Abstract
Four interesting sequoiatones stereoisomers (1–4) were isolated from a wetland soil-derived fungus Talaromyces flavus by chiral HPLC. On the basis of comprehensive NMR and mass analyses, their planar structures were elucidated as the same as that of sequoiatone B. Among them, 1 and 3 (or 2 and 4) were a pair of enantiomers, and 1 and 2 (or 3 and 4) were a pair of stereoisomers with epimerization at C-12, which indicated that sequoiatione-type metabolites exist as enantiomers rather than as optically pure compounds in some strains. With the quantum chemical ECD calculations, the absolute configurations of C-8 in 1–4 were determined, which is the first report to establish the absolute configuration of C-8 in sequoiatones. However, the absolute configurations of C-12 in sequoiatones are still unsolved.
KEY WORDS: Wetland soil-derived fungus, Talaromyces flavus, Stereoisomers, Sequoiatone, Electronic circular dichroism
Graphical abstract
In our investigation, four stereoisomers of sequoiationes were isolated by chiral HPLC, whose planar structures were the same as that of sequoiatione B. Among them, 1 and 3 (or 2 and 4) are a pair of enantiomers, and 1 and 2 (or 3 and 4) are a pair of stereoisomers with epimerization at C-12, which indicated that sequoiatione-type metabolites exist as enantiomers rather than as optically pure compounds in some strains. Based on the analyses of experimental and quantum chemical ECD, it was found that the absolute configuration of C-8 in sequoiationes can be determined by ECD, which is the first time to discuss the absolute configuration of C-8 in sequoiationes.
1. Introduction
Sequoiatones, a kind of fungal metabolites, possess an octahydrocyclopenta[c]pyran skeleton with a long conjugated system1, 2, 3, which is similar to the azaphilones monascorubrin and monochaetin4, 5. To date, only few sequoiatones, such as sequoiatones A-F, were isolated from fungi Aspergillus parasiticus and Penicillium sp.1, 2, 3, and their structural diversity depend on diversiform ring systems (dicyclic or tricyclic systems), the position of CH3 in pyran ring (at C-5 or C-6), the unsaturation of olefin (C-6–C-7), and the hydroxylation at C-18.
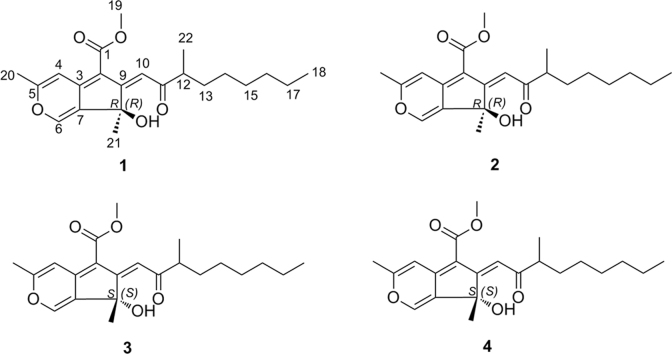
In the course of our search for bioactive secondary metabolites from wetland fungi6, 7, 8, 9, 10, the chemical investigation of metabolites from Talaromyces flavus (AHK07-3) was carried out, which led to the isolation of four stereoisomers (1–4) (Fig. 1) of sequoiatones with chiral HPLC (Fig. 2), whose planar structures were the same as that of sequoiatone B. According to the analyses of NMR, optical rotation, and electronic circular dichroism (ECD) data, 1 and 3 (or 2 and 4) were a pair of enantiomers, and 1 and 2 (or 3 and 4) were a pair of stereoisomers with epimerization at C-12. In addition, the absolute configurations of C-8 in 1–4 were determined by quantum chemical ECD calculations. Detail of the isolation and structural elucidations of compounds 1–4 are presented herein.
Figure 1.
Chemical structures of 1−4.
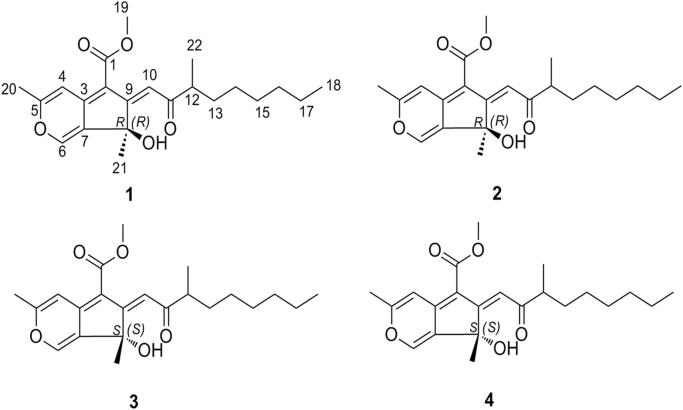
Figure 2.
HPLC analysis of 1–4 (A: the analysis of 1–4 on routine ODS HPLC; B: the analysis of 1–4 on chiral HPLC).
2. Results and discussion
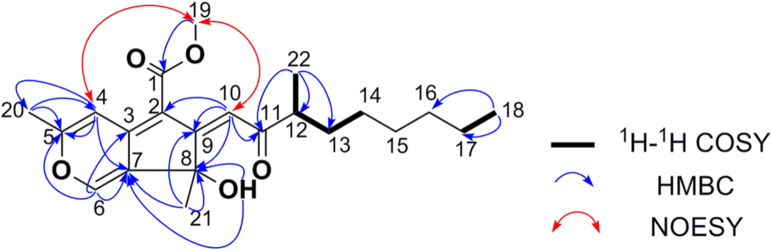
Compound 1 was obtained as a yellow oil. The quasi-molecular ion at m/z 375.2177 [M+H]+ by HR-ESI-MS indicated the molecular formula of 1 was C22H30O5 with 8 degrees of unsaturation. The 13C NMR spectrum combined with the DEPT-135 spectrum showed 22 signals (Table 1), assigned to seven sp2 quaternary carbons [including one ketone carbonyl (δC 208.0), one ester carbonyl (δC 165.4), and five olefinic carbons (δC 168.7, 163.9, 152.7, 135.4 and 106.7)], three sp2 methine carbons (δC 141.9, 113.2 and 106.6), one sp3 oxygenated quaternary carbon (δC 77.7), one sp3 methine carbon (δC 47.4), five sp3 methylene carbons (δC 34.0, 31.7, 29.4, 27.4 and 22.6), and five methyl carbons (δC 50.9, 27.3, 20.3, 17.4 and 14.1). In the 1H NMR spectrum of 1 (Table 1), the characteristic signals of one exchangeable proton [δH 7.54 (1H, s)], three aromatic or olefinic protons [δH 7.40 (1H, s), 7.21 (1H, s) and 6.96 (1H, s)], one sp3 methine proton [δH 2.67 (1H, m)], and five methyl protons [δH 3.84 (3H, s), 2.26 (3H, s), 1.53 (3H, s), 1.14 (3H, d, J=6.9 Hz) and 0.86 (3H, t, J=6.8 Hz)] were observed. The non-exchangeable proton resonances were associated with the directly attached carbon atoms in the HSQC experiment (Table 1). The analysis of the 1H–1H COSY experiment and the coupling values of the protons indicated the presence of one subunit (C-22–C-12–C-13–C-14–C-15–C-16–C-17–C-18) as shown in Fig. 3. Combined with the above deduced subunit and molecular formula, the HMBC correlations from H3-18 to C-16/C-17, from H3-19 to C-1, from H3-20 to C-4/C-5, from H3-21 to C-7/C-8/C-9, from H3-22 to C-11/C-12/C-13, from H-4 to C-5/C-7/C-20, from H-6 to C-3/C-5/C-7, from H-10 to C-2/C-8/C-9/C-11, from 8-OH to C-7/C-8, and the NOESY correlations between H3-19 and H-4/H-10, revealed the planer structure of 1 (Fig. 3). The assignments of all proton and carbon resonances are provided in Table 1. Furthermore, the key NOESY correlation between H3-19 and H-10 also suggested the Z configuration of the trisubstituted olefin (C-9–C-10) in 1.
Table 1.
1H NMR (400 MHz) and 13C NMR (100 MHz) spectral data of 1—4 (δ in ppm, J in Hz, CDCl3).
| No. |
1 |
2 |
3 |
4 |
||||
|---|---|---|---|---|---|---|---|---|
| δC | δHa | δC | δHa | δC | δHa | δC | δHa | |
| 1 | 165.4 | 165.4 | 165.4 | 165.4 | ||||
| 2 | 106.7 | 106.7 | 106.7 | 106.7 | ||||
| 3 | 152.7 | 152.8 | 152.7 | 152.8 | ||||
| 4 | 106.6 | 6.96, s | 106.6 | 6.96, s | 106.6 | 6.96, s | 106.6 | 6.96, s |
| 5 | 163.9 | 163.9 | 163.9 | 163.9 | ||||
| 6 | 141.9 | 7.40, s | 141.9 | 7.41, s | 141.9 | 7.40, s | 141.9 | 7.41, s |
| 7 | 135.4 | 135.4 | 135.4 | 135.4 | ||||
| 8 | 77.7 | 77.6 | 77.7 | 77.6 | ||||
| 9 | 168.7 | 168.6 | 168.7 | 168.6 | ||||
| 10 | 113.2 | 7.21, s | 113.4 | 7.21, s | 113.2 | 7.21, s | 113.4 | 7.21, s |
| 11 | 208.0 | 208.0 | 208.0 | 208.0 | ||||
| 12 | 47.4 | 2.67 | 47.3 | 2.69 | 47.4 | 2.67 | 47.3 | 2.69 |
| 13 | 34.0 | 1.68, Ha; 1.38, Hb | 34.0 | 1.69, Ha; 1.40, Hb | 34.0 | 1.68, Ha; 1.38, Hb | 34.0 | 1.69, Ha; 1.39, Hb |
| 14 | 27.4 | 1.28b | 27.4 | 1.28b | 27.4 | 1.28b | 27.4 | 1.28b |
| 15 | 29.4 | 1.28b | 29.4 | 1.28b | 29.4 | 1.28b | 29.4 | 1.28b |
| 16 | 31.7 | 1.28b | 31.7 | 1.28b | 31.7 | 1.28b | 31.7 | 1.28b |
| 17 | 22.6 | 1.25 | 22.6 | 1.25 | 22.6 | 1.25 | 22.6 | 1.25 |
| 18 | 14.1 | 0.86, t (6.8) | 14.1 | 0.86, t (6.8) | 14.1 | 0.86, t (6.8) | 14.1 | 0.86, t (6.8) |
| 19 | 50.9 | 3.84, s | 50.9 | 3.84, s | 50.9 | 3.84, s | 50.9 | 3.84, s |
| 20 | 20.3 | 2.26, s | 20.4 | 2.26, s | 20.3 | 2.26, s | 20.4 | 2.26, s |
| 21 | 27.3 | 1.53, s | 27.3 | 1.53, s | 27.3 | 1.53, s | 27.3 | 1.53, s |
| 22 | 17.4 | 1.14, d (6.9) | 17.1 | 1.12, d (6.8) | 17.4 | 1.14, d (6.9) | 17.1 | 1.12, d (6.8) |
| OH-8 | 7.54, s | 7.58, br s | 7.53, s | 7.57, br s | ||||
Indiscernible signals owing to overlapping or having complex multiplicity are reported without designating multiplicity.
The assignments in each column may be interchanged.
Figure 3.
Key 1H–1H COSY, HMBC, and NOESY correlations of 1.
Compounds 2–4 were also obtained as yellow oils. Based on the analysis of HR-ESI-MS data, the molecular formulas of 2–4 were the same as 1. Furthermore, the 1D and 2D NMR data revealed that 2–4 had the same planer structure as 1, which were stereoisomers of 1. In addition, the irradiation of H3-19 causing enhancements of H-10 in the selective 1D NOESY experiment of 2–4 revealed the Z configurations of the trisubstituted olefin (C-9–C-10) in 2–4, which were the same as 1 (see Supplementary Information Figs. S14, S21 and S27).
In the structures of 1–4, there are two stereogenic centers (C-8 and C-12) in the skeleton, which are isolated by three carbons. Detailed analyses of the 1D NMR data of 1–4 presented that the NMR data of 1 and 3 are identical except for the exchangeable proton (Table 1). The similar observation was in NMR data of 2 and 4, and there is only 0.01 ppm offset at Hb-13. Two sets of NMR data with little offset (0.01/0.1 ppm) at few protons/carbons (except for exchangeable protons) can be recognized as identical11. For the identical NMR data and the similar absolute values of optical rotations but in opposite directions of 1 and 3 (or 2 and 4), 1 and 3 (or 2 and 4) should be a pair of enantiomers.
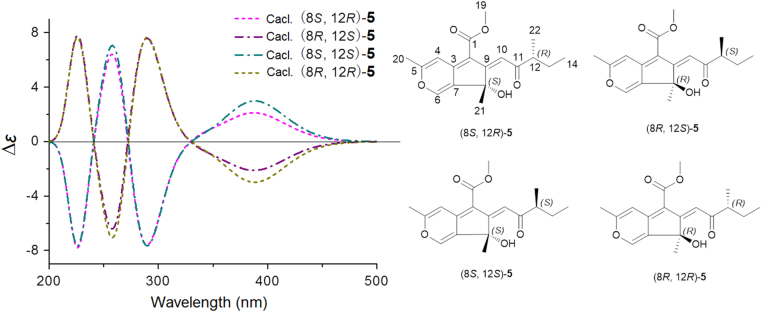
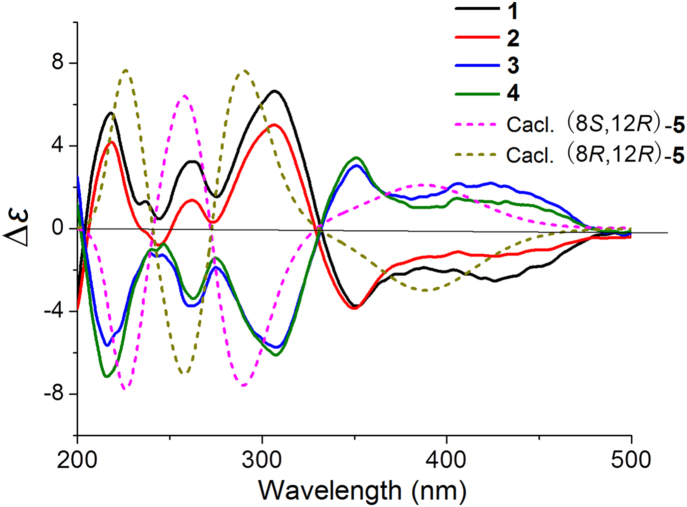
Between 1 and 2 (or 3 and 4), there were several noticeable offsets at C-10 (0.2 ppm), H-12 (0.02 ppm), and C-22 (0.3 ppm)/H3-22 (0.02 ppm) (Table 1), which resulted from the epimerization at C-8 or C-12. In ECD experiments, the ECD curves of 1 and 2 were similar with each other, which were opposite to those of 3 and 4. To investigate the effect of different configurations of C-8 and C-12 to ECD of 1–4, the ECD calculations were carried out. Since the flexible side chain at C-12 has negligible effect on the ECD of 1–4, the simplified structures (8S,12R)-5, (8R,12S)-5, (8R,12R)-5, and (8S,12S)-5 were used for ECD calculations12, 13, 14, 15, 16. The calculations (Fig. 4) showed that the signs of major cotton effects of these structures were apparently controlled by the absolute configuration of C-8. The experimental data of 1 and 2 were similar to the predicted ECD curve of (8R,12R)-5 (Fig. 5), which suggested that the absolute configurations of C-8 in 1 and 2 were R. The experimental data of 3 and 4 were similar to the predicted ECD curve of (8S,12R)-5 (Fig. 5), which suggested that the absolute configurations of C-8 in 3 and 4 were S. Therefore, the epimerization between 1 and 2 (or 3 and 4) was at C-12. However, the configurations of C-12 were not determined.
Figure 4.
The calculated ECD spectra of (8S,12R)-5, (8R,12S)-5, (8S,12S)-5, and (8R,12R)-5 (band width σ=0.3 eV).
Figure 5.
The experimental ECD spectra of 1−4 and calculated ECD spectra of (8S,12R)-5 and (8R,12R)-5 (UV correction=0 nm, band width σ=0.3 eV).
3. Conclusions
Up to now, only few sequoiatones have been reported. Except the configuration of sequoiatone A had been established by X-ray crystallography1, the absolute configurations of C-8 and C-12 in other sequoiatones were not unambiguously investigated. In our investigation, four stereoisomers of sequoiationes were isolated by chiral HPLC, whose planar structures were the same as that of sequoiatione B1. Among them, 1 and 3 (or 2 and 4) are a pair of enantiomers, and 1 and 2 (or 3 and 4) are a pair of stereoisomers with epimerization at C-12, which indicated that sequoiatione-type metabolites exist as enantiomers rather than as optically pure compounds in some strains. Based on the analyses of experimental and quantum chemical ECD, it was found that the absolute configuration of C-8 in sequoiationes can be determined by ECD, which is the first time to discuss the absolute configuration of C-8 in sequoiationes. The absolute configurations of C-12 in sequoiationes are still unsolved.
4. Experimental
4.1. General experimental procedures
Optical rotations were measured on a JASCO P1020 digital polarimeter (Jasco International Co., Ltd., Tokyo, Japan). UV data were recorded using a JASCO V-550 UV/vis spectrometer (Jasco International Co., Ltd., Tokyo, Japan). IR data were recorded on a JASCO FT/IR-480 plus spectrometer (Jasco International Co., Ltd., Tokyo, Japan). ECD spectrum was recorded on a JASCO J-810 spectrophotometer using MeOH as the solvent (Jasco International Co., Ltd., Tokyo, Japan). The ESI-MS spectra were performed on a Finnigan LCQ Advantage MAX mass spectrometer (Finnigan MAT GmbH, Bremen, Germany). The HR-ESI-MS spectra were obtained on a Micromass Q-TOF mass spectrometer (Waters Corporation, Milford, MA, USA). The NMR spectra were measured with Bruker AV-400 spectrometer (Bruker BioSpin Group, Faellanden, Switzerland) using solvent signals (CDCl3: δH 7.26/δC 77.0) as internal standard. The analytical HPLC was performed on a Dionex HPLC system equipped with an Ultimate 3000 pump, an Ultimate 3000 diode array detector (DAD), an Ultimate 3000 Column Compartment, and an Ultimate 3000 autosampler (Thermo Fisher Scientific, Inc., Waltham, MA, USA) using a Phenomenex Gemini C18 column (250 mm×4.6 mm, 5 μm) (Phenomenex Inc., Los Angeles, CA, USA). The semi-preparative HPLC was performed on a Shimadzu LC-6-AD Liquid Chromatography with an SPD-20A Detector using a Phenomenex Gemini C18 column (250 mm×10.0 mm, 5 μm) (Phenomenex Inc., Los Angeles, CA, USA). The chiral HPLC was performed on a Shimadzu LC-6-AD Liquid Chromatography with an SPD-20A Detector using a Phenomenex Lux Cellulose-2 chiral column (250 mm×4.6 mm, 3 μm) (Phenomenex Inc., Los Angeles, CA, USA). The medium pressure liquid chromatography (MPLC) was performed on ODS (60–80 μm, YMC Co., Ltd., Tokyo, Japan) and equipped with a dual pump gradient system, a UV preparative detector, and a Dr Flash II fraction collector system (Lisui E-Tech Co., Ltd., Shanghai, China).
4.2. Fungus material
The strain AHK07-3 was isolated from the soil, collected at the wetland of Ahongkou, Sinkiang Province, China. The strain was identified as Talaromyces flavus based on the morphological characters and gene sequence analysis. The ribosomal internal transcribed spacer (ITS) and the 5.8S rRNA gene sequences (ITS1-5.8S-ITS2) of the strain have been deposited at GenBank as KX011167. The fungus was cultured on slants of potato dextrose agar at 25 °C for 5 days. Agar plugs were used to inoculate four Erlenmeyer flasks (250 mL), each containing 100 mL of potato dextrose broth. Four flasks of the inoculated media were incubated at 25 °C on a rotary shaker at 200 rpm for 5 days to prepare the seed culture. Fermentation was carried out in 20 Erlenmeyer flasks (500 mL), each containing 70 g of rice. Distilled H2O (105 mL) was added to each flask, and the rice was soaked overnight before autoclaving at 120 °C for 30 min. After cooling to room temperature, each flask was inoculated with 5.0 mL of the spore inoculum and incubated at room temperature for 50 days.
4.3. Extraction and isolation
The culture was extracted three times with EtOAc, and the organic solvent was removed under reduced pressure to yield crude extract (19.7 g). The crude extract was dissolved in 90% v/v aqueous MeOH (400 mL) and partitioned against the same volume of cyclohexane to afford a cyclohexane fraction (C, 4.6 g) and an aqueous MeOH fraction (W, 14.7 g). The aqueous MeOH fraction (W, 14.7 g) was separated by MPLC (195 mm×31.2 mm) eluting with MeOH−H2O (30:70 and 100:0, v/v) and CHCl3−MeOH (50:50, v/v) to afford three fractions (W1 to W3). The fraction W2 (5.9 g) was separated by MPLC (127 mm×26.8 mm) with a gradient of MeOH−H2O (60%−100%, v/v) to yield four subfractions (W2a to W2d). The subfraction W2c (521.8 mg) was further separated by MPLC (76 mm×21.4 mm) with a gradient of MeOH−H2O (60%−90%, v/v) to yield three sub-subfractions (W2c1 to W2c3). Sub-subfraction W2c2 (100.6 mg) was purified by semi-preparative HPLC purification using MeOH–H2O (73:27, v/v) with 0.1% formic acid at a flow rate of 3 mL/min to yield an isolated peak W2c2-3 (18.6 mg) and it was separated by chiral HPLC using MeCN–H2O (78:22, v/v) at a flow rate of 1 mL/min to afford 1 (tR: 10.2 min, 3.0 mg), 2 (tR: 11.4 min, 4.2 mg), 3 (tR: 13.9 min, 3.7 mg), and 4 (tR: 17.4 min, 2.8 mg), respectively.
Compound 1 Yellow oil; [α]D25 −109.8 (c 0.50, MeOH); UV (MeOH) λmax (logε) 204 (3.25), 253 (3.17), 314 (3.08), 426 (3.53) nm; CD (c 2.1×10−4 mol/L, MeOH) λmax (Δε) 218 (+5.55), 262 (+3.27), 306 (+6.63), 352 (−3.75), 426 (−2.59) nm; IR (KBr) νmax 3440, 2925, 1664, 1541, 1501, 1459, 1172, 1127, 1057 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) see Table 1; ESI-MS (positive): m/z 375 [M+H]+, 397 [M+Na]+, HR-ESI-MS (positive): m/z 375.2177 [M+H]+ (Calcd. for C22H31O5, 375.2171).
Compound 2 Yellow oil; [α]D25 −51.2 (c 0.50, MeOH); UV (MeOH) λmax (logε) 204 (3.19), 254 (3.10), 314 (3.02), 426 (3.51) nm; CD (c 2.1×10−4 mol/L, MeOH) λmax (Δε) 218 (+4.21), 262 (+1.39), 306 (+5.06), 350 (−3.84), 426 (−1.34) nm; IR (KBr) νmax 3440, 2925, 1670, 1541, 1507, 1457, 1172, 1114, 1064 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) see Table 1; ESI-MS (positive): m/z 375 [M+H]+, 397 [M+Na]+, HR-ESI-MS (positive): m/z 375.2182 [M+H]+ (Calcd. for C22H31O5, 375.2171).
Compound 3 Yellow oil; [α]D25 +113.8 (c 0.50, MeOH); UV (MeOH) λmax (logε) 204 (3.27), 253 (3.17), 314 (3.08), 426 (3.53) nm; CD (c 2.1×10−4 mol/L, MeOH) λmax (Δε) 216 (−5.59), 262 (−3.75), 307 (−5.72), 351 (+3.05), 426 (+2.20) nm; IR (KBr) νmax 3440, 2925, 1664, 1541, 1501, 1459, 1172, 1127, 1057 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) see Table 1; ESI-MS (positive): m/z 375 [M+H]+, 397 [M+Na]+, HR-ESI-MS (positive): m/z 375.2177 [M+H]+ (Calcd. for C22H31O5, 375.2171).
Compound 4 Yellow oil; [α]D25 +57.2 (c 0.50, MeOH); UV (MeOH) λmax (logε) 204 (3.20), 254 (3.10), 314 (3.02), 426 (3.51) nm; CD (c 2.1×10−4 mol/L, MeOH) λmax (Δε) 216 (−7.11), 262 (−3.40), 307 (−6.08), 351 (+3.41), 426 (+1.30) nm; IR (KBr) νmax 3440, 2925, 1670, 1541, 1507, 1457, 1172, 1114, 1064 cm−1; 1H (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) see Table 1; ESI-MS (positive): m/z 375 [M+H]+, 397 [M+Na]+, HR-ESI-MS (positive): m/z 375.2185 [M+H]+ (Calcd. for C22H31O5, 375.2171).
4.4. ECD calculation
Before molecular initial 3D structures were generated with CORINA version 3.4, the molecules of (8S, 12R)-5, (8R, 12S)-5, (8S, 12S)-5, and (8R, 12R)-5 were converted into SMILES codes, respectively. Conformer databases were generated in CONFLEX version 7.0 using the MMFF94s force-field, with an energy window for acceptable conformers (ewindow) of 5 kcal/mol above the ground state, a maximum number of conformations per molecule (maxconfs) of 100, and an RMSD cutoff (rmsd) of 0.5 Å. Then each conformer of the acceptable conformers was optimized with HF/6-31G(d) method in Gaussian0917. Further optimization at the APFD/6-31G(d) level led the dihedral angles to be got. After that, thirteen lowest energy conformers of (8S, 12R)-5 and (8R, 12S)-5 were found out (see Supplementary Information Table S5 and Fig. S1). Fourteen lowest energy conformers of (8S, 12S)-5 and (8R, 12R)-5 were found out (see supporting information Table S6 and Fig. S2). The optimized conformers were taken for the ECD calculations, which were performed with Gaussian09 (APFD/6-311++G(2d,p)). The solvent effect was taken into account by the polarizable-conductor calculation model (IEFPCM, methanol as the solvent). Comparisons of the experimental and calculated spectra were done with the software SpecDis18, 19. It was also used to apply a UV shift to the ECD spectra, Gaussian broadening of the excitations, and Boltzmann weighting of the spectra.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (Nos. 81422054 and 81172945), the Guangdong Natural Science Funds for Distinguished Young Scholar (S2013050014287), Guangdong Special Support Program (2014TQ01R420), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (Hao Gao, 2014).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http:dx.doi.org/10.1016/j.apsb.2016.07.005.
Contributor Information
Guodong Chen, Email: chgdtong@163.com.
Hao Gao, Email: tghao@jnu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Stierle A.A., Stierle D.B., Bugni T. Sequoiatones A and B: novel antitumor metabolites isolated from a redwood endophyte. J Org Chem. 1999;64:5479–5484. doi: 10.1021/jo990277l. [DOI] [PubMed] [Google Scholar]
- 2.Stierle A.A., Stierle D.B., Bugni T. Sequoiatones C-F, constituents of the redwood endophyte Aspergillus parasiticus. J Nat Prod. 2001;64:1350–1353. doi: 10.1021/np010022e. [DOI] [PubMed] [Google Scholar]
- 3.Lin Z.J., Zhu T.J., Fang Y.C., Gu Q.Q., Zhu W.M. Polyketides from Penicillium sp. JP-1, an endophytic fungus associated with the mangrove plant Aegiceras corniculatum. Phytochemistry. 2008;69:1273–1278. doi: 10.1016/j.phytochem.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Fielding B.C., Haws E.J., Holker J.S.E., Powell A.D.G., Robertson A., Stanway D.N. Monascorubrin. Tetrahedron Lett. 1960;1:24–27. [Google Scholar]
- 5.Steyn P.S., Vleggaar R. A reinvestigation of the structure of monochaetin, a metabolite of Monochaetia compta. J Chem Soc Perkin Trans 1. 1986;1986:1975–1976. [Google Scholar]
- 6.He J.W., Mu Z.Q., Gao H., Chen G.D., Zhao Q., Hu D. New polyesters from Talaromyces flavus. Tetrahedron. 2014;70:4425–4430. [Google Scholar]
- 7.He J.W., Qin D.P., Gao H., Kuang R.Q., Yu Y., Liu X.Z. Two new coumarins from Talaromyces flavus. Molecules. 2014;19:20880–20887. doi: 10.3390/molecules191220880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J.W., Liang H.X., Gao H., Kuang R.Q., Chen G.D., Hu D. Talaflavuterpenoid A, a new nardosinane-type sesquiterpene from Talaromyces flavus. J Asian Nat Prod Res. 2014;16:1029–1034. doi: 10.1080/10286020.2014.933812. [DOI] [PubMed] [Google Scholar]
- 9.Bao Y.R., Chen G.D., Wu Y.H., Li X.X., Hu D., Liu X.Z. Stachybisbins A and B, the first cases of seco-bisabosquals from Stachybotrys bisbyi. Fitoterapia. 2015;105:151–155. doi: 10.1016/j.fitote.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Bao Y.R., Chen G.D., Gao H., He R.R., Wu Y.H., Li X.X. 4, 5-seco-probotryenols A-C, a new type of sesquiterpenoids from Stachybotrys bisbyi. RSC Adv. 2015;5:46252–46259. [Google Scholar]
- 11.Wang C.X., Chen G.D., Feng C.C., He R.R., Qin S.Y., Hu D. Same data, different structures: diastereoisomers with substantially identical NMR data from nature. Chem Commun. 2016;52:1250–1253. doi: 10.1039/c5cc07141k. [DOI] [PubMed] [Google Scholar]
- 12.Ren J.W., Zhang F., Liu X.Y., Li L., Liu G., Liu X.Z. Neonectrolide A, a new oxaphenalenone spiroketal from the fungus Neonectria sp. Org Lett. 2012;14:6226–6229. doi: 10.1021/ol302979f. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Li C., Si Y.K., Yin D.L. Absolute configuration of buagafuran: an experimental and theoretical electronic circular dichroism study. Chin Chem Lett. 2013;24:500–502. [Google Scholar]
- 14.Wu Z.Y., Wu Y., Chen G.D., Hu D., Li X.X., Sun X. Xylariterpenoids A–D, four new sesquiterpenoids from the Xylariaceae fungus. RSC Adv. 2014;4:54144–54148. [Google Scholar]
- 15.Jia Y.L., Wei M.Y., Chen H.Y., Guan F.F., Wang C.Y., Shao C.L. (+)- and (−)-Pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro[oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Org Lett. 2015;17:4216–4219. doi: 10.1021/acs.orglett.5b01995. [DOI] [PubMed] [Google Scholar]
- 16.Chen G.D., Xiao G.K., Yao X.S., Gao H. Electronic circular dichroism calculation method in the assignment of absolute configuration of natural products. J Int Pharm Res. 2015;42:738–743. [Google Scholar]
- 17.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R. Gaussian, Inc; Wallingford CT: 2010. Gaussian 09, revision D.01. [Google Scholar]
- 18.Bruhn T., Schaumlöffel A., Hemberger Y., Bringmann G. University of Würzburg; Würzburg, Germany: 2013. SpecDis, version 1.61. [Google Scholar]
- 19.Bruhn T., Schaumlöffel A., Hemberger Y., Bringmann G. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality. 2013;25:243–249. doi: 10.1002/chir.22138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material