Abstract
A 56-year-old man being treated for dilated cardiomyopathy presented with epigastralgia. He was diagnosed with ventricular tachycardia and Philadelphia chromosome-positive acute lymphoblastic leukemia. After treating incessant ventricular tachycardia, we commenced induction therapy for leukemia with dasatinib and prednisolone to minimize toxicity towards cardiomyocytes and the cardiac conduction system. Although dasatinib was temporarily withheld because of a recurrence of ventricular tachycardia, we rechallenged dasatinib while using bisoprolol and amiodarone and achieved a complete hematological response three weeks later. Although drug interactions between dasatinib and amiodarone were of concern, the blood concentration of each drug remained within the safe range after concomitant use, and there were no adverse cardiac effects such as QT prolongation after rechallenging dasatinib. Induction therapy with dasatinib and prednisolone may be an acceptable therapeutic option for Philadelphia chromosome-positive acute lymphoblastic leukemia with severe cardiac complications.
1. Introduction
Dasatinib, a second-generation tyrosine kinase inhibitor (TKI), has profoundly improved the prognosis for Philadelphia chromosome-positive leukemia [1]. Combined with steroids, dasatinib has been used to effectively treat Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) [2]. It is well known that TKIs have cardiovascular toxicity and can cause ventricular tachycardia (VT) because of QT prolongation [3–5]. In a randomized phase-3 trial, drug-related adverse cardiac effects occurred in 17 of 258 leukemia patients treated with dasatinib [6]. Dasatinib was also discontinued after QT prolongation in one patient among 26 Japanese leukemia patients [7]. In patients with advanced solid tumors, a high dasatinib blood concentration has been associated with prolongation of the QTc interval [8]. We should be aware of adverse cardiac effects when using TKIs. However, there are few data regarding treatments for Ph+ ALL patients with cardiomyopathy or life-threatening ventricular arrhythmias. Although it has been reported that drug interactions between dasatinib and amiodarone can occur [9], there has not yet been much clinical data. Herein, we describe induction therapy with dasatinib plus prednisolone with concomitant use of bisoprolol and amiodarone for a patient with Ph+ ALL who had dilated cardiomyopathy and incessant VT.
2. Case
A 56-year-old man who had been treated for dilated cardiomyopathy and atrial fibrillation presented at the hospital with epigastralgia. His electrocardiogram showed nonsustained VT, which developed into VT storms even after starting amiodarone (Figure 1). Nifekalant hydrochloride and lidocaine were also administered, and the ventricular arrhythmias then decreased, and atrial flutter was seen. After the hemodynamics had been stabilized, we started bisoprolol with a dose of 0.625 mg daily. While treating the cardiac complications, we assessed the patient's blood sample. Severe anemia and blastoid cells were observed in the peripheral blood. The bone marrow contained 96% lymphoid blast cells with a 46, XY, t(9;22)(q34;q11.2) karyotype, and minor bcr-abl mRNA transcripts were detected at 7.0 × 105 copies/μg RNA. The patient was diagnosed with Ph+ ALL and received induction therapy with dasatinib 50 mg twice daily and prednisolone 100 mg daily to minimize toxicity towards cardiomyocytes and the cardiac conduction system. We started dasatinib at a lower dose because of concerns about cardiotoxicity. Five days after initiating dasatinib and prednisolone, dasatinib was discontinued because of increments in nonsustained VT although the QTc interval had not become significantly prolonged (455 ms). We gradually increased the dose of bisoprolol to 3.75 mg daily. After being withdrawn for 22 days, dasatinib was rechallenged, and we could continue without the appearance of nonsustained VT or the prolongation of QTc intervals (Figures 2 and 3). The serum concentration of dasatinib one hour after oral administration (C1 hr) and the plasma concentrations of amiodarone and desethylamiodarone approximately 12 hours after oral administration on the 10th day of concomitant use were 45.7 ng/mL, 660.6 ng/mL, and 449.0 ng/mL, respectively. Three weeks after retreatment with dasatinib, we confirmed a complete hematological response and a 2-log reduction in minor bcr-abl mRNA transcripts in the patient's bone marrow (Table 1). He wished to be discharged from hospital and refused a dose-escalation of dasatinib or chemotherapy. On the 88th day after his admission, blastoid cells appeared in the peripheral blood. We discontinued dasatinib at that time because of the refractoriness of his leukemia. Although VT recurred and lasted for five days, it disappeared following the initiation of steroid pulse therapy and furosemide. After that, VT did not recur. We did not add any other TKIs or chemotherapy except for dexamethasone and the short-term use of mercaptopurine. Eventually, he died from leukemia on the 183rd day after his admission.
Figure 1.
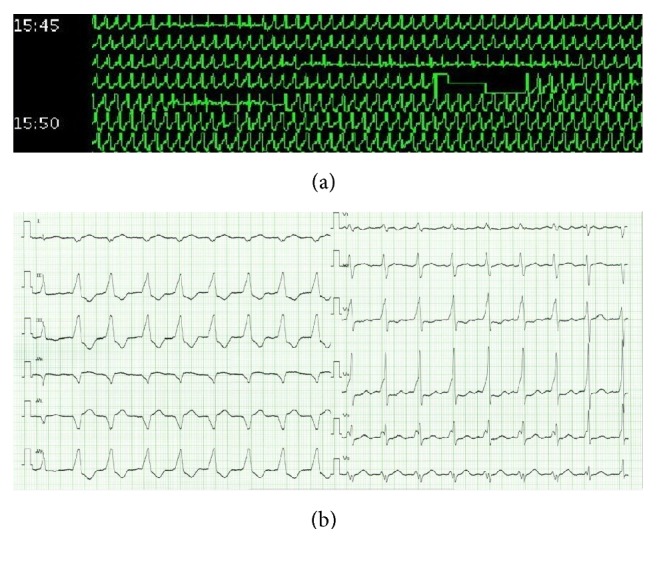
The 24-hour and twelve-lead electrocardiograms on admission. The 24-hour electrocardiogram shows a ventricular arrhythmia persisting for more than five minutes, and the twelve-lead electrocardiogram on admission shows incessant ventricular tachycardia.
Figure 2.
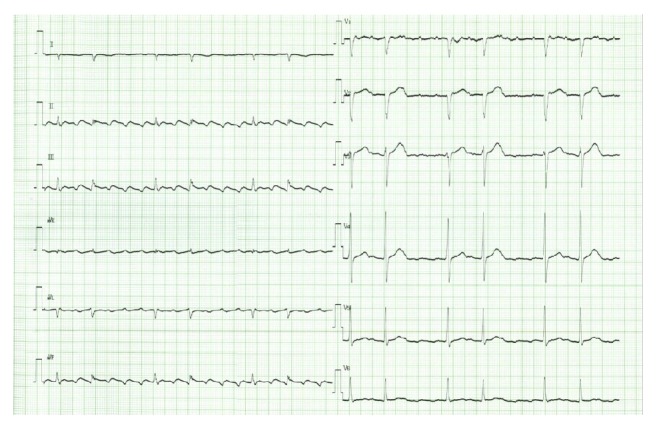
Electrocardiogram on the 22nd day after dasatinib retreatment. Potentially fatal ventricular arrhythmias have been replaced by atrial flutter.
Figure 3.
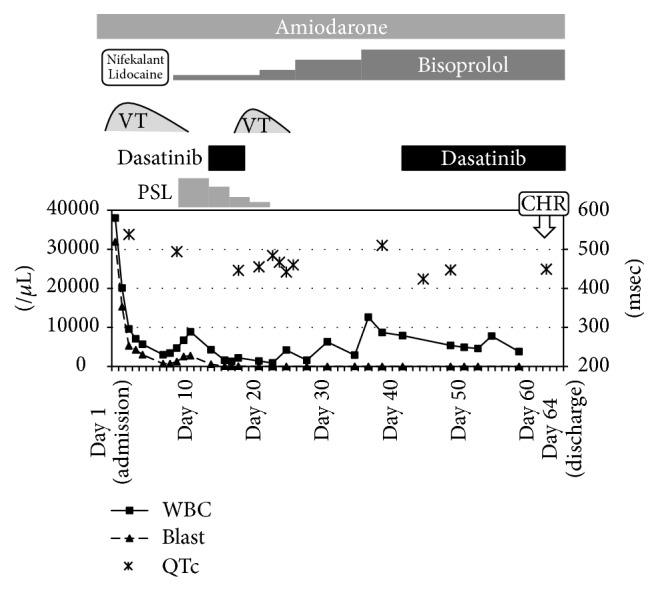
Clinical course before and after dasatinib treatment. VT, ventricular tachycardia; PSL, prednisolone; CHR, complete hematological response.
Table 1.
Laboratory data.
| Admission to hospital | Before retreatment with dasatinib | 22 days after retreatment | |
|---|---|---|---|
| Peripheral blood samples | |||
| Leukocytes (×102/μL) | 380 | 79 | 78 |
| Neutrophils (%) | 4 | 67 | 65 |
| Lymphocytes (%) | 7 | 28 | 15 |
| Monocytes (%) | 0 | 2 | 7 |
| Blastoid cells (%) | 84 | 0 | 0 |
| Hemoglobin (g/dL) | 7.6 | 9.4 | 8.2 |
| Platelet (×104/μL) | 5.4 | 19.8 | 6.8 |
| Bone marrow samples | |||
| Total nucleated cells (/μL) | 74500 | 11000 | |
| Blastoid cells (%) | 96 | 0.4 | |
| Minor bcr-abl mRNA (copy/μg RNA) | 7.0 × 105 | 7.9 × 103 | |
| Karyotype by G-banding | 46XY, t(9;22)(q34;q11.2) [17/20] |
46XY [20/20] |
3. Discussion
We encountered a Ph+ ALL patient with dilated cardiomyopathy accompanied by incessant VT and achieved a complete hematological response with dasatinib and prednisolone treatment without worsening the cardiomyopathy and ventricular arrhythmias.
Since chemotherapy including anthracycline confers a high risk of congestive heart failure [10], we avoided cardiotoxic antitumor agents and used dasatinib plus prednisolone instead. Dasatinib may also induce congestive heart failure because of fluid retention, pulmonary arterial hypertension, and ventricular arrhythmias [11]. In particular, we should be aware of adverse cardiac effects in a patient who has cardiac complications prior to therapy. In our case, we achieved a complete hematological response without fluid retention, pulmonary arterial hypertension, or ventricular arrhythmias, although this might have been affected by the short duration of dasatinib therapy.
We also continued dasatinib without exacerbating the VT. Spechbach et al. described a case of ventricular arrhythmia induced by dasatinib and the successful continuation of dasatinib with concomitant use of antiarrhythmic agents [12]. In the current case, we used bisoprolol and amiodarone to control VT during dasatinib therapy. Bisoprolol restrains excessive myocyte activity by inhibiting the myocardial beta 1 receptor [13], and amiodarone prolongs phase 3 cardiac action potential [14]. Dasatinib is not thought to directly harm cardiac mitochondrial function but to affect Purkinje-fiber assays and human ether-a-go-go-related gene potassium ion channels (HERG K+) [5, 15], which is why we might have been able to continue dasatinib without increments in VT during treatment with bisoprolol and amiodarone.
Dasatinib and amiodarone are both thought to be metabolized mainly by CYP3A4, and the concurrent use of these two drugs can elevate the concentration of each drug [9]. The actual dasatinib C1 hr was not particularly high, and the plasma concentration of amiodarone stayed within the safe range. It was speculated that the drug concentrations might have not reached a high enough range to inhibit CYP3A4 [16, 17].
Since a low plasma concentration of dasatinib can cause the bcr-abl T315I point mutation, we should use a substantial dose of dasatinib, if possible [18]. In the present case, there was a possibility that T315I mutations had occurred at the time of relapse, though this could not be assessed. The pharmacokinetics of dasatinib vary widely in individuals [8, 18]. Thus, we could consider a dose-escalation of dasatinib to prevent the occurrence of bcr-abl point mutations while monitoring the dasatinib blood concentration. We should have increased the dose of dasatinib to 140 or 180 mg daily and tried to maintain the dasatinib Cmax above 50 ng/mL [18, 19].
In conclusion, induction therapy with dasatinib and prednisolone may be an acceptable therapeutic option for Ph+ ALL patients with dilated cardiomyopathy and life-threatening ventricular arrhythmias. We can consider escalating the dose of dasatinib while monitoring its blood concentration. More cases should be accumulated to establish the optimal therapy for Ph+ ALL with severe cardiac complications.
Acknowledgments
The authors would like to thank T. Katayama for managing and processing the stored samples.
Competing Interests
Masayuki Hino received a research grant from Bristol-Myers Squibb and Astellas. Hirohisa Nakamae received a research grant from Bristol-Myers Squibb. All other authors have declared no financial interests/relationships relating to the topic of this article.
References
- 1.Talpaz M., Shah N. P., Kantarjian H., et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. New England Journal of Medicine. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 2.Foà R., Vitale A., Vignetti M., et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 3.Chen M. H., Kerkelä R., Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118(1):84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Force T., Krause D. S., Van Etten R. A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature Reviews Cancer. 2007;7(5):332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 5.Strevel E. L., Ing D. J., Siu L. L. Molecularly targeted oncology therapeutics and prolongation of the QT interval. Journal of Clinical Oncology. 2007;25(22):3362–3371. doi: 10.1200/JCO.2006.09.6925. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H. M., Shah N. P., Cortes J. E., et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa S., Nakamae H., Ogura M., et al. Efficacy and safety of dasatinib versus imatinib in Japanese patients with newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP): subset analysis of the DASISION trial with 2-year follow-up. International Journal of Hematology. 2014;99(2):141–153. doi: 10.1007/s12185-013-1470-1. [DOI] [PubMed] [Google Scholar]
- 8.Johnson F. M., Agrawal S., Burris H., et al. Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer. 2010;116(6):1582–1591. doi: 10.1002/cncr.24927. [DOI] [PubMed] [Google Scholar]
- 9.Haouala A., Widmer N., Duchosal M. A., Montemurro M., Buclin T., Decosterd L. A. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood. 2011;117(8):e75–e87. doi: 10.1182/blood-2010-07-294330. [DOI] [PubMed] [Google Scholar]
- 10.Shan K., Lincoff A. M., Young J. B. Anthracycline-induced cardiotoxicity. Annals of Internal Medicine. 1996;125(1):47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 11. Dasatinib Prescribing Information, http://www.sprycel.com.
- 12.Spechbach H., Morel P., Lorenzini K. I., et al. Reversible ventricular arrythmia induced by dasatinib. Clinical Case Reports. 2013;1(1):20–25. doi: 10.1002/ccr3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechat P., Brunhuber K. W., Hofmann R., et al. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. The Lancet. 1999;353(9146):9–13. doi: 10.1016/s0140-6736(98)11181-9. [DOI] [PubMed] [Google Scholar]
- 14.Yabek S. M., Kato R., Singh B. N. Effects of amiodarone and its metabolite, desethylamiodarone, on the electrophysiologic properties of isolated cardiac muscle. Journal of Cardiovascular Pharmacology. 1986;8(1):197–207. doi: 10.1097/00005344-198601000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Will Y., Dykens J. A., Nadanaciva S., et al. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicological Sciences. 2008;106(1):153–161. doi: 10.1093/toxsci/kfn157. [DOI] [PubMed] [Google Scholar]
- 16.Ohyama K., Nakajima M., Suzuki M., Shimada N., Yamazaki H., Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. British Journal of Clinical Pharmacology. 2000;49(3):244–253. doi: 10.1046/j.1365-2125.2000.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., He Y., Ruiz C. H., Koenig M., Cameron M. D. Characterization of dasatinib and its structural analogs as CYP3A4 mechanism-based inactivators and the proposed bioactivation pathways. Drug Metabolism and Disposition. 2009;37(6):1242–1250. doi: 10.1124/dmd.108.025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi N., Miura M., Scott S. A., Niioka T., Sawada K. Pharmacokinetics of dasatinib for Philadelphia-positive acute lymphocytic leukemia with acquired T315I mutation. Journal of Hematology & Oncology. 2012;5, article 23 doi: 10.1186/1756-8722-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biological and Pharmaceutical Bulletin. 2015;38(5):645–654. doi: 10.1248/bpb.b15-00103. [DOI] [PubMed] [Google Scholar]


