Abstract
The Ranunculaceae genus Anemone (order Ranunculales), comprising more than 150 species, mostly herbs, has long been used in folk medicine and worldwide ethnomedicine. Various medicinal compounds have been found in Anemone plants, especially triterpenoid saponins, some of which have shown anti-cancer activities. Some Anemone compounds and extracts display immunomodulatory, anti-inflammatory, antioxidant, and antimicrobial activities. More than 50 species have ethnopharmacological uses, which provide clues for modern drug discovery. Anemone compounds exert anticancer and other bioactivities via multiple pathways. However, a comprehensive review of the Anemone medicinal resources is lacking. We here summarize the ethnomedical knowledge and recent progress on the chemical and pharmacological diversity of Anemone medicinal plants, as well as the emerging molecular mechanisms and functions of these medicinal compounds. The phylogenetic relationships of Anemone species were reconstructed based on nuclear ITS and chloroplast markers. The molecular phylogeny is largely congruent with the morphology-based classification. Commonly used medicinal herbs are distributed in each subgenus and section, and chemical and biological studies of more unexplored taxa are warranted. Gene expression profiling and relevant “omics” platforms could reveal differential effects of phytometabolites. Genomics, transcriptomics, proteomics, and metabolomics should be highlighted in deciphering novel therapeutic mechanisms and utilities of Anemone phytometabolites.
KEY WORDS: Anemone, Ethnomedicine, Chemodiversity, Bioactivity, Molecular mechanism, Pharmacophylogeny
Graphical abstract
The Ranunculaceae genus Anemone (order Ranunculales), comprising more than 150 species, mostly herbs, has long been used in folk medicine and worldwide ethnomedicine. Various medicinal compounds have been found in Anemone plants, especially triterpenoid saponins, some of which have shown anti-cancer activities. This paper summarizes ethnomedicine knowledge and recent progress of chemical and pharmacological diversity of Anemone medicinal plants, as well as emerging molecular machineries and functions of medicinal compounds.
1. Introduction
Anemone is a genus of more than 150 species of flowering plants in the family Ranunculaceae, native to the temperate zones of both Northern and Southern hemispheres. It is closely related to Pulsatilla, Clematis, and Hepatica morphologically and phytochemically1. More than 50 species have ethnopharmacological uses, which provide clues for modern drug discovery. Some traditional claims of Anemone species have been validated scientifically by pre-clinical and clinical studies2, 3. The state-of-the-art methods have been adopted to isolate bioactive chemical constituents from Anemone species following bioactivity-directed fractionation4, 5. Some modes of action of bioactive extracts or compounds of Anemone species have been established6, 7. However, a comprehensive review of the Anemone medicinal resources is lacking. In the present article, we summarize the ethnomedical knowledge and the recent progress in uncovering the chemical and pharmacological diversity of Anemone medicinal plants, as well as the emerging molecular machineries and functions of these medicinal compounds. Gaps are also pointed out and further work is suggested.
Exhaustive literature search in NCBI PubMed, Google, Bing, and CNKI (http://cnki.net/) has been performed to outline the progress of Anemone research during the last three decades. Search terms “anti-cancer”, “anti-inflammatory”, “antioxidant”, “saponin”, “triterpene”, “polysaccharide”, etc. were used, combined with “Anemone” and the names of species.
2. Ethnopharmacology
More than 50 Anemone species are used in various traditional medical systems (Table 1, Fig. 1). Fifty-three species, 9 subspecies and 36 varieties are found in China, which are distributed in most provinces except Guangdong and Hainan. According to our field survey, at least 38 species/varieties have ethnopharmacological uses. In traditional Chinese medicine (TCM) and folk medicine, Anemone is used in heat-clearing and detoxification [traditional remedy index (TRI) 424, distribution density of ethnopharmacological use (β) 30], wind-dispersing and damp-eliminating (TRI 476, β 35), warming and orifice-opening (TRI 700, β 15), pesticide (TRI 400, β 30), dysentery (TRI 1 051, β 46), malaria (TRI 356, β 30), tinea (TRI 445, β 46), ulcers and sores (TRI 1 932, β 84), arthritis (TRI 896, β 76), traumatic injury (TRI 930, β 53), pharyngolaryngitis (TRI 327, β 7), parasitic disease (TRI 424, β 30), and hepatitis (TRI 445, β 7)8. Correspondingly, a broad spectrum of pharmacological activities, including antitumor, antimicrobial, anti-inflammatory, sedative and analgesic activities, and anti-convulsant and anti-histamine effects have been observed9. For instance, Anemone raddeana, distributed in Far East, is commonly used in Northeast China for rheumatism, arthritis, and skin infection, etc. Liang Tou Jian (Zhu Jie Xiang Fu in Chinese), the rhizome of A. raddeana, is recorded in China Pharmacopoeia 2015 version. Anemone altaica, extensively distributed in Europe and Northern Asia, is used in Northwest China for epilepsy, neurosis, and rheumatic arthralgia. The rhizome of A.altaica is sometimes called “Jiu Jie Chang Pu”, but actually “Jiu Jie Chang Pu” originally referred to the rhizome of Acorus tatarinowii (Araceae), which has distinct therapeutic components10. Anemone vitifolia (wild cotton) of the pan-Himalaya region is used in Southwest China for traumatic injury, rheumatic arthralgia, enteritis, dysentery, and ascariasis, etc.
Table 1.
Ethnopharmacological uses of Anemone species.
| Species | Medicinal part | Therapeutic efficacy | Distribution | Note |
|---|---|---|---|---|
| A. altaica | Rhizome | Tranquilizing, orifice-opening, wind-expelling, damp-eliminating, detoxifying, pain-relieving; high fever, delirium, epilepsy, deafness with qi stagnation, dreaminess forgetfulness, chest tightness, abdominal distension, anorexia, rheumatism pain, ulcer, scabies | Europe; North Asia; China (Hubei, Henan, Shanxi, Shaanxi, Chongqing) | |
| A. amurensis | Whole plant, rhizome | Diaphoresis, liver/kidney tonifying (whole plant); Korean medicine (paralysis, menoxenia, stomachache, pertussis (rhizome) | Russia Far East; North Korea; China (Liaoning, Jilin, Heilongjiang) | |
| A. anhuiensis | Rhizome | Traumatic injury, rheumatic arthritis | China (Anhui) | |
| A. baicalensis | Leaf | Detoxifying, vermifuge | Siberia; Korea; China (Sichuan, Gansu, Shaanxi, Qinling Mountains, Liaoning, Jilin, Heilongjiang) | |
| A. begoniifolia | Whole plant | Wind-expelling, damp-eliminating, detoxification, pain-relieving; rheumatism, urticaria, carbuncle sore | China (Yunnan, Guangxi, Guizhou, Sichuan, Chongqing) | |
| A. biflora | Bulb | Styptic, antiphlogistic; boils, burns, cuts and wounds | Kashmir Himalaya | |
| A. canadensis | Root, leaf | Anthelmintic, antiaphonic, antiseptic, astringent, ophthalmic, styptic; pain in the lumbar region, crossed eyes, twitches and eye poisoning, wounds, nose bleed, sore, headache and dizziness, clear the throat | Eastern and Central North America | |
| A. cathayensis | Rhizome | Cancer, inflammation, analgesic, convulsion | Korea; China (Shanxi, Hebei) | |
| A. chosenicola var. schantungensis | Root | Styptic, damp-eliminating, heat-clearing, detoxification | China (Shandong) | |
| A. cylindrica | Root, leaf, stem, fruit | Antiseptic; sore eyes (stem, fruit); headache, dizziness, wounds (root); burns (leaf) | Western North America | |
| A. davidii | Rhizome | Blood-activating, pain-relieving, subduing swelling, detoxicating; traumatic injury, arthritis pain, lumbar muscle strain; Tujia medicine (arthritis pain, intercostal neuralgia, traumatic injury, hematemesis, hemafecia) | China (Chongqing, Tibet, Yunnan, Sichuan, Guizhou, Hunan, Hubei) | |
| A. delavayi | Rhizome | Blood-activating, stasis-scattering, tonifying kidney | China (Yunnan, Sichuan) | |
| A. demissa | Root, fruit, whole plant | Whole plant: rheumatism, dysentery, help digestion, dyspepsia, gonorrhea, wind-cold-dampness arthralgia, joint yellow water; fruit: damp-clearing, mass-scattering, detoxifying, all kinds of cold, lump boil, snakebite | Himalayas; China (QTP East margin) | Tibetan medicine |
| A. demissa var. major | Root, fruit, whole plant | Whole plant: rheumatism, dysentery, help digestion, dyspepsia, gonorrhea, wind-cold-dampness arthralgia, joint yellow water; fruit: damp-clearing, mass-scattering, detoxifying, all kinds of cold, lump boil, snakebite | China (QTP East margin) | Tibetan medicine |
| A. demissa var. villosissima | Root, fruit, whole plant | Whole plant: rheumatism, dysentery, help digestion, dyspepsia, gonorrhea, wind-cold-dampness arthralgia, joint yellow water; fruit: damp-clearing, mass-scattering, detoxifying, all kinds of cold, lump boil, snakebite | Himalayas; China (QTP East margin) | Tibetan medicine |
| A. dichotoma | Rhizome | Muscle-relaxing, blood-activating, heat-clearing, detoxification, traumatic injury, dysentery, rheumatoid joint pain; skin ulcer; sore throat, cough with copious phlegm, lymphnoditis | North Asia; Europe; China (Jilin, Heilongjiang) | |
| A. drummondii | Root, seed | Abrasions, toothed ache, rheumatism; antibacterial; sex related difficulties; melancholy (root); headache (seed) | Western North America | |
| A. flaccida | Rhizome | Wind-expelling, dampness-eliminating, muscle-relaxing, blood-activating; traumatic injury, arthritis pain, lumbar muscle strain | Japan; Russia Far East; China (Yunnan, Sichuan, Guizhou, Hubei, Chongqing, Hunan, Jiangxi, Zhejiang, Jiangsu, Shaanxi, Gansu) | |
| A. flaccida var. hofengensis | Rhizome | Wind-expelling, dampness-eliminating, muscle-relaxing, blood-activating; traumatic injury, arthritis pain, lumbar muscle strain | China (Chongqing) | |
| A. fulingensis | Rhizome | Wind-expelling, dampness-eliminating, muscle-relaxing, blood-activating; traumatic injury, arthritis pain, lumbar muscle strain | China (Chongqing) | |
| A. griffithii | Rhizome, seed | Blood-activating, pain-relieving, subduing swelling, detoxicating; traumatic injury, arthritis pain, lumbar muscle strain; Tibet medicine (stomach worms, sharp pain, snakebite, cold tumor, gonorrhoea, joint yellow water (seed) | Sikkim; Bhutan; Nepal; China (Tibet, Sichuan, Chongqing) | Tibetan medicine |
| A. hupehensis | Rhizome, root, stem, leaf, whole plant | Heat-clearing, diuresis, detoxification, vermifuge, stasis-scattering, detumescence; dysentery, mallnutrition and indigestion of children, malaria, acute jaundice hepatitis, ascariasis, furuncle carbuncle, scrofula, traumatic injury | China (Chongqing, Southern Shaanxi, Gansu, Zhejiang, Jiangxi, Western Hubei, Northern Guangdong, Northern Guangxi, Sichuan, Guizhou, Eastern Yunnan) | |
| A. hupehensis f. alba | Rhizome | Heat-clearing, diuresis, detoxification, vermifuge, stasis-scattering, detumescence; dysentery, mallnutrition and indigestion of children, malaria, acute jaundice hepatitis, ascariasis, furuncle carbuncle, scrofula, traumatic injury | China (Chongqing) | |
| A. hupehensis var. japonica | Rhizome | Heat-clearing, diuresis, detoxification, vermifuge, stasis-scattering, detumescence; dysentery, mallnutrition and indigestion of children, malaria, acute jaundice hepatitis, ascariasis, furuncle carbuncle, scrofula, traumatic injury | China (Chongqing) | |
| A. imbricata | Root, fruit, whole plant, flower, stem, leaf, seed | Whole plant: expelling wind-damp, dysentery, help digestion, gonorrhea, wind-cold-dampness arthralgia, joint yellow water; fruit: damp-expelling, mass-eliminating, detoxifying, all kinds of cold, lump boil, snakebite; stomach worms, sharp pain, snakebite, cold tumor, gonorrhoea, joint yellow water (seed); gonorrhoea, joint yellow water, hypothermia, emetic (leaf); anti-inflammatory, burn (stem, leaf and flower) | China (QTP East margin) | Tibetan medicine |
| A. multifida | Root, seed | Abrasions, toothed ache, rheumatism; antibacterial; sex related difficulties; melancholy (root); headache (seed) | Central and Western North America | |
| A. narcissiflora | Leaf, root, seed | Abrasions, toothache, rheumatism; antibacterial; sex related difficulties; melancholy (root); headache (seed) | Europe; Asia; North America | |
| A. nemorosa | Various parts | Headaches, tertian agues and rheumatic gout (various parts), leprosy (leaf), bring away watery and phlegmatic humours (root), lethargy, eye inflammation, malignant and corroding ulcers (root) | UK; Europe; West Asia | |
| A. nikoensis | Leaf | Edible use | Japan | |
| A. obtusiloba | Seed, aboveground part, root, fruit | Diuresis detumescence, enriching blood, warming body, wound healing, pus drainage; antirheumatic; emetic (seed); ophthalmic; rubefacient; contusion (root); ill health, hypothermia, sore throat, chronic bronchitis, tonsillitis, hepatitis, gastric disease, dysentery, gonorrhea, arthritis pain, peripheral nerve paralysis, snakebite, stubborn dermatitis, impetigo, joint yellow water | Himalayas; China (QTP East margin) | Tibetan medicine |
| A. obtusiloba ssp. ovalifolia | Whole plant, aboveground part, root, fruit | Diuresis detumescence, enriching blood, warming body, wound healing, pus drainage; styptic (whole plant), ill health, hypothermia, sore throat, chronic bronchitis, tonsillitis, hepatitis, gastric disease, dysentery, gonorrhea, arthritis pain, peripheral nerve paralysis, snakebite, stubborn dermatitis, impetigo, joint yellow water | China (Taibai mountain, QTP East margin) | Tibetan medicine |
| A. parviflora | Root, seed | Abrasions, toothache, rheumatism; antibacterial; sex related difficulties; melancholy (root); headache (seed) | North America | |
| A. parviflora (A. pulsatilla) | Whole plant | Diaphoretic, diuretic, nervine, rubefacient; eye ailments, earache, stress, anxiety, tension, skin eruptions, rheumatism, leukorrhea, obstructed menses, bronchitis, coughs, asthma | UK; Europe | |
| A. quinquefolia | Root | Rubefacient; rheumatism, gout, fever; vesicant, corns | Eastern North America | |
| A. raddeana | Rhizome | Mongolian medicine: rheumatism, low back and leg pain, phlebitis; subduing inflammation; arthralgia, chill cold, cough with copious phlegm, joint pain | China (Northeastern Shandong, Liaoning, Jilin, Heilongjiang); Korea; Russia Far East | Chinese Pharmacopoeia |
| A. reflexa | Rhizome | Open the orifices with aroma, wind-expelling, dampness-eliminating, appetite-stimulating; high fever, delirium, epilepsy, deafness with qi stagnation, dreaminess forgetfulness, chest tightness, abdominal distension, anorexia, rheumatism pain, ulcer, scabies | North Korea; Siberia; Eastern Europe; China (Shaanxi, Eastern Jilin, Taibai mountain) | |
| A. rivularis | Rhizome, leaf, seed; aboveground part, root, fruit | Heat-clearing, detoxification, blood-activating, muscle-relaxing, swell-dispersing, pain-relieving; diuresis detumescence, enriching blood, warming body, wound healing, pus drainage; mumps, scrofula, carbuncle, malaria, cough, jaundice, arthritis pain, traumatic injury, stomachache, toothache; ill health, hypothermia, sore throat, chronic bronchitis, tonsillitis, hepatitis, gastric disease, dysentery, gonorrhea, arthritis pain, peripheral nerve paralysis, snakebite, stubborn dermatitis, impetigo, joint yellow water | China (Chongqing, Tibet, QTP East margin); Himalayas; Sri Lanka | Tibetan medicine |
| A. rivularis var. flore-minore | Rhizome | Heat-clearing, detoxification, blood-activating, muscle-relaxing, swell-dispersing, pain-relieving; sore throat, mumps, scrofula, carbuncle, malaria, cough, jaundice, arthritis pain, traumatic injury, stomachache, toothache | China (Chongqing) | |
| A. rockii var. pilocarpa | Rhizome | Wind-expelling, dampness-removing, muscle-relaxing, blood-activating; arthritis pain, traumatic injury | China (Chongqing) | |
| A. rupicola | Seed | Stomach worms, sharp pain, snakebite, cold tumor, gonorrhoea, joint yellow water | China (Northwestern Yunnan, Western Sichuan, Southeastern and Southern Tibet); Bhutan; Nepal; Northern India | Tibetan medicine |
| A. silvestris | Rhizome | Relieving oppression and masses, pus drainage, rot-eliminating, insecticide | Europe; Asia; China (Liaoning, Hebei, Heilongjiang, Jilin, Xinjiang, Inner Mongolia) | Mongolian medicine “Xiriwusu” |
| A. stolonifera | Leaf, stem | Edible use | China; Japan | |
| A. taipaiensis | Rhizome | Cancer | China (Taibai mountain) | |
| A. tetrasepala | Seed | Stomach worms, sharp pain, snakebite, cold tumor, gonorrhoea, joint yellow water | China (Southern Tibet); Kashmir; Afghanistan | Tibetan medicine |
| A. tibetica | Seed | Stomach worms, sharp pain, snakebite, cold tumor, gonorrhoea, joint yellow water | China (Tibet) | Tibetan medicine |
| A. tomentosa | Rhizome | Dissipating phlegm stasis, relieving dyspepsia, detoxification, vermifuge; eparsalgia cough, traumatic injury, mallnutrition and indigestion of children, malaria, dysentery, sore furuncle carbuncle, stubborn dermatitis | China (Chongqing, Sichuan, Gansu, Henan, Shanxi) | |
| A. trullifolia | Root, flower | Muscle-relaxing, blood-activating, antitussive; chronic bronchitis, peripheral nerve paralysis, neuralgia, tendon complex pain | China (QTP East margin, Southern Tibet); Sikkim; Bhutan | Tibetan medicine |
| A. trullifolia var. linearis | Root, flower | Muscle-relaxing, blood-activating, antitussive; chronic bronchitis, peripheral nerve paralysis, neuralgia, tendon complex pain | China (QTP East margin) | Tibetan medicine |
| A. tuberosa | Anxiolytic | Southwest America | ||
| A. virginiana | Root, seed | Astringent; emetic; expectorant; TB, whooping cough, diarrhea; boils | Central and Eastern North America | |
| A. vitifolia | Root, leaf, rhizome | Traumatic injury, rheumatic arthralgia, enteritis, dysentery, ascariasis (rhizome); antirheumatic and vermifuge, dysentery, relieve tooth pain and headache, scabies (root), head lice (leaf) | Europe; Himalayas |
Figure 1.
(A) Habitat of Anemone; (B) whole plant and flower of Anemone rivularis, taken in the Alpine Botanic Garden of Shangri-La, Yunnan, China; (C) fruit of Anemone, taken by the side of the Ni Yang river, Tibet, China.
In Southwest China, Anemone has been undergoing rapid diversification following the uplift of Qinghai Tibet Plateau in the Quaternary Period and the emergence of “sky islands”11. Many alpine Anemone species are used in Tibetan medicine (Table 1)12, 13. For instance, Suga is the ripe seed of Anemone rivularis, A. rivularis var. floreminore, A. obtusiloba, A. obtusiloba ssp. ovalifolia, and A. demissa, and is used in diuresis, detumescence, enriching blood, warming the body, wound healing, and pus drainage13, 14. Burchin, the root and flower of Anemone trullifolia and A. trullifolia var. linearis, is used for muscle-relaxation, blood-activation, and as an antitussive. Suga Angbo, the root, fruit, and whole plant of A.demissa, A. demissa var. major, A. demissa var. villosissima, and A. imbricata, is efficient in anti-rheumatism, helping digestion, dysentery (whole plant), eliminating chill and dampness, dissolving masses, and detoxification (fruit).
Various Anemone species are also used in the ethnomedicine of India, Korea, Mongolia, America, and Europe, etc. (Table 1). For instance, Anemone biflora bulb is styptic and is applied on boils, burns, cuts and wounds as an antiphlogistic15. The root and leaves of Anemone canadensis was one of the most highly esteemed medicines of the Omaha and Ponca Indians (http://plants.for9.net/edible-and-medicinal-plants). A decoction of the root was used as an anthelmintic and to treat pain in the lumbar region. An infusion of the root was used as an eye wash to treat crossed eyes, twitches and eye poisoning. In Korean medicine, the rhizome of Anemone amurensis is useful in paralysis, menoxenia, stomach ache, and pertussis16. Anemone stolonifera and A. nikoensis are for edible use in East Asia.
3. Phytochemical components
Identified Anemone compounds include triterpenoids, saponins, steroids, lactones, fats and oils, saccharides, and alkaloids, etc9, 17, 18. Oleanolic acid triterpene saponin is abundant in Anemone species (Supplementary Table S1). Anemone contains ranunculin, anemonin, and protoanemonin, which are characteristic constituents of Pulsatilla and illustrate the close relationship between these two genera. Anemone also contains coumarins and flavonoids.
3.1. Saponin
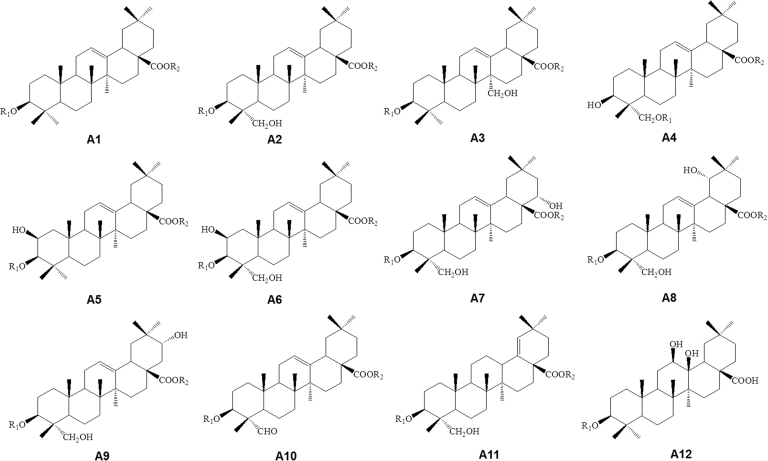
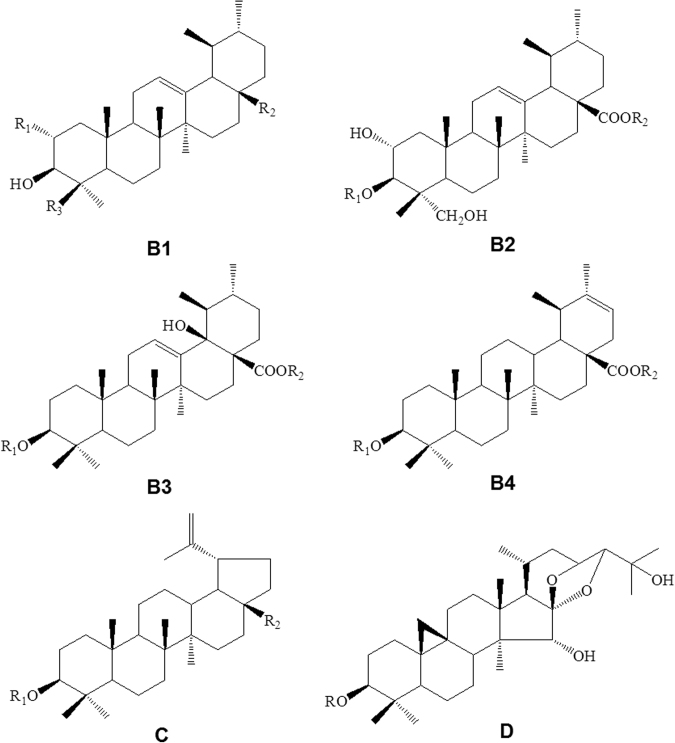
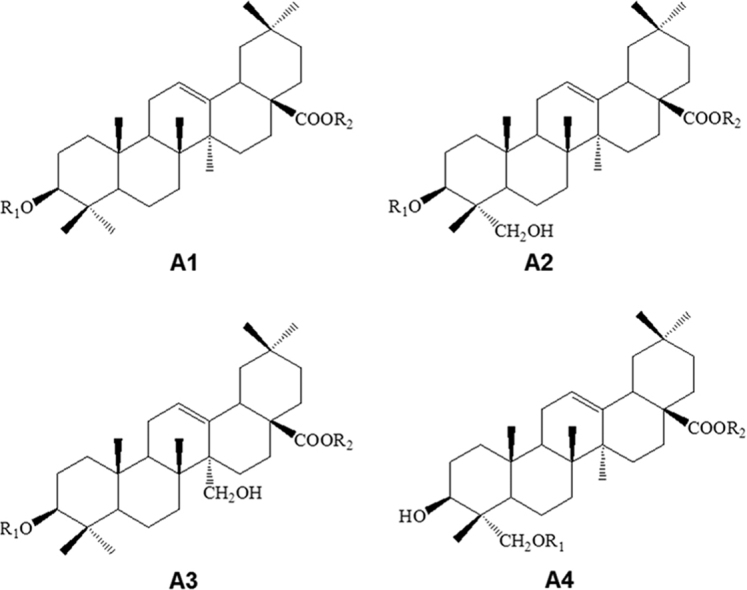
Saponins are abundant in Ranunculaceae, especially in Clematis, Pulsatilla, Anemone, and Cimicifugeae1, 19, 20, 21, which usually exert anticancer activity via cell cycle arrest and apoptosis induction. The aglycones of Clematis pentacyclic triterpene saponins mainly belong to oleanolic type (A), olean-3β, 28-diol type (B), hederagenin type (C) or hederagenin-11,13-dien type (D), where types A and C are predominant20. In Anemone, A type (Fig. 2 and Supplementary Table S1) is predominant, and ursane-type triterpenoids (B type), lupane-type triterpenoids (C type), and cycloartane-type tetracyclic triterpenoids (D type) are also present (Fig. 3 and Supplementary Table S2).
Figure 2.
Basic skeletons of oleanane-type triterpenoids (A type) from Anemone species.
Figure 3.
Basic skeletons of other type triterpenoids (B and C types) from Anemone species.
3.2. Essential oil, volatile compounds and others
Nineteen essential oil compounds were identified from the roots of A. rivularis, representing 96.1% of the total oil22 (Figure 4, Figure 5, and Supplementary Table S3). The major constituents were acetophenone (55.9%), 3-ethyl-2-methyl-hexane (16.2%), 5,6-dimethyl-decane (5.9%) and 4,5-diethyl-octane (4.4%).
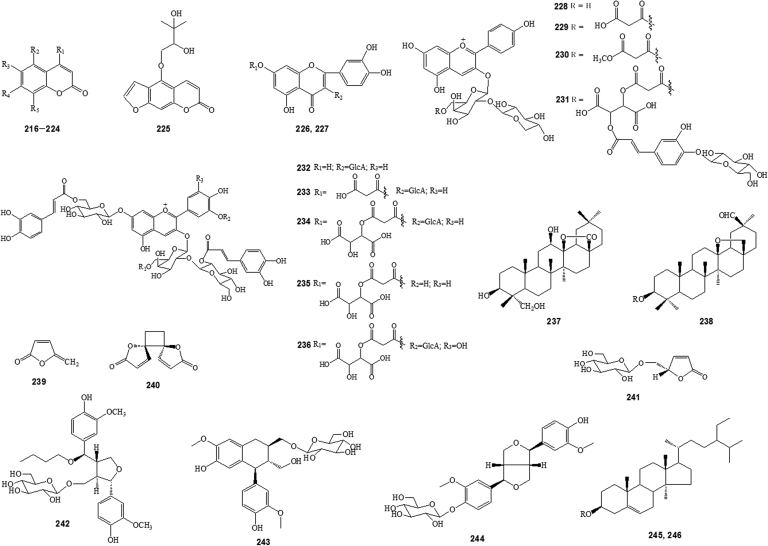
Figure 4.
Constituents isolated from Anemone species (See substituent groups Rn in Supplementary Table S3).
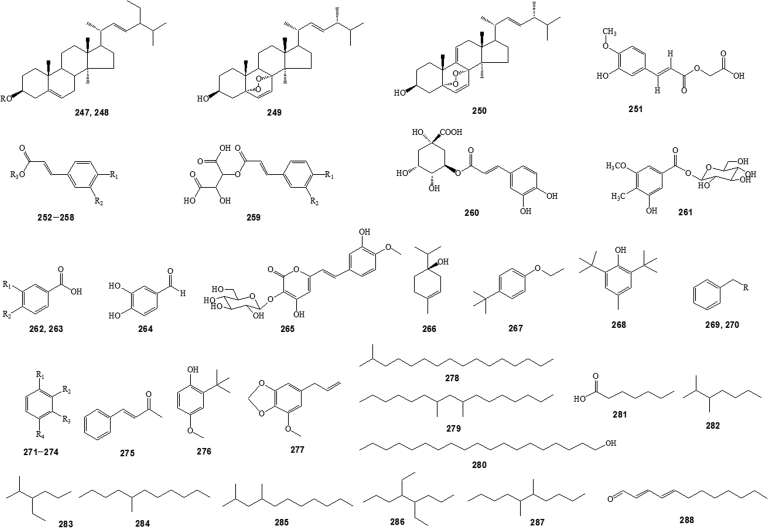
Figure 5.
Other constituents isolated from Anemone species (See substituent groups Rn in Supplementary Table S3).
The dominant benzenoid compounds in A. sylvestris anther were 2-phenylethanol and phenylacetaldehyde23. Other abundant compound classes in A. sylvestris were fatty acid derivatives (41.8%), especially pentadecane and nonanal, and sesquiterpenoids (8.0%), e.g., (E,E)-α-farnesene. Relatively low amounts of the repellent protoanemonin were found in A. sylvestris.
Coumarins, flavonoids, lactones, lignans, steroids, phenolic compounds, and other compounds are also detected (Figure 4, Figure 5, and Supplementary Table S3).
4. Bioactivities
4.1. Anticancer activity: cell death pathways and anticancer targets
The genus Anemone, evolutionarily closely related to Pulsatilla, is also rich in therapeutic saponins1. Raddeanin A, a pentacyclic triterpene saponin from A. raddeana (Liang Tou Jian in TCM), inhibits proliferation and induces apoptosis of multiple cancer cell lines7, 24, 25. Raddeanin A increased BAX expression, reduced BCL-2, BCL-xL and survivin expression, and significantly activated caspase-3, caspase-8, caspase-9 and poly-ADP ribose polymerase (PARP)25. Saponins B, 1, and 6 of A. taipaiensis exhibit significant anticancer activity against human leukemia, glioblastoma multiforme (GBM), and HCC26, 27, 28, 29, 30, 31. Saponin 1 caused characteristic apoptotic morphological changes in GBM cells26, which was confirmed by DNA ladder electrophoresis and flow cytometry. Saponon 1 also caused a time-dependent decrease in the expression and nuclear location of NF-κB. The expression of inhibitors of apoptosis (IAP) family members, e.g., survivin and XIAP, was significantly decreased by saponin 1. Moreover, saponin 1 caused a decrease in the BCL-2/BAX ratio and initiated apoptosis by activating caspase-9 and caspase-3 in the GBM cell lines. Thus, saponin 1 inhibits cell growth of GBM cells at least partially by inducing apoptosis and inhibiting survival signaling mediated by NF-κB.
Saponin B blocked the cell cycle at the S phase31. Saponin B induced chromatin condensation of U87MG GBM cells and led to the formation of apoptotic bodies. Annexin V/PI assay suggested that phosphatidylserine (PS) externalization was apparent at higher drug concentrations. Saponins B and 6 activated the receptor-mediated pathway of apoptosis via the activation of FAS-l28. These saponins increased the BAX and caspase-3 ratio and decreased the protein expression of BCL-2.
Triterpenoid saponins of A. flaccida induce apoptosis in human BEL-7402, HepG2 hepatoma cell lines, and lipopolysaccharide (LPS) stimulated HeLa cells via COX-2/PGE2 pathway32. Flaccidoside II, one of the triterpenoid saponins of A. flaccida, induced apoptosis by downregulating heme oxygenase (HO)-1 via extracellular signal-regulated kinase (ERK)-1/2 and p38 mitogen-activated protein kinase (MAPK) pathways33.
Raddeanin A significantly inhibited human umbilical vein endothelial cell (HUVEC) proliferation, motility, migration, and tube formation7. Raddeanin A dramatically reduced angiogenesis in chick embryo chorioallantoic membrane, restrained the trunk angiogenesis in zebrafish, and suppressed angiogenesis and growth of human HCT-15 colorectal cancer xenograft in mice. Raddeanin A suppressed VEGF-induced phosphorylation of VEGFR2 and its downstream protein kinases including PLCγ1, JAK2, FAK, Src, and Akt. In a molecular docking simulation, Raddeanin A formed hydrogen bonds and hydrophobic interactions within the ATP-binding pocket of VEGFR2 kinase domain.
Raddeanin A significantly inhibited the invasion, migration and adhesion of the BGC-823 human gastric cancer cells25. Raddeanin A could up-regulate the expression of reversion inducing cysteine rich protein with Kazal motifs (RECK) and E-cadherin, and down-regulate the expression of matrix metalloproteinase-2 (MMP-2), MMP-9, MMP-14 and RhoC.
In a screen of 70 species of medicinal plants, the aqueous extract of A. altaica (AAE) had the best ability to suppress the viability of HOS and U2OS human osteosarcoma cells in a concentration-dependent manner34. AAE suppressed the growth of HOS and U2OS through the intrinsic apoptotic pathway, but it had no significant influence on human osteoblast hFOB cells. The high mRNA levels of apoptosis-related factors (PPP1R15A, SQSTM1, HSPA1B, and DDIT4) and cellular proliferation markers (SKA2 and BUB1B) were significantly altered by the AAE treatment. AAE could up-regulate the expression of a cluster of genes, especially those in the apoptosis-related factor family and caspase family.
4.2. Immunomodulatory activity
ARS, the saponins extracted from the rhizome of A. raddeana, showed a slight hemolytic effect and enhanced significantly the specific antibody and cellular response against ovalbumin in mice35. A neutral polysaccharide fraction (ARP) from the rhizome of A.raddeana extraordinarily promotes splenocyte proliferation, NK cell and CTL activity, as well as serum IL-2 and TNF-α production in HCC-bearing mice2. ARP had no toxicity to body weight, liver, and kidney. Moreover, it could reverse the hematological parameters induced by 5-fluorouracil to near normal.
4.3. Anti-inflammatory and antioxidant activities
Ranunculaceae tribes and genera, such as Ranunculus, Anemoneae, Cimicifuga, Helleborus, Nigella, Delphinieae, Semiaquilegia, Coptis, and Hydrastis, are rich in both anti-inflammatory and anticancer phytometabolites1, 19. Anemonin and ranunculin, the potent anti-inflammatory and anticancer compounds, are abundant in tribes Ranunculeae and Anemoneae1, 36.
A. flaccida (Di Wu in Chinese) crude triterpenoid saponins (AFSs) inhibited redness and swelling of the right hind paw in the type II collagen-induced arthritis (CIA) model in rats4. The inflammatory responses were reduced by AFS treatment. The serum pro-inflammatory cytokines TNF-α and IL-6 were decreased in AFS-treated CIA rats at the dose of 200 and 400 mg/kg/day. AFS and its main compounds, including hederasaponin B, flaccidoside II, and hemsgiganoside B, significantly inhibited TNF-α and IL-6 production in LPS-treated RAW264.7 cells, respectively.
Osteoclasts are bone-specialized multinucleated cells and are responsible for bone-destructive diseases, such as rheumatoid arthritis and osteoporosis. In RAW264.7 cells and CIA rats, the total saponin (TS) of A. flaccida concentration-dependently inhibited receptor activator of NF-κB ligand (RANKL)-induced osteoclast formation and bone marrow-derived macrophages (BMMs), as well as decreased extent of actin ring formation and lacunar resorption6, 37. The RANKL-stimulated expression of osteoclast-related transcription factors was also diminished by TS, while the expression of osteoprotegerin (OPG), at both mRNA and protein levels increased, and the ratio of RANKL to OPG in inflamed joints and sera of CIA rats decreased37. TS blocked the RANKL-triggered TRAF6 expression, phosphorylation of MAPKs and IκB-α, and inhibited NF-κB p65 DNA binding activity. TS almost abrogated the nuclear factor of activated T cells (NFATc1) and c-Fos expression. TS suppresses RANKL-induced osteoclast differentiation and inflammatory bone loss via the down-regulation of TRAF6 level, suppression of c-jun N-terminal kinase (JNK) and p38 MAPK and NF-κB activation, and subsequently decreased expression of c-Fos and NFATc1. The triterpenoid saponin W3 of A. flaccida had similar effects38. Therefore, TS and the saponins thereof may be useful for lytic bone diseases and further in vivo studies and clinical trials are warranted.
Two coumarins of A. raddeana had inhibitory effect against human leukocyte elastase39. 3-Acetyloleanolic acid (AOA), oleanolic acid (OA), raddeanoside 12 (Rd12) and Rd13, isolated from A. raddeana, suppressed the superoxide generation induced by N-formyl-methionyl-leucyl-phenylalanine (fMLP) in a concentration-dependent manner40. Eleutheroside K (EK) and Rd10 significantly enhanced fMLP-induced superoxide generation in low concentration (0.5–0.75 μmol/L), while these compounds more efficiently suppressed superoxide generation than the other four compounds in other concentrations. Rd12 dose-dependently inhibited fMLP-induced tyrosyl phosphorylation of 123.0, 79.4, 60.3, 56.2 and 50.1 kDa proteins in human neutrophil, while Rd10 and EK enhanced the tyrosyl phosphorylation of these proteins at a low concentration range.
Superoxide generation induced by fMLP was significantly suppressed by betulin and lupeol, extracted from the roots of A. raddeana, depending on the concentration of the triterpenoids41. The suppressive effect of betulinic acid was low. The phorbol 12-myristate 13-acetate (PMA)-induced superoxide generation was suppressed by betulin in a concentration-dependent manner, but not by lupeol and betulinic acid. However, superoxide generation induced by arachidonic acid (AA) was suppressed by lupeol, while betulin and betulinic acid weakly enhanced AA-induced superoxide generation. Lupeol and betulin suppressed tyrosyl phosphorylation of a 45.0-kDa protein in fMLP-treated human neutrophils, but betulinic acid did not. Lupeol, betulin and betulinic acid showed no hemolytic effect even at the concentration of 500 μmol/L.
Five oleanolic acid triterpenoid saponins (OTSs), isolated from the rhizome of A. raddeana, suppressed fMLP-induced superoxide generation in a concentration-dependent manner42. OTS-1, 2 and 4 suppressed PMA- and AA-induced superoxide generation in a concentration-dependent manner, but OTS-3 and -5 showed no effect. fMLP- and PMA-induced tyrosyl or serine/threonine phosphorylation, and fMLP-, PMA- and AA-induced translocation of p67 (phox), p47 (phox) and Rac to plasma membrane were in parallel with the suppression of the stimulus-induced superoxide generation.
4.4. Antimicrobial activity
The antioxidant essential oil, obtained from the roots of A. rivularis, had antibacterial activity22. The inhibition zones at 100 µg/disc and minimum inhibitory concentration (MIC) values for four bacterial strains were in the range of 11.0—20.0 mm and 125—250 µg/mL, respectively.
5. Taxonomy and pharmacophylogeny
The distribution of anticancer compounds within Ranunculaceae is not random but phylogeny-related43, 44, 45. For instance, Ranunculus, Clematis, Pulsatilla, Anemone, and Nigella are rich in pentacyclic triterpene saponins. Pulsatilla, Anemone, and Clematis belong to the tribe Anemoneae, and Pulsatilla is evolutionarily more close to Anemone than to Clematis1. Clematis is closer to Naravelia and Anemoclema than to Anemone and Pulsatilla. Hepatica is basal to all other genera of Anemoneae46. The sister group relationship between Ranunculeae and Anemoneae is revealed by two independent groups46, 47.
Previous phylogenies based on molecular data indicated that segregate genera from both the Northern and Southern Hemispheres (Hepatica, Pulsatilla, Knowltonia, Oreithales, and Barneoudia) are embedded within Anemone and should be subsumed within the genus. Based on a new phylogeny that substantially increases the sampling of the austral anemones (especially from Africa), Hoot et al.48 analyzed combined sequence data (chloroplast atpB-rbcL spacer and nuclear ITS regions) for 55 species of Anemone, using Bayesian inference, maximum likelihood (ML), and maximum parsimony. The segregate genera, Oreithales and Barneoudia, nest within Anemone and are included in a well-supported clade (subgenus Anemone, section Pulsatilloides) consisting largely of Southern Hemisphere species. The Mexican A. mexicana is sister to all remaining members of section Pulsatilloides (Supplementary Fig. S1), which consists of two clades: a poorly supported South American and Tasmanian clade (A. sellowii, A. helleborifolia, A. rigida, Barneoudia and Oreithales species, and A. crassifolia) and a highly supported Southern African clade including 9 species of Knowltonia and 8 species of Anemone. A. antucensis (Chile, Argentina) falls in a separate clade (subgenus and section Anemonidium) that is sister to A. tenuicaulis (New Zealand). A. thomsonii (Eastern Africa) and A. somaliensis (Somalia) are in a clade (subgenus and section Anemone) composed largely of Northern Hemisphere species. A. somaliensis is further associated with other Mediterranean tuberous anemones in subsection and series Anemone (A. coronaria, A. hortensis, and A. pavonina). The topology of both sections Pulsatilloides and Anemonidium suggests that anemones originated in the Northern Hemisphere and subsequently spread to the Southern Hemisphere, a pattern that is shared with other members of Ranunculaceae.
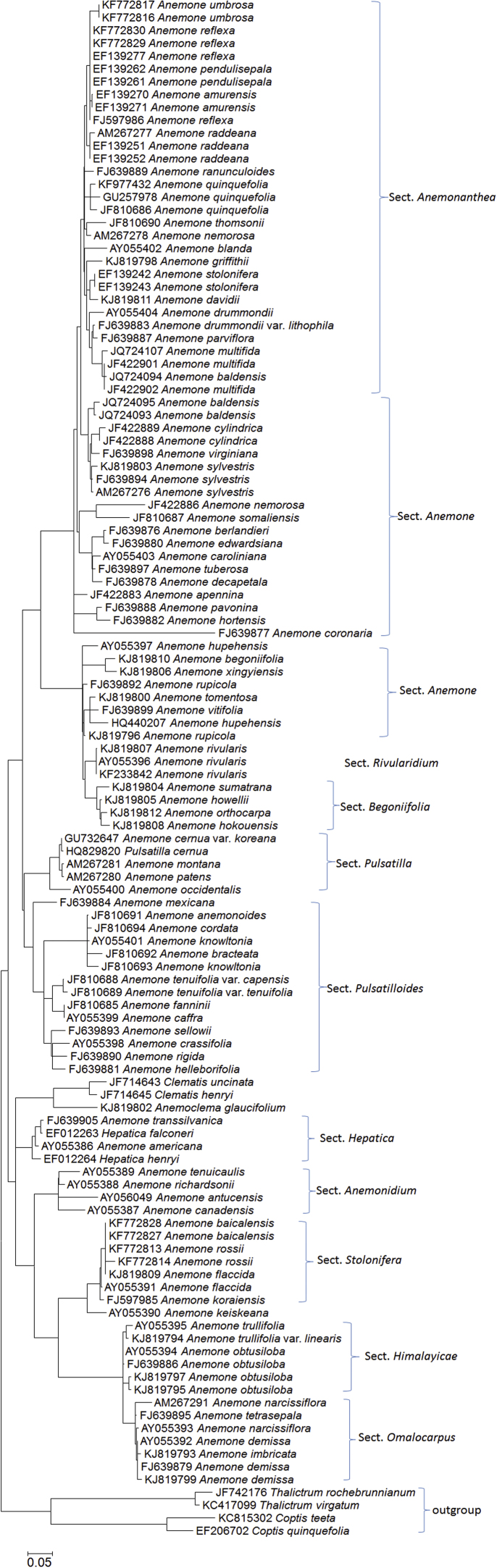
The taxonomic relationship of many Chinese species is elusive, we thus retrieved ITS sequences of more Anemone species from the NCBI GenBank and reconstructed their phylogenetic relationship (Fig. 6). Anemone umbrosa, A. reflexa, A. amurensis, A. raddeana, A. griffithii, A. stolonifera, and A. davidii, belonging to the section Anemonanthea, cluster on the top of the phylogenetic tree, which is congruent to the morphological classification. Some non-Chinese species, e.g., Anemone virginiana, A. sylvestris, A. somaliensis, and A. tuberosa, belonging to the section Anemone, are below the Anemonanthea species. However, some Chinese species of the section Anemone, e.g., Anemone hupehensis, A. begoniifolia, A. xingyiensis, and A. rupicola, are closer to A. rivularis (Sect. Rivularidium), A. orthocarpa, and A. hokouensis (Sect. Begoniifolia). All above taxa belong to the morphological subgenus Anemone. The sections Pulsatilla and Pulsatilloides, belonging to the subgenus Anemone, have no Chinese taxa. On the other hand, five sections, belonging to the subgenus Anemonidium, form another major clade. The section Himalayicae is closer to the section Omalocarpus than to the section Stolonifera. The non-Chinese sections Hepatica and Anemonidium are basal to these sections. The sections Himalayicae and Omalocarpus, evolutionarily younger than other sections, have some important Tibetan medicinal plants, e.g., Anemone trullifolia, A. obtusiloba, A. demissa, and A. imbricata. These two groups are still in the process of rapid radiation, corresponding to the extensive uplift of Qinghai–Tibet Plateau (QTP) during Quaternary45. The numerous morphologically and phytochemically distinct species should be investigated in detail to facilitate the sustainable development of Anemone-based clinical therapy.
Figure 6.
Phylogenetic relationship of Anemone ITSs inferred by ML (maximum likelihood) method. Scale bar represents 0.05 substitutions per site.
6. Transcriptomics, proteomics and metabolomics
Transcriptome sequencing and proteomic techniques were combined to comprehensively analyze the triterpenoid saponin biosynthetic pathway in Anemone flaccida49. A total of 126 putative cytochromes P450 (CYP) and 32 UDP glycosyltransferases were selected from 46,962 unigenes as candidates for triterpenoid saponin modifiers. Four CYPs were annotated as genes of CYP716A subfamily, the key enzyme in the oleanane-type saponin biosynthetic pathway. Based on RNA-Seq, iTRAQ proteome analysis, and quantitative RT-PCR verification, the expression level of genes and proteins committed to triterpenoids biosynthesis in the leaf and the rhizome could be compared. De novo transcriptome and proteome profiling are powerful in the discovery of candidate genes, which are related to the biosynthesis of phytometabolites in a non-model plant. The transcriptome of A. coronaria50, following infection with rust, is available, allowing for comparative transcriptomic studies. Twenty taxonomically related benzylisoquinoline-alkaloid-producing plants, belonging to Ranunculaceae and closely related families, were subjected to the transcriptome sequencing and analysis51. These essential data resources can be used to isolate and discover functional homologues and novel catalysts within the metabolism of medicinal compounds. Orthologs could be extracted for transcriptome-based phylogeny reconstruction52, 53.
Metabolomics studies provide imperative insight into the primary biochemical networks behind specialized metabolism and contribute key resources for metabolic engineering, gene discovery, and elucidation of regulatory mechanisms54. Future comprehensive and thorough metabolomics investigations of Anemone species are warranted.
7. Conclusion and future prospects
Are triterpenoid saponins of Anemone plants epiphany molecules for cancer patients? Not yet. Much more in vivo evidence has to be collated in experimental animals and humans, while at present there is a lack of pharmacokinetic and pharmacodynamic data on Anemone anticancer compounds. The structure—activity relationships should be investigated for understanding the molecular mechanisms and rational drug design. Some new Anemone taxa have been identified in Southwest China55, 56, 57, the biodiversity center of Ranunculaceae plants. A majority of Anemone species have not been probed with respect to their unique biosynthetic pathways and chemodiversity. A trade-off should be recognized between the conservation of endangered Anemone species and the utilization of Anemone pharmaceutical resources. The advent of the genomic era has provided important and surprising insights into the deducted genetic composition of Anemone species. Various innovative “omics” platforms would be of great help in deciphering biosynthetic pathways of Anemone phytometabolites, which will provide a solid foundation for future synthetic biology manipulations and also help protect Anemone medicinal resources for sustainable utilization.
Acknowledgments
This work is supported by Natural Science Fund of Liaoning Province (No. 2015020663).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.apsb.2016.12.001.
Appendix A. Supplementary material
Supplementary material
References
- 1.Hao D.C., Xiao P.G., Ma H., Peng Y., He C.N. Mining chemodiversity from biodiversity: pharmacophylogeny of medicinal plants of the Ranunculaceae. Chin J Nat Med. 2015;13:507–520. doi: 10.1016/S1875-5364(15)30045-5. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y., Li Y., Yang W., Zhang L., Cao G. Anti-hepatoma activity in mice of a polysaccharide from the rhizome of Anemone raddeana. Int J Biol Macromol. 2012;50:632–636. doi: 10.1016/j.ijbiomac.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Han L.T., Li J., Huang F., Yu S.G., Fang N.B. Triterpenoid saponins from Anemone flaccida induce apoptosis activity in HeLa cells. J Asian Nat Prod Res. 2009;11:122–127. doi: 10.1080/10286020802573818. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q., Zhu X.Z., Feng R.B., Liu Z., Wang G.Y., Guan X.F. Crude triterpenoid saponins from Anemone flaccida (Di Wu) exert anti-arthritic effects on type II collagen-induced arthritis in rats. Chin Med. 2015;10:20. doi: 10.1186/s13020-015-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito N., Toki K., Moriyama H., Shigihara A., Honda T. Acylated anthocyanins from the blue-violet flowers of Anemone coronaria. Phytochemistry. 2002;60:365–373. doi: 10.1016/s0031-9422(02)00097-3. [DOI] [PubMed] [Google Scholar]
- 6.Kong X., Wu W., Yang Y., Wan H., Li X., Zhong M. Total saponin from Anemone flaccida Fr. Schmidt abrogates osteoclast differentiation and bone resorption via the inhibition of RANKL-induced NF-κB, JNK and p38 MAPKs activation. J Transl Med. 2015;13:91. doi: 10.1186/s12967-015-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan Y.Y., Liu H.J., Luan X., Xu J.R., Lu Q., Liu Y.R. Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine. 2015;22:103–110. doi: 10.1016/j.phymed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Xiao P.G., Wang L.W., Lv S.J., Qiu G.S. Statistical analysis of the ethnopharmacologic data based on Chinese medicinal plants by electronic computer I. Magnoliidae. Chin J Integr Trad West Med. 1986;6:253–256. [PubMed] [Google Scholar]
- 9.Sun Y.X., Liu J.C., Liu D.Y. Phytochemicals and bioactivities of Anemone raddeana Regel: a review. Pharmazie. 2011;66:813–821. [PubMed] [Google Scholar]
- 10.Wang W., Liu H., Song Y., Zhang B., Xu J. Bencaological studies on Changpu. Chin Trad Herb Drug. 1995;26:263–265. [Google Scholar]
- 11.He K., Jiang X. Sky islands of Southwest China. I. An overview of phylogeographic patterns. Chin Sci Bull. 2014;59:585–597. [Google Scholar]
- 12.Gong H.D. Investigation on traditional Tibetan medicine plant resources of Anemone in the Eastern of Qinghai-Tibet Plateau. J Anhui Agri Sci. 2011;39:6388–6391. [Google Scholar]
- 13.Li M., Lei Z.Q., Zhong G.Y. Analysis of varieties and standards of Ranunculaceae medicinal plants used in Tibetan medicine. Trad Chin Drug Res Clin Pharmacol. 2015;26:133–137. [Google Scholar]
- 14.Pentso T.T. In: Jing Zhu Ben Cao. Mao J.Z., editor. Shanghai Scientific and Technical Publishers; Shanghai: 2012. [Google Scholar]
- 15.Kumar K., Sharma Y.P., Manhas R.K., Bhatia H. Ethnomedicinal plants of Shankaracharya hill, Srinagar, J&K, India. J Ethnopharmacol. 2015;170:255–274. doi: 10.1016/j.jep.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Lv C.N., Fan L., Wang J., Qin R.L., Xu T.Y., Lei T.L. Two new triterpenoid saponins from rhizome of Anemone amurensis. J Asian Nat Prod Res. 2015;17:132–137. doi: 10.1080/10286020.2014.983091. [DOI] [PubMed] [Google Scholar]
- 17.Cao P., Wu F.E., Ding L.S. Advances in the studies on the chemical constituents and biologic activities for Anemone species. Nat Prod Res Dev. 2004;16:581–584. [Google Scholar]
- 18.Zou Z.J., Liu H.X., Yang J.S. Phytochemical components and pharmacological activities of the genus Anemone. Chin Pharm J. 2004;39:493–495. [Google Scholar]
- 19.Hao D.C., Gu X.J., Xiao P.G. Elsevier-Woodhead; Oxford: 2015. Medicinal plants: chemistry, biology and omics. [Google Scholar]
- 20.Hao D.C., Gu X.J., Xiao P.G., Peng Y. Chemical and biological research of Clematis medicinal resources. Chin Sci Bull. 2013;58:1120–1129. [Google Scholar]
- 21.Hao D.C., Gu X.J., Xiao P.G., Liang Z.G., Xu L.J., Peng Y. Recent advance in chemical and biological studies on Cimicifugeae pharmaceutical resources. Chin Herb Med. 2013;5:81–95. [Google Scholar]
- 22.Shi B., Liu W., Gao L., Chen C., Hu Z., Wu W. Chemical composition, antibacterial and antioxidant activity of the essential oil of Anemone rivularis. J Med Plant Res. 2012;6:4221–4224. [Google Scholar]
- 23.Jürgens A., Dötterl S. Chemical composition of anther volatiles in Ranunculaceae: genera-specific profiles in Anemone, Aquilegia, Caltha, Pulsatilla, Ranunculus, and Trollius species. Am J Bot. 2004;91:1969–1980. doi: 10.3732/ajb.91.12.1969. [DOI] [PubMed] [Google Scholar]
- 24.Wang M.K., Ding L.S., Wu F.E. Antitumor effects of raddeanin A on S180, H22 and U14 cell xenografts in mice. Chin J Cancer. 2008;27:910–913. [PubMed] [Google Scholar]
- 25.Xue G., Zou X., Zhou J.Y., Sun W., Wu J., Xu J.L. Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem Biophys Res Commun. 2013;439:196–202. doi: 10.1016/j.bbrc.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Tang H., Zhang Y., Tang C., Li B., Wang Y. Saponin 1 induces apoptosis and suppresses NF-κB—mediated survival signaling in glioblastoma multiforme (GBM) PLoS One. 2013;8:e81258. doi: 10.1371/journal.pone.0081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji C., Cheng G., Tang H., Zhang Y., Hu Y., Zheng M. Saponin 6 of Anemone taipaiensis inhibits proliferation and induces apoptosis of U87 MG cells. Chin J Cell Mol Immunol. 2015;31:484–486. [PubMed] [Google Scholar]
- 28.Ji C.C., Tang H.F., Hu Y.Y., Zhang Y., Zheng M.H., Qin H.Y. Saponin 6 derived from Anemone taipaiensis induces U87 human malignant glioblastoma cell apoptosis via regulation of FAS and BCL‑2 family proteins. Mol Med Rep. 2016;14:380–386. doi: 10.3892/mmr.2016.5287. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Zhang W., Gao K., Lu Y., Tang H., Sun X. Oleanane-type saponins from Anemone taipaiensis and their cytotoxic activities. Fitoterapia. 2013;89:224–230. doi: 10.1016/j.fitote.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Wang X.Y., Gao H., Zhang W., Li Y., Cheng G., Sun X.L. Bioactive oleanane-type saponins from the rhizomes of Anemone taipaiensis. Bioorg Med Chem Lett. 2013;23:5714–5720. doi: 10.1016/j.bmcl.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Tang H., Zhang Y., Li J., Li B., Gao Z. Saponin B, a novel cytostatic compound purified from Anemone taipaiensis, induces apoptosis in a human glioblastoma cell line. Int J Mol Med. 2013;32:1077–1084. doi: 10.3892/ijmm.2013.1500. [DOI] [PubMed] [Google Scholar]
- 32.Han L.T., Fang Y., Li M.M., Yang H.B., Huang F. The antitumor effects of triterpenoid saponins from the Anemone flaccida and the underlying mechanism. Evid Based Complement Alternat Med. 2013;2013:517931. doi: 10.1155/2013/517931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han L.T., Fang Y., Cao Y., Wu F.H., Liu E., Mo G.Y. Triterpenoid saponin flaccidoside II from Anemone flaccida triggers apoptosis of NF1-associated malignant peripheral nerve sheath tumors via the MAPK-HO-1 pathway. Onco Targets Ther. 2016;9:1969–1979. doi: 10.2147/OTT.S95597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang I.C., Chiang T.I., Lo C., Lai Y.H., Yue C.H., Liu J.Y. Anemone altaica induces apoptosis in human osteosarcoma cells. Am J Chin Med. 2015;43:1031–1042. doi: 10.1142/S0192415X15500597. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y., Li M., Liu J. Haemolytic activities and adjuvant effect of Anemone raddeana saponins (ARS) on the immune responses to ovalbumin in mice. Int Immunopharmacol. 2008;8:1095–1102. doi: 10.1016/j.intimp.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Lee T.H., Huang N.K., Lai T.C., Yang A.T., Wang G.J. Anemonin, from Clematis crassifolia, potent and selective inducible nitric oxide synthase inhibitor. J Ethnopharmacol. 2008;116:518–527. doi: 10.1016/j.jep.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Liu C., Yang Y., Sun D., Wang C., Wang H., Jia S. Total saponin from Anemone flaccida Fr. Schmidt prevents bone destruction in experimental rheumatoid arthritis via inhibiting osteoclastogenesis. Rejuvenation Res. 2015;18:528–542. doi: 10.1089/rej.2015.1688. [DOI] [PubMed] [Google Scholar]
- 38.Kong X., Yang Y., Wu W., Wan H., Li X., Zhong M. Triterpenoid saponin W3 from Anemone flaccida suppresses osteoclast differentiation through inhibiting activation of MAPKs and NF-κB pathways. Int J Biol Sci. 2015;11:1204–1214. doi: 10.7150/ijbs.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren F.Z., Chen S.H., Zheng Z.H., Zhang X.X., Li L.H., Dong A.H. Coumarins of Anemone raddeana Regel and their biological activity. Acta Pharm Sin. 2012;47:206–209. [PubMed] [Google Scholar]
- 40.Lu J., Sun Q., Sugahara K., Sagara Y., Kodama H. Effect of six compounds isolated from rhizome of Anemone raddeana on the superoxide generation in human neutrophil. Biochem Biophys Res Commun. 2001;280:918–922. doi: 10.1006/bbrc.2000.4183. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita K., Lu H., Lu J., Chen G., Yokoyama T., Sagara Y. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin Chim Acta. 2002;325:91–96. doi: 10.1016/s0009-8981(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 42.Wei S., He W., Lu J., Wang Z., Yamashita K., Yokoyama M. Effects of five oleanolic acid triterpenoid saponins from the rhizome of Anemone raddeana on stimulus-induced superoxide generation, phosphorylation of proteins and translocation of cytosolic compounds to cell membrane in human neutrophils. Fitoterapia. 2012;83:402–407. doi: 10.1016/j.fitote.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Hao D.C., Xiao P.G., Liu M., Peng Y., He C.N. Pharmaphylogeny vs. pharmacophylogenomics: molecular phylogeny, evolution and drug discovery. Acta Pharm Sin. 2014;49:1387–1394. [PubMed] [Google Scholar]
- 44.Hao D.C., Xiao P.G., Liu L.W., Peng Y., He C.N. Essentials of pharmacophylogeny: knowledge pedigree, epistemology and paradigm shift. China J Chin Mater Med. 2015;40:3335–3342. [PubMed] [Google Scholar]
- 45.Hao D.C., Xiao P.G. Genomics and evolution in traditional medicinal plants: road to a healthier life. Evol Bioinform Online. 2015;11:197–212. doi: 10.4137/EBO.S31326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W., Lu A.M., Ren Y., Endress M.E., Chen Z.D. Phylogeny and classification of Ranunculales: evidence from four molecularloci and morphological data. Perspect Plant Ecol Evol Syst. 2009;11:81–110. [Google Scholar]
- 47.Cossard G., Sannier J., Sauquet H., Damerval C., de Craene L.R., Jabbour F. Subfamilial and tribal relationships of Ranunculaceae: evidence from eight molecular markers. Plant Syst Evol. 2016;302:419–431. [Google Scholar]
- 48.Hoot S.B., Meyer K.M., Manning J.C. Phylogeny and reclassification of Anemone (Ranunculaceae), with an emphasis on austral species. Syst Bot. 2012;37:139–152. [Google Scholar]
- 49.Zhan C., Li X., Zhao Z., Yang T., Wang X., Luo B. Comprehensive analysis of the triterpenoid saponins biosynthetic pathway in Anemone flaccida by transcriptome and proteome profiling. Front Plant Sci. 2016;7:1094. doi: 10.3389/fpls.2016.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laura M., Borghi C., Bobbio V., Allavena A. The effect on the transcriptome of Anemone coronaria following infection with rust (Tranzschelia discolor) PLoS One. 2015;10:e0118565. doi: 10.1371/journal.pone.0118565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagel J.M., Morris J.S., Lee E.J., Desgagné-Penix I., Bross C.D., Chang L. Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants. BMC Plant Biol. 2015;15:227. doi: 10.1186/s12870-015-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao D.C., Ma P., Mu J., Chen S.L., Xiao P.G., Peng Y. De novo characterization of the root transcriptome of a traditional Chinese medicinal plant Polygonum cuspidatum. Sci China Life Sci. 2012;55:452–466. doi: 10.1007/s11427-012-4319-6. [DOI] [PubMed] [Google Scholar]
- 53.Han F., Peng Y., Xu L., Xiao P.G. Identification, characterization, and utilization of single copy genes in 29 angiosperm genomes. BMC Genomics. 2014;15:504. doi: 10.1186/1471-2164-15-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagel J.M., Mandal R., Han B., Han J., Dinsmore D.R., Borchers C.H. Metabolome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants. BMC Plant Biol. 2015;15:220. doi: 10.1186/s12870-015-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W.T., Liu B. A new section with a new species of Anemone (Ranunculaceae) from Mt. Xiaowutai, China. J Syst Evol. 2008;46:738–741. [Google Scholar]
- 56.Yuan Q., Yang Q.E. Anemone xingyiensis (Ranunculaceae), a new species from Guizhou, China. Bot Studi. 2009;50:493–498. [Google Scholar]
- 57.Wang W.T. Three new species of Anemone (Ranunculaceae) from Xizang. Plant Diversity Resour. 2014;36:449–452. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material