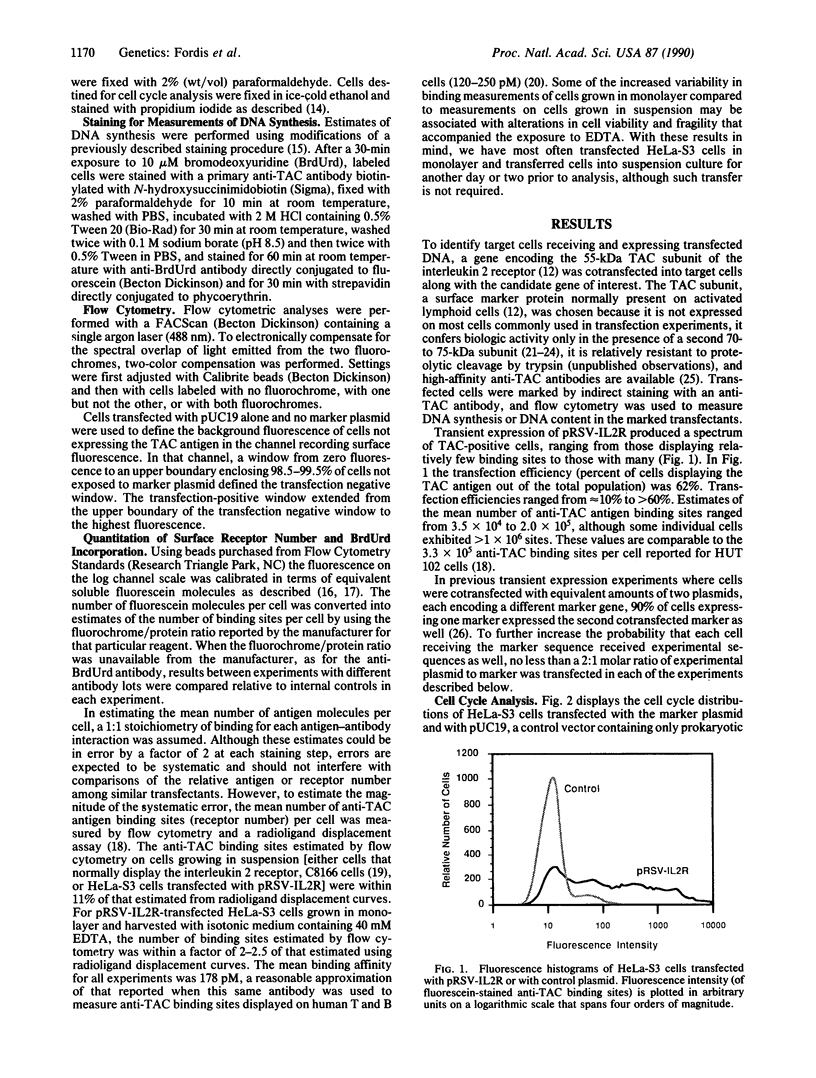
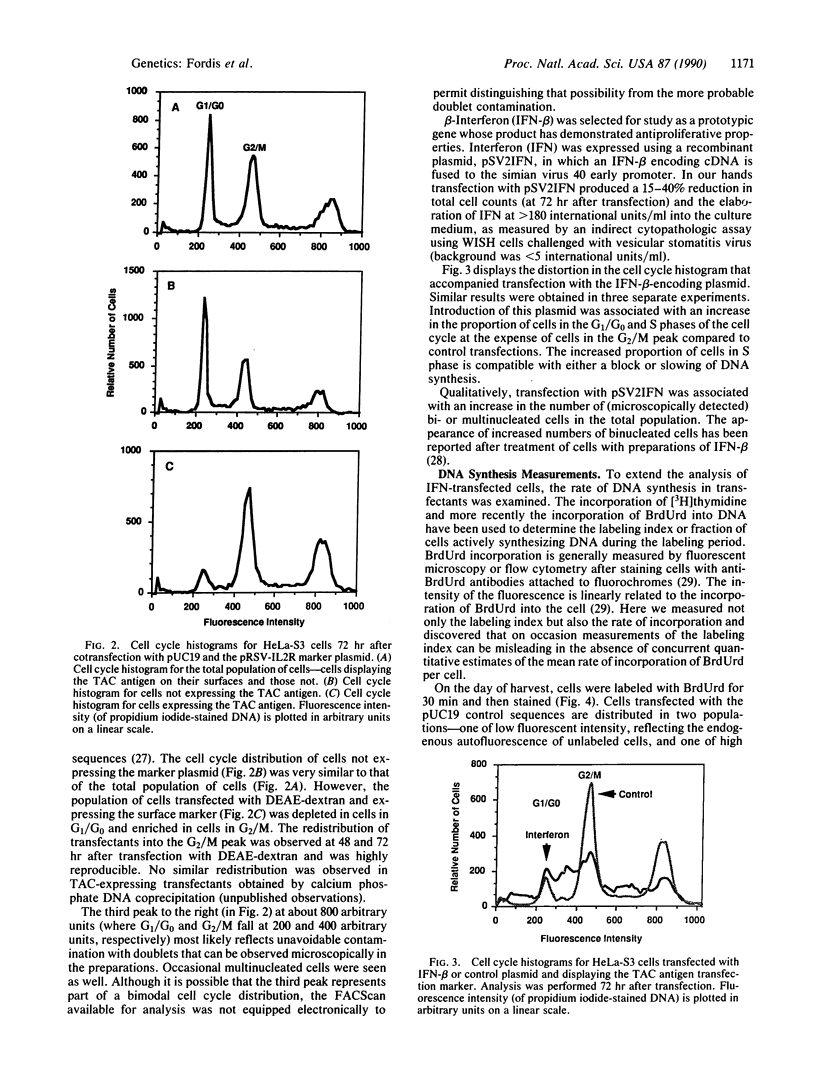
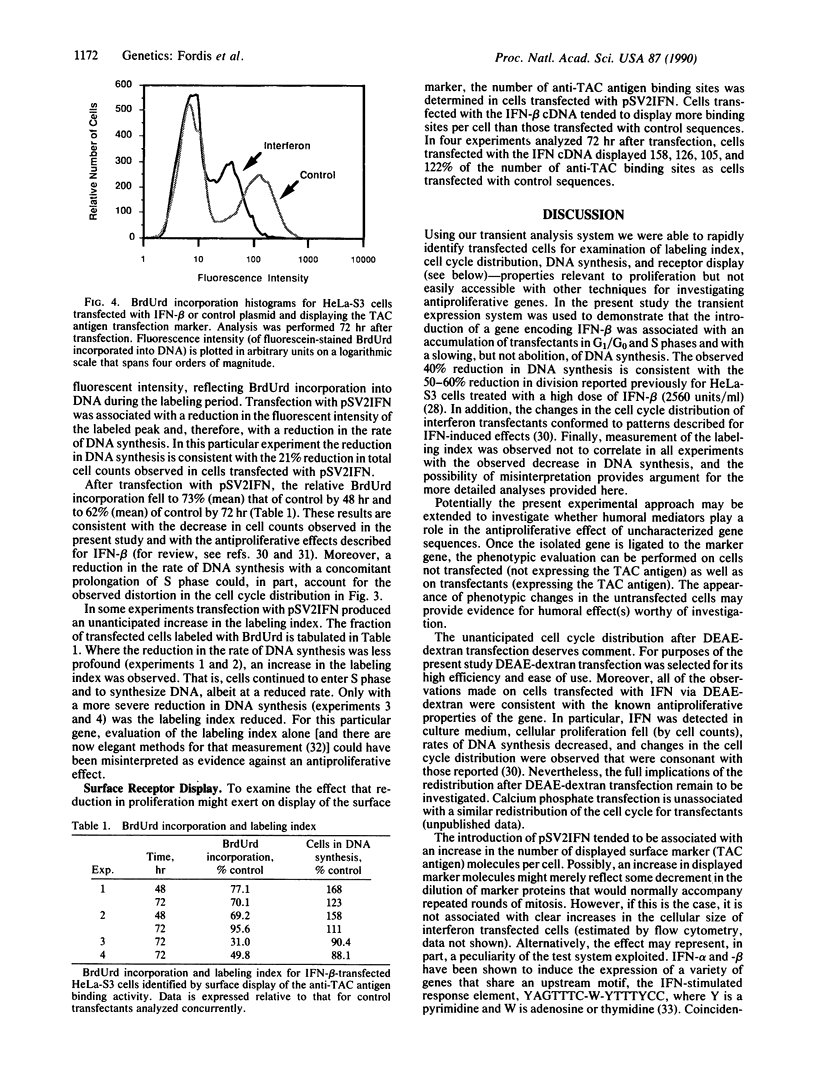
Abstract
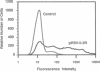
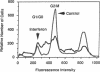
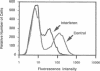
A subset of tumor suppressor genes presumably functions by the inhibition of cellular proliferation; however, antiproliferative activity after transfection with putative suppressor genes has been difficult to demonstrate and often requires lengthy selection either in nude mice or in vitro. A rapid alternative is presented here that utilizes a gene encoding a surface marker protein to identify transfectants in a transient expression assay. In this assay the labeling index, rate of DNA synthesis, cell-cycle distribution, and surface receptor display are measured by flow cytometry. Human beta-interferon, a gene with proven antiproliferative activity, was studied using the transient analysis system. The beta-interferon gene was introduced into human tumor cells along with the marker gene encoding the 55-kDa subunit of the human interleukin 2 receptor. Within a few days of transfection, analysis of transfectants by flow cytometry revealed a decrease in the fraction of cells in G2/M and an increase in the fraction of cells in G1/G0 and S phases. The distortion of the cell cycle was accompanied by as much as a 69% reduction in the rate of DNA synthesis and, in some experiments, an unanticipated increase in the labeling index. Therefore, cells accumulating in S phase were not blocked but continued to synthesize DNA although at a reduced rate. These studies on DNA synthetic rates revealed the caveat that screening for antiproliferative candidate genes with a labeling index alone could, in certain circumstances, exclude potentially interesting sequences from further consideration. Although this transient analysis system was developed for studies on cellular proliferation, it may prove suitable for phenotypic assays on other genes as well.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dolbeare F., Gratzner H., Pallavicini M. G., Gray J. W. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5573–5577. doi: 10.1073/pnas.80.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Dryja T. P., Weinberg R. A. Oncogenes and tumor-suppressing genes. N Engl J Med. 1988 Mar 10;318(10):618–622. doi: 10.1056/NEJM198803103181007. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Svetlik P. B., Rusk C. M., Depper J. M., Leonard W. J. Stable expression of cDNA encoding the human interleukin 2 receptor in eukaryotic cells. J Exp Med. 1985 Jul 1;162(1):363–368. doi: 10.1084/jem.162.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. Malignant tumours generated by recessive mutations. Nature. 1986 Oct 16;323(6089):582–583. doi: 10.1038/323582a0. [DOI] [PubMed] [Google Scholar]
- Hirakawa T., Ruley H. E. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter W., Fordis C. M., Howard B. H. Efficient gene transfer by sequential treatment of mammalian cells with DEAE-dextran and deoxyribonucleic acid. Exp Cell Res. 1989 Oct;184(2):546–551. doi: 10.1016/0014-4827(89)90353-4. [DOI] [PubMed] [Google Scholar]
- Holter W., Goldman C. K., Casabo L., Nelson D. L., Greene W. C., Waldmann T. A. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J Immunol. 1987 May 1;138(9):2917–2922. [PubMed] [Google Scholar]
- Houck D. W., Loken M. R. Simultaneous analysis of cell surface antigens, bromodeoxyuridine incorporation and DNA content. Cytometry. 1985 Nov;6(6):531–538. doi: 10.1002/cyto.990060607. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Wang X. F., Weinberg R. A., Cheifetz S., Massagué J. Absence of TGF-beta receptors and growth inhibitory responses in retinoblastoma cells. Science. 1988 Apr 8;240(4849):196–199. doi: 10.1126/science.2895499. [DOI] [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P. P., Mishal Z., Lemonnier F. A., Kourilsky F. M. Quantification by flow cytofluorimetry of HLA class I molecules at the surface of murine cells transformed by cloned HLA genes. J Immunol Methods. 1983 Jul 29;61(3):301–315. doi: 10.1016/0022-1759(83)90224-7. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Noda M., Kitayama H., Matsuzaki T., Sugimoto Y., Okayama H., Bassin R. H., Ikawa Y. Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):162–166. doi: 10.1073/pnas.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan R., Corsico C. D., Howard T. H., Holter W., Fordis C. M., Willingham M., Howard B. H. Purification of transiently transfected cells by magnetic affinity cell sorting. Anal Biochem. 1988 May 1;170(2):341–348. doi: 10.1016/0003-2697(88)90640-9. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Zanetti M., King R., Delia D., Ansorge W., Philipson L., Schneider C. Automatic microinjection system facilitates detection of growth inhibitory mRNA. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6748–6752. doi: 10.1073/pnas.85.18.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Murphy J. S., Tamm I. Interferon effects on the growth and division of human fibroblasts. Exp Cell Res. 1979 Jun;121(1):111–120. doi: 10.1016/0014-4827(79)90450-6. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Shearer M., Taylor-Papadimitriou J. Regulation of cell growth by interferon. Cancer Metastasis Rev. 1987;6(3):199–221. doi: 10.1007/BF00144264. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]