Abstract
Introduction:
Brucella is a zoonotic infection commonly diagnosed by isolation of the organism from blood culture or positive serological testing. It is an uncommon cause of a pyrexia of unknown origin in the United Kingdom.
Case presentation:
We describe the case of a 14-year-old girl with no history of travel who presented with pyrexia, weight loss, arthralgia, multiple splenic abscesses and a subsequent pleural effusion, the latter of which isolated a Brucella species on 16S rRNA PCR. The patient responded well to initiation of treatment for brucellosis and on repeat imaging, after 3 months, the splenic abscesses had resolved.
Conclusion:
This unique case demonstrates uncommon complications of brucellosis and the challenges of diagnosing the organism, the latter of which can be alleviated by the utilization of molecularbased technologies. This patient had a negative serology result for brucellosis, which highlights the need to interpret serology results with caution in non-endemic regions for brucellosis.
Keywords: Brucella, splenic abscesses, pleural effusion, 16S PCR, brucella serology
Introduction
Brucellosis is a zoonotic infection characteristically presenting with an undulating fever. Thirty per cent of infections can result in localized infection (Aygen et al., 2002; Hasanjani Roushan et al., 2004) of which splenic abscesses represent a small minority of that. In the paediatric group, there are only two documented cases (Vallego et al., 1996; Parande et al., 2010).
We describe a rare case of splenic and pulmonary brucellosis in a patient with no recent travel. This interesting case demonstrates the complex nature of diagnosing brucellosis and the benefits of molecular testing in a disease normally exclusively diagnosed by serology and blood culture.
Case report
A 14-year-old British born girl presented with a 1 month history of fever, weight loss, lethargy, arthralgia and diarrhoea. She had been investigated a month previously for an iron deficiency anaemia with no cause found. Travel history consisted of a trip to Israel 2 years ago.
Upon examination, she was pyrexial (38.5 °C) and tachycardic with a soft systolic murmur. There was a palpable splenic tip below the left costal margin.
Investigations
Bloods demonstrated haemoglobin of 6.1 g dl−1, mean cell volume of 72 fl, erythrocyte sedimentation rate of 73 mm h−1 and C-reactive protein of 149 mg L−1. All other blood parameters were normal. A presumptive diagnosis of inflammatory bowel disease was made. Upper and lower gastro-intestinal endoscopies were unremarkable whereas a magnetic resonance imaging (MRI) of the abdomen showed splenomegaly of 14.8 cm with multiple small, hypogenic splenic abscesses (Fig. 1).
Fig. 1.
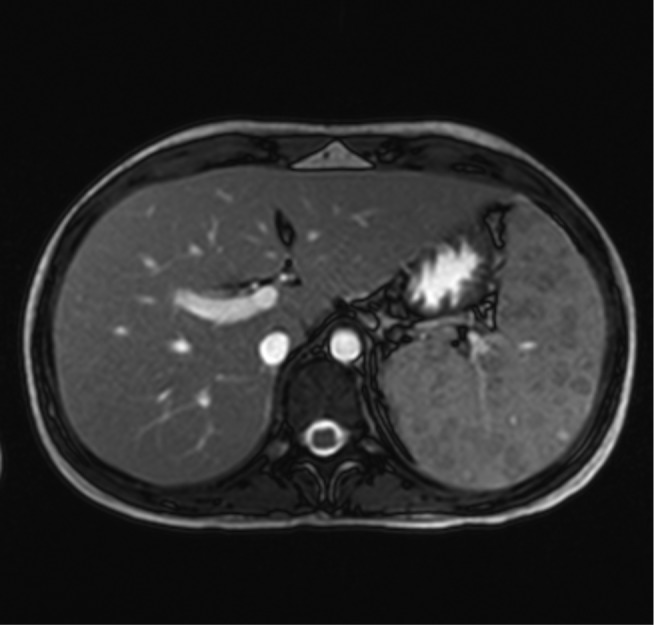
MRI of the abdomen demonstrating multiple hypogenic splenic abscesses.
A total of four sets of blood cultures taken over a period of 5 days were negative. The initial antibiotic regimen of ciprofloxacin 500 mg bd was changed to ceftriaxone 2 g od and flucloxacillin 1 g qds on day 7 in order to broaden cover for a potential salmonella or staphylococcal infection.
Diagnosis
One month into admission, the patient remained pyrexial despite antimicrobial therapy. A transthoracic echocardiogram was negative and a repeat ultrasound of her spleen showed no change in the size of the abscesses, resulting in consideration of a splenec tomy. The patient subsequently developed a left-sided pleural effusion. Pleural aspirate was negative on culture and for acid-fast bacilli but was positive for a Brucella species following 16S PCR. Of note, serology was negative using the Brucellacapt immunocapture assay.
Treatment
Treatment consisted of a 12-week course of rifampicin and doxycycline in conjunction with the addition of gentamicin for the first 14 days.
Outcome and follow-up
The patient had a good clinical response with complete regression of her splenic abscesses on follow-up ultrasound imaging. In the absence of any recent foreign travel, the case was notified to Public Health of England as a probable UK acquisition.
Discussion
Brucellosis is caused by small, non-motile Gram-negative coccobacilli. The four species of Brucella that are pathogenic to humans are Brucella melitensis, Brucella abortus, Brucella suis and Brucella canis (Franco et al., 2007; Mansur et al., 2008). Infection can be acquired from inhalation of aerosolized bacteria or the consumption of products such as unpasteurized milk and cheese or meats from infected animals (Aygen et al., 2002; Franco et al., 2007). Brucellosis is endemic to areas including the Mediterranean Basin and Middle East. In the UK, an average of 10 cases are reported each year, nearly all of which are acquired from abroad (Health Protection Agency, October 2013).
This case demonstrates the complexity of obtaining a diagnosis of brucellosis whereupon multiple sets of blood cultures are culture negative. This may reflect prior antimicrobial treatment with ceftriaxone and ciprofloxacin, both of which have activity against Brucella (Falagas & Bliziotis, 2006; Baykam et al., 2004). In addition, the detection of Brucella from blood cultures has previously been demonstrated to have low sensitivity in focal and chronic infections (Azira, 1996).
In the presence of this low yield from blood cultures, serology has become the mainstay of diagnosis with the standard tube agglutination (STA) test being used worldwide as the gold standard for acute brucellosis. A titre value of 1 : 80 in non-endemic regions and 1 : 320 in endemic regions is regarded as a positive threshold for the diagnosis of brucellosis (Christopher et al., 2010). A fourfold increase in titre from the acute to convalescent sample is considered diagnostic. In regions of high seropositivity, isolated low-level STA titres can be difficult to interpret. The ELISA test measuring IgA, IgM and IgG has demonstrated higher sensitivity in the detection of very acute and chronic brucellosis than the STA, although some studies suggest that the overall specificity is lower (Mantur et al., 2010). The Brucellacapt, an immunocapture agglutination test, has the advantage over the aforementioned tests of detecting both non-agglutinating and agglutinating antibodies. It has been shown in studies to detect higher titre values in acute and evolving infections than the STA or Coombs test (Orduña et al., 2000), possibly due to its ability to detect IgM, IgG and IgA and blocking antibodies. The Brucellacapt has shown good correlation with the Coombs test with superior sensitivity to the STA (Casao et al., 2004). The Brucellacapt and the Coombs test are deemed superior serology tests for prolonged active infection and relapsing disease (Bosilkovski et al., 2010).
In the UK, the Brucellacapt test has superseded the STA for diagnosing Brucella due to its ability to detect both acute and chronic infections (Baykam et al., 2004). However, the variable sensitivity of serology tests, especially in non-endemic settings (Fadeel et al., 2011), highlights the need for caution in advocating the widespread use of serology tests alone in diagnosing focal brucellosis.
In the present case, Brucella was identified from a pleural fluid sample using broad-spectrum 16S rRNA gene PCR. The benefit of 16S PCR for the identification of bacterial pathogens from pleural fluid when compared to culture alone has been previously reported (Saglani et al., 2005). It has also been proposed that PCR assays are more sensitive at detecting relapses in brucellosis when compared to serological testing (Mitka et al., 2007). At the present time, 16S rRNA PCR has the ability to accurately identify to a genus level but, as in our case, has difficulty speciating Brucella. Nevertheless, the current case demonstrates the advantages of utilizing 16S PCR methodology in situations of diagnostic uncertainty and, to the author’s knowledge, represents the first isolation of Brucella from a pleural fluid sample.
Splenic abscesses in the context of brucellosis are rare with an incidence reported to be approximately 2–3 % and predominantly associated with chronic hepatosplenic brucellosis (Ariza et al., 2001). Medical treatment alone can be successful as in the aforementioned case but surgical intervention is often required (Solera et al., 1997). Respiratory involvement in brucellosis is also a rare occurrence as demonstrated by Pourbagher et al. (2006) who reported the presence of pleural effusions in 2.8 % of patients. Consequently, this case demonstrates the presence of two rare complications arising from brucellosis in a single patient.
Treatment of brucellosis historically consists of dual therapy with rifampicin and doxycycline being advocated by the World Health Organisation for the treatment of uncomplicated cases. A systematic review demonstrated an aminoglycoside for 2 weeks with rifampicin and doxycycline for at least 12 weeks to be superior to standard dual therapy (Skalsky et al., 2008).
The difficulty in diagnosing brucellosis has been consistently reported. This present case adds to this literature by demonstrating the importance of employing molecular techniques in the investigation of unusual pathogens such as Brucella from sterile samples. It is the subsequent opinion of the authors that molecular testing for the detection of acute infection and relapses with brucellosis should be encouraged.
Acknowledgements
We thank Nelofar Obaray and Mark Wilks, who performed the 16S PCR testing.
Abbreviations:
- MRI
magnetic resonance imaging
- STA
standard tube agglutination
References
- Ariza J., Pigrau C., Cañas C., Marrón A., Martínez F., Almirante B., Corredoira J. M., Casanova A., Fabregat J., Pahissa A.(2001). Current understanding and management of chronic hepatosplenic suppurative brucellosis. Clin Infect Dis 321024–1033. 10.1086/319608 [DOI] [PubMed] [Google Scholar]
- Aygen B., Doğanay M., Sümerkan B., Yildiz O., Kayabaş Ü.(2002). Clinical manifestations, complications and treatment of brucellosis: a retrospective evaluation of 480 patients. Med Malad Infect 32485–493. 10.1016/S0399-077X(02)00403-1 [DOI] [Google Scholar]
- Azira J.(1996). Brucellosis. Curr. Opin.Infect.Dis 9126–131. [Google Scholar]
- Baykam N., Esener H., Ergönül O., Eren S., Celikbas A. K., Dokuzoguz B.(2004). In vitro antimicrobial susceptibility of Brucella species. Int J Antimicrob Agents 23405–407. 10.1016/j.ijantimicag.2003.09.024 [DOI] [PubMed] [Google Scholar]
- Bosilkovski M., Katerina S., Zaklina S., Ivan V.(2010). The role of Brucellacapt test for follow-up patients with brucellosis. Comp Immunol Microbiol Infect Dis 33 435. 10.1016/j.cimid.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Casao M. A., Navarro E., Solera J.(2004). Evaluation of Brucellacapt for the diagnosis of human brucellosis. J Infect 49102–108. 10.1016/j.jinf.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Christopher S., Umapathy B. L., Ravikumar K. L.(2010). Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. J Lab Physicians 255–60. 10.4103/0974-2727.72149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel M. A., Hoffmaster A. R., Shi J., Pimentel G., Stoddard R. A.(2011). Comparison of four commercial IgM and IgG ELISA kits for diagnosing brucellosis. J Med Microbiol 601767–1773. 10.1099/jmm.0.033381-0 [DOI] [PubMed] [Google Scholar]
- Falagas M. E., Bliziotis I. A.(2006). Quinolones for treatment of human brucellosis: critical review of the evidence from microbiological and clinical studies. Antimicrob Agents Chemother 5022–33. 10.1128/AAC.50.1.22-33.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M. P., Mulder M., Gilman R. H., Smits H. L.(2007). Human brucellosis. Lancet Infect Dis 7775–786. 10.1016/S1473-3099(07)70286-4 [DOI] [PubMed] [Google Scholar]
- Hasanjani Roushan M. R., Mohrez M., Smailnejad Gangi S. M., Soleimani Amiri M. J., Hajiahmadi M.(2004). Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, Northern Iran. Epidemiol Infect 1321109–1114. 10.1017/S0950268804002833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Protection Agency (2013). England 30 October 2013 www.hpa.org.uk.
- Mantur B., Parande A., Amarnath S., Patil G., Walvekar R., Desai A., Parande M., Shinde R., Chandrashekar M., Patil S.(2010). ELISA versus conventional methods of diagnosing endemic brucellosis. Am J Trop Med Hyg 83314–318. 10.4269/ajtmh.2010.09-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantur B. G., Mulimani M. S., Bidari L. H., Akki A. S., Tikare N. V.(2008). Bacteremia is as unpredictable as clinical manifestations in human brucellosis. Int J Infect Dis 12303–307. 10.1016/j.ijid.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Mitka S., Anetakis C., Souliou E., Diza E., Kansouzidou A.(2007). Evaluation of different PCR assays for early detection of acute and relapsing brucellosis in humans in comparison with conventional methods. J Clin Microbiol 451211–1218. 10.1128/JCM.00010-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduña A., Almaraz A., Prado A., Gutierrez M. P., Garcia-Pascual A., Dueñas A., Cuervo M., Abad R., Hernández B., et al. (2000). Evaluation of an immunocapture-agglutination test (Brucellacapt) for serodiagnosis of human brucellosis. J Clin Microbiol 384000–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parande A. M., Mantur B. G., Kore M., Palled E.(2010). Splenic abscess due to Brucella melitensis - a rare pediatric complication. J Lab Physicians 2105–108. 10.4103/0974-2727.72212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbagher M. A., Pourbagher A., Savas L., Turunc T., Demiroglu Y. Z., Erol I., Yalcintas D.(2006). Clinical pattern and abdominal sonographic findings in 251 cases of brucellosis in southern Turkey. AJR Am J Roentgenol 187W191–W194. 10.2214/AJR.05.0241 [DOI] [PubMed] [Google Scholar]
- Saglani S., Harris K. A., Wallis C., Hartley J. C.(2005). Empyema: the use of broad range 16S rDNA PCR for pathogen detection. Arch Dis Child 9070–73. 10.1136/adc.2003.042176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky S., Yahav D., Bishara J., Pitlik S., Leibovici L., Paul M.(2008). Treatment of human brucellosis: systematic review and meta- analysis of randomised controlled trials BMJ. 336701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solera J., Martínez-Alfaro E., Espinosa A.(1997). Recognition and optimum treatment of brucellosis. Drugs 53245–246. 10.2165/00003495-199753020-00005 [DOI] [PubMed] [Google Scholar]
- Vallejo J. G., Stevens A. M., Dutton R. V., Kaplan S. L.(1996). Hepatosplenic abscesses due to Brucella melitensis: report of a case involving a child and review of the literature. Clin Infect Dis 22485–489. 10.1093/clinids/22.3.485 [DOI] [PubMed] [Google Scholar]


