornix. The lower eyelid was retracted and scarred to the underlying implant. After assessment, the patient was scheduled left orbit hardware removal and a patient specific implant orbital reconstruction.
Fig. 1.
A: Portrait preoperative photo; B: Worm's eye view of patient, appreciable entropion; C: Bird's eye view of patient; D: Right profile view; E: Left profile view; F: Limited eye motion in the superior direction; G: Purulent inferior orbital rim of left eye.
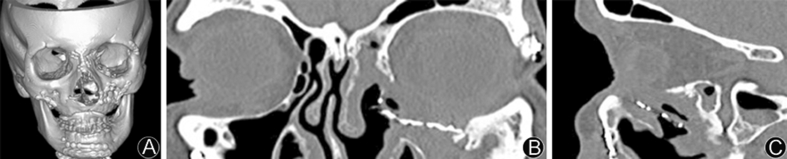
Fig. 2.
A: Preoperative CT reconstruction reveals prior extensive facial reconstruction; B: Preoperative axial CT showing malplaced orbital plate with soft tissue displacement from infection; C: Preoperative sagittal CT showing defective hardware intruding maxillary sinus space.
Patient specific implant
The CT scan image data (0.5 mm slice cuts) was converted to a DICOM format and sent to the manufacturer, DePuySynthes (Synthes® Maxillofacial 1302 Wrights Lane East, West Chester, PA), who digitally planned and designed the surgical models and implant (Fig. 3). The surgical team discussed the implant fabrication with a design engineer prior to implant and 3D model manufacturing via web meeting. The implant and models were then sterilized before the surgery.
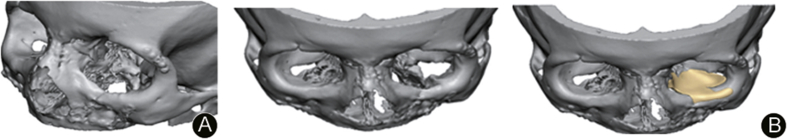
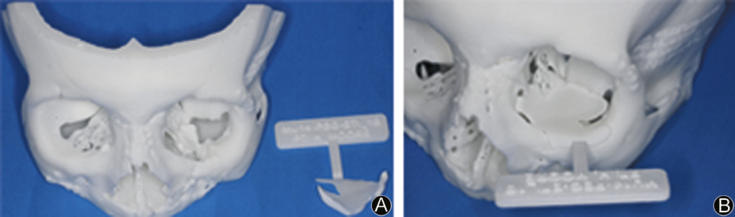
Fig. 3.
A: Reconstructed 3D model showing orbital floor defect; B: Reconstructed 3D model with PSI virtually placed.
Fusion, intraoperative navigation
Computerized navigation was used to assist in the both removing of the infected hardware and in the reconstructive phase. During the management of the orbital floor, navigation provided consistent help to the surgeon, providing guidance for the patient's native anatomy to identify the exact position of anatomical structures; decreasing the risk of damage to critical arterial, venous, and nervous structures in the area (Fig. 4).
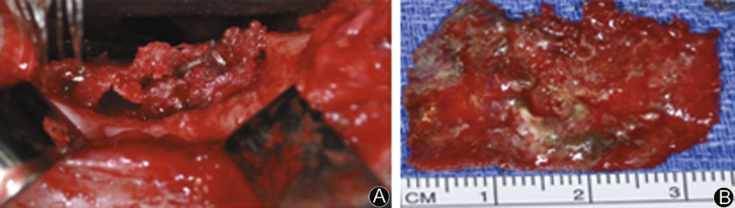
Fig. 4.
A: Intraoperative photo shows tissue ingrown metal plate on the inferior floor of the orbit; B: Excised defective hardware.
For the reconstruction, intraoperative navigation was used to verify the 3D implant orientation and position planned. After the last preoperative check on the Stereolithic model (Fig. 5) the 3D implant was inserted in the bone defect according to the virtual position shown on the screen (Fig. 6).
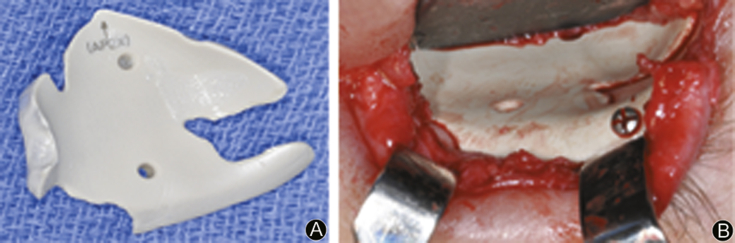
Fig. 5.
A: Stereolithic model and implant; B: Stereolithic model with implant adaptation.
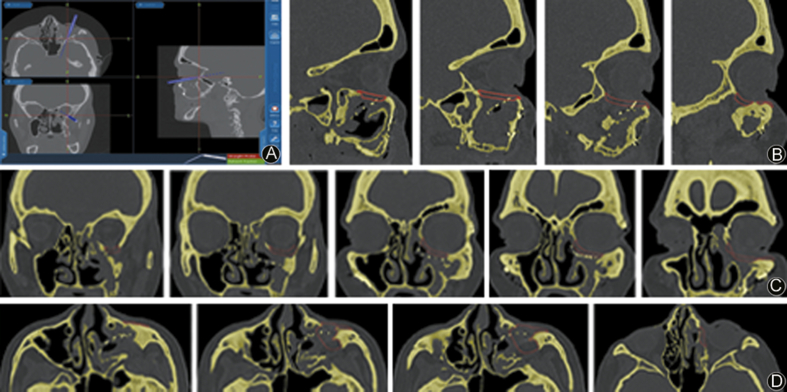
Fig. 6.
A: Intraoperative fusion navigation screenshot with probe indicating; B: Virtual surgical planning: preoperative position of the 3D implant in sagittal section; C: Virtual surgical planning: preoperative position of the 3D implant in coronal section; D: Virtual surgical planning: preoperative position of the 3D implant in transversal section.
The 3D implant position was verified, checking the virtual gap between the planned position and the achieved position (Fig. 7). Advancement of a lower eyelid flap was used to reconstruct the missing tissue of the lower eyelid and treat the presenting entropion.
Fig. 7.
A: Patient 3D implant, removed from the stereolithic model; B: The placement of the 3D implant in the bone defect.
Results
Postoperative CT showed excellent positioning of the implant, especially when compared to the unaffected side (Fig. 8). Both orbital and maxillary sinus volume and borders have been re-established (Fig. 9).
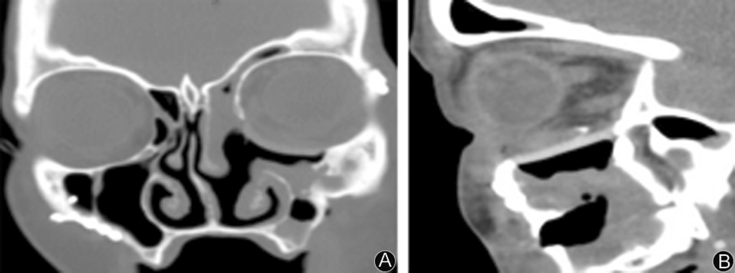
Fig. 8.
A: 5 weeks post-op CT, a coronal scan, appreciable adaptation of implant to mesial wall; B: 5 weeks Post-op CT, a sagittal scan, appreciable adaptation of implant to posterior stop.
Fig. 9.
A: Postoperative photo showing complete closure of patient's eyelid; B: Postoperative photo showing complete closure of patient's eyelid.
Discussion
Orbital bone fractures represent one of the most common sequela of craniomaxillofacial trauma.13, 14 The external orbit can be involved in several different types of facial fractures, either in isolation or as a component of complex midfacial or upper facial injury. The orbital area contains many important structures and neurovascular complexes that could potentially be involved and damaged in a traumatic event. In this regard, advances in computer imaging have improved the ability to visualize, in real time, the orbital anatomy during surgery, reducing the risk of iatrogenic damage. Orbital floor fractures can be viewed in isolation or associated with several different traumas, like panfacial trauma. Often they are reported together with the fracture of the medial orbital wall. The main objective in the treatment of orbital trauma is to restore and maintain the original orbital volume. In this case report, a secondary surgery was needed due to hardware infection occurring after initial treatment of the left traumatized orbit. Hardware removal has been a debated topic in recent years, due to the numerous variables involved in the procedure. Vos et al15 in a recent review concluded that the indication to remove implants after fracture healing should exist only in symptomatic patients and after a proper informed consent. In our case the evident infection and the non-union of the bone segments represented valid and unquestionable indications to operate a second time. Thoren et al,16 in a retrospective review, investigated the features of hardware removal in different patients. In a sample composed by 238 patients, only 20.2% underwent a hardware removal, and in this sub-group, wound infection represented the most common reason (20.8%). They also concluded that orbital rim plates had a higher risk of being removed than maxillary or frontal bone plates (p = 0.02).
Other investigations reported removal rates ranging from 3.7% to 27.2%.17, 18 Though not common, hardware removal is still a complication that must be taken in consideration by the surgeon during the patient's healing. When a secondary surgery is performed, the need for higher precision, better device reliability, and short surgical time is required in order to reduce the complication risk in a patient that has already faced an adverse event. VSP and patient specific implants can find appreciable application in these cases, improving both the surgical planning and intraoperative phase. In Section Fusion, intraoperative navigation, VSP was used to establish the size and position of the orbital implant required for reconstruction of a posttraumatic defect involving the orbital floor. In other studies it has also be extended to large and irregular defects.19
In order to assess the exact orbital volume, the contralateral orbit was used for comparison. The postoperative radiographic scan showed a correct positioning of the implant along the orbital floor and medial wall, and a remarkable improvement in globe position, with a complete resolution of the initial entropion.
Several papers describe many advantages of VSP and 3D implants. Cohen et al7 showed reduced operative times when using VSP for plate bending and bone graft contouring in mandibular reconstruction. Furthermore, they observed how VSP was associated to higher surgical precision and reduced exposure to general anesthesia and blood loss. Suenaga et al20 underlined how VSP is able to simulate the surgical phases in a Le Fort 1 osteotomy report, making the whole procedure easier. Stranix21 described how VSP could be used in the reconstructive surgery of large craniofacial defects, associated with several different techniques and procedures. Wang et al22 not only used VSP to observe a higher precision, but they also measured the difference between two different groups, with or without the aid of VSP, in a mandibular reconstruction using vascular fibular graft. The positions of the fibular grafts, including the vertical and horizontal positions, were significantly more accurate in the virtual planning group than those in the conventional surgery group (p < 0.05). In Section Case report the surgical team opted for the placement of a 3D printed patient specific implant. 3D printing was developed over 30 years ago, however the technology has recently became popular in craniofacial reconstruction and vascular surgery.2, 7, 23 Many authors have already described different application of 3D implants. Mazzoni et al,2 in a mandibular reconstruction study, compared traditional techniques and VSP with 3D surgical models used as cutting guides for mandibular osteotomies. The authors found VSP to be a safe method of mandibular reconstruction that allowed for accurate reconstruction of mandibular contour and reduced operative times. Other studies reported successfully clinical applications of 3D implants for reconstruction of large craniofacial defects.19 In Section Fusion, intraoperative navigation we used a bioplastic composed of PEEK. The material, while possessing good mechanical properties, must also have absolute biocompatibility and absence of immunoreaction. Furthermore, Inzana et al24 demonstrated how some 3D printed implants could also be used as scaffolds to deliver local antibiotics in the treatment of bony infections. In any case, strict regulation and testing should be required to ensure tissue compatibility with these engineered products. Finally, 3D surgical models could also be used as an effective educational tool for patients and students to better understand the surgery phases and the plan for reconstruction.4, 6
In conclusion, virtual surgical planning is a beneficial tool that may be used to help the surgeon in the presurgical and intraoperative phase. VSP can improve the precision and accuracy of implant and hardware placement, preserving the neurovascular complex of the orbital area. Using VSP in conjunction with 3D, patient specific implants can give surgeons an effective therapeutic solution in treating complex shape defects or secondary surgery as demonstrated in the present case.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
Contributor Information
Alan Scott Herford, Email: aherford@llu.edu.
Fabrizio Signorino, Email: fabroski@hotmail.it.
References
- 1.Neubert J., Bitter K., Somsiri S. Refined intraoperative repositioning of the osteotomized maxilla in relation to the skull and TMJ. J Craniomaxillofacial Surg. 1988;16:8–12. doi: 10.1016/s1010-5182(88)80006-4. [DOI] [PubMed] [Google Scholar]
- 2.Mazzoni S., Badiali G., Lancellotti L. Simulation-guided navigation: a new approach to improve intraoperative three-dimensional reproducibility during orthognathic surgery. J Craniofacial Surg. 2010;21:1698–1705. doi: 10.1097/SCS.0b013e3181f3c6a8. [DOI] [PubMed] [Google Scholar]
- 3.Lauritano F., Runci M., Cervino G., Fiorillo L., Bramanti E., Cicciù M. Three-dimensional evaluation of different prosthesis retention systems using finite element analysis and the Von Mises stress test. Minerva Stomatol. 2016 Dec;65(6):353–367. [PubMed] [Google Scholar]
- 4.Cicciù M., Cervino G., Bramanti E. FEM analysis of mandibular prosthetic overdenture supported by dental implants: evaluation of different retention methods. Comput Math Methods Med. 2015;2015:943839. doi: 10.1155/2015/943839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chim H., Schantz J.T. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. 2005;116:1726–1741. doi: 10.1097/01.prs.0000182386.78775.cd. [DOI] [PubMed] [Google Scholar]
- 6.Emmez H., Küçüködük İ., Börcek A.Ö. Effectiveness of skull models and surgical simulation: comparison of outcome between different surgical techniques in patients with isolated brachycephaly. Childs Nerv Syst. 2009;25:1605–1612. doi: 10.1007/s00381-009-0939-y. [DOI] [PubMed] [Google Scholar]
- 7.Cohen A., Laviv A., Berman P. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:661–666. doi: 10.1016/j.tripleo.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Erickson D.M., Chance D., Schmitt S. An opinion survey of reported benefits from the use of stereolithographic models. J Oral Maxillofacial Surg. 1999;57:1040–1043. doi: 10.1016/s0278-2391(99)90322-1. [DOI] [PubMed] [Google Scholar]
- 9.Susarla S.M., Duncan K., Mahoney N.R. Virtual surgical planning for orbital reconstruction. Middle East Afr J Ophthalmol. 2015;22:442–446. doi: 10.4103/0974-9233.164626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofacial Surg. 2014;4:9–18. doi: 10.4103/2231-0746.133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scolozzi P. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by “mirroring” computational planning. Aesthet Plast Surg. 2012;36:660–665. doi: 10.1007/s00266-011-9853-2. [DOI] [PubMed] [Google Scholar]
- 12.Camarini E.T., Tomeh J.K., Dias R.R. Reconstruction of frontal bone using specific implant polyether-ether-ketone. J Craniofacial Surg. 2011;22:2205–2207. doi: 10.1097/SCS.0b013e3182326f2c. [DOI] [PubMed] [Google Scholar]
- 13.Cruz A.A., Eichenberger G.C. Epidemiology and management of orbital fractures. Curr Opin Ophthalmol. 2004;15:416–421. doi: 10.1097/01.icu.0000136113.56288.87. [DOI] [PubMed] [Google Scholar]
- 14.Holt G.R., Holt J.E. Incidence of eye injuries in facial fractures: an analysis of 727 cases. Otolaryngol Head Neck Surg. 1983;91:276–279. doi: 10.1177/019459988309100313. [DOI] [PubMed] [Google Scholar]
- 15.vos D.I., Verhofstad M.H. Indications for implant removal after fracture healing: a review of the literature. Eur J Trauma Emerg Surg. 2013;39:327–337. doi: 10.1007/s00068-013-0283-5. [DOI] [PubMed] [Google Scholar]
- 16.Thorén H., Snäll J., Kormi E. Symptomatic plate removal after treatment of facial fractures. J Craniomaxillofacial Surg. 2010;38:505–510. doi: 10.1016/j.jcms.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Chaushu G., Manor Y., Shoshani Y. Risk factors contributing to symptomatic plate removal in maxillofacial trauma patients. Plast Reconstr Surg. 2000;105:521–525. doi: 10.1097/00006534-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Rallis G., Mourouzis C., Papakosta V. Reasons for miniplate removal following maxillofacial trauma: a 4-year study. J Craniomaxillofacial Surg. 2006;34:435–439. doi: 10.1016/j.jcms.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Steinbacher D.M. Three-dimensional analysis and surgical planning in craniomaxillofacial surgery. J Oral Maxillofacial Surg. 2015;73:S40–S56. doi: 10.1016/j.joms.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Suenaga H., Taniguchi A., Yonenaga K. Computer-assisted preoperative simulation for positioning of plate fixation in Lefort I osteotomy: a case report. J Formos Med Assoc. 2016;115:470–474. doi: 10.1016/j.jfma.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Stranix J.T., Monaco C., Brecht L.E. Preoperative head and neck surgical planning with computer-assisted design and modeling. Curr Surg Rep. 2016;4:1–8. [Google Scholar]
- 22.Wang Y.Y., Fan S., Zhang H.Q. Virtual surgical planning in precise maxillary reconstruction with vascularized fibular graft after tumor ablation. J Oral Maxillofacial Surg. 2016;74:1255–1264. doi: 10.1016/j.joms.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Gillis J.A., Morris S.F. Three-dimensional printing of perforator vascular anatomy. Plast Reconstr Surg. 2014;133:80e–82e. doi: 10.1097/01.prs.0000436523.79293.64. [DOI] [PubMed] [Google Scholar]
- 24.Inzana J.A., Trombetta R.P., Schwarz E.M. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur Cell Mater. 2015;30:232–247. doi: 10.22203/ecm.v030a16. [DOI] [PMC free article] [PubMed] [Google Scholar]