Abstract
Myosin is a ubiquitous eukaryotic contractile protein that generates the force responsible for such diverse cellular movements as muscle contraction and cytokinesis. Although there have been numerous studies of sarcomeric myosin heavy chain (MHC) genes, no molecular clones have been reported that encode mammalian nonmuscle MHC. This study presents the molecular genetic characterization of a human nonmuscle MHC that is expressed in fibroblasts, endothelial cells, and macrophages. Human nonmuscle MHC amino acids are weakly homologous (33%) to sarcomeric MHC but are approximately 72% identical to smooth muscle MHC. In contrast to vertebrate sarcomeric MHCs, which generate diversity through the expression of members of a multigene family, an alternative polyadenylylation site is used in the nonmuscle MHC gene to generate multiple transcripts that encode the same protein. We have mapped this gene to chromosome 22. It is thus unlinked to either of the sarcomeric MHC gene clusters on human chromosomes 14 and 17.
Full text
PDF


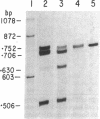
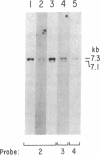
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Burridge K., Bray D. Purification and structural analysis of myosins from brain and other non-muscle tissues. J Mol Biol. 1975 Nov 25;99(1):1–14. doi: 10.1016/s0022-2836(75)80154-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Citi S., Kendrick-Jones J. Regulation of non-muscle myosin structure and function. Bioessays. 1987 Oct;7(4):155–159. doi: 10.1002/bies.950070404. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Bernstein S. I. Molecular genetics of myosin. Annu Rev Biochem. 1987;56:695–726. doi: 10.1146/annurev.bi.56.070187.003403. [DOI] [PubMed] [Google Scholar]
- Engel J., Gunning P., Kedes L. Human cytoplasmic actin proteins are encoded by a multigene family. Mol Cell Biol. 1982 Jun;2(6):674–684. doi: 10.1128/mcb.2.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. D., Stark G. R., Bloom B. R. Molecular cloning of a gene selectively induced by gamma interferon from human macrophage cell line U937. Mol Cell Biol. 1989 May;9(5):1922–1928. doi: 10.1128/mcb.9.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali R., Leinwand L. A. Molecular genetic characterization of a developmentally regulated human perinatal myosin heavy chain. J Cell Biol. 1989 May;108(5):1791–1797. doi: 10.1083/jcb.108.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kavinsky C. J., Umeda P. K., Sinha A. M., Elzinga M., Tong S. W., Zak R., Jakovcic S., Rabinowitz M. Cloned mRNA sequences for two types of embryonic myosin heavy chains from chick skeletal muscle. I. DNA and derived amino acid sequence of light meromyosin. J Biol Chem. 1983 Apr 25;258(8):5196–5205. [PubMed] [Google Scholar]
- Kiehart D. P., Mabuchi I., Inoué S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982 Jul;94(1):165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Leinwand L. A., Fournier R. E., Nadal-Ginard B., Shows T. B. Multigene family for sarcomeric myosin heavy chain in mouse and human DNA: localization on a single chromosome. Science. 1983 Aug 19;221(4612):766–769. doi: 10.1126/science.6879174. [DOI] [PubMed] [Google Scholar]
- Leinwand L. A., Saez L., McNally E., Nadal-Ginard B. Isolation and characterization of human myosin heavy chain genes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3716–3720. doi: 10.1073/pnas.80.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R., Larson D. M., Periasamy M. Characterization of a mammalian smooth muscle myosin heavy chain cDNA clone and its expression in various smooth muscle types. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1047–1051. doi: 10.1073/pnas.85.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Myers J. C. Characterization of a pro-alpha 2(I) collagen gene mutation by nuclease S1 mapping. Methods Enzymol. 1987;145:213–222. doi: 10.1016/0076-6879(87)45011-8. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Differential processing of RNA transcribed from the single-copy Drosophila myosin heavy chain gene produces four mRNAs that encode two polypeptides. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2128–2132. doi: 10.1073/pnas.83.7.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez L. J., Gianola K. M., McNally E. M., Feghali R., Eddy R., Shows T. B., Leinwand L. A. Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res. 1987 Jul 10;15(13):5443–5459. doi: 10.1093/nar/15.13.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez L., Leinwand L. A. Characterization of diverse forms of myosin heavy chain expressed in adult human skeletal muscle. Nucleic Acids Res. 1986 Apr 11;14(7):2951–2969. doi: 10.1093/nar/14.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Shohet R. V., Conti M. A., Kawamoto S., Preston Y. A., Brill D. A., Adelstein R. S. Cloning of the cDNA encoding the myosin heavy chain of a vertebrate cellular myosin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7726–7730. doi: 10.1073/pnas.86.20.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Haley L. L., Byers M. G., Eddy R. L., Cooper E. S., Goggin A. P. Assignment of the beta-glucuronidase structural gene to the pter leads to q22 region of chromosome 7 in man. Cytogenet Cell Genet. 1978;21(1-2):99–104. doi: 10.1159/000130882. [DOI] [PubMed] [Google Scholar]
- Shows T., Eddy R., Haley L., Byers M., Henry M., Fujita T., Matsui H., Taniguchi T. Interleukin 2 (IL2) is assigned to human chromosome 4. Somat Cell Mol Genet. 1984 May;10(3):315–318. doi: 10.1007/BF01535253. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Wydro R. M., Nguyen H. T., Gubits R. M., Nadal-Ginard B. Characterization of sarcomeric myosin heavy chain genes. J Biol Chem. 1983 Jan 10;258(1):670–678. [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]