Abstract
Cimicifugae Rhizoma (Sheng ma) is a Ranunculaceae herb belonging to a composite family and well known in China. has been widely used in traditional Chinese medicine. The Pharmacopoeia of the People׳s Republic of China contains three varieties (Cimicifuga dahurica (Turcz.), Cimicifuga foetida L. and Cimicifuga heracleifolia Kom.) which have been used clinically as “Sheng-ma”. However, the chemical constituents of three components of “Sheng-ma” have never been documented. In this study, a rapid method for the analysis of the main components of “Sheng-ma” was developed using ultra-high performance liquid chromatography with quadrupole-time-of-flight mass spectrometry (UPLC/Q-TOF-MS). The present study reveals the major common and distinct chemical constituents of C. dahurica, C. foetida and C. heracleifolia and also reports principal component and statistical analyses of these results. The components were identified by comparing the retention time, accurate mass, mass spectrometric fragmentation characteristic ions and matching empirical molecular formula with that of the published compounds. A total of 32 common components and 8 markers for different “Sheng-ma” components were identified. These findings provide an important basis for the further study and clinical utilities of the three “Sheng-ma” varieties.
KEY WORDS: Sheng-ma, Chemical profiling, UPLC/Q-TOF-MS, Chemical marker, Cimicifuga, Cimicifugae Rhizoma
Graphical abstract
A rapid method for the analysis of the main components of “Sheng-ma” was developed using ultra-high performance liquid chromatography with quadrupole-time-of-flight mass spectrometry (UPLC/Q-TOF-MS). The present study reveals the major common and distinct chemical constituents of Cimicifuga dahurica (Turcz.), Cimicifuga foetida L. and Cimicifuga heracleifolia Kom., and also reports principal component and statistical analyses of these results. A total of 32 common components and 8 markers for different “Sheng-ma” components were identified. These findings provide an important basis for the further study and clinical utilities of the three “Sheng-ma” varieties.
1. Introduction
Cimicifugae Rhizoma (“Sheng-ma”), a traditional Chinese medicine derived from the genus Cimicifuga (Ranunculaceae family), has a long history of clinical use. Currently, this rhizome, which encompasses three species (Cimicifuga dahurica (Turcz.), Cimicifuga foetida L. and Cimicifuga heracleifolia Kom.), is listed in the Chinese Pharmacopoeia and used for anti-inflammatory, antipyretic, analgesic and wound-healing actions in traditional Chinese medicine1, 2, 3. C. foetida naturally grows in southern China (e.g. Yunnan and Sichuan province), whereas the other two varieties are mainly distributed in the northeastern China. Due to the different species and growing conditions, there are chemical differences between the three species, which may result in the improper clinical usage. However, since these have been used under the same drug name in clinical prescriptions for ages, it is necessary to clarify their differences in composition.
Other crude preparations of traditional Chinese medicines have been clarified by the use of modern analytical chemical methods4, 5. For example, the black cohosh herbal has been distinguished with the other 4 different groups of Actaea racemosa, Asian species, A. racemosa, and North American species by using UPLC/TOF-ESI-MS technique and principal component analysis. These efforts can ensure the safe usage of the black cohosh. In addition, a phytochemical method was developed to distinguish four different groups of Actaea, including: species other than A. racemosa, Asian species, A. racemosa, and North American species other than A. racemosa using HPLC/TOF-ESI-MS technique and principal component analysis. This method was used to distinguish black cohosh products from among different plant species for quality control purposes6, 7. According to literature studies, markers based on available standards to distinguish the three different “Shengma” species have never been found. Therefore, two key advances are required in order to develop good manufacturing practices of “Sheng-ma” products, which are the development of methods for the correct identification of C. dahurica (XSM), C. foetida (SM) and C. heracleifolia (DSM), and discovery of suitable marker compounds to distinguish among various “Sheng-ma” ingredients.
These three “Sheng-ma” have similar chemical properties because they are homologous, and it is difficult to distinguish them with conventional spectroscopic methods. Ultra-high performance liquid chromatography (UHPLC) coupled to quadrupole, hybrid orthogonal acceleration time-of-fight tandem mass spectrometry (Q-TOF-MS), which is a powerful hyphenated technique for the structural characterizations of constituents, has been increasingly used in the analysis of the chemical constituents of Chinese medicinal herbs8, 9, 10. The Q-TOF-MS spectrometer can produce exact mass measurements and high energy collision–induced dissociation (CID), which enable the UPLC/Q-TOF-MS to be a powerful tool to identify the chemical composition11. The components were identified by comparing the retention time, accurate mass, mass spectrometric fragmentation characteristic ions and matching empirical molecular formula with that of the published compounds. In this paper, UPLC/Q-TOF-MS was used to rapidly detect and identify the common compounds in DSM, SM and XSM and to identify the marker compounds through principal component analysis (PCA) and statistical t-test analysis.
2. Experimental
2.1. Materials and reagents
The standardized C. dahurica (XSM) and C. heracleifolia (DSM) were collected in Jilin province in September, 2015 and C. foetida (SM) were purchased from Nanjing Haichang Chinese Medicine Group Corporation (Nanjing, China). All samples were identified by Prof. Jianwei Chen (Nanjing University of Chinese Medicine, Nanjing, China). Caffeic acid, ferulic acid and isoferulic acid were obtained from the Chinese Authenticating Institute of Material and Biological Products (Beijing, China). Acetonitrile (HPLC/MS grade) and formic acid (HPLC grade) were purchased from Merck Company (Darmstadt, Germany). HPLC-grade formic acid with a purity of 99% was obtained from Anaqua chemicals supply (Wilmington, DE, USA). HPLC grade methanol was purchased from ANPEL Scientific Instrument Co., Ltd. (Shanghai, China). Purified water was acquired from a Milli-Q system (Millipore, Bedford, MA, USA). All other reagents were of analytical grade and obtained from Nanjing Chemical Reagent Company (Nanjing, China).
2.2. Preparation of C. dahurica, C. foetida and C. heracleifolia samples
DSM, XSM and SM samples were dried at 60 °C until the moisture content remained constant. Dried samples were ground to powders using an electric grinder and passed through a 40-mesh sieve. The powders were extracted twice by the reflux extraction in 80% ethanol (v/v) for 120 min. Finally, the extracts were filtered, and then concentrated to 10 mL at 60 °C under vacuum by using a rotary evaporator. The obtained solution was filtered through a 0.22-mm membrane filter before injection into the UPLC/Q-TOF-MS system for analysis.
2.3. Chromatographic separation
2.3.1. Liquid chromatography
Chromatographic analysis was performed on an UPLC system (Shimadzu, Kyoto, Japan) with an LC-30AD binary pump, an autosampler (Model SIL-30SD), an on-line DGU-20A5R degasser, and a temperature controller compartment for the column (CTO-30A). Separation was performed on an extend C18 column (100 mm×2.1 mm, 1.8 μm), held at 35 °C and the flow rate was 0.3 mL/min. The optimal mobile phase consisted of A (HCOOH/H2O, 0.1:100, v/v) and B (CH3CN). The optimized UPLC gradient elution conditions were as follows: 0—5 min, 15%–25% B; 5—10 min, 25%–40% B; 10—30 min, 40%–55% B. The injection volume was 1 μL.
2.3.2. Mass spectrometry (MS)
MS detections were performed on a hybrid quadrupole time of flight tandem mass spectrometry (Triple TOF™ 5600, AB SCIEX, Foster City, CA, USA) with negative and positive electrospray (ESI) modes, and its sufficient sensitivity could ensure as many putative compounds as possible to be identified. TOF-MS was scanned with the mass ranges of m/z 100—1200, and experiments were run with 200 ms accumulation time for TOF-MS. Positive and negative ionization were tested and negative ion mode was selected for better sensitivity. The conditions used for the ESI source were as follows: capillary voltage, 3.0 kV (negative mode); sampling cone, 25 V; extraction cone, 4 V; source temperature, 120 °C; desolvation temperature, 450 °C. For ESI-MS (±), the operating parameters were as follows: ion source GS1, 55 psi; ion Source GS2, 55 psi; curtain gas (CUR), 35 psi; temperature (TEM), 550 °C (—)/550 °C (+); ion spray voltage floating (ISVF), —4500 V/+5500 V; declustering potential (DP), —60 V/+60 V; collision energy (CE), —10 V/+10 V; collision energy ramp, 25–45 eV. Acquiring data in this manner can provide for the collection information of the precursor ions as well as fragment ions.
2.4. Data processing and analysis strategy
For data processing, Peak View™ was used for qualitative analyses and Extract Ions Using Dialog (XIC) and MS Library were used to find the target compounds. Firstly, a formula database of target compounds, which includes names, molecular formulas, accurate molecular weights, and chemical structures, was established for the target compounds, and the database showed above had been reported by Chemspider, Pubmed and Chinese National Knowledge Infrastructure (CNKI). Secondly, the formula database of target compounds was then imported into the tool of XIC system in Peak View™ to accomplish the screening of target compounds. After screening, the compounds which matched the above requirements of the target compounds in the formula database would be extracted and their purity scores would be obtained by matching their MS/MS fragment to the experimental MS/MS spectra. Their purity scores were based on the relative intensity of the parent ion and products. Finally, the compounds could be identified when their purity scores were all above 75%. Through this way, the common compounds existing in DSM, XSM and SM could be identified12, 13, 14. Principal component analysis (PCA), a non-biased statistical technique, was applied to investigate the marker components of DSM, XSM and SM, according to their differences in chemical compositions by Marker View. The Students׳ t-test was done subsequently by Marker View to assess significant differences between these markers. To identify these markers found in both of the above methods, the tools of IDA Explorer, Formula Finder, and Fragment and Neutral Loss Filter were applied in Peak View by setting these compounds as non-target compounds. For the standard unavailable compound, their structures were presumed mainly based on accurate mass and the mass fragmentation by Analyst TF 1.6 software. Finally, fragment ions were used to further confirm the chemical structures15, 16.
Taking an example, the precise molecular weight of a compound was 194.0579, and the main fragment ions analyzed by MS/MS screening were observed at m/z 193.0508 and 179.0351 in the negative ion mode. The calculated molecular formula was speculated to be C10H10O4 based on the analysis of its elemental composition and fractional isotope abundance, and after screening the target compounds in the formula database, the purity scores was 90%. So this ion was then identified as isoferulic acid.
3. Results and discussion
3.1. Mass fragmental analysis of standards
For each “Sheng-ma” species extract sample, Q-TOF-MS spectra were recorded in both positive and negative ion modes, whereas, the most useful ion information was obtained in negative mode. So, the negative ion mode was selected for this analysis.
3.2. Identification of common compounds in C. dahurica, C. foetida and C. heracleifolia
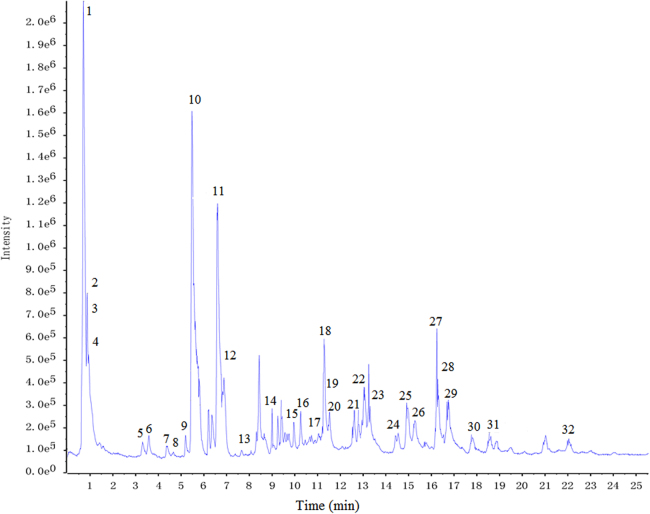
Under the optimal chromatographic and MS conditions, a total of 32 common components were well detected and identified in DSM, XSM and SM by using the analysis method for target compounds mentioned above. The major components in three “Sheng-ma” samples were well separated and detected within 30 min. Thirty-two components including phenolic acids, triterpenoids and chromone were tentatively identified by elemental composition analysis within an error of 5 ppm. The representative chromatograms obtained in negative ion modes are presented in Fig. 1. Corresponding retention time and MS data of all the main chromatographic peaks are summarized in Table 1.
Figure 1.
UPLC-MS base peak intensity chromatograms of “Sheng-ma” in negative mode.
Table 1.
Identification of common components in C. dahurica, C. foetida and C. Heracleifolia using UPLC/Q-TOF-MS in negative ion mode.
| No. | tR (min) | Extraction mass (Parent ion) | Mass | Formula | Characteristic fragment ion | Error (ppm) | Name |
|---|---|---|---|---|---|---|---|
| 1 | 0.80 | 445.1140 | 446.1213 | C22H22O10 | 165[C9H9O3]− | 3.9 | 2-Isoferulic piscidic acid-1-metyl ester |
| 193[C10H9O4]− | |||||||
| 2 | 0.86 | 271.0459 | 272.0532 | C11H12O8 | 271[C11H11O8]− | −1.5 | Fukinolic acid |
| 195[C9H7O5]− | |||||||
| 163[C5H7O6]− | |||||||
| 3 | 0.88 | 315.1085 | 316.1158 | C14H20O8 | 153[C8H9O3]− | −3.8 | Cimidahurinine |
| 123[C7H7O2]− | |||||||
| 109[C6H5O2]− | |||||||
| 4 | 0.91 | 255.0510 | 256.0583 | C11H12O7 | 179[C9H7O4]− | −0.7 | Piscidic acid |
| 193[C10H9O4]− | |||||||
| 165[C9H9O3]− | |||||||
| 5 | 3.29 | 179.0350 | 180.0423 | C9H8O4 | 179[C9H7O4]− | 0.9 | Caffeic acid |
| 109[C6H5O2]− | |||||||
| 6 | 3.52 | 193.0506 | 194.0579 | C10H10O4 | 193[C10H9O4]− | −2.4 | Ferulic acid |
| 7 | 3.58 | 504.1875 | 505.1948 | C25H31NO10 | 342[C19H20NO5]− | 1.0 | Cohosh amide |
| 8 | 3.58 | 193.0506 | 194.0579 | C10H10O4 | 193[C10H9O4]− | 1.0 | Isoferulic acid |
| 9 | 4.38 | 417.0827 | 418.0900 | C20H18O10 | 417[C20H17O10]− | −1.8 | Acimicifugic acid C |
| 10 | 5.48 | 447.0933 | 448.1006 | C21H20O11 | 447[C21H19O11]− | −1.5 | Acimicifugic acid A |
| 253[C11H9O7]− | |||||||
| 191[C10H7O4]− | |||||||
| 109[C6H5O2]− | |||||||
| 11 | 6.19 | 461.1089 | 462.1162 | C22H22O11 | 461[C22H21O11]− | −1.4 | 2-Isoferuloyl fukinolic acid-1-metyl ester |
| 253[C11H9O7]− | |||||||
| 181[C9H9O4]− | |||||||
| 109[C6H5O2]− | |||||||
| 12 | 7.38 | 431.0984 | 432.1057 | C21H20O10 | 431[C21H19O10]− | −3.1 | 2-Feruloyl piscidic acid |
| 193[C10H9O4]− | |||||||
| 149[C9H9O2]− | |||||||
| 13 | 7.38 | 461.1089 | 462.1162 | C22H22O11 | 461[C22H21O11]− | −3.1 | 2-Feruloyl fukinolic acid-1-metyl ester |
| 253[C11H9O7]− | |||||||
| 181[C9H9O4]− | |||||||
| 191[C10H7O4]− | |||||||
| 14 | 9.08 | 695.4012 | 696.4085 | C37H60O12 | 695[C37H59O12]− | −1.2 | 24-Epi-7β-hydroxy–24–O–acetyl-hydrogen cohosh alcohol -3-O-β-D-xyl |
| 649[C35H53O11]− | |||||||
| 545[C32H49O7]− | |||||||
| 15 | 10.25 | 721.4169 | 722.4241 | C39H62O12 | 721[C39H61O12]− | −0.9 | Beesioside II |
| 679[C37H59O11]− | |||||||
| 601[C35H53O8]− | |||||||
| 16 | 10.28 | 943.4908 | 944.4981 | C47H76O19 | 943[C47H75O19]− | −0.3 | Cimicifuga alcohol-3-O-β-d- glu (1–2)β-D-glu (1–2) β-d- xyl |
| 781[C41H65O14]− | |||||||
| 17 | 11.30 | 635.3801 | 636.3874 | C35H56O10 | 635[C35H55O10]− | −0.5 | 22-β-Hydroxy cohosh alcohols-3-O-β-D-xyl |
| 577[C32H49O9]− | |||||||
| 18 | 11.52 | 683.4012 | 684.4085 | C36H60O12 | 683[C36H59O12]− | −2.7 | Beesioside O |
| 637[C35H57O10]− | |||||||
| 19 | 11.53 | 637.3957 | 638.4030 | C35H58O10 | 637[C35H57O10]− | −2.8 | Beesioside E |
| 579[C31H47O10]− | |||||||
| 20 | 12.95 | 637.3957 | 638.4030 | C35H58O10 | 637[C35H57O10]− | −2.8 | Beesioside B |
| 579[C31H47O10]− | |||||||
| 21 | 12.25 | 781.4380 | 782.4453 | C41H66O14 | 781 [C41H65O14]− | −0.7 | Cimicifuga glycosides II |
| 619[C35H55O9]− | |||||||
| 22 | 13.06 | 707.4012 | 708.4085 | C38H60O12 | 707[C38H59O12]− | −0.9 | 24-Epi-24-O-acetyl-7,8-dehydro cohosh alcohol-3-O-β-d-gal |
| 661[C36H53O11]− | |||||||
| 619[C35H55O9]− | |||||||
| 469[C30H45O4]− | |||||||
| 23 | 14.00 | 823.4486 | 824.4558 | C43H68O15 | 823[C43H67O15]− | 0.5 | 25-O-Acetyl alcohol cimicifuga-3-O-β-d-glu(1–3)β-D-xyl |
| 24 | 14.56 | 747.3961 | 748.4034 | C40H60O13 | 701[C38H53O12]− | −0.8 | 23-O-Acetyl cohosh alcohol -3-O-(2′-O- malonyl)-β-D-xyl |
| 659[C37H55O10]− | |||||||
| 641[C37H53O9]− | |||||||
| 25 | 14.96 | 659.3801 | 660.3874 | C37H56O10 | 659[C37H55O10]− | −0.9 | 27-Deoxy Arcot hormone |
| 617[C35H53O9]− | |||||||
| 559[C32H47O8]− | |||||||
| 26 | 15.29 | 661.3957 | 662.4030 | C37H58O10 | 661[C37H57O10]− | −1.7 | 23-O-Acetyl alcohol Cimicifuga-3-O-β-D-xyl |
| 619[C35H55O9]− | |||||||
| 601[C35H53O8]− | |||||||
| 27 | 16.26 | 677.3906 | 678.3979 | C37H58O11 | 677[C37H57O11]− | 0.5 | 7,8-Deoxy cohosh alcohol-24-O-acetyl alcohol-ara |
| 617[C35H53O9]− | |||||||
| 28 | 16.36 | 649.3957 | 650.4030 | C36H58O10 | 649[C36H57O10]− | −0.2 | Cimicifuga alcohol-3-O-β-d-glu |
| 29 | 16.74 | 679.4063 | 680.4136 | C37H60O11 | 679[C37H59O11]− | −0.8 | 24-O-Acetyl-hydrogen cohosh alcohol-3-O-β-d-xyl |
| 619[C35H55O9]− | |||||||
| 30 | 17.78 | 701.3906 | 702.3979 | C39H58O11 | 701[C39H57O11]− | −0.4 | 2′-O-2-Deoxy-acyl-27-prime Arcot |
| 659[C37H55O10]− | |||||||
| 641[C37H53O9]− | |||||||
| 31 | 18.59 | 665.3906 | 666.3979 | C36H58O11 | 665[C36H57O11]− | −1.5 | 12β-Hydroxy cohosh alcohol -3-O-β-d-gal |
| 619[C34H51O10]− | |||||||
| 543[C32H47O7]− | |||||||
| 32 | 22.05 | 601.3746 | 602.3819 | C35H54O8 | 601[C35H53O8]− | −1.6 | 25-Deoxy cimicifuga alcohol-3-O-β-d-xyl |
| 543[C32H47O7]− | |||||||
| 525[C32H45O6]− |
Note: RT, retention time; [M−H]−, the deprotonated and protonated molecular ions in the negative ion modes; extracted mass and masses were obtained by PeakView software.
3.3. Discovery and identification of marker compounds in C. dahurica, C. foetida and C. heracleifolia
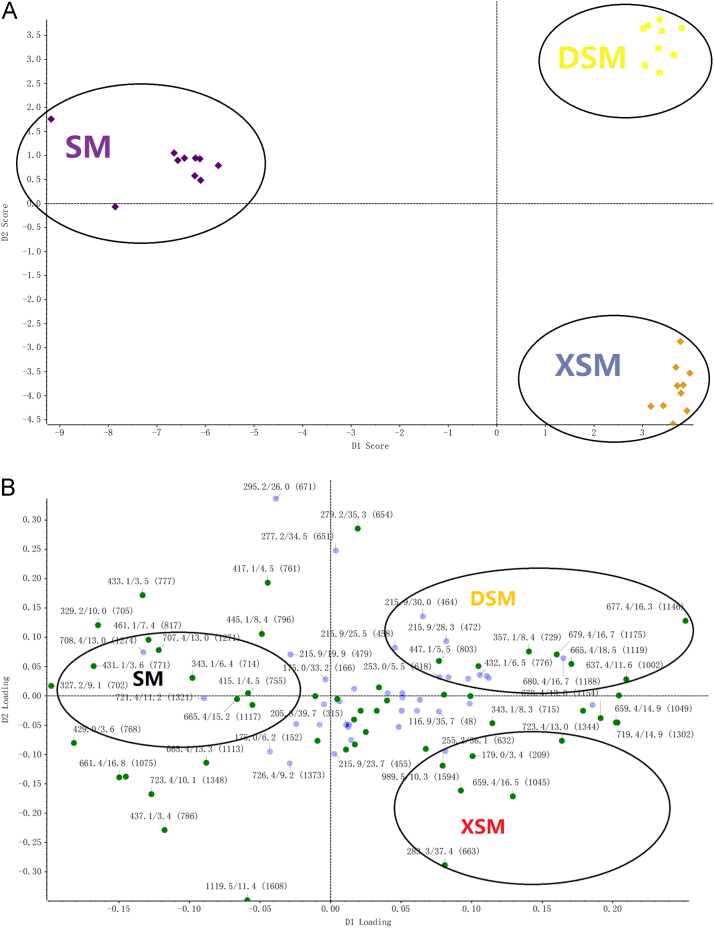
To discover and identify marker compounds, PCA, was used to investigate whether DSM, XSM and SM could be separated according to their different chemical compositions. This was followed by t-tests to identify the candidate compounds and display P-values one variety from the other two. For PCA analysis, all data were displayed as scores and loadings in a coordinate system of principal components, which resulted from data dimensionality reduction. As shown in Fig. 2A, a three-dimensional PCA score plot showed a tendency to separate DSM, XSM and SM. In Fig. 2B, we can see a three-dimensional PCA loading plot, which can help find markers to further distinguish the different varieties. In order to find and identify more compounds with significantly changed structures or contents besides, t-tests were performed. When P-values lower than 0.001% are obtained, the confidence level for a correct identification is more than 99%.
Figure 2.
Score plot (A) and loading plot (B) of principal component analysis.
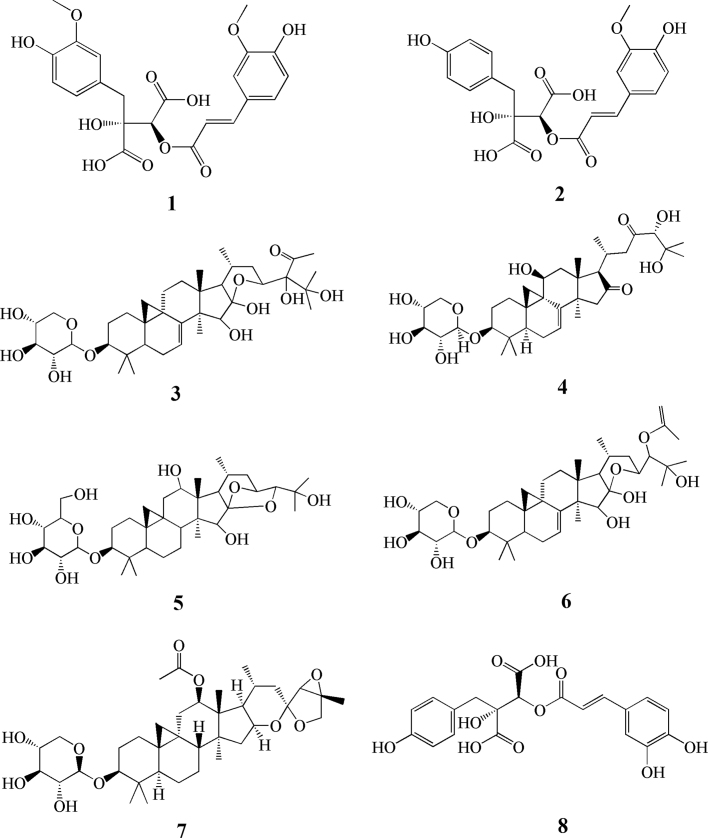
As seen in Fig. 2A, three kinds of “Sheng-ma” were distributed in different coordinate positions, thereby showing significant differences among the three varieties. In order to distinguish these different compositions and to find markers, the PCA loading plot showed above was used to screen analyses. In Fig. 2B, eight ionic compounds were far from the origin and were tentatively identified (see Table 2). All compounds had a large contribution for PCA analysis, therefore were considered to be different species markers. Corresponding to Fig. 2A, SM ion markers can be seen to be located in the left area of the PCA axis, whereas the DSM and XSM ion markers are located in the right area of the PCA axis. The DSM ion compounds are mainly located in the upper half of the axis and the XSM ion compounds located in lower part. The structures of the 8 marker compounds are shown in Fig. 3.
Table 2.
Identification of markers from C. dahurica, C. foetida and C. heracleifolia.
| Compd. | tR (min) | Extractionmass | Mass | Formula | Characteristicfragment ion | Error(ppm) | Name | t-value | P-value | From |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.40 | 461.1089 | 462.1162 | C22H22O11 | 461[C22H21O11]− | −3.1 | 2-Feruloyl fukinolic acid-1-metyl ester | 6.74 | 3.12e−7 | C. foetida |
| 253[C11H9O7]− | ||||||||||
| 181[C9H9O4]− | ||||||||||
| 191[C10H7O4]− | ||||||||||
| 2 | 6.60 | 431.0984 | 432.1056 | C21H20O10 | 431[C21H19O10]− | −3.1 | 2-Feruloyl piscidic acid | 7.64 | 3.19e−8 | C. foetida |
| 193[C10H9O4]− | ||||||||||
| 149[C9H9O2]− | ||||||||||
| 3 | 13.03 | 665.3906 | 666.3979 | C37H58O11 | 677[C37H57O11]− | 3.9 | 7,8-Didehydro cimigenol-24-O-cimicifuga alcohol-3-O-β-D-xyl | −7.66 | 3.08e−8 | C. foetida |
| 617[C35H53O9]− | ||||||||||
| 4 | 14.90 | 634.3717 | 633.3644 | C35H54O10 | 633[C35H53O10]− | 3.2 | Cimicifugoside H-2 | −9.54 | 3.86e−10 | C. foetida |
| 5 | 16.70 | 679.4063 | 680.4136 | C37H60O11 | 679[C37H59O11]− | −0.8 | 12β-Hydroxy cohosh alcohol-3-O-β-D-gal | 6.06 | 1.79e−6 | C. heracleifolia |
| 619[C35H55O9]− | ||||||||||
| 6 | 16.26 | 677.3906 | 678.3979 | C37H58O11 | 677[C37H57O11]− | 0.5 | 7,8-Deoxy cohosh alcohol-24-O-acetyl alcohol-ara | 11.10 | 1.44e−11 | C. heracleifolia |
| 617[C35H53O9]− | ||||||||||
| 7 | 16.50 | 659.3801 | 660.3873 | C37H56O10 | 659[C37H55O10]− | −0.9 | 27-Deoxy Arcot hormone | 15.89 | 3.18e-15 | C. dahurica |
| 617[C35H53O9]− | ||||||||||
| 559[C32H47O8]− | ||||||||||
| 8 | 4.38 | 417.0827 | 418.0900 | C20H18O10 | 417[C20H17O10]− | −1.8 | Acimicifugic acid D | −5.85 | 3.14e−6 | C. dahurica |
Note: Compounds 1−4 were markers of C. foetida; Compounds 5−6 were markers of C. heracleifolia; Compounds 7−8 were markers of C. dahurica.
Figure 3.
The structures of the identified compounds of C. dahurica, C. foetida and C. heracleifolia. (1) 2-Feruloyl fukinolic acid-1-metyl ester; (2) 2-feruloyl piscidic acid; (3) 7,8-didehydro cimigenol-24-O-cimicifuga alcohol-3-O-β-D-xyl; (4) cimicifugoside H-2; (5) 12β-hydroxy cohosh alcohol-3-O-β-D-gal; (6) 7,8-deoxy cohosh alcohol-24-O-acetyl alcohol-ara; (7) 27-deoxy Arcot hormone; (8) acimicifugic acid D.
4. Conclusions
The increased incidence of the adulteration of botanical supplements is an ongoing concern which can lead to therapeutic failures or toxicity. The present study describes a rapid and effective UPLC/Q-TOF-MS method for identification of major compounds in three kinds of “Sheng-ma”. A total of 32 common components were detected and identified in three varieties of “Sheng-ma” samples by using the target compound analysis method. Eight marker compounds were identified by statistical analysis methods of PCA and t-tests. The identification and structural elucidation of the chemical constituents provided essential data for further pharmacological and clinical studies on different species of DSM, XSM and SM. The UPLC/Q-TOF-MS method established in the present study can be used for quality control of “Sheng-ma”, and provide a useful tool for the further study of the pharmacology and mechanisms of action for these three “Sheng-ma” varieties.
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province, China (Grant No. 20141093).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Vol I. Beijing: China Medical Science Press; 2015.
- 2.Kusano A., Seyama Y., Nagai M., Shibano M., Kusano G. Effects of fukinolic acid and cimicifugic acids from Cimicifuga species on collagenolytic activity. Biol Pharm Bull. 2001;24:1198–1201. doi: 10.1248/bpb.24.1198. [DOI] [PubMed] [Google Scholar]
- 3.Li JX, Yu ZY. Cimicifugae Rhizoma: from origins, bioactive constituents to clinical outcomes. Curr Med Chem. 2006;13:2927–2951. doi: 10.2174/092986706778521869. [DOI] [PubMed] [Google Scholar]
- 4.Kolniak-Ostek J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC–PDA–Q/TOF-MS. J Food Comp Anal. 2016;49:65–77. [Google Scholar]
- 5.Wang X., Zhang A., Yan G., Han Y, Sun H. UHPLC—MS for the analytical characterization of traditional Chinese medicines. TrAC Trend Anal Chem. 2014;63:180–187. [Google Scholar]
- 6.He K., Zheng B., Kim C.H., Rogers L., Zheng QY. Direct analysis and identification of triterpene glycosides by LC/MS in black cohosh, Cimicifuga racemosa, and in several commercially available black cohosh products. Planta Med. 2000;66:635–640. doi: 10.1055/s-2000-8619. [DOI] [PubMed] [Google Scholar]
- 7.Ma C., Kavalier A.R., Jiang B., Kennelly EJ. Metabolic profiling of Actaea species extracts using high performance liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. J Chromatogr A. 2011;1218:1461–1476. doi: 10.1016/j.chroma.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S.L., Song J.Z., Qiao C.F., Yan Z., Qian KD., Lee KH. A novel strategy to rapidly explore potential chemical markers for the discrimination between raw and processed Radix Rehmanniae by UHPLC–TOF-MS with multivariate statistical analysis. J Pharm Pharmacol. 2010;51:812–823. doi: 10.1016/j.jpba.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X., Shi P., Cheng Y., Qu H. Rapid analysis of a Chinese herbal prescription by liquid chromatography–time-of-flight tandem mass spectrometry. J Chromatogr A. 2008;1206:140–146. doi: 10.1016/j.chroma.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Kolniak-Ostek J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC–PDA–Q/TOF–MS. J Food Compos Anal. 2016;49:65–77. [Google Scholar]
- 11.Wan J.B., Bai X., Cai X.J., Rao Y, Wang YS, Wang YT. Chemical differentiation of Da-Cheng-Qi-Tang, a Chinese medicine formula, prepared by traditional and modern decoction methods using UPLC/Q-TOFMS-based metabolomics approach. J Pharm Pharmacol. 2013;83:34–42. doi: 10.1016/j.jpba.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Yan G., Sun H., Sun W., Meng X., Wang X. Rapid and global detection and characterization of aconitum alkaloids in Yin Chen Si Ni Tang, a traditional Chinese medical formula, by ultra performance liquid chromatography–high resolution mass spectrometry and automated data analysis. J Pharm Pharmacol. 2010;53:421–431. doi: 10.1016/j.jpba.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Cao G., Zhang Y., Feng J., Cai H, Zhang C, Ding M. A Rapid and sensitive assay for determining the main components in processed Fructus corni by UPLC–Q-TOF-MS. Chromatographia. 2011;73:135–141. [Google Scholar]
- 14.Deng P., You T., Chen X., Yuan T, Huang H, Zhong D. Identification of amiodarone metabolites in human bile by ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Drug Metab Dispos. 2011;39:1058–1069. doi: 10.1124/dmd.110.037671. [DOI] [PubMed] [Google Scholar]
- 15.Montoro P., Teyeb H., Masullo M., Mari A, Douki W, Piacente S. LC–ESI-MS quali-quantitative determination of phenolic constituents in different parts of wild and cultivated Astragalus gombiformis. J Pharm Pharmacol. 2013;72:89–98. doi: 10.1016/j.jpba.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Zhang A., Ying Z., Sun H, Meng X, Yan G. Application of ultra-performance liquid chromatography with time-of-flight mass spectrometry for the rapid analysis of constituents and metabolites from the extracts of Acanthopanax senticosus harms leaf. Pharmacogn Mag. 2015;46:145–152. doi: 10.4103/0973-1296.177902. [DOI] [PMC free article] [PubMed] [Google Scholar]