Abstract
An effective herbal medicinal prescription of Shengjiang Xiexin decoction (SXD) was used in treating the inflammatory bowel disease in clinic. In this study, an ultrafast liquid chromatography—tandem mass spectrometry (UFLC—MS/MS) method was developed to separate and to simultaneously determine 14 major active ingredients in SXD. Chromatographic separation was successfully accomplished on an Acquity BEH C18 (100 mm×2.1 mm, 1.7 μm) column using gradient elution with 0.1% (v/v) formic acid water (A) and 0.1% (v/v) formic acid in methanol (B). Negative and positive electrospray ionization tandem mass spectrometry was used to detect the 14 analytes using its selective reaction monitoring (SRM) mode. A good linear regression relationship for each analyte was obtained over the range from 3.88 ng/mL to 4080 ng/mL. The precision was evaluated by intra- and inter-day assays with a relative standard deviation (RSD) of less than 6.25%. The recovery measured at three concentration levels varied from 98.72% to 103.47%. The overall limits of quantification (LOQ) ranged from 2.05 ng/mL to 4.72 ng/mL. The method was successfully implemented in the qualitative and quantitative analyses of the 14 chemical constituents in SXD. The results showed that the developed UFLC—MS/MS method was linear and accurate. The method could be used reliably as a quality control method for SXD.
KEY WORDS: Inflammatory bowel disease, Quality control, UFLC–MS/MS, Determination, Shengjiang Xiexindecoction
Graphical abstract
This work reports that optimized UFLC–MS/MS could quantify 14 compounds in Shengjiang Xiexin decoction. The method can be used for fast, accurate, quality control of Shengjiang Xiexin decoction. Some trace, yet effective components, were observed and simultaneously determined.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory and destructive disease of the gastrointestinal tract. The chronically active inflammation causes ulcerations, stricture formations, and perforations; it is also a risk factor for the development of colorectal cancer. The current treatments for IBD are not always effective, because they are non-specific, and often accompanied by serious side effects1, 2, 3. At present, there is an urgent need to identify new therapeutics to replace the traditional therapies. Shengjiang Xiexin decoction (SXD), a traditional Chinese medicine prescribed in Shang Han Lun, is commonly used to treat gastroenteritis, gastrointestinal mucositis, diarrhea, digestive ulcers, and functional dyspepsia in integrated traditional and western medicine departments4, 5. SXD has been proven to be a potential prescription to manage IBD, and to date, no side effects have been seen in clinical presentation6.
The favorable efficacy is likely a result of the synergistic effect of multiple components in the preparation. Pharmacological studies7, 8, 9, 10, 11, 12, 13, 14 have shown that 6-gingerol from Zingiberis Rhizoma and Zingiberis Recens Rhizoma, baicalin, baicalein and wogonin from Scutellariae Radix, berberine, epiberberine and palmatine from Coptidis Rhizoma, trigonelline from Pinelliae Rhizoma, liquiritin from Glycyrrhizae Radix et Rhizoma, and lobetyolin from Codonopsis Radix play important roles in anti-viral, anti-oxidative, anti-microbial, antibacterial, and anti-inflammatory activities, which all contribute to the IBD therapy. Rutin, oleanolic acid, betulinic acid, and ursolic acid from Jujubae Fructus were medicine food homology that can also produce remarkable pharmacological effects15, 16, 17, 18, 19.
Other reports20, 21, 22 also indicated that some components in SXD may contribute significantly to the anti-inflammatory bowel disease efficacy of this prescription. However, a detailed study on the profile of constituents of SXD formula has not yet been conducted. To investigate the relationship between the efficacy and chemical contents, as well as quality control of SXD, the active constituents of SXD should be determined.
Several methods have been described in previous publications for the detection of the quantity of some of the active ingredients in SXD. The HPLC method has been described to measure baicalin, glycyrrhizic acid, berberine, palmatine, and wogonin in kampo medicines23. GC—MS technology has also been applied in the quantification of volatile constituents derived from Glycyrrhizae Radix et Rhizoma24. However, other active constituents, such as 6-gingerol, baicalein, epiberberine, trigonelline, liquiritin, lobetyolin, rutin, oleanolic acid, betulinic acid, and ursolic acid—all having notable pharmacological effects—have not been investigated to date. Furthermore, an ion-pair HPLC method has been reported for the simultaneous determination of baicalin, baicalein, wogonoside, wogonin, berberine, coptisine, jatrorrhizine, palmatine, and glycyrrhizin in some widely used kampo medicines in Japan, such as Hangeshashin-to25, 26. However, the reported HPLC method was time-consuming and insensitive. Thus, the qualitative and quantitative analyses of components in SXD are still a challenge. Recently, the ultra-performance liquid chromatography tandem—mass spectrometry (UPLC—MS/MS) has been used to separate and quantitatively analyze the active components of the kampo medicines. The UPLC—MS/MS system utilizes UPLC and triple quadrupole detection, and has the benefit of the high resolution and high selectivity due to ultra-pressured elution, high peak capacity, and multiple ion detection based on selective ion fragmentation27, 28, 29, 30. In this study, a simple and sensitive UFLC—MS/MS method was established and validated for the simultaneous determination of the active constituents in SXD, including 6-gingerol, baicalin, baicalein, wogonin, epiberberine, trigonelline, liquiritin, lobetyolin, rutin, oleanolic acid, betulinic acid, ursolic acid, berberine, and palmatine, which has the advantages of high selectivity, short analysis time, high sample throughput and low limit of quantitation.
2. Experimental sections
2.1. Chemicals and materials
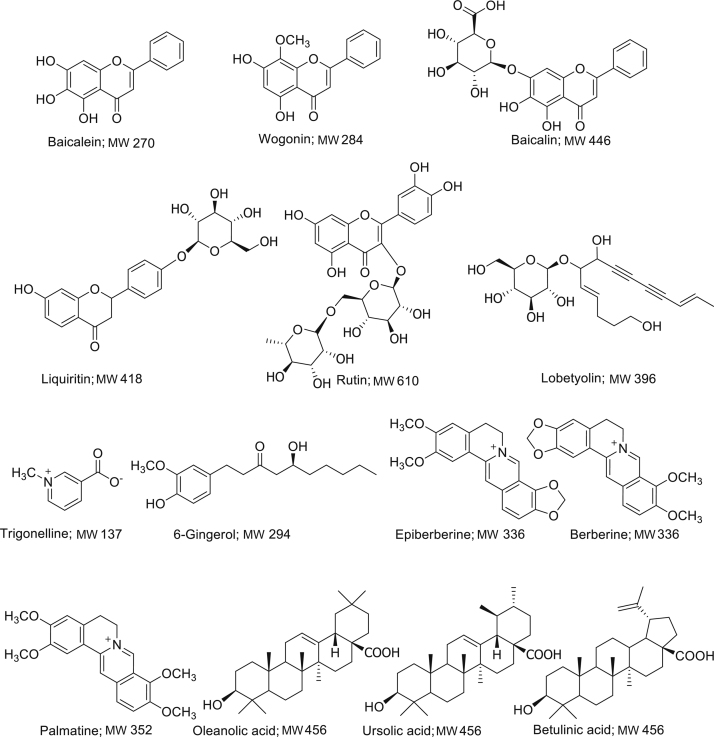
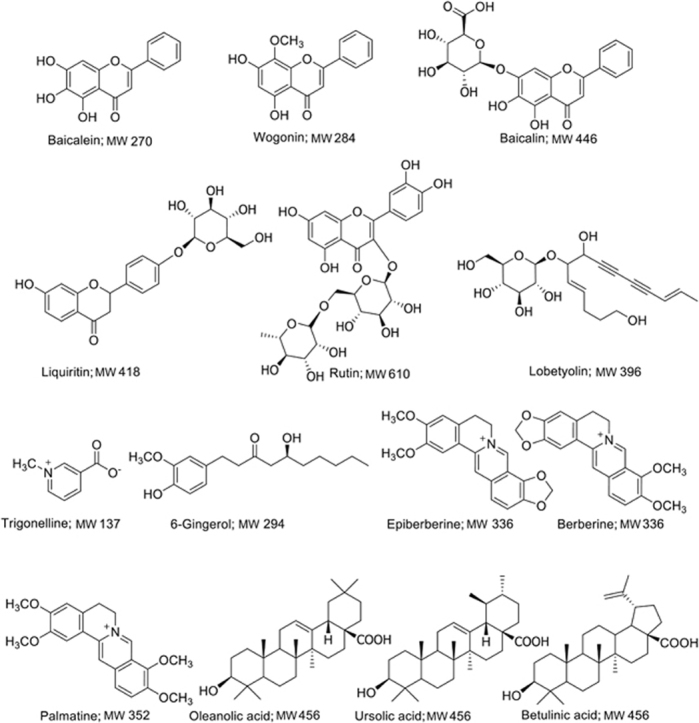
Analytically standard 6-gingerol, baicalin, baicalein, wogonin, epiberberine, trigonelline, liquiritin, lobetyolin, rutin, oleanolic acid, betulinic acid, ursolic acid, berberine, palmatine, and carbamazepine (internal standard) with purities of over 98.0% were purchased from the Chinese National Institute for Control of Pharmaceutical and Biological Products (Beijing, China). The chemical structures of these compounds are shown in Fig. 1. Formic acid and methanol of HPLC-grade were purchased from Merck (Darmstadt, Germany). All other reagents were also of analytical grade. Deionized water for UFLC analysis was prepared with a Millipore water purification system (Millipore, Bedford, MA, USA). Zingiberis Rhizoma and Zingiberis Recens Rhizoma (collected in Shandong Province, China), Jujubae Fructus (collected in Henan Province, China), Codonopsis Radix (collected in Shanxi Province, China), Scutellariae Radix (collected in Hebei Province, China), Pinelliae Rhizoma (collected in Hebei Province, China), Glycyrrhizae Radix et Rhizoma (collected in Ningxia Province, China), and Coptidis Rhizoma (collected in Hubei Province, China) were purchased from Beijing Tong Ren Tang Group Co., Ltd. (Beijing, China), and were authenticated by Dr. Yulin Lin. The test decoctions of SXD1 (batch No. 20150622), SXD2 (batch No. 20150710), SXD3 (batch No. 20150719), SXD4 (batch No. 20150730), SXD5 (batch No. 20150812), and SXD6 (batch No. 20150820) were prepared by our own laboratory technicians from different batches of eight crude drugs.
Figure 1.
Chemical structures of the 14 compounds.
2.2. UFLC—MS/MS conditions
The separation was carried out on an Acquity BEH C18 (100 mm×2.1 mm, 1.7 μm; Waters, Milford, MA, USA) analytical column coupled with a column filter. The column and auto-sampler were maintained at 30 °C and 10 °C, respectively. Mobile phase was a mixture of 0.1% (v/v) formic acid water (A) and 0.1% (v/v) formic acid in methanol (B) at a flow rate of 0.2 mL/min. The gradient program consisted of an initial linear increase from 20% to 70% of B over 2 min and increased to 90% of B over 4 min, which was maintained for 6 min, followed by a return to the initial conditions over 3 min. The samples were kept at 10 °C in the auto-sampler, and a volume of 10 μL was injected using full loop injection.
The UFLC system was coupled with an AB SCIEX 5500 Q-Trap mass detector operating in both negative and positive ion modes. Each analyte was set up for MS/MS detection in different time segments. The experimental conditions for the operation of the instrument were optimized by direct infusion of the 14 compounds individually. Optimal conditions determined were as the follows: ion spray voltage, 5500 V; curtain gas pressure, 20 psi; nebulizer gas (Gas 1), 40 psi; auxiliary gas (Gas 2), 60 psi, source temperature, 550 °C; and channel electron multiplier (CEM), 2100 V. The quadrupole was set to maximum resolution: precursor ion to production transitions were detected from m/z 136.0/66 (trigonelline) to m/z 609.2/300.0 (rutin), the parameters of a collision energy (−2 to−9 V), entrance potential (−12.5 to −3.8 V), collision cell exit potential (−42 to −6 V), and declustering potential (−289 to −21 V) were set in the negative ion mode, and the positive ion mode for epiberberine, berberine and palmatine were set at collision energy (30–40 V), entrance potential (8–10 V), collision cell exit potential (10–15 V), and declustering potential (80–100 V) (Table 1). All data were recorded and processed using an Analyst 1.6 software (AB SCIEX).
Table 1.
Optimal conditions for SRM transitions of 14 components and carbamazepine (I.S.).
| Compd. | m/z | DP | EP | CE | CXP |
|---|---|---|---|---|---|
| Rutin | 609.2–300.0 | −234 | −8.5 | −48 | −13 |
| Baicalein | 269.0–136.9 | −218 | −7.2 | −40 | −16 |
| Trigonelline | 136.0–66.0 | −289 | −6.7 | −18 | −6 |
| Lobetyolin | 395.4–226.9 | −21 | −11.8 | −33 | −24 |
| Oleanolic acid | 455.3–407.5 | −201 | −8.3 | −54 | −8 |
| Ursolic acid | 455.4–373.1 | −196 | −11.2 | −62 | −42 |
| Betulinic acid | 455.4–255.3 | −185 | −10.3 | −10 | −17 |
| Epiberberine | 336.2–263.0 | 100 | 10 | 30 | 15 |
| Baicalin | 445.2–269.0 | −128 | −10.5 | −32 | −13 |
| Wogonin | 283.3–268.0 | −114 | −3.8 | −24 | −12 |
| Berberine | 336.1–320.2 | 100 | 8.3 | 39 | 10.4 |
| Liquiritin | 417.1–254.7 | −55 | −7.3 | −20 | −24 |
| Palmatine | 352.2–336.2 | 80 | 8 | 40 | 10 |
| 6-Gingerol | 293.1–135.0 | −166 | −10.5 | −30 | −10 |
| Carbamazepine | 235.1–198.8 | −108 | −12.5 | −9 | −10 |
| Carbamazepine | 237.1–194.1 | 90 | 15 | 22 | 13 |
DP: declustering potential; EP: entrance potential; CE: collision energy; CXP: collision cell exit potential.
2.3. Preparation of standard solutions
Stock standard solutions were prepared by dissolving each standard of the SXD components in methanol. Standard mixtures of baicalin, baicalein, wogonin, epiberberine, trigonelline, liquiritin, lobetyolin, rutin, oleanolic acid, betulinic acid, ursolic acid, berberine, and palmatine were prepared separately by diluting the individual stock standard solution in a 65% methanol solution to provide a series of concentrations in the ranges of 3.88–970 ng/mL to 16.32–4080 ng/mL. The internal standard carbamazepine was added to each vial at a concentration of 100.0 ng/mL. Calibration curves were plotted after the linear regression of the ratios of the peak areas of the analytes to those of the internal standard. Each line was based on six standard concentrations.
2.4. Sample preparation
Eight crude herbal drugs (human daily dose of SXD: Zingiberis Recens Rhizoma 12 g, Codonopsis Radix 9 g, Zingiberis Rhizoma 3 g, Scutellariae Radix 9 g, Coptidis Rhizoma 3 g, Pinelliae Rhizoma 9 g, Glycyrrhizae Radix et Rhizoma 9 g and Jujubae Fructus 12 g) were immersed in water for 15 min, and decocted with 10-fold amount water for 30 min. After filtration, two decoctions were combined and evaporated, and the mixture was lyophilized. The lyophilized powder (0.5 g) was extracted with 25 mL of 65% methanol in an ultrasonic bath for 30 min, then the solution (20 mg/mL) was diluted with water to 100-fold. The obtained solution was filtered through a membrane filter (0.22 μm pore size) prior to injection. Six sample preparations (SXD1–SXD6) were made with different batches of eight crude drugs in the same way.
The samples of Zingiberis Recens Rhizoma, Codonopsis Radix, Zingiberis Rhizoma, Scutellariae Radix, Coptidis Rhizoma, and Glycyrrhizae Radix et Rhizoma were prepared following the same procedures for the negative control samples of SXD.
2.5. Linearity-calibration range
The calibration curves were analyzed at six concentration levels by the standard analysis. The mixed solutions were established by mixing each level of the component standard solutions with the same amount of internal standard (100 ng/mL). The LOD and LOQ were calculated based on the signal to noise (S/N) ratio. The LODs and LOQs were determined at an S/N ratios of 3 and 10, respectively.
2.6. Precision and accuracy
The analysis of intra- and inter-day precision and repeatability were carried out by six repetitive injections. The intra- and inter-day test was carried out in the same day and for three consecutive days. The recovery test was performed by adding an accurate amount of 14 standards to SXD in triplicate. The total concentrations of the 14 components were determined at 75%, 100% and 125%, respectively. Concentration determination of each component could not exceed the calibration range. The recovery percentage (%) for all 14 components was calculated according to the following equation: (Detected amount−Original amount)/Spiked amount × 100.
2.7. Repeatability and stability
Six samples were prepared to evaluate the repeatability. Sample stability was assessed by analyzing three injections of a diluted SXD sample at 0, 4, 8, 12 and 24 h, which was stored at 4 °C.
3. Results and discussion
3.1. Optimization of MS/MS conditions
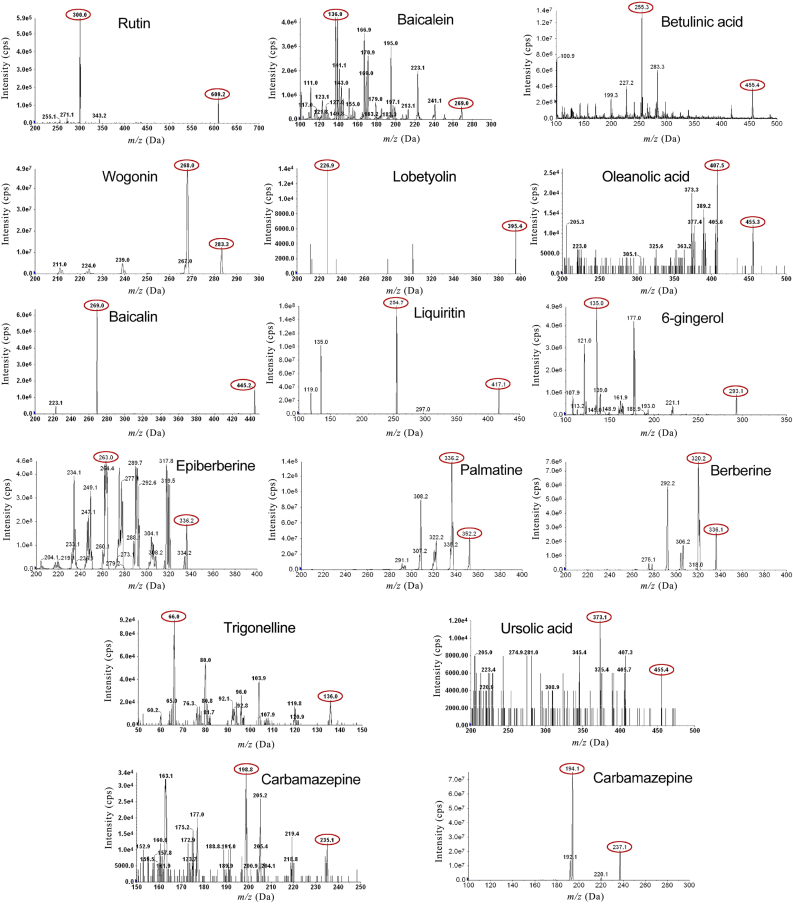
Mass spectrometric analysis was performed using an ESI interface, and the SRM mode was applied for mass spectrometry signal acquisition. Since the polarity of 14 analytes was quite different, the ionization of these compounds was a challenge. Full scanning in both positive and negative ion modes was investigated. It was found that epiberberine, berberine, and palmatine were more sensitive in the positive ion mode, and the molecular ions [M+H]+ provided higher intensity. Therefore, the base peak (the highest peak) at m/z 336.2, 336.1, and 352.2 was selected as the precursor ions for the detection of epiberberine, berberine, and palmatine, respectively. The high-intensity product ions of other components apart from berberine, epiberberine and palmatine were observed in the negative mode. And, the mass response of [M–H]− in negative ion mode was more sensitive. Hence, [[M–H]− was employed as the precursor ion for 6-gingerol, baicalin, baicalein, wogonin, trigonelline, liquiritin, lobetyolin, rutin, oleanolic acid, betulinic acid, and ursolic acid. The positive/negative ion-switching function was used to simultaneously monitor the 14 active constituents in SXD in the same analytic cycle (Fig. 2).
Figure 2.
Mass spectra for 14 standards and carbamazepine (I.S.).
The collision energy was optimized to obtain the most abundant product ions. The components produced [[M–H]− with a collision energy (−62 to −9 V) determined by optimization trials applied to 6-gingerol, baicalin, baicalein, wogonin, trigonelline, liquiritin, lobetyolin, rutin, oleanolic acid, betulinic acid, and ursolic acid. But molecular ion [M+H]+ with a collision energy (30–40 V) determined by optimization trials applied to berberine, epiberberine and palmatine ionized well in the positive ion mode (Table 1).
3.2. Optimization of UFLC conditions
Based on the structure and polarity of the components in SXD, an Acquity BEH C18 analytical column and a gradient elution with mobile phase consisting of methanol and 0.1% formic acid were chosen for separation. The formic acid was more effective in the ionization of those compounds detected in both negative and positive ESI modes, compared with the acetic acid. Thus, different concentrations (0.05%, 0.1% and 0.2%) of formic acid were investigated and 0.1% of formic acid was selected to be the optimal concentration for ionization.
In general, the complete separation of all components is not necessary in MS/MS detection. In this study, oleanolic acid, betulinic acid and ursolic acid had the same molecular weight and were detected by the different ions fragment. Therefore, a series of water—methanol ratios in the mobile phase were necessary. Unfortunately, the separation effect of oleanolic acid, betulinic acid, or ursolic acid only improved slightly. After assaying the SXD samples, the 14 components of SXD were finally separated on a micro-bore C18 column with a particle size of 1.7 μm using the mobile phase of water and methanol containing 0.1% formic acid. Finally, the optimized gradient program consisted of an initial linear increase from 20% to 70% of B over 2 min and increased to 90% of B over 4 min, which was maintained for 6 min, followed by a return to the initial conditions over 3 min. Separation of the 14 components was achieved in a reasonable time.
3.3. Optimization of the sample preparation
The concentrations of 60%, 65%, and 70% (v/v) methanol were compared as the extracting solvent. It was found that there were no significant differences among samples. All of the solvents were able to produce complete extraction of all components. However, the extraction efficiency was found higher when using 65% methanol than using the other two concentrations. Therefore, 65% methanol was chosen for further extractions in this study. In addition, extraction time (15, 30, and 60 min) and extraction repetitions (1–3 times) were the optimized conditions when using ultrasonication. It was found that ultrasound extraction with 65% methanol for 30 min was the most effective condition that leads to a complete extraction of SXD samples.
3.4. Method validation
3.4.1. Interference trial
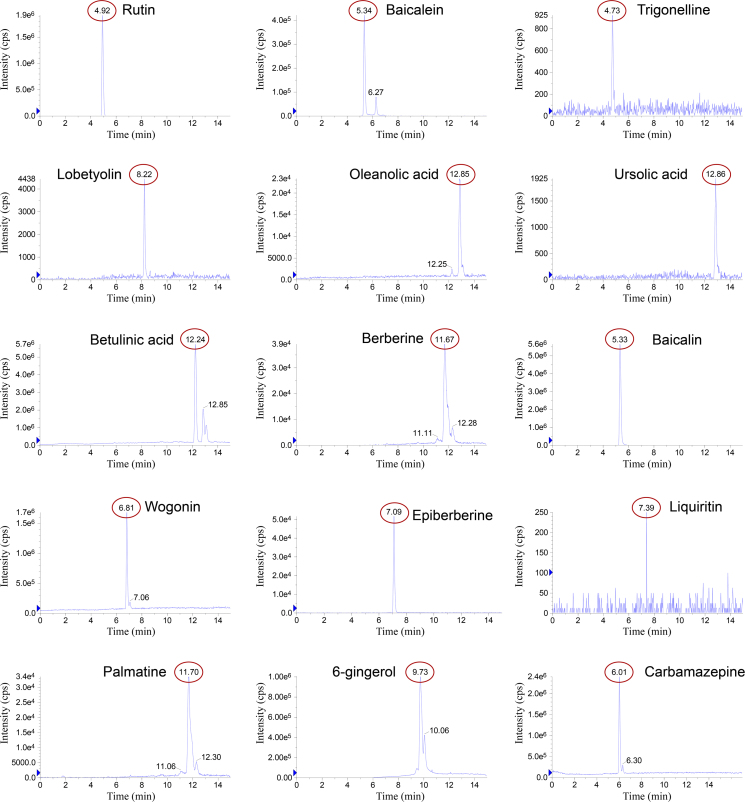
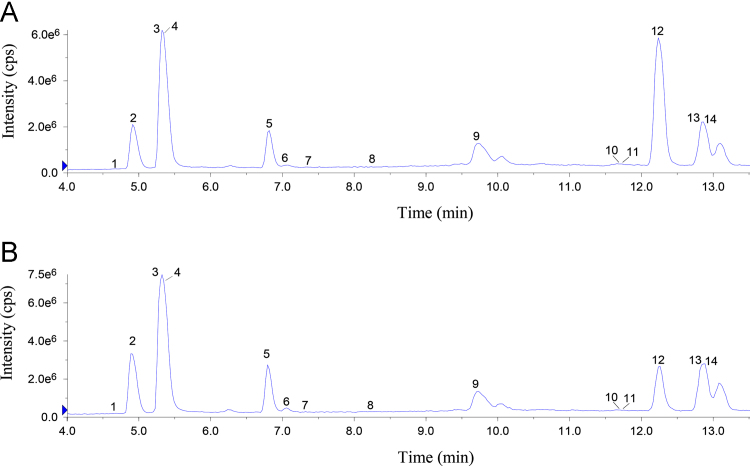
The typical SRM chromatograms and the total ion chromatogram (TIC) profiles obtained from standard solutions and extraction SXD samples are shown in Figure 2, Figure 3, Figure 4, respectively. The 14 components were proven to be sufficient for separation, and determined in 15 min. The negative control samples of SXD barely interfered with the SXD samples in the UFLC–MS/MS conditions described previously.
Figure 3.
SRM chromatograms of 14 analytes of the SXD1 sample mixed with carbamazepine (I.S.).
Figure 4.
Total ion chromatogram of 14 analytes of the (A reference compounds and (B) SXD1 sample: (1) trigonelline; (2) rutin; (3) baicalin; (4) baicalein; (5) wogonin; (6) epiberberine; (7) liquiritin; (8) lobetyolin; (9) 6-gingerol; (10) berberine; (11) palmatine; (12) betulinic acid; (13) oleanolic acid; (14) ursolic acid.
3.4.2. Linearity–calibration range
Table 2 shows the equations of the calibration curves and LOQs of those components determined. All calibration curves showed good linear regression (r2>0.99) within the test ranges from 3.88–970 ng/mL to 16.32–4080 ng/mL. The assay data proved to be linear and suitable for the quantitative analysis of SXD.
Table 2.
Linear range, r2 and LOQ of calibration curve used in the determination of the 14 analytes (n=6).
| Analyte | Linear range (ng/mL) | Calibrtion curve | r2 | LOQ (ng/mL) |
|---|---|---|---|---|
| Rutin | 4.04–1010 | y=1.2x+4.99 | 0.9958 | 2.74 |
| Baicalein | 4.04–1010 | y=0.235x+1.14 | 0.9979 | 2.63 |
| Trigonelline | 4.00–1000 | y=0.000396x+0.0153 | 0.9981 | 2.41 |
| Lobetyolin | 3.96–990 | y=0.00017x+0.0336 | 0.9919 | 2.05 |
| Oleanolic acid | 4.00–1000 | y=0.0163x+0.0371 | 0.9956 | 2.66 |
| Ursolic acid | 3.88–970 | y=0.00104x+0.00828 | 0.9998 | 3.13 |
| Betulinic acid | 3.96—1000 | y=4.76x+125 | 0.9909 | 3.25 |
| Epiberberine | 3.95–1010 | y=0.0546x+82.6 | 0.9960 | 2.66 |
| Baicalin | 4.04–1010 | y=4.55x+28.1 | 0.9995 | 2.16 |
| Wogonin | 16.32–4080 | y=0.231x+1.6 | 0.9912 | 4.72 |
| Berberine | 4.05—1020 | y=0.0131x+4.05 | 0.9957 | 2.78 |
| Liquiritin | 4.10–1030 | y=0.000217x+0.0316 | 0.9983 | 2.47 |
| Palmatine | 4.08–1010 | y=0.0524x+76.3 | 0.9979 | 2.92 |
| 6-Gingerol | 4.15–1000 | y=0.729x+764 | 0.9950 | 2.85 |
3.4.3. Precision and accuracy
Table 3 shows the results of precision and accuracy tests. Calculated by the calibration curves using an internal standard, it is clearly shown that all the relative standard deviation (RSD) values for the concentrations of the 14 compounds calculated by the calibration curves using an internal standard were within 6.25%. The data indicate that the precision of the method is acceptable.
Table 3.
Intra- and inter-day precision for quantification of Shengjiang Xiexin decoction.
| Analyte | Intra-day (n=6) |
Inter-day (n=3) |
||
|---|---|---|---|---|
| Mean (ng/mL) | RSD (%) | Mean (ng/mL) | RSD (%) | |
| Rutin | 509.33 | 2.13 | 497.15 | 2.67 |
| Baicalein | 503.27 | 2.89 | 512.17 | 3.17 |
| Trigonelline | 513.83 | 4.71 | 504.33 | 5.09 |
| Lobetyolin | 501.09 | 3.46 | 506.67 | 4.20 |
| Oleanolic acid | 515.17 | 5.80 | 518.67 | 6.25 |
| Ursolic acid | 492.67 | 3.69 | 501.02 | 4.28 |
| Betulinic acid | 510.18 | 3.51 | 504.65 | 3.91 |
| Epiberberine | 501.61 | 3.77 | 511.50 | 4.23 |
| Baicalin | 498.55 | 2.89 | 508.45 | 3.55 |
| Wogonin | 2077.50 | 5.20 | 2093.98 | 5.89 |
| Berberine | 500.83 | 3.10 | 502.67 | 4.24 |
| Liquiritin | 503.92 | 4.23 | 496.02 | 5.57 |
| Palmatine | 501.98 | 3.44 | 501.15 | 4.15 |
| 6-Gingerol | 517.32 | 3.36 | 511.83 | 4.50 |
3.4.4. Repeatability and stability
Table 4 indicates that the RSD of repeatability tested is less than 5.21% (RSD<5.21%). The stability of the 14 components at 4, 8, 12 and 24 h was acceptable with all samples having an RSD less than 5.63% (RSD<5.63%). These results indicate that the sample solution was stable for 24 h at 4 °C. The recovery of the developed method was acceptable with good accuracy over the range 98.72% to 103.47%.
Table 4.
Accuracy, repeatability, and stability levels of the 14 analytes in Shengjiang Xiexin decoction.
| Analyte | Accuracya |
Repeatabilityb |
Stabilityb |
|
|---|---|---|---|---|
| Recovery (%) | RSD (%) | RSD (%) | RSD (%) | |
| Rutin | 101.17 | 2.41 | 3.73 | 4.27 |
| Baicalein | 103.40 | 1.86 | 3.29 | 3.81 |
| Trigonelline | 98.81 | 4.53 | 3.51 | 3.89 |
| Lobetyolin | 102.15 | 1.69 | 2.31 | 3.62 |
| Oleanolic acid | 102.86 | 2.91 | 2.46 | 3.38 |
| Ursolic acid | 98.72 | 2.27 | 1.85 | 3.24 |
| Betulinic acid | 100.62 | 2.88 | 2.11 | 3.62 |
| Epiberberine | 102.27 | 4.09 | 2.87 | 4.17 |
| Baicalin | 102.29 | 2.74 | 3.52 | 4.14 |
| Wogonin | 103.47 | 2.44 | 2.03 | 3.57 |
| Berberine | 100.55 | 4.35 | 3.36 | 4.52 |
| Liquiritin | 99.73 | 5.76 | 5.21 | 5.63 |
| Palmatine | 103.15 | 3.23 | 2.76 | 3.86 |
| 6-Gingerol | 99.91 | 3.48 | 2.64 | 3.19 |
n=3;
n=6.
3.5. Quantitative analysis of the 14 components in SXD
The described UFLC–MS/MS method was subsequently applied to the evaluation of the 14 active compounds in SXD (SXD 1, 2, 3, 4, 5 and 6) using the same pretreatment process. Table 5 shows the means and standard deviations of six injections for each sample spiked with the internal standard.
Table 5.
Contents of the 14 analytes in Shengjiang Xiexin decoction (mg/g, n=6).
| Analyte | SXD1 | SXD2 | SXD3 | SXD4 | SXD5 | SXD6 |
|---|---|---|---|---|---|---|
| Rutin | 0.0514±0.0031 | 0.0500±0.0057 | 0.0530±0.0062 | 0.0480±0.0035 | 0.0501±0.0073 | 0.0510±0.0065 |
| Baicalein | 15.3000±0.2200 | 15.0010±0.2100 | 15.5010±0.2600 | 14.9010±0.3900 | 15.1000±0.1800 | 15.1980±0.1700 |
| Trigonelline | 3.9420±0.0320 | 3.8900±0.0240 | 3.9300±0.0410 | 3.8800±0.0310 | 3.9000±0.0190 | 3.9600±0.0370 |
| Lobetyolin | 4.3920±0.0350 | 4.4080±0.0210 | 4.3760±0.0450 | 4.3330±0.0590 | 4.3910±0.0370 | 4.4040±0.0290 |
| Oleanolic acid | 0.2090±0.0038 | 0.2080±0.0026 | 0.2110±0.0033 | 0.2010±0.0030 | 0.2100±0.0023 | 0.2120±0.0019 |
| Ursolic acid | 0.1720±0.0005 | 0.1690±0.0014 | 0.1730±0.0005 | 0.1700±0.0009 | 0.1710±0.0015 | 0.1750±0.0012 |
| Betulinic acid | 0.2920±0.0061 | 0.2880±0.0059 | 0.2920±0.0052 | 0.2870±0.0042 | 0.2950±0.0037 | 0.2900±0.0054 |
| Epiberberine | 7.2300±0.0370 | 7.2100±0.0380 | 7.2400±0.0450 | 7.2600±0.0310 | 7.2000±0.0330 | 7.2200±0.0460 |
| Baicalin | 6.6200±0.0570 | 6.6610±0.0590 | 6.6300±0.0710 | 6.6000±0.0650 | 6.6200±0.0500 | 6.5800±0.0640 |
| Wogonin | 60.5520±1.3600 | 60.8800±1.3100 | 60.2400±1.4900 | 59.7500±1.5400 | 60.1380±1.1900 | 60.8200±1.2700 |
| Berberine | 7.2200±0.0420 | 7.1800±0.0280 | 7.2500±0.0370 | 7.1600±0.0580 | 7.2200±0.0190 | 7.2400±0.0340 |
| Liquiritin | 2.3300±0.0130 | 2.3200±0.0230 | 2.3400±0.0170 | 2.2900±0.0210 | 2.3300±0.0140 | 2.3600±0.0250 |
| Palmatine | 6.6650±0.0290 | 6.6600±0.0320 | 6.6620±0.0310 | 6.5920±0.0330 | 6.6620±0.0270 | 6.6670±0.0220 |
| 6-Gingerol | 6.2100±0.0540 | 6.2300±0.0450 | 6.2400±0.0480 | 6.1900±0.0510 | 6.2000±0.0590 | 6.2400±0.0490 |
The analytical results demonstrate that all 14 compounds were detected from the six batches of SXD samples, although their contents differed greatly from each other. Among the tested components, 6-gingerol (6.210 mg/g), baicalin (6.620 mg/g), baicalein (15.300 mg/g), wogonin (60.552 mg/g), epiberberine (7.230 mg/g), trigonelline (3.942 mg/g), liquiritin (2.330 mg/g), lobetyolin (4.392 mg/g), berberine (7.220 mg/g), and palmatine (6.665 mg/g) were determined as the main active compounds in SXD1. Comparatively, the components such as rutin (0.0514 mg/g), oleanolic acid (0.209 mg/g), betulinic acid (0.292 mg/g), and ursolic acid (0.172 mg/g) that have relatively low concentrations were observed and determined simultaneously. The differences in the content of the compounds between different decoction batches might have primarily resulted from the extraction time or temperature variability during the decocting process. As a result, prevention measures were implemented to avoid these effects. Samples were filtered, evaporated and freeze-dried continuously immediately upon the completion of boiling. The data were analyzed using the SPSS 17.0 software. No significant differences were observed in the content of the 14 compounds between the 6 SXD batches.
4. Conclusions
Fourteen active components in SXD were accurately quantified using the UFLC—MS/MS method established above. For the first time, the active components of SXD from eight crude herbal drugs were thoroughly and simultaneously quantitatively analyzed by UFLC—MS/MS. The developed method allows faster analysis and offers a greater selectivity compared with the conventional HPLC or GC methods used for the quantification of some of the compounds discussed. This study has demonstrated the benefits of establishing quality standards for Chinese medicinal preparations containing the same crude drugs, which could be useful for future pharmaceutical studies of SXD.
Acknowledgments
This work was supported by Beijing Municipal Natural Science Foundation (Grant No. 7142109), National Natural Science Foundation of China (Grant No. 81274054), Science and Technology Major Programmer for Major Drug Discovery (Grant No. 2012ZX09301002-001-028).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Lin W.C., Wong J.M., Tung C.C., Lin C.P., Chou J.W., Wang H.Y. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015;21:13566–13573. doi: 10.3748/wjg.v21.i48.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadnia-Afrouzi M., Hosseini A.Z., Khalili A., Abediankenari S., Amari A., Aghili B. Altered microRNA expression and immunosuppressive cytokine production by regulatory T cells of ulcerative colitis patients. Immunol Invest. 2016;45:63–74. doi: 10.3109/08820139.2015.1103749. [DOI] [PubMed] [Google Scholar]
- 3.Hellström A.E., Färkkilä M., Kolho K.L. Infliximab-induced skin manifestations in patients with inflammatory bowel disease. Scand J Gastroenterol. 2016;5:563–571. doi: 10.3109/00365521.2015.1125524. [DOI] [PubMed] [Google Scholar]
- 4.Chen G., Yang Y., Liu M., Teng Z., Ye J., Xu Y. Banxia xiexin decoction protects against dextran sulfate sodium—induced chronic ulcerative colitis in mice. J Ethnopharmacol. 2015;166:149–156. doi: 10.1016/j.jep.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Wu J.S., Shi R., Lu X., Ma Y.M., Cheng N.N. Combination of active components of Xiexin decoction ameliorates renal fibrosis through the inhibition of NF-κB and TGF-β1/Smad pathways in db/db diabetic mice. PLoS One. 2015;10:e0122661. doi: 10.1371/journal.pone.0122661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Jia L.Q., Tan H.Y., Pan L., Yu L.L., Deng B. Effect of Shengjiang xiexin decoction on the repair of damaged rat intestinal mucosa after irinotecan chemotherapy. Chin J Integr Tradit Wes Med. 2015;35:1236–1243. [PubMed] [Google Scholar]
- 7.An K., Zhao D., Wang Z., Wu J., Xu Y., Xiao G. Comparison of different drying methods on Chinese ginger (Zingiberofficinale Roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016;197:1292–1300. doi: 10.1016/j.foodchem.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Ji S., Li R., Wang Q., Miao W.J., Li Z.W., Si L.L. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J Ethnopharmacol. 2015;176:475–484. doi: 10.1016/j.jep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Jiang S., Wang Y., Ren D., Li J., Yuan G., An L. Antidiabetic mechanism of Coptis chinensis polysaccharide through its antioxidant property involving the JNK pathway. Pharm Biol. 2015;53:1022–1029. doi: 10.3109/13880209.2014.952838. [DOI] [PubMed] [Google Scholar]
- 10.Kumagai T., Müller C.I., Desmond J.C., Imai Y., Heber D., Koeffler H.P. Scutellaria baicalensis, a herbal medicine: anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res. 2007;31:523–530. doi: 10.1016/j.leukres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q.S., Zhu X.N., Jiang H.L., Wang G.F., Cui Y.L. Protective effects of alginate-chitosan microspheres loaded with alkaloids from Coptis chinensis Franch. and Evodia rutaecarpa (Juss.) Benth. (Zuojin Pill) against ethanol-induced acute gastric mucosal injury in rats. Drug Des Devel Ther. 2015;9:6151–6165. doi: 10.2147/DDDT.S96056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H.L., Zhao T.F., Wu H., Pan Y.Z., Zhang Q., Wang K.L. Pinellia ternata lectin exerts a pro-inflammatory effect on macrophages by inducing the release of pro-inflammatory cytokines, the activation of the nuclear factor–κB signaling pathway and the overproduction of reactive oxygen species. Int J Mol Med. 2015;36:1127–1135. doi: 10.3892/ijmm.2015.2315. [DOI] [PubMed] [Google Scholar]
- 13.Gong H., Zhang B.K., Yan M., Fang P.F., Li H.D., Hu C.P. A protective mechanism of licorice (Glycyrrhiza uralensis): isoliquiritigenin stimulates detoxification system via Nrf2 activation. J Ethnopharmacol. 2015;162:134–139. doi: 10.1016/j.jep.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Chen J., Li E., Fan Q., Wang D., Li P. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int Immunopharmacol. 2015;24:299–305. doi: 10.1016/j.intimp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Hoshyar R., Mohaghegh Z., Torabi N., Abolghasemi A. Antitumor activity of aqueous extract of Ziziphus jujube fruit in breast cancer: an in vitro and in vivo study. Asian Pac J Reprod. 2015;4:116–122. [Google Scholar]
- 16.Hamedi S., Arian A.A., Farzaei M.H. Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/ethanol-induced gastric mucosal injury in rats. J Tradit Chin Med. 2015;35:666–670. doi: 10.1016/s0254-6272(15)30157-6. [DOI] [PubMed] [Google Scholar]
- 17.Periasamy S., Liu C.T., Wu W.H., Chien S.P., Liu M.Y. Dietary Ziziphus jujuba fruit influence on aberrant crypt formation and blood cells in colitis-associated colorectal cancer in mice. Asian Pac J Cancer Prev. 2015;16:7561–7566. doi: 10.7314/apjcp.2015.16.17.7561. [DOI] [PubMed] [Google Scholar]
- 18.Chi A., Kang C., Zhang Y., Tang L., Guo H., Li H. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus jujube on chronic fatigue syndrome rats. Carbohydr Polym. 2015;122:189–196. doi: 10.1016/j.carbpol.2014.12.082. [DOI] [PubMed] [Google Scholar]
- 19.Hoshyar R., Jamali S., Fereidouni M., Abedini M.R. The cytotoxic activity of Ziziphus jujube on cervical cancer cells: in vitro study. Cell Mol Biol (Noisy-Le-Grand) 2015;61:128–130. [PubMed] [Google Scholar]
- 20.Mori K., Kondo T., Kamiyama Y., Kano Y., Tominaga K. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51:403–406. doi: 10.1007/s00280-003-0585-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen P., Zhou X., Zhang L., Shan M., Bao B., Cao Y. Anti-inflammatory effects of Huangqin tang extract in mice on ulcerative colitis. J Ethnopharmacol. 2015;162:207–214. doi: 10.1016/j.jep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi O., Kawashima A., Shiono A., Maeno Y., Ishikawa R., Masumoto A. Hange-Shashin-to for preventing diarrhea during afatinib therapy. Gan Kagaku Ryoho. 2015;42:581–583. [PubMed] [Google Scholar]
- 23.Okamura N., Miki H., Ishida S., Ono H., Yano A., Tanaka T. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, berberine, coptisine, palmatine, jateorrhizine and glycyrrhizin in kampo medicines by ion-pair high-performance liquid chromatography. Biol Pharm Bull. 1999;22:1015–1021. doi: 10.1248/bpb.22.1015. [DOI] [PubMed] [Google Scholar]
- 24.Gao X., Zheng X., Li Z., Zhou Y., Sun H., Zhang L. Metabonomic study on chronic unpredictable mild stress and intervention effects of Xiaoyaosan in rats using gas chromatography coupled with mass spectrometry. J Ethnopharmacol. 2011;137:690–699. doi: 10.1016/j.jep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Dewani A.P., Bakal R.L., Kokate P.G., Chandewar A.V., Patra S. Development of a single ion pair HPLC method for analysis of terbinafine, ofloxacin, ornidazole, clobetasol, and two preservatives in a cream formulation: application to in vitro drug release in topical simulated media-phosphate buffer through rat skin. J AOAC Int. 2015;98:913–920. doi: 10.5740/jaoacint.14-189. [DOI] [PubMed] [Google Scholar]
- 26.Nojavan Y., Kamankesh M., Shahraz F., Hashemi M., Mohammadi A. Ion pair—based dispersive liquid—liquid microextraction followed by high performance liquid chromatography as a new method for determining five folate derivatives in foodstuffs. Talanta. 2015;137:31–37. doi: 10.1016/j.talanta.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Wei L., Wang X., Zhang P., Sun Y., Jia L., Zhao J. An UPLC—MS/MS method for simultaneous quantitation of two coumarins and two flavonoids in rat plasma and its application to a pharmacokinetic study of Wikstroemia indica extract. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1008:139–145. doi: 10.1016/j.jchromb.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Wang M., Jiang N., Xian H., Wei D., Shi L., Feng X. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2016;1429:22–29. doi: 10.1016/j.chroma.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Lin Q., Pang T., Shi J. Determination of 12 flavonoids in tobacco leaves using ultra-high performance liquid chromatography—tandem mass spectrometry. Chin J Chrom. 2015;33:746–752. doi: 10.3724/sp.j.1123.2015.03001. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Xu R., Xiao J., Zhang J., Wang X., An R. Quantitative analysis of flavonoids, alkaloids and saponins of Banxia Xiexin decoction using ultra-high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2014;88:525–535. doi: 10.1016/j.jpba.2013.10.002. [DOI] [PubMed] [Google Scholar]