Abstract
Introduction:
An outbreak of Streptococcus pneumoniae (pneumococcal) infection complicated by concomitant influenza A on an elderly care ward was detected.
Case presentation:
Thirteen patients with hospital-acquired respiratory infections were investigated during the course of the outbreak investigation. Six had a positive BinaxNOW S. pneumoniae urinary antigen test and two patients had culture-confirmed pneumococcal bacteraemia and a positive urine antigen test. Five patients gave positive influenza A PCR results of which two were also positive for S. pneumoniae antigen.
Conclusion:
The concurrence of influenza and pneumococcal infections made tracking the course of the infection difficult. This case study shows how the use of a sensitive, S. pneumoniae serotype-specific urine antigen assay, in the absence of cultured isolates, helped determine whether patients were infected with the same pneumococcal serotype. This was particularly useful when additional respiratory symptoms were seen following the administration of chemoprophylaxis.
Keywords: Diagnosis, influenza, outbreak, Pneumococcus, respiratory, urine antigen
Introduction
The National Reference Laboratory for Streptococcus pneumoniae, the Respiratory and Vaccine Preventable Bacteria Reference Unit (RVPBRU) of Public Health England, Colindale, UK, was alerted to a suspected nosocomial outbreak of S. pneumoniae infections on an elderly care ward at a hospital in the south-east of England.
Outbreaks of S. pneumoniae disease are relatively rare but can occur in closed communities (Birtles et al., 2005, Gupta et al., 2008, Nuorti et al., 1998, Pichon et al., 2010). This report describes a complicated outbreak of both S. pneumoniae and influenza A on a hospital ward and the use of a sensitive, serotype-specific urine antigen test to determine the course of the pneumococcal outbreak and effectiveness of control methods (use of antibiotics to halt onward transmission of S. pneumoniae). The characteristics of the incident were a limited number (n = 2) of S. pneumoniae culture-confirmed cases, a background of respiratory infections caused by influenza and a likelihood of co-incidental pneumococcal infection. Due to the persistence of respiratory symptoms in some patients, despite completion of the course of antibiotics, the use of the serotype-specific urinary antigen assay helped to inform whether the control measures had been successful.
Case report
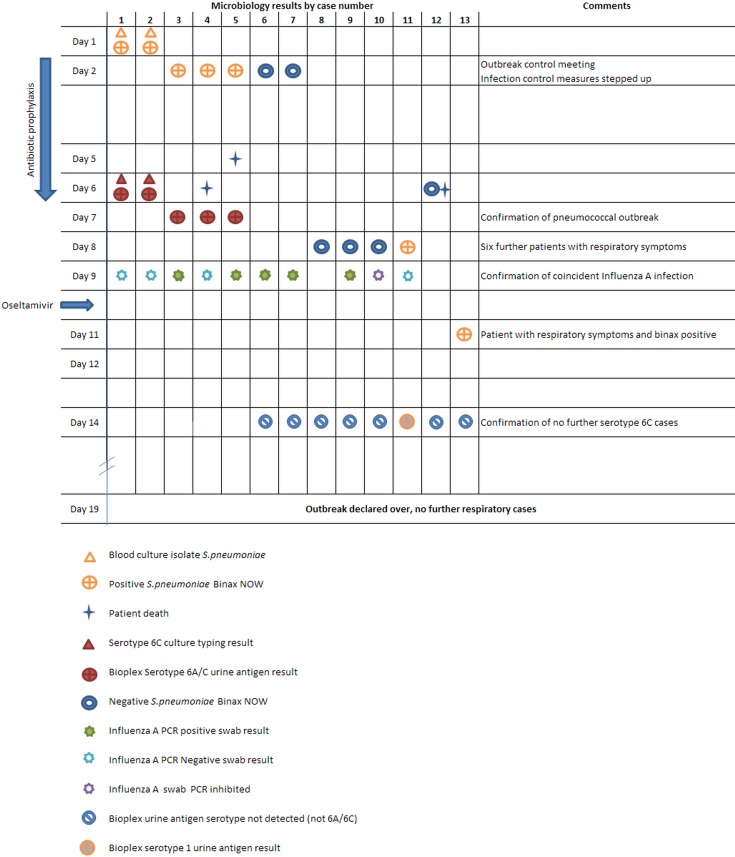
A diagrammatic representation of the cases with the microbiology result time line in shown in Fig. 1.
Fig. 1.
Diagrammatic representation of the cases with the microbiology result time line.
Day 1: outbreak recognition
Two patients in a 28-bed care-of-the-elderly ward were identified with blood culture-confirmed pneumococcal bacteraemia. Urine samples from both patients also gave a positive result with the BinaxNow S. pneumoniae urinary antigen test (Alere). Case 1 (Fig. 1) had been an inpatient for over 1 month following a collapse secondary to cardiac arrhythmia. On day 1, the patient complained of a 1-day history of cough and fever, and a diagnosis of healthcare-associated pneumonia was made and piperacillin/tazobactam treatment was initiated. Case 2 (Fig. 1) had also been an inpatient for 1 month following admission with a septic episode and collapse. The patient developed respiratory symptoms and fever on day 1 and was also commenced on piperacillin/tazobactam for a suspected healthcare-associated pneumonia. Cases 1 and 2 subsequently tested negative for influenza RNA on PCR testing of throat swabs; the cause for the sudden onset of invasive pneumococcal infection in case 1 remains unknown.
Day 2
Investigations identified a further six patients on the ward with respiratory symptoms and a clinical diagnosis of pneumonia, with some having radiological confirmation. Urine specimens from three additional patients tested positive for pneumococcal antigen using the BinaxNOW test. The urine specimens and S. pneumoniae isolates were sent to the reference laboratory (RVPBRU) for typing. One patient (case 7, Fig. 1 – the probable index case for influenza) had been admitted 1 month previously with community-acquired pneumonia but the BinaxNOW test was negative for S. pneumoniae antigen when tested on day 2.
The ward was closed to admissions, transfers and discharges to anywhere other than the patients' own homes. The patients had a range of underlying illnesses and reasons for admission, and no common intervention or equipment was identified.
Chemoprophylaxis was started for the patients on the ward, consisting of a 5-day course of oral amoxicillin given to patients not already on antibiotics appropriate for pneumococcal treatment, and augmented with intravenous benzyl penicillin for those patients who were already on antibiotic therapy that would not cover pneumococcal infection.
Ward staff were risk assessed for significant exposure and underwent oropharyngeal swabbing to detect carriage of pneumococci, and infection control measures and cleaning on the ward were stepped up. Patients, staff and visitors were provided with information about pneumococcal disease by the hospital infection control team.
Day 6
An outbreak control meeting involving hospital staff, the Health Protection Team and Public Health England reference laboratory staff and expert advisors was convened. There had been no new respiratory cases seen on the ward over the weekend.
Seven patients who had recently been discharged from the ward were followed up and given chemoprophylaxis: five were treated at home with oral antibiotics and two were readmitted to hospital for treatment of respiratory illness and antimicrobial cover appropriate for pneumococcal disease.
There had been three deaths on the ward (one on day 5 and two on day 6). The cause of death in these patients was bronchopneumonia secondary to carcinomatosis (case 4, Fig. 1), acute coronary syndrome (case 5, Fig. 1) and sepsis secondary to pneumonia (case 12, Fig. 1), respectively. Ante-mortem urine samples were tested with S. pneumoniae BinaxNOW from these three patients, two of whom were positive; the urine from the patient with sepsis secondary to pneumonia was negative. The BinaxNOW-negative urine was later sent for serotype-specific antigen testing by Bio-Plex (Bio-Rad, Hemel Hempstead UK) and was negative.
Day 7
Serotyping results became available from two patients with blood culture isolates, which were both S. pneumoniae serotype 6C, and the five BinaxNOW-positive urine specimens (two from the culture-positive patients), which were positive for serotype 6A/6C in the serotype-specific Bio-Plex assay (serotypes 6A and 6C cannot be distinguished in the Bio-Plex assay). These results confirmed that an outbreak of serotype 6C pneumococcal infection had occurred on the ward.
Day 8
Further patients on the ward showed respiratory symptoms: urine samples tested from five of these patients were negative with the BinaxNOW test and one was positive. This positive result raised the possibility of failure of the infection control measures and prophylaxis. Due to the higher sensitivity of the Bio-Plex assay, all urine samples including those found previously to be BinaxNow negative were sent to RVPBRU for testing using this assay to ensure additional pneumococcal 6C cases were not missed.
Day 9
A further outbreak control meeting was held. Results from PCR testing confirmed influenza A in five of eight patients, and two of these also had laboratory-confirmed pneumococcal disease. Patients on the ward were given oseltamivir prophylaxis or treatment depending on their clinical condition. The General Practitioners of the patients who had already been discharged home were notified about the additional potential for influenza infection.
All of the screening swabs collected from members of staff were culture negative for pneumococci. The ward staff were instructed to liaise with the occupational health department if they developed a ‘flu-like’ illness for testing and treatment as appropriate.
Day 11
A urine sample from one further patient on the ward gave a positive BinaxNOW result, and the urine was sent to RVPBRU for serotype-specific antigen detection to determine whether this could be due to failure of the control measures for the 6C outbreak.
Day 14
Urine sample serotype Bio-Plex antigen results were obtained from the six additional patients that were BinaxNOW negative and one Binax NOW-positive sample. All were negative for pneumococcal serotype 6A/6C antigen, which helped to confirm the end of the serotype 6C outbreak. One BinaxNOW weakly positive sample gave a positive result for pneumococcal serotype 1.
Day 19
The outbreak was declared over after a week of monitoring and no further respiratory cases. In total, of the symptomatic patients, nine had blood cultures taken during the course of their illness of which only the first two cases cultured an organism (S. pneumoniae).
Investigations
The S. pneumoniae BinaxNOW test kit (Alere) was used following the manufacturer's instructions for detection of common polysaccharide antigen in the urine specimens.
A sensitive and specific Bio-Plex (Luminex technology) bead-based multiplex immunoassay was used to determine serotype-specific antigen from the urine specimens obtained during the outbreak. The multiplex assay panel contains mAbs against the following S. pneumoniae serotypes: 1, 3, 4, 5, 6A/6C, 6B, 7A/7F, 8, 9V and 14, and serogroup 18, 19A, 19F and 23F (Sheppard et al., 2011).
Influenza testing was carried out based on probes and primers supplied by Public Health England testing for influenza A matrix and 2009 H1N1 influenza virus (swine flu).
Discussion
The use of a serotype-specific urine antigen assay confirmed the presence of the pneumococcal outbreak by detecting infections with the same serotype in three cases in addition to the original two isolates. The concurrence of the influenza A infections at the same time as the pneumococcal outbreak made tracing the course of the bacterial infection difficult and increased the probability of spread, with many patients exhibiting respiratory symptoms. When the influenza results were available, they showed that some patients had both influenza and pneumococcal infections, some had evidence of pneumococcal infection only and some of influenza infection only. The presence of a concomitant influenza infection probably aided the dissemination of pneumococcus around the ward environment, allowing such a significant outbreak to develop.
The identification of further respiratory symptoms and two pneumococcal BinaxNOW-positive results after the course of antibiotic prophylaxis, together with the results of the serotype-specific urine antigen assay provided the physicians with the confidence that the therapy had worked and that the serotype 6C outbreak was not continuing. One of the post-prophylaxis-positive BinaxNow samples was negative for a detectable serotype, while the other was positive for serotype 1 and was probably a coincidence in this susceptible age group as no cross-reaction between the assays for serotype 1 and serotype 6C has been reported.
The serotype-specific Bio-Plex assay developed in our laboratory has demonstrated its utility previously in pneumococcal outbreak or cluster situations. It has played a major role in several such investigations in the past few years including school (Gupta et al., 2008) and nursing home (Thomas et al., 2015) clusters, but our study illustrates the first application in a rare, nosocomial dual-infection outbreak setting. In its current form, the assay is limited in its serotype repertoire but could be expanded by sourcing further mAbs to serotypes not currently included. Since the introduction of pneumococcal conjugate vaccination, there has been a large shift in serotype distribution, with a reduction in the proportion of vaccine types such as those available in the multiplex assay compared with non-vaccine types. Inclusion of further serotype-specific mAbs would expand its existing utility for both outbreak investigation and epidemiological surveillance in the vast number of cases of pneumococcal disease from which an isolate is not obtained but a urine specimen is available.
Acknowledgements
Samples were taken and analysed for routine diagnostic purposes and analyses were performed as appropriate for this purpose only. The authors would like to acknowledge Ella Campion and Gurkiran Mankoo for serotyping of pneumococcal isolates and Professor Liz Miller for assistance and advice throughout the outbreak. M.P.E.S. has received fees for Advisory Boards and Speaking Engagements from Pfizer, GSK, Sanofi Pasteur and MSD. The other authors have no conflicts of interest to declare.
References
- Birtles A., McCarthy N., Sheppard C. L., Rutter H., Guiver M., Haworth E., George R. C. Multilocus sequence typing directly on DNA from clinical samples and a cultured isolate to investigate linked fatal pneumococcal disease in residents of a shelter for homeless men. J Clin Microbiol. 2005;43:2004–2008. doi: 10.1128/JCM.43.4.2004-2008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Khaw F. M., Stokle E. L., George R. C., Pebody R., Stansfield R. E., Sheppard C. L., Slack M., Gorton R., Spencer D. A. Outbreak of Streptococcus pneumoniae serotype 1 pneumonia in a United Kingdom school. BMJ. 2008;337:a2964. doi: 10.1136/bmj.a2964. [DOI] [PubMed] [Google Scholar]
- Nuorti J. P., Butler J. C., Crutcher J. M., Guevara R., Welch D., Holder P., Elliott J. A. An outbreak of multidrug-resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med. 1998;338:1861–1868. doi: 10.1056/NEJM199806253382601. [DOI] [PubMed] [Google Scholar]
- Pichon B., Moyce L., Sheppard C., Slack M., Turbitt D., Pebody R., Spencer D. A., Edwards J., Krahé D., George R. Molecular typing of pneumococci for investigation of linked cases of invasive pneumococcal disease. J Clin Microbiol. 2010;48:1926–1928. doi: 10.1128/JCM.02054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard C. L., Harrison T. G., Smith M. D., George R. C. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific Streptococcus pneumoniae antigen in urine samples. J Med Microbiol. 2011;60:49–55. doi: 10.1099/jmm.0.023150-0. [DOI] [PubMed] [Google Scholar]
- Thomas H. L., Gajraj R., Slack M.P.E., Sheppard C., Hawkey P., Gossain S., Drew C. M., Pebody R. G. An explosive outbreak of Streptococcus pneumoniae serotype-8 infection in a highly vaccinated residential care home, England, summer 2012. Epidemiol Infect. 2015;143:1957–1963. doi: 10.1017/S0950268814002490. [DOI] [PMC free article] [PubMed] [Google Scholar]