Abstract
Stem cells are undifferentiated cells that can differentiate into specialized cells. Recently, enormous growth has been seen in the recognition of stem cell-based therapies, which have the potential to ameliorate the life of patients with conditions that span from Parkinson's disease to cardiac ischemia to bone or tooth loss. This research has produced new but unexplored possibilities in the regeneration of different organs and tissues. Presently, research is focused on the proficiency of stem cells and their utilization in dentistry, which is gaining interest. The tooth is nature's “esteem” for these precious stem cells and there are a number of these cells in permanent and primary teeth, as well as in the wisdom teeth. Dental stem cells are easy, convenient, and affordable to collect. They hold promise for a range of very potential therapeutic applications, such as in the treatment of cancer, spinal cord injury, brain damage, myocardial infarction, hearing loss, diabetes, wound healing, baldness, etc. Since these cells were used to regenerate damaged tissue in medical therapy successfully, it is possible that the dentist in future might use stem cell to regenerate lost or damaged dental and periodontal structures. This paper reviews the current concepts, characteristics of stem cells in regeneration, and its subsequent uses in dentistry.
Keywords: Stem cells, Tooth regeneration, Dental stem cells, Regeneration endodontics
1. Introduction
Stem cells are unique type of cells that have specialized capacity for self-renewal and potency, and can give rise to one and sometimes many different cell types. They are found in many of the multicellular organisms and have the ability to renew through mitotic cell division and even maintain the original undifferentiated state.1 On cell divison, each new cell has the potential to either remain a stem cell or become another type of cell with a more specialized function, such as a cardiac muscle cell, skeletal muscle cell, liver cell, a red blood cell, a brain cell, etc.2 Stem cells have two paramount characteristics that differentiate it from other cells. The first is “self-renewal,” i.e., the ability of renewing themselves through cell division, sometimes after long periods of inactivity. The second is “potency,” i.e., they can be induced to become tissue-specific cells with special functions, under certain physiologic or experimental conditions. In some organs, such as the bone marrow, they regularly divide to repair and replace worn out or damaged tissues.3
In medical therapy, stem cells have been used for engineering many tissues and organs. Stem cell therapy has also been used to treat diseases including Parkinson's and Alzheimer's diseases, stroke, burns, heart diseases, diabetes, osteoarthritis, and rheumatoid arthritis.4
Recently, scientists have started to search applications of stem cells for the regeneration and repair of dentofacial and dental structures.5, 6
At present, teeth can only be replaced with prostheses, i.e., removable prostheses, fixed prostheses, or implants, with prior bone augmentation if necessary. Stem cell biology and tissue engineering may present new options for replacing damaged or lost teeth, or even individual tooth structures. The promise of such treatment possibilities puts stem cells in the focus of dental research.7
2. Dental stem cells
Existence of stem cells in the teeth is an oaken phenomenon and is required for odontogenesis. In the early fetal developmental stages, teeth arise from the neural crest cells through a series of interactions between neural, mesenchymal, and epithelial tissues.8 The developed tooth can be thought of as an encapsulated population of quiescent stem cells.9 The finding of stem cells in natal teeth,10 supernumerary teeth,11 and odontoma12 reinforces the concept that stem cells play a key role in the formation of every tooth. It has also been shown that the pluripotency of dental stem cells may be a function of the age of the tooth or the age of the donor. It means, the younger the tooth, the more is the number of dental stem cells.13 In other words, primary teeth, molars, and wisdom teeth of young adults all contain potent sources of dental stem cells.
3. Dental sources of adult stem cells14, 15 (Fig. 1 and Table 1)
Fig. 1.
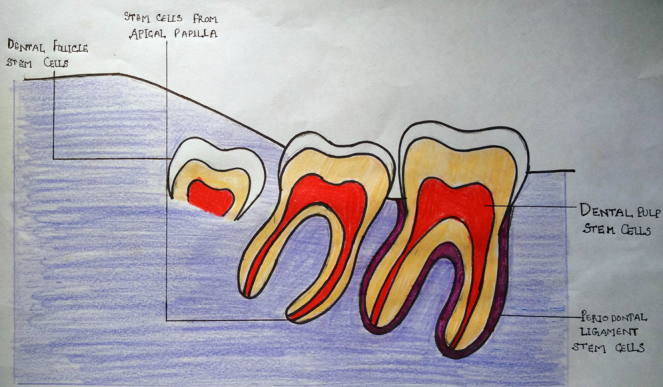
Dental stem cells.
Table 1.
Classification and characterization of dental stem cells.
| Properties | DFSC | SHED | DPSC | PDLSC | SCAP |
|---|---|---|---|---|---|
| Full name | Dental follicle stem cells | Stem cells of human exfoliated deciduous teeth | Dental pulp stem cells | Periodontal ligament stem cells | Stem cells of the (dental) apical papilla |
| Isolation | From the follicles of impacted third molars | Exfoliated deciduous teeth and coronal pulp | From dental pulp | Root from extracted teeth | Impacted third molars |
| Location | Dental follicle of developing tooth | Exfoliated deciduous tooth pulp | Permanent tooth pulp | Periodontal ligament | Apical papilla of developing root |
| Proliferative rate | High | High | Moderate | High | High |
| Heterogeneity | Yes | Yes | Yes | Yes | Yes |
| Multipotentially | Odontoblast osteoblast neurocytes | Odontoblast osteoblast chondrocytes myocytes neurocytes adipocytes, induced pluripotent stem cell | Odontoblast osteoblast chondrocytes myocytes neurocytes adipocytes, corneal epithelial cell, melanoma cell, induced pluripotent stem cell | Odontoblast osteoblast chondrocyte, cementoblast, neurocytes | Odontoblast Osteoblast neurocytes adipocytes, induced pluripotent stem cell |
| Tissue repair | Bone regeneration, periodontal regeneration | Bone regeneration, neuroregeneration, tubular dentin | Bone regeneration, neuroregeneration, myogenic regeneration, dentin pulp regeneration | Bone regeneration, root formation, periodontal regeneration | Bone regeneration, neuroregeneration, dentin pulp regeneration, root formation |
| Population Doubling | Not determined | >70 | 60 to >120 | Not determined | >140 |
They are divided into two groups with respect to their major differentiation potential.
4. Uses of stem cells in dentistry
-
1.Regeneration of dental hard tissues
-
i.Enamel regeneration
-
ii.Dentin regeneration
-
i.
-
2.Regeneration of dental soft tissues
-
i.Pulp regeneration
-
ii.Periodontal tissue regeneration
-
i.
-
3.
Whole tooth regeneration
5. Regeneration of dental hard tissues
5.1. Enamel regeneration
Dental enamel is the hardest tissue of the body. Regeneration of enamel is dependent on ameloblasts, which are lost as soon as the tooth erupts in the mouth. The enamel spends the remainder of its lifetime vulnerable to wear, damage and decay.
Although researches have shown positive results in producing enamel-like and tooth-like tissues, still there are problems, which remain to be solved before the technology can be tested in humans. One of the major problems has been to produce a sufficient number of enamel-forming cells in culture. There have been reports that a new technique is being developed for culturing cells that have the capacity to produce enamel.16
5.2. Dentin regeneration
In response to any injury or trauma, dental pulp tissue has the regenerative potential to form dentin, which is known as the reparative dentin. Dentin formation was observed in immunocompromised mice when pulp stem cells were cultivated with hydroxyapatite or tricalcium phosphate scaffold and implanted in them. Reparative dentin was formed when stem cells were combined with recombinant human bone morphogenetic protein-2 (BMP-2) on adulterated pulp in experimental studies on animal models.17
6. Regeneration of dental soft tissues
6.1. Regeneration of pulp
Regenerative pulp procedures are biologically based procedures, which are designed to replace mutilated structures including dentin and root structures, as well as the cells of the pulp-dentin complex.
Research regarding regenerative endodontic techniques is:
-
a)
Postnatal stem cell therapy: In teeth with closed apex, the root canal space is disinfected after the apex is opened and then postnatal stem cells are injected. This is the simplest method to administer the cells of appropriate regenerative potential.18
-
b)
Scaffold implantation: In pulpally involved teeth, dentin fragments are used to stimulate reparative dentin. These fragments may act as a matrix for stem cell attachment and may also serve as a reservoir of growth factors. This process can be accomplished by cultivating pulp stem cells on a porous polymer scaffold. The natural reparative activity of the pulp stem cells in response to the dentin fragments provides some support for the use of scaffolds to regenerate the pulp dentin complex.19
-
c)
Injectable scaffold delivery: Soft 3D scaffold matrix-like hydrogels are used and tissue engineered pulp tissue is seeded into them. These hydrogels are then delivered by a syringe. They have the potential to be noninvasively and easily delivered into the root canal systems. These injectable scaffolds have the potential to serve as a substrate for cell proliferation and differentiation into an organized tissue structure. Even after these advances, the research is at its preliminary stage and is yet to be proven in in vivo studies.
-
d)
Gene therapy: The most recent therapy used as a means of delivering genes for growth factors, morphogens, transcription factors, and extracellular matrix molecules locally to the somatic cells of individuals and which has shown good therapeutic effect is the Gene therapy. These genes induce a biological process and form the tissue of interest by expressing the molecules that are involved in the regenerative response. In endodontic procedures, gene therapy can be used to deliver mineralizing genes into the pulp tissues and thus promote tissue mineralization.20
6.2. Periodontal regeneration
For regenerating periodontal tissue, the use of growth and differentiation factors is one of the most popular tissue engineering approaches. Till date, several growth factors including transforming growth factors-β (TGF-β) superfamily members, such as, BMP-2, BMP-6, BMP-7, BMP-12, TGF-β, basic fibroblast growth factors (bFGF), and platelet-derived growth factors (PDGF) have been used. These factors have been used as a protein-based approach for periodontium regeneration.
Nagatomo et al. in their experimental studies found that periodontium can be regenerated by using PDL cells that have stem cell properties.
Iwata et al. did a similar in vitro study wherein PDL cells were harvested and expanded. He also made transplantable constructs of primary canine containing PGA scaffold and PDL cell sheets. Regeneration of periodontal structures that included cementum, alveolar bone, and periodontal fibers was induced by the transplanting constructs in combination with porous bTCP (b-tricalcium phosphate).17
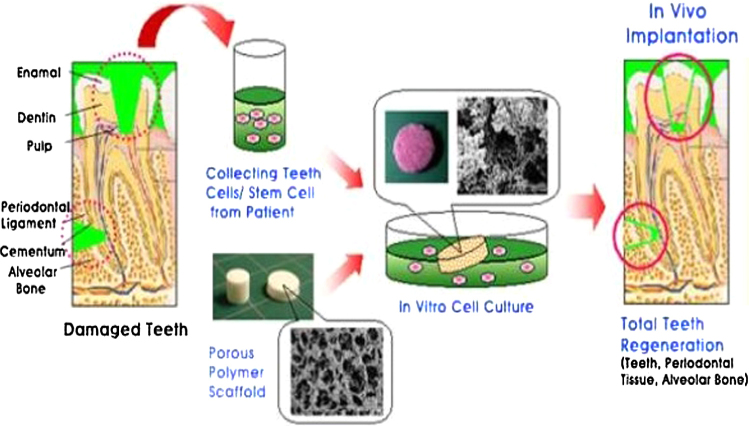
7. Whole tooth regeneration
Tooth-like tissues have been regenerated by the cultivation of different cell types on biodegradable scaffolds. Stem cells are harvested, expanded, and differentiated in vitro, seeded onto scaffolds, and then implanted in vivo. These scaffolds, in some cases, are then reimplanted into an extracted tooth socket or the jaw.
In 2009, Ikeda et al. reported a successful and fully operating tooth replacement in an adult mouse. This was accomplished by transplanting a bioengineered tooth germ into the alveolar bone in the lost tooth region. This methodology was proposed as a model for future organ replacement therapies.
7.1. Process of tooth regeneration
Step 1: Dental stem cell isolation and identification
This is one of the foremost and critical steps in tooth regeneration. DPSC have been isolated from human teeth, and swine and rat dental pulp. In addition to these, a new cell culture system was obtained from human exfoliated deciduous teeth (SHED) (6–10 years of age).
Step 2: Culturing of stem cells in association with scaffold materials
In addition to stem cells, tissue regenerative treatment requires another key element during culturing: a suitable inductive carrier, i.e. a scaffold material. A scaffold material is required in regenerative dentinogenesis so as to provide optimal conditions for cell adhesion, migration, proliferation, and differentiation of stem cells into their multipotent forms. The selection of an appropriate scaffold material is thus of vital importance for appropriate formation of new dentin matrix. An ideal scaffold material should be biocompatible and nontoxic, possess good physical properties in terms of tensile, compressive, and flexural strength, convictive for odontoblast-like cells, bioresorbable, and bioactive.
Step 3: Delivery and effect of growth factors
The next key element of the tissue engineering triad is the regulation of cellular proliferation and differentiation in the omnipresence of growth factors. It is of utmost importance to liberate growth factor in a regulated manner from the scaffold. Major signaling growth factors implicated as mediators in tooth development include BMPs, TGF-β1, bFGF-2, insulin growth factor-I, activin, Wnt (Wingless), retinoic acid, PDGF, hedgehog, etc.
BMPs have been implicated in tooth development and associated with the differentiation of odontoblasts and ameloblasts responsible for dentin and enamel formation, respectively by inducing mRNA expression of dentin sialophosphoprotein (responsible for mineralization of dentin) after the implantation onto the dental papilla in organ culture.
TGF-β1 is a major component of the extracellular matrix of dental pulp and is theorized to be involved in differentiation process of odontoblasts and in dentin and predentin formation in human tooth.
The partially bioengineered tooth bud is finally transplanted into a surgically prepared anatomical site in the oral cavity of animal models (as no current evidence of human experiments exists)21 (Fig. 2).
Fig. 2.
Tooth regeneration.
Advantages of dental stem cell
The advantages of dental stem cells are that:
-
•
These cells have high plasticity.
-
•
Dental stem cells are ideal for stem cell banking as they can be preserved for a longer period.
-
•
These cells have shown a favorable response with scaffold and growth factors.
Risk factors of stem cell therapy
-
1.
Teratogenic: Stem cell may be potential candidates for malignant transformation as they resemble features of cancer cells.
-
2.
Immune responses: Stem cells may have a modulating effect on the immune system and thus affect the host immune response.
-
3.
Adventitious agents: Viral and microbial safety is a major risk factor that is associated with the use of stem cells, as the manufacturing of these cell-based products does not include sterilization or viral removal and its inactivation thus may lead to life-threatening reactions.22
8. Conclusion
“Stem cells are miracles to humanity and have the ability to save thousands.”
Regeneration of the dental tissues endeavors a spectacular alternative to more conventional restorative approaches because the damaged tissue is replaced by natural tissue. Stem cell therapy has got a cardinal role as a forthcoming treatment modality in dentistry. The ultimate goal of tooth regeneration is to replace the lost teeth by a natural tooth. Stem cell-based tooth engineering is conceived as an upcoming approach in the making of a biological tooth (biotooth). A biological tooth made from autogenous DPSC should be the best choice for clinical tooth reconstruction. However, replacing dental tissues with either stem cell or gene-based therapy may be complicated. The novel approach of stem cell regeneration requires more research to obtain successful treatment modalities and the opportunities for their utilization in dental tissue regeneration should be made more clearer, so that they escort us for considerable benefits in the management of the effects of dental disease.
Conflicts of interest
The authors have none to declare.
References
- 1.Murray P.E., Garcia-Godoy F., Hargreaves K.M. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Bluteau G., Luder H.U., De Bari C., Mitsiadis T.A. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y., Jahagirdar B.N., Reinhardt R.L. Pluripotency of mesenchymal stem cells derived from adult marrow. J Dent Res. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Bonassar L.J., Vacanti C.A. Tissue engineering: the first decade and beyond. J Cell Biochem Suppl. 1998;30(31):297–303. [PubMed] [Google Scholar]
- 5.Shaikh R. Therapeutic potential of stem cells in regenerative dentistry; a review of literature. Int J Stud Res. 2013;1(May (4)):22–30. [Google Scholar]
- 6.Gupta A.S., Gupta S., Singaraju S., Singaraju M. Role of dental adult stem cells in regenerative medicine. J Orofac Res. 2013;3(2):115–120. [Google Scholar]
- 7.Vanishree N., Chaithra V., Prabbla A. A tooth for a tooth: dental stem cell banking in India. Ann Essences Dent. 2011;3(4):90–93. [Google Scholar]
- 8.Sharpe P.T. Neural crest and tooth morphogenesis. Adv Dent Res. 2001;15:4–7. doi: 10.1177/08959374010150011001. [DOI] [PubMed] [Google Scholar]
- 9.Yalvac M.E., Ramazanoglu M., Rizvanov A.A., Sahin F., Bayrak O.F., Salli U. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo-, adipo- and neurogenesis. Pharmacogenomics J. 2010;10:105–113. doi: 10.1038/tpj.2009.40. [DOI] [PubMed] [Google Scholar]
- 10.Karaoz E., Dogan B.N., Aksoy A., Gacar G., Akyuz S., Ayhan S. Isolation and in-vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2009;133:95–112. doi: 10.1007/s00418-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 11.Huang A.H., Chen Y.K., Lin L.M. Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med. 2008;37:571–574. doi: 10.1111/j.1600-0714.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 12.Song J.S., Stefanik D., Damek-Poprawa M., Alawi F., Akintoye S.O. Differentiation and regenerative capacities of human odontoma-derived mesenchymal cells. Differentiation. 2009;77(1):29–37. doi: 10.1016/j.diff.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda T., Tezuka Y., Horiuchi M. Characterization of dental pulp stem cells of human tooth germs. J Dent Res. 2008;87:676–681. doi: 10.1177/154405910808700716. [DOI] [PubMed] [Google Scholar]
- 14.Seo B.M., Sooyama W., Yamaza T., Kikuiri T., Akiyama K., Lee J.S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–434. doi: 10.1111/j.1601-0825.2007.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irma T., Mark T. Stem cells and tissue engineering: prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12(1):43–50. doi: 10.1159/000069840. [DOI] [PubMed] [Google Scholar]
- 16.Den Besten P.K., Mathews C.H., Gao C., Li W. Primary culture and characterization of enamel organ epithelial cells. Connect Tissue Res. 1998;38:3–8. doi: 10.3109/03008209809017011. [DOI] [PubMed] [Google Scholar]
- 17.Guleria M., Dua H., Rohila S., Sharma A.K. Stem cells in dentistry. Indian J Dent Sci. 2014;4(6):107–111. [Google Scholar]
- 18.Dewan R.G., Kochhar R., Bhandari P.P., Tyagi N. Regenerative endodontics in the light of recent research. Indian J Dent Sci. 2013;2(5):132–135. [Google Scholar]
- 19.Torvi S.J., Munniswamy K. Regenerative dentistry: current and future perspectives to rejuvenate and reclaim dental tissues. J Int Clin Dent Res Organ. 2014;6(2):112–117. [Google Scholar]
- 20.Murray P.E., Godoy F.G., Hargreaves K.M. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(3):77–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Jolly M., Jolly A., Singh N., Rathore M., Tandon S. Stem cells: regeneration and transplantation of teeth. J Investig Dent Sci. 2014;1(1):1–7. [Google Scholar]
- 22.Herberts C.A., Kwa M.S., Hermsen H.P. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]