Abstract
Cordyceps is a famous traditional Chinese medicine (TCM) that has been used in China for hundreds of years. In the present study a multi-column liquid chromatography (MC-LC) system was developed for the qualitative analysis of macromolecules and micromolecules in Cordyceps. The MC-LC system includes a size exclusion pre-column, a size exclusion column (SEC) and a reversed phase column (RP) which were controlled by column-switching valves. The sample was separated by the size exclusion pre-column into two fractions (macromolecules and micromolecules). These fractions were further separated on SEC and RP columns, respectively. A diode array detector (DAD) and a mass spectrometer (MS) were used to detect the components. This MC-LC method was utilized for analysis of Cordyceps samples. Two macromolecular peaks and 15 micromolecular peaks were found in Cordyceps, and 11 of the micromolecular peaks were identified as adenosine-5′-monophosphate (AMP), phenylalanine, uridine, hypoxanthine, inosine, guanine, guanosine, deoxyadenosine-5′-monophosphate (dAMP), adenosine, adenine and cordycepin (or its isomer). This method is useful for quality control of Cordyceps.
KEY WORDS: Cordyceps, Macromolecules, Micromolecules, Multi-column, Size exclusion column
Graphical abstract
In the present study a multi-column liquid chromatography (MC-LC) system was developed for the qualitative analysis of macromolecules and micromolecules in Cordyceps. The MC-LC system includes a size exclusion pre-column, a size exclusion column (SEC) and a reversed phase column (RP) which were controlled by column-switching valves. Two macromolecular peaks and 15 micromolecular peaks were found in Cordyceps, and 11 of the micromolecular peaks were identified.

1. Introduction
Cordyceps, one of the famous and valuable traditional Chinese medicines (TCM), is commonly used to improve kidney and lung function, stanch bleeding and resolve phlegm1. Modern studies have shown that Cordyceps has many biological activities, including immunoregulation, anti-tumor, anti-oxidant, anti-inflammatory, anti-apoptosis, anti-ageing and renoprotective activities2, 3, 4, 5. In previous reports many qualitative and quantitative analytical methods for control of the quality of Cordyceps have been reported6, 7, 8. Thin layer chromatography (TLC) analysis of nucleosides9, high performance liquid chromatography–diode array detector (HPLC–DAD) analysis of nucleosides10 and sterols11, high performance liquid chromatography–mass spectrometer (HPLC–MS) analysis of nucleosides12, high performance liquid chromatography–evaporative light scattering detector (HPLC–ELSD) analysis of carbohydrates13, ultra-performance liquid chromatography (UPLC) analysis of nucleosides14, capillary electrophoresis (CE) and capillary electrochromatography (CEC) analysis of nucleosides15, 16. These reported methods were mainly focused on analysis of one type of compound and could not reflect the overall quality of Cordyceps. There are macromolecules (polysaccharides and proteins) and micromolecules (nucleosides and sterols) that are believed to serve as bioactive components in Cordyceps3, 4, 5. Therefore, simultaneous analysis of both macromolecules and micromolecules is a reasonable approach for quality evaluation of Cordyceps. However, it is difficult to separate macromolecules and micromolecules by a single column HPLC system. Fortunately, the column-switching technique allows separation of analytes on different types of chromatographic columns. In previous reports, the multi-column liquid chromatography system had been successfully applied in analysis of different types of components in TCM, such as Notoginseng and Ganoderma17, 18.
In the present study, a new multi-column liquid chromatography (MC-LC) system, including a size exclusion pre-column, a size exclusion column (SEC) and a reversed phase column (RP), was developed for the simultaneous analysis of micromolecules and macromolecules in Cordyceps.
2. Materials and methods
2.1. Chemicals, reagents and materials
Phenylalanine, uridine, hypoxanthine, inosine, guanine and guanosine were purchased from Sigma (St. Louis, MO, USA), and the purity of each compound was more than 98% as determined by HPLC. Dextran was purchased from Sigma (St. Louis, MO, USA). Bovine serum albumin was obtained from Amersham Biosciences (USA). Acetonitrile was HPLC-grade from Merck (Darmstadt, Germany). Ammonium acetate was purchased from Fluka (Buchs, France) and deionized water was purified by a Milli-Q purification system (Millipore, Bedford, MA, USA). Cordyceps sample was collected from Sichuan province, China. The botanical origin of materials was identified by the corresponding author. The voucher specimens were deposited at the Institute of Chinese Medical Sciences, University of Macau, Macao, China.
2.2. Sample preparation
Syncore Reactor (BUCHI-Syncore, Flawil, Switzerland) was used for the extraction of sample. Dried powder of the sample (0.5 g) was put into the heating tube and extracted with 10 mL water at 100 °C for 30 min, with a vigorous mixing at 100 rpm (5415 D, Eppendorf AG). The extract was cooled to room temperature and Milli-Q water was added to compensate for lost weight. After centrifugation at 13,000 rpm for 5 min, the supernatant fraction was filtered through a 0.45 µm nylon membrane filter (Whatman, UK) prior to injection into the MC-LC system.
2.3. Chromatographic equipment
The online MC-LC system was constructed with a column-switching format. Fig. 1 shows the schematic diagram of MC-LC system, which includes a quaternary pump (Agilent G1354A), two switching valves (Rheodyne 2 position 6 port switching valve), a pre-column (BioSep-SEC-S2000 30 mm×4.6 mm, 5 μm), a SEC column (TSK G-4000 PWXL column, 300 mm×7.8 mm, 10 μm), a RP column (Zorbax SB-Aq column, 250 mm×4.6 mm, 5.0 µm), a diode array detector (Agilent G1315A DAD), mass spectrometer (Agilent ion trap MS) and an evaporative light-scattering detector (Alltech 2000 ELSD). The chromatographic data was collected and processed by Agilent chemstation software (Rev. B. 03. 02).
Figure 1.
The schematic diagrams of the MC-LC system for analysis of Cordyceps. (A) Macromolecules loading; (B) macromolecules analyzing; (C) micromolecules loading; (D) micromolecules analyzing.
2.4. Chromatographic conditions
2.4.1. Chromatographic conditions of pre-column LC analysis
The analysis was performed on an Agilent 1100 series LC system (Agilent Technologies, Palo Alto, CA, USA) coupled with an evaporative light-scattering detector (ELSD). The separation was achieved on a BioSep-SEC-S2000 (30 mm×4.6 mm, 5 μm). Isocratic elution with 5 mmol/L ammonium acetate aqueous solution was used at a flow rate of 0.2 mL/min with the column at room temperature (25 °C). The signal from the Alltech ELSD 2000 (Alltech, Deerfield, IL, USA) was transmitted to the Chemstation for processing through an Agilent 35900E interface. The parameters of ELSD were set as follows: the drift tube temperature was 110 °C and nebulizer nitrogen gas flow rate was at 3.0 L/min, impact factor off mode. An aliquot of 5 µL solution was injected for analysis.
2.4.2. Chromatographic conditions of MC-LC analysis
A pre-column (BioSep-SEC-S2000 30 mm×4.6 mm, 5 μm) was used for pre-separation of macromolecules and micromolecules in the Cordyceps extract. Mobile phase consisted of 5 mmol/L ammonium acetate aqueous solution at a flow rate of 0.2 mL/min and column temperature of 25 °C. The SEC column (TSK G-4000 PWXL column, 300 mm×7.8 mm, 10 μm) was used for macromolecule analysis. Mobile phase (Table 1) was 20 mmol/L aqueous ammonium acetate (C), a flow rate 0.6 mL/min and column temperature was room temperature at 25 °C. The RP column (Zorbax SB-Aq column, 250 mm×4.6 mm, 5.0 µm) was used for micromolecule analysis. The mobile phase was 5 mmol/L aqueous ammonium acetate (A) and acetonitrile (B) gradient elution, with a flow rate 0.7 mL/min and the column at 25 °C. The elution program for MC-LC analysis is shown in Table 1. The column switching program was as follow: 0–2.4 min macromolecule loading (Fig. 1A); 2.4–30.5 min macromolecule separation (Fig. 1B); 30.5–35 min micromolecule loading (Fig. 1C); 35–90 min micromolecules separation (Fig. 1D). The detection wavelength was set at 260 nm. An aliquot of 15 μL was injected for analysis.
Table 1.
The elution program of MC-LC analysis.
| Time (min) | A (%) | B (%,) | C (%) | Flow (mL/min) |
|---|---|---|---|---|
| 0 | 100 | 0 | 0 | 0.2 |
| 2.4 | 100 | 0 | 0 | 0.2 |
| 2.6 | 0 | 0 | 100 | 0.6 |
| 29 | 0 | 0 | 100 | 0.6 |
| 30.5 | 100 | 0 | 0 | 0.2 |
| 35 | 100 | 0 | 0 | 0.2 |
| 36 | 100 | 0 | 0 | 0.7 |
| 45 | 100 | 0 | 0 | 0.7 |
| 60 | 95 | 5 | 0 | 0.7 |
| 75 | 80 | 20 | 0 | 0.7 |
| 85 | 60 | 40 | 0 | 0.7 |
| 90 | 20 | 80 | 0 | 0.7 |
A, 5mmol/L NH4Ac; B, ACN; C, 20 mmol/L NH4Ac.
MS analysis was performed on an Agilent MSD Trap system (Palo Alto, CA, USA) equipped with an ESI interface. MC-LC effluent was introduced into MS detector in a post-column splitting ratio of 1:1. MS analysis was monitored in positive mode. The mass range was set at 100–2000 U (0–31 min) and 100–400 U (31–90 min). The conditions of ESI source were as follows: drying gas (N2) flow rate, 10 L/min; drying gas temperature, 350 °C; nebulizer, 40 psi. ESI-MS/MS conditions: isolation width, 4; fragment amplification, 1.5 V.
3. Results and discussion
3.1. Optimization of MC-LC conditions
In this study an MC-LC system was developed for the analysis of macromolecules and micromolecules in Cordyceps. Firstly, the size exclusion pre-column was used for separating the macromolecules and micromolecules. The LC conditions are described in Section 2.4.1. The results (Fig. 2) indicate that there are two major components in Cordyceps; part 1 is macromolecular compounds (MW>5000) and part 2 is micromolecular compounds (MW<5000). The retention time of the dextran standard (MW=5000) was used as a marker for determination of the column switching time for separation of macromolecules and micromolecules. The further separation of macromolecules was carried out on an SEC column and the chromatographic conditions of the column were modified from Ref. 19. Micromolecules were usually separated on an RP column. However, because components in the water extraction with high polarity are difficult to separate on a common RP column, a Zorbax SB-Aq column was used, which is effective at separating polar components in high ratio of aqueous mobile phase. In order to improve the chromatographic separation and MS response of nucleosides, 5 mmol/L ammonium acetate aqueous solution and acetonitrile were selected as the mobile phases. The gradient elution program was modified from previous reports17, 20. The MC-LC analysis process was as follows:
Step 1 (0–2.4 min): Loading the macromolecules on SEC column (Fig. 1A, macromolecules loading). The extraction of Cordyceps was separated by pre-column, where macromolecules were eluted from the pre-column and loaded onto the SEC column, while the micromolecules were retained on the pre-column.
Step 2 (2.4–30.5 min): Separation of the macromolecules on the SEC column (Fig. 1B, macromolecules analyzing). After loading, the pre-column was cut off from the MC-LC system and macromolecules were separated on the SEC column.
Step 3 (30.5–35 min): Loading micromolecules onto the RP column (Fig. 1C, micromolecules loading). After the separation of macromolecules, the SEC column was cut off from MC-LC system and the pre-column and RP column were added onto the MC-LC system. Micromolecules were eluted from the pre-column and loaded onto the RP column.
Step 4 (35–90 min): Separating micromolecules on the RP column (Fig. 1D, micromolecules analyzing). After all compounds were eluted from the pre-column, the pre-column was cut off from MC-LC system and micromolecules were separated on the RP column.
Figure 2.
LC–ELSD chromatograms of (A) dextran (MW=5000) and (B) Cordyceps. Part 1: macromolecular components (MW>5000); Part 2: micromolecular components (MW<5000).
Based on the characteristic UV absorption of components (Table 2)12,21,22 and previous reports10, the detection wavelength was set at 260 nm. The MS condition was modified from Refs. 17, 20.
Table 2.
MS and UV data of components.
| Peak No. | Identification | Retention time (min) | MW | MS data (m/z) | MS2 data (m/z) | UV λmax (nm) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Unknown | 38 | – | 310 | 292, 274, 226 | 265 | – |
| 2 | Unknown | 39 | – | 266 | 248, 230 | 250 | – |
| 3 | Unknown | 40 | – | 156 | 118 | 260 | – |
| 4 | Unknown | 41 | – | 292 | 175, 118 | 260 | – |
| 5 | AMP | 42 | 347 | 348 [M+H]+ | 136 [M+H-Rib-H3PO4]+ | 270 | 21 |
| 6 | Phenylalanine | 43 | 165 | 166 [M+H]+ | 120 [M–CO2H]+ | 260 | – |
| 7 | Uridine | 47 | 244 | 267 [M+Na]+, 245 [M+H]+ | 113 [M+H-Rib]+ | 260 | – |
| 8 | Hypoxanthine | 61 | 136 | 137 [M+H]+ | – | 250 | – |
| 9 | Inosine | 65 | 268 | 269 [M+H]+ | 137 [M+H-Rib]+ | 250 | – |
| 10 | Guanine | 68 | 151 | 152 [M+H]+ | – | 255 | – |
| 11 | Guanosine | 69 | 283 | 284 [M+H]+ | 152 [M+H-Rib]+ | 255 | – |
| 12 | dAMP | 74 | 329 | 330 [M+H]+ | 136 [M+H-dRib-H3PO4]+ | 255 | 22 |
| 13 | Adenosine | 78 | 267 | 268 [M+H]+ | 136 [M+H-Rib] + | 260 | 12,21 |
| 14 | Adenine | 80 | 135 | 136 [M+H]+ | – | 260 | 12,21 |
| 15 | Cordycepin orisomer | 81 | 251 | 252 [M+H]+ | 136 [M+H-dRib]+ | 260 | 12,21 |
Rib: ribose; dRib: deoxyribose.– Not applicable.
3.2. Qualitative analysis of components in Cordyceps
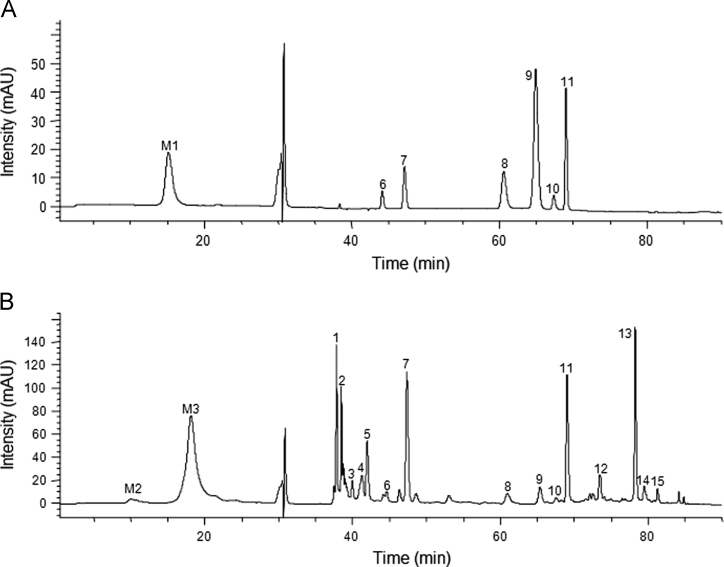
The developed MC-LC system was first applied in separation of mixed standards including 1 macromolecule and 6 micromolecules. As a result (Fig. 3A), the macromolecule (peak M1) was separated on the SEC column and micromolecules (peak 6–11) were separated on the RP column. This result indicated that the method could be used to analyze macromolecules and micromolecules in a complex sample. The Cordyceps samples were submitted to this MC-LC system. The chromatogram of a Cordyceps sample is shown in Fig. 3B. In macromolecule separation, peaks M2 and M3 were detected in the Cordyceps sample, which is consistent with a previous report 23. In previous reviews4, 5, 6, polysaccharide, protein and polypeptide were believed as the major macromolecular components in Cordyceps. In these components, protein, polypeptide or their derivatives could be detected by 260 nm, so peaks M2 and M3 were tentatively identified as protein, polypeptide or their derivatives. In the micromolecule analysis, 15 peaks were found in the Cordyceps sample. In order to identify the micromolecular components, an MS experiment was carried out. The MS and UV detection of major micromolecular chromatographic peaks (1–15) are presented in Table 2. Peaks 6–11 were positively identified as phenylalanine (6), uridine (7), hypoxanthine (8), inosine (9), guanine (10), guanosine (11) by compared with retention time, UV data and MS data with reference compounds. Peaks 5 and 12–15 were tentatively identified as AMP (5), dAMP (12), adenosine (13), adenine (14), cordycepin or isomer (15) by comparison of MS data with previous reports12, 22, 23. These results are similar to those reported in the literature22, with the major nucleosides including AMP, uridine, guanosine and adenosine, all found in Cordyceps.
Figure 3.
Typical MC-LC chromatograms of (A) mixed standards and (B) Cordyceps. (M1) bovine serum albumin, (M2 and M3) macromolecules in Cordyceps, (1-4) unknown, (5) AMP, (6) phenylalanine, (7) uridine, (8) hypoxanthine, (9) inosine, (10) guanine, (11) guanosine, (12) dAMP, (13) adenosine, (14) adenine, (15) cordycepin or isomer.
In this study, an MC-LC method for simultaneous analysis of macromolecular and micromolecular components in Cordyceps was developed. This method is helpful to the overall quality control of Cordyceps. However, the analytical time of developed method was a bit long, which was 90 min. In future study, the rapid LC column, such as sub 2 μm column and core shell column, would be applied in MC-LC system to reduce the run time.
4. Conclusions
In this study an MC-LC method for the analysis of macromolecules and micromolecules in Cordyceps was developed, which is a novel and comprehensive quality control method for Cordyceps standardization.
Acknowledgments
This work was supported by grant from the Science and Technology Development Fund of Macao (FDCT 059/2011/A3).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Chinese Pharmacopoeia Commission . Vol I. China Medical Science Press; Beijing: 2015. (Pharmacopoeia of the People׳s Republic of China.). [Google Scholar]
- 2.Liu H.J., Hu H.B., Chu C., Li Q., Li P. Morphological and microscopic identification studies of Cordyceps and its counterfeits. Acta Pharm Sin B. 2011;1:189–195. [Google Scholar]
- 3.Chen P.X., Wang S.N., Nie S.P., Marcone M. Properties of Cordyceps sinensis: a review. J Funct Foods. 2013;5:550–569. doi: 10.1016/j.jff.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Wang J., Wang W., Zhang H., Zhang X., Han C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid Based Complement Altern Med. 2015;2015:575063. doi: 10.1155/2015/575063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X.W., Gong Z.H., Su Y., Lin J., Tang K.X. Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61:279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 6.Li S.P., Yang F.Q., Tsim K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–1584. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J., Xie J., Wang L.Y., Li S.P. Advanced development in chemical analysis of Cordyceps. J Pharm Biomed Anal. 2014;87:271–289. doi: 10.1016/j.jpba.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Qian Z.M., Liao N., Li W.Q., Ai Z., Tian Y., Li W.J. Application of modern instrumental analytical techniques in quality evaluation of Cordyceps sinensis. Mod Chin Med. 2016;18:682–688. [Google Scholar]
- 9.Mi L.L., Zhang S.W., Sun J.J., Wang Z.H., Hong X.K. Study of nucleotides in Cordyceps and its mycelia by TLC-scanning. Chin Trad Pat Med. 2003;25:402–405. [Google Scholar]
- 10.Li W.Q., Li W.J., Qian Z.M., Chen S.L., Xiang L. Rapid analysis of polar components in Ophiocordyceps sinensis by conventional liquid chromatography system. Chin Herb Med. 2014;6:217–221. [Google Scholar]
- 11.Li S.P., Li P., Ji H., Zhang P. RP-HPLC determination of ergosterol in natural and cultured Cordyceps. Chin JMAP. 2001;18:297–299. [Google Scholar]
- 12.Fan H., Li S.P., Xiang J.J., Lai C.M., Yang F.Q., Gao J.L. Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC–ESI-MS/MS) Anal Chim Acta. 2006;567:218–228. [Google Scholar]
- 13.Wang S., Yang F.Q., Feng K., Li D.Q., Zhao J., Li S.P. Simultaneous determination of nucleosides, myriocin, and carbohydrates in Cordyceps by HPLC coupled with diode array detection and evaporative light scattering detection. J Sep Sci. 2009;32:4069–4076. doi: 10.1002/jssc.200900570. [DOI] [PubMed] [Google Scholar]
- 14.Yang F.Q., Guan J., Li S.P. Fast simultaneous determination of 14 nucleosides and nucleobases in cultured Cordyceps using ultra-performance liquid chromatography. Talanta. 2007;73:269–273. doi: 10.1016/j.talanta.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Gong Y.X., Li S.P., Li P., Liu J.J., Wang Y.T. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis. J Chromatogr A. 2004;1055:215–221. doi: 10.1016/j.chroma.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Yang F.Q., Li S.P., Li P., Wang Y.T. Optimization of CEC for simultaneous determination of eleven nucleosides and nucleobases in Cordyceps using central composite design. Electrophoresis. 2007;28:1681–1688. doi: 10.1002/elps.200600416. [DOI] [PubMed] [Google Scholar]
- 17.Qian Z.M., Wang J.B., Zhang Q.W., Li S.P. Simultaneous determination of nucleobases, nucleosides and saponins in Panax notoginseng using multiple columns high performance liquid chromatography. J Pharm Biomed Anal. 2008;48:1361–1367. doi: 10.1016/j.jpba.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Qian Z.M., Zhao J., Li D.Q., Hu D.J., Li S.P. Analysis of global components in Ganoderma using liquid chromatography system with multiple columns and detectors. J Sep Sci. 2012;35:2725–2734. doi: 10.1002/jssc.201200441. [DOI] [PubMed] [Google Scholar]
- 19.Guan J., Li S.P. Discrimination of polysaccharides from traditional Chinese medicines using saccharide mapping—enzymatic digestion followed by chromatographic analysis. J Pharm Biomed Anal. 2010;51:590–598. doi: 10.1016/j.jpba.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Gao J.L., Leung K.S., Wang Y.T., Lai C.M., Li S.P., Hu L.F. Qualitative and quantitative analyses of nucleosides and nucleobases in Ganoderma spp. by HPLC–DAD–MS. J Pharm Biomed Anal. 2007;44:807–811. doi: 10.1016/j.jpba.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Yang F.Q., Li D.Q., Feng K., Hu D.J., Li S.P. Determination of nucleotides, nucleosides and their transformation products in Cordyceps by ion-pairing reversed-phase liquid chromatography-mass spectrometry. J Chromatogr A. 2010;1217:5501–5510. doi: 10.1016/j.chroma.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 22.Borges C., Lemière F., Embrechts J., Van Dongen W., Esmans E.L. Characterisation of estrone-nucleic acid adducts formed by reaction of 3, 4-estrone-o-quinone with 2′-deoxynucleosides/deoxynucleotides using capillary liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2191–2200. doi: 10.1002/rcm.1610. [DOI] [PubMed] [Google Scholar]
- 23.Guan J., Zhao J., Feng K., Hu D.J., Li S.P. Comparison and characterization of polysaccharides from natural and cultured Cordyceps using saccharide mapping. Anal Bioanal Chem. 2011;399:3465–3474. doi: 10.1007/s00216-010-4396-y. [DOI] [PubMed] [Google Scholar]