Abstract
Introduction:
Wound infections with Vibrio alginolyticus, a Gram-negative bacterium found in all temperate oceans, are rarely reported. However, a rising incidence of wound infections caused by V. alginolyticus requires better knowledge about this infectious agent.
Case presentation:
We report the case of a 14-year-old boy suffering from a wound infection caused by V. alginolyticus and Staphylococcus lugdunensis after stepping on a sea urchin. Despite wound debridement and antibiotic therapy with cefaclor, the lesion did not heal over several weeks. After identification of the pathogens and antibiotic-susceptibility testing, antibiotic therapy was switched to ciprofloxacin, followed by trimethoprim/sulfamethoxazole. Two months after the accident the wound was re-epithelialized. Follow up after 6 months revealed a painful scar.
Conclusion:
Non-cholera vibrios like V. alginolyticus should be considered as possible causative agents in seawater-contaminated wounds. S. lugdunensis is a relevant pathogen in mixed wound infections. Early microbiological diagnosis and antibiotic-susceptibility testing is crucial to prevent therapeutic failure.
Keywords: Vibrio, Vibrio alginolyticus, coagulase-negative staphylococci, Staphylococcus lugdunensis, wound, sea urchin
Introduction
Vibrio alginolyticus is a Gram-negative halophilic bacterium found in temperate oceans all over the world. Most isolates show resistance to penicillins and second-generation cephalosporins (French et al., 1989; Li et al., 1999), and are capable of causing serious wound infections, even in association with only minor lesions (Gomez et al., 2003; Reilly et al., 2011).
Infections in humans are rarely reported, e.g. the incidence was only 0.048 per 100 000 population in the USA in 2011 (Conrad, 2013). Nevertheless, like other Vibrio-mediated infections there has been an increase in incidence over many years, possibly induced by an elevation of marine temperature due to global warming (Conrad, 2013; Vezzulli et al., 2012).
Herein, we report the clinical course and follow up of a 14-year-old immunocompetent boy who was suffering from a mixed wound infection caused by V. alginolyticus and Staphylococcus lugdunensis. In the case of wounds acquired from contact with seawater or marine organisms, clinicians should be aware of Vibrio infections. Nevertheless, these infections are unknown to many physicians even in high-incidence countries (Osaka et al., 2004) and require careful microbiological work-up.
Case report
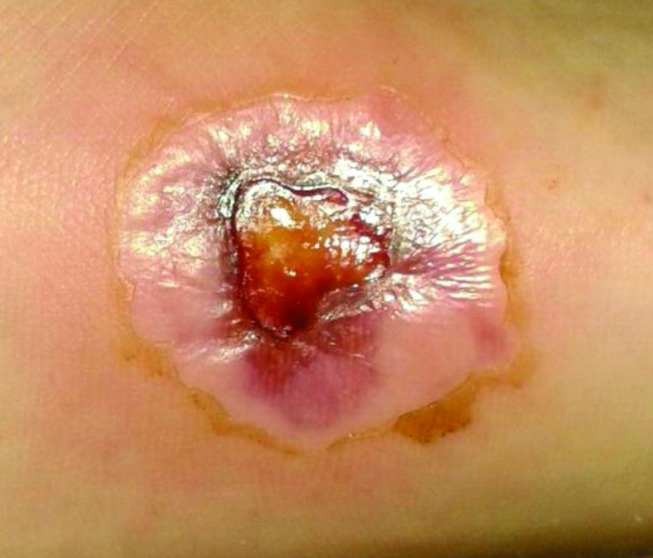
The patient was swimming in the Red Sea while on vacation in Egypt, near Hurghada, when he stepped on a sea urchin. He was injured on the medial plantar margin of his left foot (Fig. 1). The wound was treated immediately by the beach warden using hot oil and lemon juice. This treatment led to a minor burn with blistering.
Fig. 1.
Lesion on the medial margin of the sole of the left foot 28 days after the accident. Diameter approximately 2 cm.
Immediately afterwards, the patient was taken to the local hospital. There a burn blister on top of the wound was opened and disinfected with an iodine-containing ointment. The wound was covered with sterile dressing, which was changed daily. During the next 2 weeks, until the boy's departure from Egypt, the lesion became livid and kept oozing.
Back in Germany, the patient presented with the non-healing wound and an additional tonsillitis at a paediatric outpatient clinic. The wound was cleaned and empiric antibiotic therapy was started with cefaclor (500 mg three times daily). Seven days later, the patient returned to the clinic with continuing impaired wound healing, while the tonsillitis had resolved. A wound swab was submitted for microbiological analysis. The patient was then referred to the local hospital’s surgical outpatient clinic, where an extensive wound debridement was performed. Four days afterwards, the patient returned for a control examination and antibiotic therapy was changed to ciprofloxacin (200 mg twice daily).
Two bacterial isolates were obtained from the wound swab and identified as V. alginolyticus and S. lugdunensis. Numerous colonies were present up to the second (V. alginolyticus) and third (S. lugdunensis) streak area of the initial streak plates. After receiving antibiotic-susceptibility test results, therapy was switched to trimethoprim/sulfamethoxazole (160/800 mg twice daily) to which both isolates were susceptible. Antibiotic therapy with trimethoprim/sulfamethoxazole was continued for 20 days.
Investigations
Aerobic and anaerobic cultures were performed using Columbia blood agar, chocolate agar, Columbia CNA agar, MacConkey agar and Schaedler agar with/without kanamycin, using standard microbiological procedures. A Gram-negative rod and coagulase-negative staphylococci were grown and identified to the species level using appropriate VITEK 2 ID cards (VITEK 2 GN and GP-cartridge; bioMérieux) as V. alginolyticus and S. lugdunensis. Antibiotic-susceptibility testing was performed using appropriate VITEK 2 cards, AST N263 and AST P619, respectively, and interpreted according to current Clinical and Laboratory Standards Institute (CLSI) guidelines (Table 1).
Table 1. MIC and antimicrobial-susceptibility test. Interpretive categories were according to the CLSI guidelines for Vibrio spp. (CLSI, 2015a) and Staphylococcus spp. with special regard to S. lugdunensis (CLSI, 2015b).
| Antibiotic | V. alginolyticus | S. lugdunensis | ||
|---|---|---|---|---|
| MIC (mg l−1) | Interpretation | MIC (mg l−1) | Interpretation | |
| Benzylpenicillin | nt* | nt | ≥0.5 | R |
| Ampicillin | ≥32 | R | nt* | nt |
| Ampicillin/sulbactam | ≤2 | S | nt* | nt |
| Oxacillin | nt* | nt | 1 | S |
| Cefuroxime | 16 | I | nt* | nt |
| Cefotaxime | ≤1 | S | nt* | nt |
| Ceftazidime | ≤1 | S | nt* | nt |
| Ciprofloxacin | ≤0.25 | S | nt | nt |
| Levofloxacin | ≤0.12 | S | ≤0.12 | S |
| Imipenem | ≤0.25 | S | – | S |
| Meropenem | ≤0.25 | S | – | S |
| Trimethoprim/sulfamethoxazole | ≤20 | S | ≤10 | S |
I, Intermediate susceptibility; nt, not tested; R, resistant; S, susceptible.
*Testing not recommended for this species.
For confirmation of the V. alginolyticus identification, the strain was sub-cultured on Vibrio selective agar (thiosulfate-citrate-bile-sucrose agar; Becton-Dickinson) at 37 °C. After 24 h, colonies grew on the plates turning the colour of the agar to yellow as expected for V. alginolyticus. Additionally, matrix-associated laser desorption ionization-time of flight MS identification using a MALDI Biotyper (Bruker Daltonics) using software version 3.1 was performed, revealing V. alginolyticus with a score of 1.94. The next most closely related species was Vibrio mytili with a score of 1.84.
As the MALDI Biotyper revealed only an identification at the probable genus level, further confirmation was sought using 16S rRNA gene sequencing applying the method of Harmsen et al. (2003). The resulting 0.5 kbp amplicons were sequenced with a 3500xL Genetic Analyzer (Thermo Fisher Scientific). Using the curated database of EZbioCloud (Kim et al., 2012) and criteria for microbial identification using DNA target sequences (CLSI, 2008),similarities larger than 99 % were found for numerous species of the genus Vibrio, including V. alginolyticus, without sufficient discrimination for identification at the species level. Similarly, the 16S rRNA gene sequence was analysed using blastn 2.2.26 and the DNA Database of Japan (DDBJ) due to its large number of well-documented Vibrio spp. genome sequences (http://ddbj.nig.ac.jp/blast/; Altschul et al., 1997). More than 200 strains of Vibrio spp. shared the best-reached similarity of 98 % to our isolate, including 10 different species (Vibrio fischeri, Vibrio parahaemolyticus, Vibrio harveyi, V. alginolyticus, Vibrio campbellii, Vibrio communis, Vibrio orientalis, Vibrio rotiferianus, Vibrio owensii and 'Vibrio antiquarius').
In the next step, additional multiplex PCR for the conserved transcriptional regulator genes VptoxR, VctoxR and VvtoxR (Osorio & Klose, 2000), according to Bauer & Rørvik (2007), was performed. There was a negative result for all toxR genes, leading to an exclusion of the species V. parahaemolyticus, Vibrio cholerae and Vibrio vulnificus from the identification.
Finally, rpoB sequencing applying the method and primers of Tarr et al. (2007) delivered two sequences of the rpoB gene (456 bp upstream, 528 bp downstream), which were analysed using DDBJ and blastn as described above (http://ddbj.nig.ac.jp/blast/; Altschul et al., 1997). Our isolate showed identity of 100 % to more than 50 strains of V. alginolyticus (upstream) and 99 % to more than 200 strains of V. alginolyticus (downstream). There was one 99 % match with V. harveyi (upstream and downstream) and one with V. parahaemolyticus (upstream). The sequence data were analysed with Bionumerics (Applied Maths, version 7.1; Applied Maths) and compared to previously published sequences of the most common pathogenic Vibrio spp. in the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/nuccore). To visualize the phylogenetic relationship, the unweighted pair group method with arithmetic mean based on multiple alignments between the rpoB sequences was used (Fig. 2).
Fig. 2.
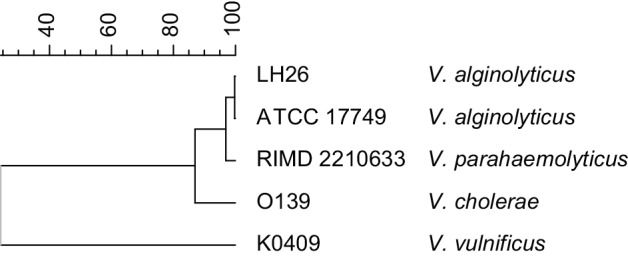
Phylogenetic tree including our isolate (V. alginolyticus LH26), V. cholerae O139 (NCBI reference sequence no. NZ_AWWA01000041.1), V. parahaemolyticus RIMD 2210633 (NCBI reference sequence no. NC_004603.1), V. vulnificus K0409 (GenBank accession no. EF064441.1) and V. alginolyticus ATCC 17749 (GenBank accession no. JN188438.1). The x-axis shows the percentage similarity.
Because of the former biochemical identification results and the large number of perfect homologies to strains identified in various taxonomic studies (Ki et al., 2009; Oberbeckmann et al., 2011), we accepted V. alginolyticus as the final identification. For coagulase-negative staphylococci, biochemical identification is widely used and commonly accepted (Becker et al., 2014). Therefore, we accepted the Vitek 2-based identification of S. lugdunensis described above.
Diagnosis
V. alginolyticus and S. lugdunensis co-infection of a sea urchin-induced wound.
Outcome and follow-up
In the following months, wound healing continued slowly until the wound was epithelialized about 2 months later. When examined for follow-up 6 months after the initial accident, it was noticed that there remained an induration of the former wound with tenderness on palpation.
Discussion
In this case, we identified three major reasons for the prolonged, complicated wound infection. First of all, insufficient first aid and the resulting burn necrosis led to an environment where V. alginolyticus and S. lugdunensis could survive repeated debridement and disinfection. Lack of protection because of burned skin enables secondary bacterial infections.
Second, V. alginolyticus is well known for its numerous chromosomal and plasmid-mediated antibiotic-resistance determinants (French et al., 1989; Li et al., 1999). Many of the expressed β-lactamases lead to resistance to ampicillin and second-generation cephalosporins, as seen in our isolate (Li et al., 1999). Resistance to trimethoprim/sulfamethoxazole is commonly reported (Li et al., 1999). Some isolates also show resistance to third-generation cephalosporins and fluoroquinolones, as reported by Ye et al. (2016). According to this evolution of antibiotic resistance and because of the typical mixed flora in chronic wound infections (like S. lugdunensis in our case; Altoparlak et al., 2004), early antibiotic-susceptibility testing is important to prevent therapeutic failure.
Lastly, the presence of S. lugdunensis may have triggered the progression of the disease. Compared to many other coagulase-negative staphylococci, S. lugdunensis has higher pathogenic potential. It can cause serious infections, i.e. soft tissue and wound infections as well as infective endocarditis, and has to be considered as a relevant pathogen (Becker et al., 2014).
When treating the patient, the chosen therapy in the hospital with ciprofloxacin was an appropriate choice for the infection. However, fluoroquinolone use in children is still off label for many indications (except, for example, cystic fibrosis), especially if there is an alternative treatment (Bradley et al., 2011). Therefore, therapy was changed successfully to trimethoprim/sulfamethoxazole.
Altogether, this case and its course are an example of the need to consider Vibrio-mediated infections in similar circumstances. Even if it is a rare disease at present, a rising incidence has been observed, as indicated above. Warming of the oceans will probably make this a global trend as the first cases from northern shores suggest (Reilly et al., 2011; Schets et al., 2006). Identification of V. alginolyticus is less than straightforward and requires a combination of classical biochemical identification methods, as well as appropriate selective media and advanced molecular identification methodology.
Abbreviations:
- CLSI
Clinical and Laboratory Standards Institute
- DDBJ
DNA Database of Japan
References
- Altoparlak U., Erol S., Akcay M. N., Celebi F., Kadanali A.(2004). The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 30660–664. 10.1016/j.burns.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J.(1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 253389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Rørvik L. M.(2007). A novel multiplex PCR for the identification of Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. Lett Appl Microbiol 45371–375. 10.1111/j.1472-765X.2007.02195.x [DOI] [PubMed] [Google Scholar]
- Becker K., Heilmann C., Peters G.(2014). Coagulase-negative staphylococci. Clin Microbiol Rev 27870–926. 10.1128/CMR.00109-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2008). Interpretive Criteria for Identification of Bacteria and Fungi by DNA Target Sequencing; Approved Guideline, MM18-A. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- CLSI (2015a). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; 3rd edn, M45 Wayne, PA: Clinical and Laboratory Standards Institute. [DOI] [PubMed] [Google Scholar]
- CLSI (2015b). Performance Standards for Antimicrobial Susceptibility Testing; 25th edn, M100S Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- Bradley J. S., Jackson M. A., the Committee on Infectious Diseases (2011). The use of systemic and topical fluoroquinolones. Pediatrics 128e1034–e1045. 10.1542/peds.2011-1496 [DOI] [PubMed] [Google Scholar]
- Conrad A.(2013). Trends in vibriosis transmission among the top four Vibrio species, United States, 1988-2012. MPH thesis, Georgia State University, Atlanta, GA, USA Available at: http://scholarworks.gsu.edu/iph_theses/314 .
- French G. L., Woo M. L., Hui Y. W., Chan K. Y.(1989). Antimicrobial susceptibilities of halophilic vibrios. J Antimicrob Chemother 24183–194. 10.1093/jac/24.2.183 [DOI] [PubMed] [Google Scholar]
- Gomez J. M., Fajardo R., Patiño J. F., Arias C. A.(2003). Necrotizing fasciitis due to Vibrio alginolyticus in an immunocompetent patient. J Clin Microbiol 413427–3429. 10.1128/JCM.41.7.3427-3429.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen D., Dostal S., Roth A., Niemann S., Rothgänger J., Sammeth M., Albert J., Frosch M., Richter E.(2003). RIDOM: comprehensive and public sequence database for identification of Mycobacterium species. BMC Infect Dis 326. 10.1186/1471-2334-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki J. S., Zhang R., Zhang W., Huang Y. L., Qian P. Y.(2009). Analysis of RNA polymerase beta subunit (rpoB) gene sequences for the discriminative power of marine Vibrio species. Microb Ecol 58679–691. 10.1007/s00248-009-9519-7 [DOI] [PubMed] [Google Scholar]
- Kim O. S., Cho Y. J., Lee K., Yoon S. H., Kim M., Na H., Park S. C., Jeon Y. S., Lee J. H., et al. (2012). Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62716–721. 10.1099/ijs.0.038075-0 [DOI] [PubMed] [Google Scholar]
- Li J., Yie J., Foo R. W. T., Ling J. M., Xu H., Woo N. Y. S.(1999). Antibiotic resistance and plasmid profiles of Vibrio isolates from cultured silver sea bream, Sparus sarba. Mar Pollut Bull 39245–249. 10.1016/S0025-326X(99)00062-4 [DOI] [PubMed] [Google Scholar]
- Oberbeckmann S., Wichels A., Maier T., Kostrzewa M., Raffelberg S., Gerdts G.(2011). A polyphasic approach for the differentiation of environmental Vibrio isolates from temperate waters. FEMS Microbiol Ecol 75145–162. 10.1111/j.1574-6941.2010.00998.x [DOI] [PubMed] [Google Scholar]
- Osaka K., Komatsuzaki M., Takahashi H., Sakano S., Okabe N.(2004). Vibrio vulnificus septicaemia in Japan: an estimated number of infections and physicians' knowledge of the syndrome. Epidemiol Infect 132993–996. 10.1017/S0950268804002407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio C. R., Klose K. E.(2000). A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J Bacteriol 182526–528. 10.1128/JB.182.2.526-528.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly G. D., Reilly C. A., Smith E. G., Baker-Austin C.(2011). Vibrio alginolyticus-associated wound infection acquired in British waters, Guernsey, July 2011. Euro Surveill 1619994. [PubMed] [Google Scholar]
- Schets F. M., van den Berg H. H., Demeulmeester A. A., van Dijk E., Rutjes S. A., van Hooijdonk H. J., de Roda Husman A. M.(2006). Vibrio alginolyticus infections in the Netherlands after swimming in the North Sea. Euro Surveill 113077. [DOI] [PubMed] [Google Scholar]
- Tarr C. L., Patel J. S., Puhr N. D., Sowers E. G., Bopp C. A., Strockbine N. A.(2007). Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol 45134–140. 10.1128/JCM.01544-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli L., Brettar I., Pezzati E., Reid P. C., Colwell R. R., Höfle M. G., Pruzzo C.(2012). Long-term effects of ocean warming on the prokaryotic community: evidence from the vibrios. ISME J 621–30. 10.1038/ismej.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Li R., Lin D., Zhou Y., Fu A., Ding Q., Chan E. W., Yao W., Chen S.(2016). Characterization of an IncA/C multidrug resistance plasmid in Vibrio alginolyticus. Antimicrob Agents Chemother 603232–3235. 10.1128/AAC.00300-16 [DOI] [PMC free article] [PubMed] [Google Scholar]