Abstract
Introduction:
Helicobacter fennelliae is an enterohepatic Helicobacter species causing bacteraemia in immunocompromised hosts. Only a few cases of recurrent H. fennelliae bacteraemia have been reported in Japan and there are no guidelines regarding antimicrobial treatment for H. fennelliae infection.
Case presentation:
H. fennelliae bacteraemia was observed in a patient receiving platinum-based chemotherapy for lung cancer. To prevent recurrence, the patient received antibiotic therapy with cefepime, amoxicillin and doxycycline for 6 weeks, which is similar to the therapy for Helicobacter cinaedi bacteraemia. Bacteraemia recurred despite the long-term antibiotic therapy. We hypothesized that the H. fennelliae bacteraemia originated from endogenous infection in the intestinal tract due to the long-term damage of the enteric mucosa by platinum-based drugs and performed selective digestive decontamination (SDD) with kanamycin. Bacteraemia did not recur after SDD.
Conclusion:
Our observations indicate that clinicians should be aware of possible recurrent H. fennelliae bacteraemia, which could be effectively prevented by SDD with kanamycin.
Keywords: recurrent bacteremia, lung cancer, chemotherapy; Helicobacter fennelliae; selective digestive decontamination, kanamycin
Introduction
Helicobacter cinaedi and Helicobacter fennelliae are spiral-shaped Gram-negative rods that are enterohepatic Helicobacter species; they inhabit the colons of humans and animals, and cause bacteraemia in immunocompromised hosts, especially in patients with human immunodeficiency virus or haematological malignancies (Kemper et al., 1993; Kiehlbauch et al., 1995; Ng et al., 1987; Hsueh et al., 1999; Rimbara et al., 2013a, b; Saito et al., 2016). H. cinaedi and H. fennelliae also cause bacteraemia in patients with chronic renal failure, autoimmune diseases and solid organ cancers (Nishine et al., 2007; Matsumoto et al., 2007; Rimbara et al., 2013b; Saito et al., 2016). There are several reports of recurrent H. cinaedi bacteraemia in immunocompromised hosts (Sullivan et al., 1997; Mammen et al., 1995; Kikuchi et al., 2012; Uçkay et al., 2006), which is treated with prolonged antibiotic therapy to prevent further recurrence (Kiehlbauch et al., 1994; Tee et al., 1996; Sullivan et al., 1997). However, only a few cases of recurrent H. fennelliae bacteraemia have been reported in Japan (Saito et al., 2016), and there are no guidelines regarding the choice or duration of antimicrobial treatment and prevention of recurrence for H. fennelliae infection. Here, we describe a case of recurrent H. fennelliae bacteraemia, which occurred in a patient receiving platinum-based chemotherapy for solid organ cancer. The recurrence of H. fennelliae bacteraemia in this case was successfully prevented by selective digestive decontamination (SDD) with oral kanamycin.
Case report
A 73-year-old man suffering from advanced lung adenocarcinoma with multiple metastases was admitted to our hospital in Shinjuku-ku, Tokyo, for a third cycle of chemotherapy. His lung adenocarcinoma diagnosed 8 years previously had gradually progressed despite surgical treatment, chemotherapy and radiation therapy. He also had a medical history of thyroid papillary carcinoma treated by thyroidectomy 7 years previously. The patient had received two cycles of carboplatin/pemetrexed (CBDCA/PEM) therapy within the 4 months prior to this admission.
On hospital day 2, 43 days after the second cycle of chemotherapy, the patient developed a fever (38.3 °C); however, no other symptoms, such as shaking chills, diarrhoea, abdominal pain or extremity pain, were observed. His physical parameters were almost normal. Laboratory examinations showed a white blood cell count of 8640 cells µl−1, a neutrophil count of 6790 cells µl−1 and a C-reactive protein level of 16.2 mg dl−1, indicating a strong inflammatory response without neutropenia. Chest X-ray findings were the same as in previous tests. The patient was treated empirically with ampicillin/sulbactam (3 g twice a day) for 2 days without clinical improvement, and then with cefepime (2 g twice a day), after which the fever gradually subsided.
After the initiation of ampicillin/sulbactam therapy, two sets of aerobic and anaerobic blood cultures were performed using BACTEC Plus Aerobic/F culture vials and BACTEC Plus Anaerobic/F culture vials (Becton, Dickinson), respectively. On hospital day 7, one of the two aerobic cultures became positive after 6 days of incubation in BACTEC 9240 medium (Becton, Dickinson), whereas both anaerobic cultures remained negative. Gram staining of the positive culture revealed spiral-shaped Gram-negative rods. Subsequent subculturing on Nissui sheep blood agar plates and Nissui modified Skirrow’s medium EX plates (Nissui Pharmaceutical) revealed thin film-forming colonies and scanty transparent colonies, respectively, after 7 days of incubation at 35 °C in a microaerobic atmosphere (6–12 % O2, 5–8 % CO2). H. cinaedi was suspected based on the colony characteristics and the biochemical properties of the isolate were tested by the API Campy system (bioMérieux).
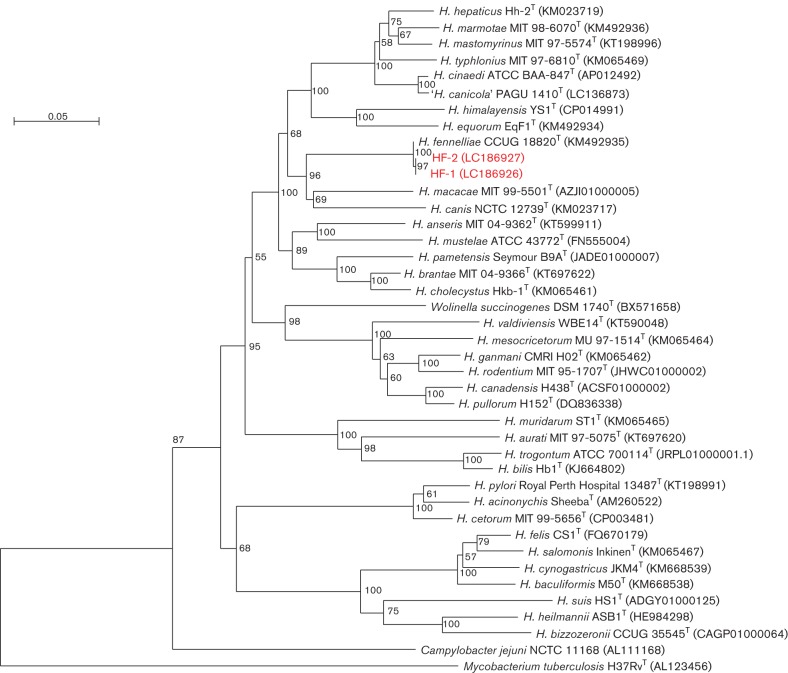
The isolate was initially identified as H. fennelliae (microcode 4401064) with a relatively low probability level of 80.2 %. However, the biochemical characteristics of nitrate reduction, alkaline phosphatase production and esterase activity were different from those of H. cinaedi. To confirm the identification, the gyrA gene of the isolate was sequenced (2450 bp) and the deduced protein sequence (811 residues) used to determine the phylogenetic relationship with the GyrA protein of H. fennelliae CCUG 18820T, whose sequence was obtained from a public database (Ménard et al., 2016; Kawamura et al., 2016). In the phylogenetic analysis based on GyrA sequences, the isolate (HF-1; accession no. LC186926) was identified as H. fennelliae (Fig. 1). Antimicrobial susceptibility was determined by the agar dilution method (Rimbara et al., 2012) Our experiments revealed low MICs of ampicillin, cefepime and doxycycline for our H. fennelliae isolate; however, the MIC of ciprofloxacin was much higher than that for H. fennelliae CCUG 18820T (Table 1), which is consistent with previous findings (Rimbara et al., 2013b).
Fig. 1.
Phylogenetic tree based on the GyrA protein sequences showing the position of our two isolates (HF-1 and HF-2) within the genus Helicobacter. GenBank/EMBL/DDBJ accession numbers and type strains are indicated. The numbers at the branching points are bootstrap percentages (based on 1000 replications). The evolutionally distances were computed using the Kimura empirical model, and the phylogenetic tree was constructed using the NJplot software. The bar represents 1 inferred amino acid substitution per 100 amino acids.
Table 1. MICs of antibiotics for H. fennelliae isolated from the lung cancer patient and the CCUG 18820 strain.
| MIC (µg ml−1) of : | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H. fennelliae isolate | Ampicillin | Amoxicillin | Cefepime | Imipenem | Kanamycin | Gentamicin | Clarithromycin | Clindamycin | Doxycycline | Minocycline | Ciprofloxacin |
| HF-1 | 0.5 | na | 1 | 0.031 | 0.5 | 0.25 | na | 0.5 | 0.25 | 0.031 | 32 |
| HF-2 | 0.5 | na | 1 | 0.031 | 0.5 | 0.25 | na | 0.5 | 0.25 | 0.031 | 32 |
| CCUG 18820* | nd | 1 | nd | 0.031 | nd | 0.5 | 0.125 | nd | nd | 0.125 | 0.5 |
na, Not attempted; nd, no data.
The patient was treated with cefepime for 15 days, and then with the oral antibiotics doxycycline (100 mg twice a day) and amoxicillin (500 mg three times a day) for 24 days. The long-term antibiotic therapy we used was similar to that used for H. cinaedi. The patient received the third cycle of chemotherapy on hospital day 15 and was discharged on hospital day 21.
After 6 weeks of oral antibiotic therapy, the patient was admitted again for a fourth cycle of CBDCA/PEM therapy. On day 7 after chemotherapy, he showed a high-grade fever (39.8 °C) and malaise with no other symptoms. Laboratory tests revealed no neutropenia. H. fennelliae bacteraemia was considered, and the patient was treated empirically with cefepime after two sets of blood cultures were performed. One aerobic blood culture was positive after 5 days of incubation, demonstrating spiral-shaped Gram-negative rods. Subcultures, phenotypic tests, gene sequence determination and phylogenetic analysis of the GyrA protein were performed as described above. This isolate (HF-2; accession no. LC186927) was identified as H. fennelliae and was the same as the HF-1 strain (Fig. 1). HF-2 showed an antimicrobial susceptibility similar to that observed in the HF-1 strain. Antibiotic therapy with cefepime continued for 14 days and the patient defervesced.
H. fennelliae bacteraemia recurred in spite of the long-term oral antibiotic therapy. The patient was still being treated with CBDCA/PEM and was at risk of recurrence. We assumed that the primary source of blood infection was the gastrointestinal (GI) tract, which is known as a common reservoir for H. cinaedi (Imafuku et al., 2016; Uçkay et al., 2006), and prescribed SDD with oral kanamycin monosulfate (500 mg four times a day) for 2 weeks. The patient did not have any further episodes of H. fennelliae bacteraemia during chemotherapy after completing the SDD.
Discussion
Here, we report a case of recurrent H. fennelliae bacteraemia during platinum-based chemotherapy. Based on the clinical course observed in the patient, we can draw three important conclusions. First, H. fennelliae bacteraemia could recur during platinum-based anticancer chemotherapy. Our patient had advanced lung adenocarcinoma, progressing despite chemotherapy. He developed a fever without neutropenia or any other symptoms 43 days after the second cycle of chemotherapy. The portal of H. fennelliae entry and the cause of bacteraemia episodes in our case were unknown. It has been reported that H. fennelliae could be recovered from stool cultures (Burnens et al., 1993; Smuts & Lastovica, 2011). CBDCA is a platinum-based anticancer chemotherapeutic agent, which sometimes causes chemotherapy-induced mucositis in the GI tract. In some cases, mucosal inflammation can persist for a long time after chemotherapy because of GI dysfunction due to enteric neuropathy associated with the platinum-based agents (Stojanovska et al., 2015). Therefore, we assumed that the H. fennelliae bacteraemia in our case originated from endogenous infection in the GI tract due to damage of the enteric mucosa caused by chemotherapy, and that persisting mucosal damage could result in the recurrence of bacteraemia.
Second, recurrent H. fennelliae bacteraemia could be prevented by SDD. We chose cefepime, amoxicillin and doxycycline for the treatment based on previous reports (Rimbara et al., 2013b; Kiehlbauch et al., 1995), and the isolate demonstrated high sensitivity to these antibiotics as evidenced by low MICs (Table 1). It should be noted that the MIC of cefepime for H. fennelliae has not been reported previously and that cefepime was clinically effective for our patient. However, H. fennelliae bacteraemia recurred despite a 6 week antibiotic therapy. Given that Helicobacter spp. had not been isolated in specimens from other patients in the same ward either before or after our patient's admission, we considered it to be a recurrence rather than nosocomial infection. Recurrent bacteraemia caused by ‘Helicobacter rappini' (now classified as Helicobacter bilis) after antibiotic therapy has been reported in a patient with X-linked (Bruton’s) agammaglobulinaemia. In addition, it was reported that in a case of recurrent H. cinaedi bacteraemia, SDD with oral kanamycin monosulfate could prevent recurrence (Imafuku et al., 2016). Given that the MICs of aminoglycosides for H. fennelliae are low (Kiehlbauch et al., 1995; Rimbara et al., 2013b; Ng et al., 1987) and that the MIC of kanamycin for our isolate was 0.5 µg ml−1, we prescribed oral kanamycin. Following SDD, no side effects, including renal dysfunction, were observed and bacteraemia did not recur despite repeated courses of chemotherapy. As kanamycin is rarely absorbed from the intestinal tract (Hewitt & Finegold, 1958), this observation also indicates that the infection originated from the GI system. Our results suggest that SDD with oral kanamycin can be used to prevent the recurrence of H. fennelliae bacteraemia. However, this is, to the best of our knowledge, the first case report of SDD used to treat recurrent H. fennelliae bacteraemia and large-scale studies are required to comprehensively investigate the application of kanamycin for SDD in such situations.
Third, fluoroquinolones may be clinically less effective for H. fennelliae infection. No standardized antimicrobial susceptibility tests are currently available for H. cinaedi and H. fennelliae. A recent study has examined a broth microdilution method, which revealed high MICs of fluoroquinolones for H. cinaedi isolated in Japan (Tomida et al., 2013). Although ciprofloxacin demonstrated low MICs for H. fennelliae CCUG 18820T and another previously described isolate (Hsueh et al., 1999), Rimbara et al. (2013b) have reported high MICs of ciprofloxacin for Japanese patients infected with H. fennelliae, which is consistent with our case and may be attributed to gene mutations. Although antimicrobial susceptibility breakpoints for H. fennelliae are yet to be determined, our isolates may be considered resistant to ciprofloxacin (Mégraud & Lehours, 2007), suggesting that at least in Japanese patients infected with H. fennelliae, fluoroquinolones, including ciprofloxacin, should be avoided.
In conclusion, our report describes a case of recurrent H. fennelliae bacteraemia in a patient with solid organ cancer undergoing platinum-based chemotherapy. Our observations indicate that clinicians should be aware of possible recurrent H. fennelliae bacteraemia, which could be effectively prevented by SDD with kanamycin.
Acknowledgements
We thank Hideki Araoka, Toranomon Hospital in Tokyo, Japan, for the consultation regarding SDD therapy. This work was supported by grants from the National Center for Global Health and Medicine (25–114 and 26-101). This report is in accordance with the Declaration of Helsinki (1975, as revised in 2008) and the regulations of the Japanese Ministry of Health, Labour and Welfare.
Abbreviations:
- CBDCA
carboplatin
- GI
gastrointestinal
- PEM
pemetrexed
- SDD
selective digestive decontamination
References
- Burnens A. P., Stanley J., Schaad U. B., Nicolet J.(1993). Novel campylobacter-like organism resembling Helicobacter fennelliae isolated from a boy with gastroenteritis and from dogs. J Clin Microbiol 311916–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt W. L., Finegold S. M.(1958). Laboratory studies with kanamycin. Ann N Y Acad 76122–128. [DOI] [PubMed] [Google Scholar]
- Hsueh P. R., Teng L. J., Hung C. C., Chen Y. C., Yang P. C., Ho S. W., Luh K. T.(1999). Septic shock due to Helicobacter fennelliae in a non-human immunodeficiency virus-infected heterosexual patient. J Clin Microbiol 372084–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imafuku A., Araoka H., Tanaka K., Marui Y., Sawa N., Ubara Y., Takaichi K., Ishii Y., Tomikawa S.(2016). Helicobacter cinaedi bacteremia in four renal transplant patients: clinical features and an important suggestion regarding the route of infection. Transpl Infect Dis 18132–136. 10.1111/tid.12480 [DOI] [PubMed] [Google Scholar]
- Kawamura Y., Tomida J., Miyoshi-Akiyama T., Okamoto T., Narita M., Hashimoto K., Cnockaert M., Vandamme P., Morita Y., et al. (2016). Proposal of Helicobacter canicola sp. nov., previously identified as Helicobacter cinaedi, isolated from canines. Syst Appl Microbiol 39307–312. 10.1016/j.syapm.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Kemper C. A., Mickelsen P., Morton A., Walton B., Deresinski S. C.(1993). Helicobacter (Campylobacter) fennelliae-like organisms as an important but occult cause of bacteraemia in a patient with AIDS. J Infect 2697–101. 10.1016/0163-4453(93)97128-K [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Tauxe R. V., Baker C. N., Wachsmuth I. K.(1994). Helicobacter cinaedi-associated bacteremia and cellulitis in immunocompromised patients. Ann Intern Med 12190–93. 10.7326/0003-4819-121-2-199407150-00002 [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Brenner D. J., Cameron D. N., Steigerwalt A. G., Makowski J. M., Baker C. N., Patton C. M., Wachsmuth I. K.(1995). Genotypic and phenotypic characterization of Helicobacter cinaedi and Helicobacter fennelliae strains isolated from humans and animals. J Clin Microbiol 332940–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Asako K., Tansho S., Ueda T., Koshio O., Ubagai T., Asahara M., Kawakami S., Ono Y.(2012). Recurrent Helicobacter cinaedi cellulitis and bacteremia in a patient with systemic lupus erythematosus. Intern Med 513185–3188. 10.2169/internalmedicine.51.8149 [DOI] [PubMed] [Google Scholar]
- Mammen M. P., Aronson N. E., Edenfield W. J., Endy T. P.(1995). Recurrent Helicobacter cinaedi bacteremia in a patient infected with human immunodeficiency virus: case report. Clin Infect Dis 211055– 1056. 10.1093/clinids/21.4.1055 [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Goto M., Murakami H., Tanaka T., Nishiyama H., Ono E., Okada C., Sawabe E., Yagoshi M., et al. (2007). Multicenter study to evaluate bloodstream infection by Helicobacter cinaedi in Japan. J Clin Microbiol 452853–2857. 10.1128/JCM.00465-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégraud F., Lehours P.(2007). Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 20280–322. 10.1128/CMR.00033-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard A., Buissonnière A., Prouzet-Mauléon V., Sifré E., Mégraud F.(2016). The GyrA encoded gene: a pertinent marker for the phylogenetic revision of Helicobacter genus. Syst Appl Microbiol 3977–87. 10.1016/j.syapm.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Ng V. L., Hadley W. K., Fennell C. L., Flores B. M., Stamm W. E.(1987). Successive bacteremias with "Campylobacter cinaedi" and "Campylobacter fennelliae" in a bisexual male. J Clin Microbiol 252008–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishine H., Kasai S., Yoshikawa M., Otsuka Y., Tokuda H.(2007). [A case of recurrent Helicobacter cinaedi-associated bacteremia in a small cell lung cancer patient during chemotherapy]. Nihon Kokyuki Gakkai Zasshi 4526–30 [in Japanese]. [PubMed] [Google Scholar]
- Rimbara E., Mori S., Matsui M., Suzuki S., Wachino J., Kawamura Y., Shen Z., Fox J. G., Shibayama K.(2012). Molecular epidemiologic analysis and antimicrobial resistance of Helicobacter cinaedi isolated from seven hospitals in Japan. J Clin Microbiol 502553–2560. 10.1128/JCM.06810-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbara E., Matsui M., Mori S., Suzuki S., Suzuki M., Kim H., Sekizuka T., Kuroda M., Shibayama K.(2013a). Draft genome sequence of Helicobacter fennelliae strain MRY12-0050, isolated from a bacteremia patient. Genome Announc 1e00512-13 10.1128/genomeA.00512-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbara E., Mori S., Kim H., Matsui M., Suzuki S., Takahashi S., Yamamoto S., Mukai M., Shibayama K.(2013b). Helicobacter cinaedi and Helicobacter fennelliae transmission in a hospital from 2008 to 2012. J Clin Microbiol 512439–2442. 10.1128/JCM.01035-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S., Tsukahara M., Ohkusu K., Kurai H.(2016). Helicobacter fennelliae bacteremia: three case reports and literature review. Medicine 95e3556. 10.1097/MD.0000000000003556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts H. E., Lastovica A. J.(2011). Molecular characterization of the 16S rRNA gene of Helicobacter fennelliae isolated from stools and blood cultures from paediatric patients in South Africa. J Pathog 2011217376. 10.4061/2011/217376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovska V., Sakkal S., Nurgali K.(2015). Platinum-based chemotherapy: gastrointestinal immunomodulation and enteric nervous system toxicity. Am J Physiol Gastrointest Liver Physiol 308G223–G232. 10.1152/ajpgi.00212.2014 [DOI] [PubMed] [Google Scholar]
- Sullivan A. K., Nelson M. R., Walsh J., Gazzard B. G.(1997). Recurrent Helicobacter cinaedi cellulitis and bacteraemia in a patient with HIV Infection. Int J STD AIDS 859–60. 10.1258/0956462971918634 [DOI] [PubMed] [Google Scholar]
- Tee W., Street A. C., Spelman D., Munckhof W., Mijch A.(1996). Helicobacter cinaedi bacteraemia: varied clinical manifestations in three homosexual males. Scand J Infect Dis 28199–203. 10.3109/00365549609049078 [DOI] [PubMed] [Google Scholar]
- Tomida J., Oumi A., Okamoto T., Morita Y., Okayama A., Misawa N., Hayashi T., Akaike T., Kawamura Y.(2013). Comparative evaluation of agar dilution and broth microdilution methods for antibiotic susceptibility testing of Helicobacter cinaedi. Microbiol Immunol 57353–358. 10.1111/1348-0421.12044 [DOI] [PubMed] [Google Scholar]
- Uçkay I., Garbino J., Dietrich P. Y., Ninet B., Rohner P., Jacomo V.(2006). Recurrent bacteremia with Helicobacter cinaedi: case report and review of the literature. BMC Infect Dis 686. 10.1186/1471-2334-6-86 [DOI] [PMC free article] [PubMed] [Google Scholar]