Summary
Listeriolysin O (LLO) has been proposed as a potential carrier or adjuvant molecule in the vaccination field. However, the cytotoxic and pro‐apoptotic effects of LLO are the major limitations for this purpose. Here, we have performed a preclinical safety evaluation and characterized a new potential adjuvant application for a non‐cytolytic LLO mutant (dtLLO) to enhance and modulate the immune response against the envelope (E) protein from dengue virus. In addition, we have studied the adjuvant effects of dtLLO on human immune cells and the role of membrane cholesterol for the binding and proinflammatory property of the toxoid. Our in‐vivo results in the murine model confirmed that dtLLO is a safer molecule than wild‐type LLO (wtLLO), with a significantly increased survival rate for mice challenged with dtLLO compared with mice challenged with wtLLO (P < 0·001). Histopathological analysis showed non‐toxic effects in key target organs such as brain, heart, liver, spleen, kidney and lung after challenge with dtLLO. In vitro, dtLLO retained the capacity of binding to plasma membrane cholesterol on the surface of murine and human immune cells. Immunization of 6–8‐week‐old female BALB/c mice with a combination of dtLLO mixed with E protein elicited a robust specific humoral response with isotype diversification of immunoglobulin (Ig)G antibodies (IgG1 and IgG2a). Finally, we demonstrated that cholesterol and lipid raft integrity are required to induce a proinflammatory response by human cells. Taken together, these findings support a potential use of the dtLLO mutant as a safe and effective adjuvant molecule in vaccination.
Keywords: adjuvant, cholesterol, dengue, listeriolysin O
Introduction
Adjuvant molecules are components in human vaccine formulations, which are added to increase the immunogenicity of antigens and to modulate the immune response against them 1. Currently there is a limited number of licensed adjuvants for human use; consequently, basic studies and characterization of new molecules are of high priority in the vaccination field 2. In recent years, listeriolysin O (LLO), the major virulence factor from Listeria monocytogenes, has been proposed as a potential microbial adjuvant/carrier molecule based on its biological properties, resulting in both the induction of the production of proinflammatory cytokines such as interleukin (IL)−1β, IL‐6, IL‐8, tumour necrosis factor (TNF)‐α 3, 4, 5, 6, 7, 8 and the capability of membrane pore‐forming activity to introduce exogenous antigens into intracellular compartments 9, 10. However, major limitations for the use of wild‐type LLO (wtLLO) in human vaccination are the previously described pro‐apoptotic effects and in‐vitro cytotoxicity of wtLLO on different cell types 11, 12, 13, 14, 15. In vivo, wtLLO is lethal to mice when they are injected intravenously (i.v.) or intraperitoneally (i.p.) with the toxin 16, 17, 18. Also, injection of wtLLO into the footpads of mice led to terminal deoxynucleotidyl transferase dUTP nick end‐labelling (TUNEL)‐positive cells in the peripheral cortex and paracortex of the draining popliteal lymph node, confirming the pro‐apoptotic activity of LLO in vivo 19. Interestingly, deletions or mutations into domain 4 of the wtLLO structure abrogate the in‐vitro cytotoxic and pro‐apoptotic activities 20, 21, 22, opening the possibility to use safer LLO variants than the wild‐type molecule. Here, we show new evidence about the safety and adjuvanticity of a previously described detoxified non‐haemolytic LLO mutant, named dtLLO 23. This toxoid has been generated by introducing mutations in three key amino acids into the undecapeptide sequence in domain 4 of the LLO structure at residues 484, 491 and 492 23. These mutations were chosen because the selected amino acids have been postulated to be important for eventual membrane pore formation 21. dtLLO has been tested previously as an effective adjuvant in tumour immunotherapy in a mouse model, and was found to potentiate anti‐tumour‐specific T cell responses. This molecule has pathogen‐associated molecular pattern (PAMP) properties, and activates the innate immune response in a Toll‐like receptor (TLR)‐4 independent mechanism 23.
There is limited evidence for the in‐vitro cytotoxic effects of dtLLO. A previous study has demonstrated the reduced lytic effect of dtLLO on sheep red blood cells (RBCs). No other studies have addressed the issue of toxic activity on other cell populations.
Interestingly, there are no published studies that address specifically the in‐vivo toxicity of dtLLO. In this paper we describe a preclinical safety evaluation using a single‐dose i.v. administration of dtLLO in the mouse model to identify potential target organs for toxicity. In addition, we have evaluated a new adjuvant application for this non‐cytotoxic LLO mutant to potentiate and modulate the humoral immune response against microbial antigens from dengue virus (DENV), a pathogen of global public health concern. Finally, we evaluated the capacity of dtLLO to induce a proinflammatory response by human immune cells such as monocyte‐derived dendritic cells (moDCs) and peripheral blood mononuclear cells (PBMCs), and found that the adjuvant effect of dtLLO on human cells is dependent upon the retention of dtLLO to bind to cholesterol.
Materials and methods
Purification of wtLLO and dtLLO
Construction of plasmids with the cloned sequences for wtLLO and dtLLO has been described previously 23. BL21 (DE3) chemically competent cells (Invitrogen, Carlsbad, CA, USA) were transformed with the recombinant protein expression plasmids and grown in Luria–Bertani (LB) medium containing 30 μg/ml of kanamycin (Sigma Aldrich, St Louis MO, USA). wtLLO and dtLLO were purified as described previously 24. Protein expression was induced in BL21 cells by the addition of 1 mM isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) and the culture was incubated, shaking at 30°C for 6 h. The purification was realized by a polyhistidine motif contained in the c‐terminal region of the proteins to allow for isolation on a nickel‐nitrilotriacetic acid (Ni‐NTA) column (Qiagen, Venlo, Limburg, the Netherlands), following the manufacturer's instructions. The purified recombinant proteins were dialyzed extensively against storage buffer [50 mM phosphate/acetate (pH 6.0), 1 M NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA) and 5 mM dithiothreitol (DTT)]. The recombinant proteins were purified further by passage over Detoxy‐Gel endotoxin removal columns (ThermoFisher Scientific, Waltham, MA, USA). The purity and integrity of the recombinant proteins were confirmed by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Endotoxin levels were measured by chromogenic Limulus amoebocyte lysate test (Lonza, Basel, Switzerland), according to the manufacturer's protocol. Endotoxin levels were < 1 EU/mg of protein.
Haemolysis assay for dtLLO
Lytic activity of wtLLO and dtLLO was measured by haemoglobin release from human RBCs (HRBCs), as described previously 25. wtLLO and dtLLO were diluted twofold serially with phosphate‐buffered saline (PBS) [pH 6·0, 0·1% bovine serum albumin (BSA)] and then 0·2% HRBCs were added for 30 min at 37°C. PBS was used as a negative control; wtLLO and dtLLO final concentrations covered a range from 25 to 0·02 μg/ml. After incubation, RBCs were spun out at 600 g to remove unlysed RBCs. The supernatants were collected and the degree of haemolysis was evaluated by measuring the absorbance of haemoglobin (405 nm) released from erythrocytes.
Ethics statement
The animal study was conducted in compliance with the Good Laboratory Practices and Use of Laboratory Animals (NOM‐062‐ZOO‐1999). The study was approved by the Ethics Committee of the Instituto de Investigaciones Médico‐Biológicas, Universidad Veracruzana. For survival experiments, mice were monitored closely for signs of illness for 10 min and were euthanized humanely when the time was reached.
Systemic toxicity of wtLLO and dtLLO in mice
Female 6‐8‐week‐old BALB/c mice were challenged i.v. with 20 μg of recombinant dtLLO or wtLLO diluted in a 100 μl volume of sterile physiological saline solution (PSS). A negative control group was included in the experiment, challenging with 100 μl volume of PSS. All animals were monitored during the first 10 min–1 h after the challenge to record mortality. Evaluation of the acute systemic toxic effects on key target organs was performed. The organ weight and histopathology were examined. Heart, liver, spleen, lungs, kidneys and brain were collected from all mice groups after they died or after the first 10 min or 1 h post‐challenge, depending on the mortality and survival of each group. Organs were fixed with 4% buffered formalin, processed and embedded in paraffin. Tissue sections were stained with haematoxylin and eosin (H&E).
TUNEL assay
Apoptosis in vivo was assessed by TUNEL assay. Lungs, liver, heart, spleen and kidneys were fixed overnight at 4°C in 10% buffered formalin and embedded in paraffin. An in‐situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN, USA) was used to carry out TUNEL staining on sections of 5‐μm thickness and fluorescence detection, according to the manufacturer's instructions. A positive control was included in the experiments for tissue sections from each organ analysed. The tissue sections were permeabilized and treated with DNAse 1 recombinant 1500 U/ml in 50 mM Tris HCl, pH 7·5, 10 mM MgCl2, 1 mg/mL BSA buffer for 10 min at +15 to +25 ºC to induce DNA strand breaks. Finally, the slides were rinsed again with PBS and analysed using a fluorescence microscope and excitation wavelength in the range of 450–500 nm and detection in the range of 515–565 nm (green).
Binding capacity of wtLLO and dtLLO to murine cholesterol cell membranes
Spleens were removed aseptically from BALB/c mice and squeezed between a cell strainer and a syringe plunger to prepare a single‐cell suspension. Cells were pooled and treated with 0·83% ammonium chloride in 170 mM Tris‐HCl (pH 7·65) to lyse erythrocytes. Cells were washed twice with RPMI‐1640 medium and suspended at 2 × 106 cells/ml density in RPMI‐1640 medium. Spleen cells (1× 106) were stimulated with dtLLO 10 µg/ml or wtLLO 10 µg/ml in RPMI‐1640 medium for 10 or 20 min at low temperature (4°C) to prevent membrane recycling and internalization of the antigens. After the incubation time was over, the cells were fixed with paraformaldehyde (PFA) 4% for 20 min. Then, spleen cells were blocked with bovine fetal serum 5% and stained with an anti‐LLO primary rabbit antibody (Abcam, Cambridge, UK), followed by an Alexa Fluor 488 anti‐rabbit IgG (Invitrogen). The stained cells were analysed in an Accuri C6 flow cytometer. To evaluate the interaction with plasma membrane cholesterol, spleen cells were treated previously with methyl‐beta cyclodextrin (mβCD) (Sigma Aldrich) 5 mM for 30 min before the stimulation with wtLLO and dtLLO;the cells were washed twice with medium and then stimulated with the proteins, and were finally fixed and stained as described previously.
In‐vivo proinflammatory effect of dtLLO
In order to determine the proinflammatory effects of dtLLO in an in‐vivo model, BALB/c mice were injected i.p. with dtLLO (20 μg/mouse), lipopolysaccharide (LPS) (20 μg/mouse) as positive control (Sigma Aldrich) or 100 μl volume of PSS as the negative control group. Serum samples were collected after 1 and 2 h post‐immunization and the levels of IL‐12 and IL‐6 were measured by commercial enzyme‐linked immunosorbent assay (ELISA) (Peprotech, Rocky Hill, NJ, USA).
Immunizations
Groups of 10 female 6–8‐week‐old BALB/c mice were immunized i.p. with an experimental vaccine, formulated by a single combination of dtLLO (20 μg) plus E protein (5 μg) from dengue virus serotype 4 (DENV4) (CTK Biotech, San Diego, CA, USA). Appropriate control groups were included: a group of mice immunized with E protein alone (5 μg) i.p. or a group of mice immunized with a combination of E protein (5 μg) mixed with alum (2% alhydrogel; Brenntag Biosector, Frederikssund, Denmark), a well‐characterized human adjuvant. All animals received a booster injection of the same dose and route on day 14. Serum samples were collected from mouse‐tail veins at 0, 14 and 28 days post‐immunization to determine humoral primary and secondary responses.
Evaluation of E‐protein‐specific antibody titres
Serum‐specific total IgG or IgG1 and IgG2a isotypes titres for E protein from DENV4 were measured by ELISA. Briefly, ELISA plates were coated with recombinant E protein at 5 μg/ml diluted in carbonate buffer, pH 9·6, incubated overnight at 4ºC. Plates were washed five times with PBS containing 0·05% Tween 20 (PBST) and blocked for 1 h with PBS containing 0·5% BSA (Sigma Aldrich). Serially diluted serum samples were incubated for 2 h at 37ºC. Plates were washed again, as described previously. Specific E protein antibodies were revealed adding horseradish peroxidase (HRP)‐labelled goat anti‐mouse IgG, IgG1 or IgG2a at a dilution of 1 : 2500 (Jackson ImmunoResearch, West Grove, PA, USA) antibodies. Plates were washed as described previously, followed by the addition of 2, 2'‐azinobis (3‐ethylbenzothiazoline‐6‐sulphonic acid)‐diammonium salt (ABTS) substrate (Sigma Aldrich). Finally, optical density (OD) was measured in an ELISA plate reader (Awareness Technology Inc., Palm City, FL, USA). Titres were calculated through linear regression equations as the reciprocals of the serum dilutions that produced OD405 nm values of 0·2 above the blank, and the titres were reported in ELISA units per ml (EU ml− 1).
Binding capacity of dtLLO to human cholesterol membranes
PBMCs were isolated from healthy blood donors using density gradient centrifugation on Lymphoprep (Axis Shield, Dundee, Scotland, UK), according to the manufacturer's instructions. PBMCs (1 × 106) were stimulated with dtLLO 1 µg/ml or wtLLO 1 µg/ml in RPMI‐1640 medium for 10 or 20 min at low temperature (on ice) to prevent membrane recycling and internalization of the antigens. After incubation, the cells were fixed with PFA 4% for 20 min. Then, PBMCs were blocked with 2% BSA–PBS and stained with an anti‐LLO primary antibody (Abcam), followed by an Alexa Fluor 488 anti‐rabbit IgG (Invitrogen). The stained cells were analysed in an Accuri C6 flow cytometer. To evaluate the interaction with cholesterol of PBMC membranes, cells were treated previously with mβCD (Sigma Aldrich) at 5 mM for 30 min before stimulation with wtLLO and dtLLO, then the cells were washed, stimulated and finally fixed and stained as described previously.
Stimulation assays with human immune cells
For human dendritic cell (DC) differentiation, PBMCs were incubated for 2 h in 75 cm2 plastic culture flasks (Corning, New York, NY, USA). The non‐adherent fraction was washed out thoroughly, and isolated adherent monocytes were incubated in RPMI‐1640 medium in the presence of human granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (1·5 ng/ml; Peprotech) and human IL‐4 (15 ng/ml; Peprotech) for 6 days. Human DCs were analysed for CD1a, CD11c, CD14 and major histocompatibility complex (MHC) II expression by flow cytometry to confirm differentiation and DC phenotype. PBMC (1 × 106 or 2 × 105) human DCs were stimulated with dtLLO 1 µg/ml or LPS 1 µg/ml (Sigma Aldrich) in the presence or absence of 50 µg/ml of polymyxin B (Invivogen, San Diego, CA, USA). After 24 h stimulation, the supernatants were collected and tested for cytokine production by ELISA. To evaluate if the interaction of dtLLO with cholesterol is required for proinflammatory property on human immune cells, PBMCs (1 × 106) were incubated with mβCD (Sigma Aldrich) at 1 and 5 mM for 30 min at 37°C, washed with medium and then stimulated with dtLLO (1 µg/ml) for 4 h at 37°C. The supernatants were collected and tested for cytokine production by ELISA.
In other experiments, stimulation of PBMCs with dtLLO (1 µg/ml) was performed in the presence of fisetin (Sigma Aldrich) at 7 and 28 μM doses. For the experiments with mβCD and fisetin, the cell viability was tested before and after the stimulation times using trypan blue, with no cytotoxic effects observed.
Analysis of cytokines
Concentrations of murine IL‐6 and IL‐12 and human TNF‐α, IL‐6, IL‐12, IL‐10 in murine serum samples, or culture supernatants from stimulated human immune cells were measured by commercial ELISA (Peprotech). Briefly, ELISA plates (Corning) were coated overnight with specific capture antibodies. Next day, the plates were blocked with 0·5% BSA (Sigma Aldrich) and washed with PBS‐Tween (PBST). Murine serum samples from in‐vivo experiments or cell culture supernatant from in‐vitro‐stimulated human cells were then added and incubated at room temperature for 2 h. A linear standard curve was generated using appropriate recombinant cytokines. After washing with PBST, specific biotin‐conjugated anti‐cytokine antibodies were added and allowed to incubate for 2 h at room temperature. Plates were washed with PBST and an avidin–HRP conjugate was added for 30 min. Finally, the plates were washed again with PBST, followed by incubation with the substrate ABTS (Sigma Aldrich). Absorbance was measured at 450 nm using an ELISA plate reader. The concentrations for each cytokine in the sample were determined by extrapolating OD values using the standard curve. The values were expressed in pg/ml.
Statistical analysis
The statistical significance of the differences between IgG, IgG1 and IgG2a titres and cytokine concentrations were analysed using the one‐way analysis of variance (anova) statistical test with Tukey's post‐test, using GraphPad Prism software version 6.01 (GraphPad Software, San Diego, CA, USA). P‐values < 0·05 were considered statistically significant. Significant P‐values for all comparisons are depicted in the figures as follows: *P < 0·05; **P < 0·01; and ***P < 0·001; non‐significant P‐values are shown as n.s.
Results
Safety evaluation for dtLLO
The biological ability of adjuvant molecules to enhance or modulate the immune response to different antigens is well documented. However, the use of adjuvants represents a potential safety risk for the host, which needs to be evaluated in preclinical studies. Thus, the first aim of our study was to evaluate the safety of dtLLO in vitro and in vivo, first as an individual molecule and then as part of an experimental vaccine formulation. Given that the cytolytic activity could be a major problem for LLO in biomedical applications, we evaluate the lytic potential of both dtLLO and wtLLO in a haemolysis assay, using human erythrocytes as substrate. Our results confirm the data reported previously for bovine and sheep RBCs, where wtLLO exhibited a highly haemolytic activity on human erythrocytes in a wide range of concentrations (25–0·01 μg/ml) (Fig. 1a). In contrast, dtLLO did not show detectable haemolytic activity, even at the highest concentrations (25–12·5 μg/ml) (Fig. 1a). In order to evaluate the safety of the dtLLO mutant, we performed further experiments to determine dtLLO safety in an in‐vivo mouse model. We know from previous studies that i.v. administration of wtLLO causes a convulsive, rapidly fatal reaction in mice 16, 17, 18, and that 200 μg of dtLLO can be administered safely to C57BL/6 mice subcutaneously (s.c.) 23. In addition, i.v. injection of wtLLO causes high lethality within 4 or 5 min. Electrocardiograms from LLO‐treated mice indicate serious alterations in heart rate and rhythm, suggesting damage to contractile and pacemaker cardiac tissue 18. On the basis of this evidence, we compared the systemic effects and lethality rate between dtLLO and wtLLO. We challenged groups of female BALB/c mice with a high and lethal dose of wtLLO (20 μg per mouse, i.v.) and compared with the group of mice challenged with the same dose of dtLLO or the group of mice injected only with PSS as a negative control. For ethical reasons, and given the previous information about the effects and time in which wtLLO causes high lethality rates 16, 18, we decided to perform these experiments with the minimum number of animals per group required to achieve statistical significance (five mice per group). All experimental mice groups were monitored for 10 min to compare the lethality rate. As expected, i.v. challenge with wtLLO resulted in a high percentage of lethality (80%) of BALB/c mice in an interval of 2–10 min (Fig. 1b). Respiratory failure and convulsions preceded death. In contrast, the group of mice challenged with dtLLO showed 100% survival (Fig. 1b). In an independent experiment, mice immunized (n = 15) i.p. with 20 μg of dtLLO were monitored for 4 weeks after dtLLO administration, with 0% lethality (data no shown). The above results confirm that the molecule dtLLO was a detoxified and safe protein, even when the toxoid was administered to the mice by systemic routes.
Figure 1.
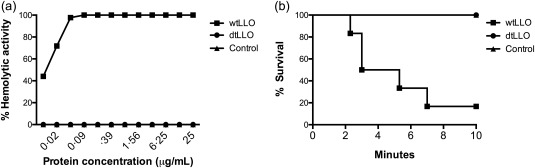
Evaluation of detoxified listeriolysin O (dtLLO) safety. Haemolytic activity of dtLLO was determined by red blood cell (RBC) lysis assay (a). Briefly, wild‐type LLO (wtLLO) and dtLLO proteins (25–0·02 μg/ml) were incubated with human RBCs. The percentage of haemolytic activity was determined by measuring released haemoglobin (optical density at 405 nm). In‐vivo safety of dtLLO (b) represents the survival curves of mice challenged intravenously (i.v.) with 20 μg of dtLLO or wtLLO. The lines show the percentage of survival after the challenge with the recombinant proteins over 10 min. One representative experiment of two independent experiments is shown.
While there is a clear description of the toxic effects of wtLLO on different tissues during an in‐vivo bacterial infection 26, little is known about histopathological findings in mice challenged with purified wtLLO, and nothing is known about the toxicity of recombinant dtLLO on key target organs. To address this issue, the severity of the tissue damage in mice was assessed through conventional histological analysis. Spleen, liver, lungs, kidney, heart and brain from mice challenged with dtLLO, wtLLO or PSS were collected during or at the first 10 min or 1 h of antigen injection (depending on the length of time the mice survived). Mice injected with wtLLO exhibited multiple focal haemorrhages, sarcoplasmic vacuoles and hypercontracted fibres in the heart. The lungs showed oedema and intra‐alveolar haemorrhage and focal subpleural haemorrhage. The kidneys showed generalized congestion, focal haemorrhage and vacuolation of epithelial cells of distal tubules. Furthermore, livers and spleen showed multiple haemorrhages and dilatation of centrilobular veins. We observed subarachnoid haemorrhage in the brain, especially in the temporal lobes and brain stem. Focal haemorrhage, neuropil oedema and vacuolation of neurones were observed in sections of brain stem. Organs from mice treated with PSS or dtLLO exhibited normal tissue structure with minimal congestion. A summary of the histopathological findings in the experimental groups is shown in Table 1. Representative pictures from the tissue damages of lungs, spleen, liver, kidney, heart and brain in the immunized mice groups with wtLLO and dtLLO are shown in Fig. 2. Because recombinant or purified wtLLO is highly lytic for the cells and may also trigger apoptotic cell death by itself in vitro or during infection by LM in vivo in the spleen, lymph nodes, liver and brain 19, 27, 28, we examined if dtLLO mutant protein retains the pro‐apoptotic effects of wtLLO. To answer this issue, we analysed the presence of apoptotic cells in the same mice tissue sections that we collected for the H&E staining after 60 min post‐challenge with dtLLO. Apoptotic cells were detected by TUNEL assay as described in the Material and methods section. No evidence of significant apoptotic cells was found in all the organs analysed, including lung, spleen, heart, kidney and liver (Fig. 3). Taken together, the mice lethality rate, immunohistochemistry analysis of the mice tissue sections and TUNEL assay results after challenge with dtLLO confirmed that this toxoid is a safe molecule without significant systemic toxic effects, such as those observed with wtLLO.
Table 1.
Summary of histopathological findings in the experimental groups
| Control | wtLLO | dtLLO | |
|---|---|---|---|
| Heart | Minimal congestion |
Severe congestion Focal haemorrhages Presence of sarcoplasmic vacuoles and hypercontracted fibres Perivascular plasma extravasation |
Minimal congestion |
| Lungs | Minimal congestion |
Severe congestion Intra‐alveolar oedema and haemorrhage Multiple subpleural focal haemorrhages |
Minimal congestion |
| Kidney | Minimal medullary and cortical congestion |
Severe congestion in medulla, cortex and glomeruli Distal tubular focal haemorrhages Focal vacuolation of epithelial cells in distal tubules |
Minimal medullary and cortical congestion |
| Liver | Minimal congestion |
Severe congestion Central vein dilatation Subcapsular haemorrhages |
Minimal congestion Mild dilatation of vessels |
| Spleen | Minimal congestion | Severe congestion | Minimal congestion |
| Brain |
Minimal capillary congestion Few ischaemic (eosinophilic) neurones (Purkinje cells and hippocampus) |
Severe congestion Focal subarachnoid haemorrhage Extravasation of proteinaceous material Ischaemic neurones in cerebellum (Purkinje cells, dentate nucleus) and hippocampus Neuropil oedema, vacuolation of neurones and focal haemorrhages (brain stem) Scarce oligodendrocytes with cytoplasmic vacuolization Vascular dilatation |
Minimal capillary congestion Few ischaemic (eosinophilic) neurones (Purkinje cells and hippocampus) |
dtLLO = detoxified listeriolysin O; wtLLO = wild‐type listeriolysin O.
Figure 2.
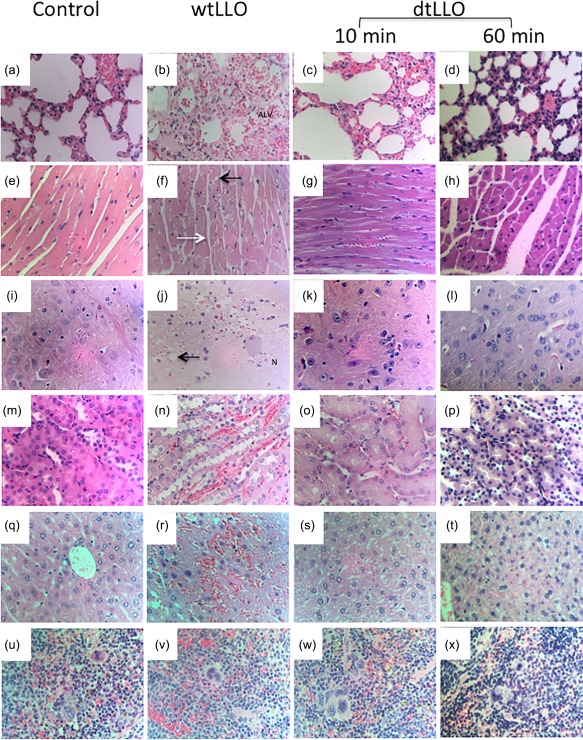
Histopathology findings in mice challenged intravenously (i.v.) with physiological saline solution (PSS) (negative control group), wild‐type listeriolysin O (wtLLO) (20 μg per mouse) or detoxified LLO (dtLLO) (20 μg per mouse) (n = 5 each group). Histological sections of lung (a–d), heart (f–h), brain (i–l), kidney (m–p), liver (q–t) and spleen (u–x) showing vascular congestion in control and toxoid groups (dtLLO) and severe changes in the toxin‐treated group (wtLLO) including oedema and alveolar haemorrhage (b), sarcoplasmic vacuoles (black arrow) and hypercontracted fibres (white arrow) in the heart (f), focal haemorrhages, neuropil oedema and vacuolation of neurones in brain sections (j), focal haemorrhages and vacuolation of epithelial cells of distal tubules in kidneys (n) and multiple haemorrhages in the liver (r) and spleen (v). Haematoxylin and eosin ×400. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
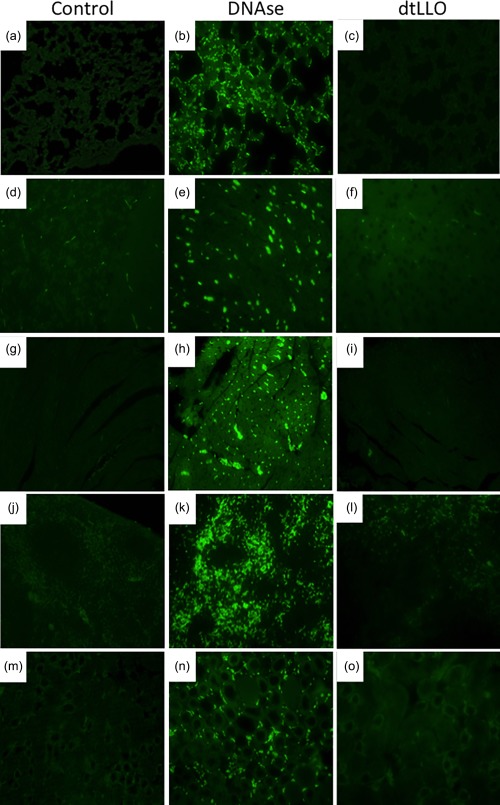
Terminal deoxynucleotidyl transferase dUTP nick end‐labelling (TUNEL) assay in mice challenged intravenously (i.v.) with detoxified listeriolysin O (dtLLO) (20 μg per mouse) or physiological saline solution (PSS) (n = 5 each group). Analysis of apoptotic cells after the challenge of mice with dtLLO was determined by TUNEL assay after 60 min post‐injection. Histological sections from lungs (a–c), liver (d–f), heart (g–i), spleen (j–l) and kidney (m–o) were fixed, processed and stained with TUNEL stain. A positive control was included in the experiments for tissue sections from each organ analysed. The positive controls were prepared from tissue sections permeabilized and treated with DNAse 1 recombinant 1500 U/ml. Tissue sections from mice injected with SSF were included as negative control. Apoptotic cells are marked as green dots after TUNEL staining, as observed under a fluorescent microscope. Green dots were not visible in the negative control or dtLLO group. Magnification ×400. [Colour figure can be viewed at wileyonlinelibrary.com]
Cholesterol‐dependent binding of dtLLO to murine cell membranes
Some of the most important biological functions of LLO depend upon the interaction with cell membranes through cholesterol, which is a key receptor. It is well known that a conserved undecapeptide sequence in domain 4 of LLO structure is considered crucial for membrane binding and cytotoxic activity 29. However, a previous report has shown that mutations in two tryptophan at positions 491 and 492 in this undecapeptide sequence do not prevent LLO from binding to cells 30. To address if dtLLO can bind to cell membranes, we performed experiments using pulsed murine splenocytes with wtLLO or dtLLO for 10 and 20 min on ice to prevent membrane recycling and the internalization of the antigens. The cells were then fixed with PFA, and evaluated by flow cytometry as described previously in the Material and methods section. As shown in Fig. 4a–d, dtLLO conserves the ability to bind with high affinity to the cell membranes of murine splenocytes, similarly to wtLLO. Interestingly, the binding property of dtLLO to the cells depend upon the presence of cholesterol in cell membranes, because pretreatment of splenocytes with mβCD, a pharmacological agent used commonly to remove membrane cholesterol, abrogates the binding of dtLLO to the splenocyte membrane significantly (Fig. 4e–h).
Figure 4.
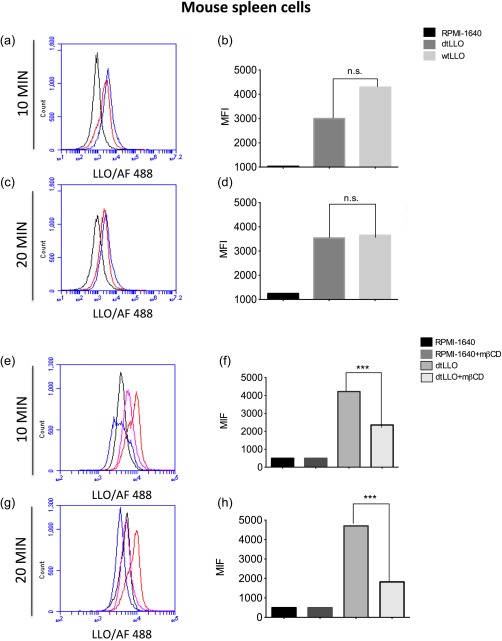
Detoxified listeriolysin O (dtLLO) preserves the binding property of LLO to the plasma membrane of murine cells. Whole spleen cells were pulsed with dtLLO (red line) or wild‐type LLO (wtLLO) (blue line) for 10 or 20 min. Non‐pulsed cells were included (black line) as a negative control. The pulsed cells were fixed and stained as described previously in the Materials and methods section and analysed by flow cytometry (a,c). Bars (b,d) represent the mean fluorescence intensity for each variable in (a) and (c). To evaluate the role of cholesterol in the binding property of the toxoid to the plasma membranes, spleen cells were treated (pink line) or not (red line) with methyl‐β‐cyclodextrin (mβCD) for 30 min at 37ºC, washed and then pulsed with dtLLO for 10 or 20 min; the cells were then fixed, stained and analysed by flow cytometry. Non‐pulsed cells were treated (blue line) or not (black line), with mβCD as controls (e–g). Bars (f,h) represent the mean fluorescence intensity for each variable in (e) and (g). One representative experiment of three independent experiments is shown. [Colour figure can be viewed at wileyonlinelibrary.com]
In‐vivo induction of proinflammatory cytokines by dtLLO
A major mechanism by which adjuvant molecules potentiate and modulate the immune response against vaccine antigens is by inducing the production of a variety of proinflammatory cytokines, which help to shape the adaptive immune response. In order to test if dtLLO retains the ability to induce a proinflammatory response we performed some in‐vivo experiments, immunizing female BALB/c mice (n = 11 each group) i.p. with the toxoid (20 μg per mouse) and comparing with a positive control (LPS). We decided to test two representative proinflammatory cytokines, IL‐6 and IL‐12. As shown in Fig. 5, dtLLO retains its proinflammatory activity in vivo. One and 2 h after the challenge with dtLLO, both IL‐6 and IL‐12 reached high serum levels. However, the inflammatory response induced by the dtLLO is considerably lower compared to the robust production of IL‐6 and IL‐12 induced by LPS. Collectively, these results suggest that dtLLO delivered i.p. acts as a safe adjuvant molecule inducing in‐vivo activation and increasing the production of key innate cytokines such as IL‐6 and IL‐12.
Figure 5.
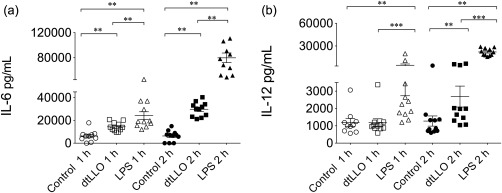
In‐vivo production of proinflammatory cytokines after the challenge of BALB/c mice with detoxified listeriolysin O (dtLLO). Groups of female BALB/c mice (n = 11 each group) were challenged intraperitoneally (i.p.) with physiological saline solution (control) dtLLO (20 μg per mouse) or lipopolysaccharide (LPS) (20 μg per mouse). The levels of interleukin (IL)‐6 and IL‐12 in the serum of immunized mice were measured by enzyme‐linked immunosorbent assay (ELISA) after 1 and 2 h post‐challenge. Significant P‐values are shown as follows: **P < 0·01; ***P < 0·001.
Evaluation of the efficacy of dtLLO as an adjuvant molecule to potentiate humoral immune response to dengue antigens
Given our previous results, confirming that dtLLO retains its binding and proinflammatory properties in the absence of adverse effects and without in‐vivo toxicity, we next evaluated the adjuvant potential of dtLLO for the E protein from DENV, a re‐emerging pathogen and a major public health concern worldwide. Recently a licensed vaccine for dengue virus has become available to protect against this viral pathogen; however, the performance and protection generated by this vaccine is controversial 31 The E protein is a key surface viral glycoprotein involved in the binding of DENV to the cell receptors. E protein is the immunodominant antigen, and elicits protective immunity through the induction of specific antibodies 32.
Using the experimental vaccination approach, consisting of a single combination of dtLLO (20 μg/ml) mixed with E protein (5 μg/ml) from DENV4, we assessed the adjuvant capacity of dtLLO to increase and modulate the humoral immune response against this viral antigen. A group of mice immunized with alum adjuvant combined with E protein (5 μg/ml) was included in the experiments to compare the humoral immune response induced by these two different adjuvants. A control mouse group immunized with E protein alone (5 μg/ml) was also included. After a single immunization, total IgG titres (Fig. 6a) and IgG1 and IgG2a (Fig. 6b,c) isotypes were measured in all experimental groups on day 14 post‐immunization (primary response). All animals received a boost dose on day 14 and the total IgG titres (Fig. 6d) and the IgG1 and IgG2a (Fig. 6e,f) isotypes were measured again on day 28 after the boost dose. For both primary and secondary responses, the dtLLO–E protein combination elicited higher titres of total specific IgG compared to E protein alone (P < 0·05 and P < 0·01, respectively), without significant differences with respect to the total IgG titres induced by the alum–E protein formulation in the primary response (P > 0·05) but with a statistically significant difference in the secondary response (P < 0·001). Furthermore, while E protein alone or mixed with alum induced a typical Th2 immunoglobulin pattern characterized by the production of E‐protein specific IgG1 titres, the formulation of E protein plus dtLLO adjuvant elicited a balanced production of T helper type 1 (Th1) (IgG2a) and Th2 (IgG1) isotypes, with higher titres of both isotypes than the E protein alone. For the IgG2a isotype, dtLLO induced a more potent response than the alum–E protein formulation in both primary (P < 0·001) and secondary responses (P < 0·01). No adverse effects of the experimental vaccine could be seen after the first and second immunizations using dtLLO as an adjuvant i.p.
Figure 6.
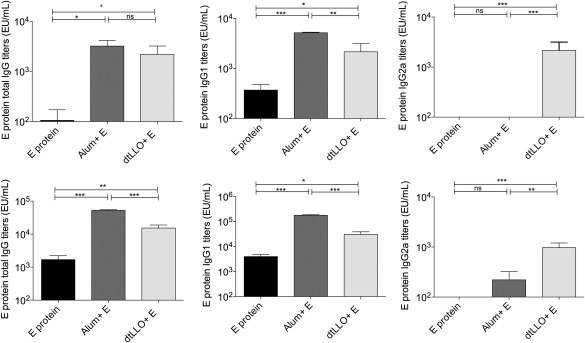
Detoxified listeriolysin O (dtLLO) enhances the specific humoral response against envelope protein from dengue virus 4 (DENV4). Groups of BALB/c mice (n = 10) were immunized intraperitoneally with E protein (5 μg per mouse), E protein (5 μg per mouse) plus alum or E protein (5 μg per mouse) plus dtLLO (20 μg per mouse). All animals received a booster injection of the same dose and route on day 14. Sera were collected from mice 14 and 28 days after the first immunization. Pre‐immune sera (day 0) were collected and used as basal levels for comparison. Comparison of primary (a) and secondary total immunoglobulin (Ig)G titres (d) using alum or dtLLO as adjuvants for E protein. Primary IgG1 (b) and IgG2a (c) antibodies titres or secondary IgG1 (E) and IgG2a (F) antibodies titres were evaluated by enzyme‐linked immunosorbent assay (ELISA). One representative experiment of two independent experiments is shown. The results are expressed as the mean ± standard deviation (s.d.). Statistical significance was determined by the analysis of variance (anova) statistical test with Tukey's post‐test. Significant P‐values for all comparisons are shown as follows: *P < 0·05; **P < 0·01; ***P < 0·001. Non‐significant P‐values are shown as n.s.
Induction of proinflammatory cytokines in PBMCs and monocyte‐derived dendritic human cells by dtLLO
In order to explore the potential use of dtLLO as an adjuvant in human vaccination we evaluated the proinflammatory capacity of dtLLO on human immune cells, such as PBMCs and DCs. PBMCs (1 × 106) or DCs (2 × 105) were stimulated in vitro with the toxoid (1 μg/ml), LPS (1 μg/ml) or medium alone for 24 h. Although endotoxin contaminant concentrations in the recombinant dtLLO batch were very low, polymyxin B (PMB), an antibiotic known to inhibit the biological activities induced by LPS, was added as an experimental control. The supernatants were harvested for assessment of cytokine secretion. The concentrations of IL‐6, TNF‐α, IL‐12 and IL‐10 were determined (Fig. 7). Strong IL‐6, TNF‐α and IL‐10 production was observed in dtLLO‐stimulated PBMCs (IL‐6 mean = 6019·25 ng/ml, TNF‐α mean = 2230·61 ng/ml, IL‐10 mean = 889·64 ng/ml), with a moderate production of IL‐12 (mean = 489·08 ng/ml) compared with the levels measured in LPS‐stimulated PBMCs. Interestingly, the stimulation of human DCs with dtLLO also induced high levels of TNF‐α (mean = 3536·71 ng/ml) and IL‐10 (mean = 930·66 ng/ml), with moderate levels of IL‐6 and IL‐12, compared with the production of these cytokines in LPS‐stimulated DCs. Taken together, the cytokine analysis in dtLLO‐stimulated human immune cells confirmed that the adjuvant property for this toxoid is conserved on human cells.
Figure 7.
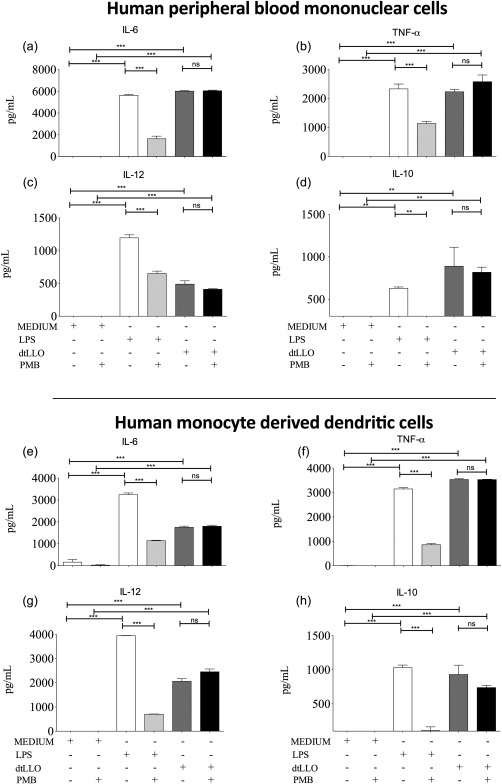
Detoxified listeriolysin O (dtLLO) promote an inflammatory response in human peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs). Human PBMCs were treated with 1 μg/ml of dtLLO, 1 μg/ml of lipopolysaccharide (LPS) or maintained with RPMI‐1640 medium as negative control in the presence or absence of polymyxin B (PMB) (a–d). Human DCs were also stimulated with dtLLO 1 μg/ml, LPS 1 μg/ml or RPMI‐1640 medium as negative control in the presence and absence of PMB (e–h). The culture supernatants were collected for screening of cytokine production, interleukin (IL)−6, tumour necrosis factor (TNF)‐α, IL‐12 and IL‐10. Statistical significance was determined by the analysis of variance (anova) statistical test with Tukey's post‐test. Significant P‐values for all comparisons are shown as follows: **P < 0·01; ***P < 0·001. Non‐significant P‐values are shown as n.s. One representative experiment of three independent experiments is shown.
Cholesterol‐dependent binding of dtLLO to human PBMCs membranes
To confirm the dtLLO capacity to bind to human PBMCs membranes, PBMCs (1 × 106) were pulsed with wtLLO (1 μg/ml) or dtLLO (1 μg/ml) on ice to prevent membrane recycling and internalization of the antigens. They were fixed later with PFA, and protein binding to the cell membrane was confirmed by flow cytometry. As shown in Fig. 8a–d, dtLLO retains the ability to bind with high affinity to human cell membranes, similar to that of wtLLO. As described previously for murine cells, pretreatment of PBMCs with methyl‐β‐cyclodextrin (mβCD) (5 mM) abrogates significantly the binding of dtLLO to human cell membrane (Fig. 8e–h), demonstrating the key role of cholesterol as a binding receptor for the toxoid.
Figure 8.
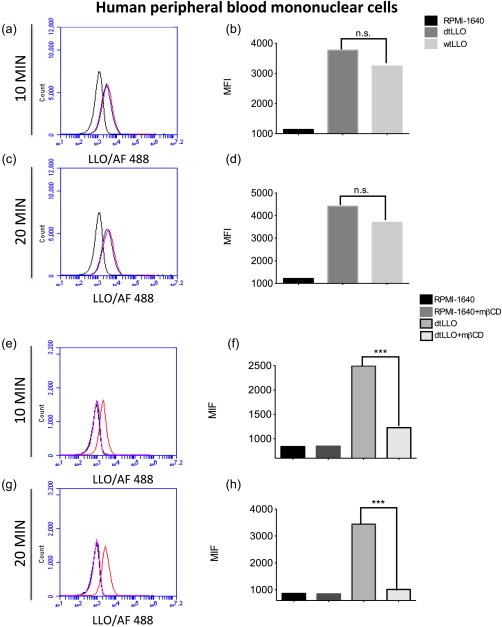
Detoxified listeriolysin O (dtLLO) preserves the binding property of LLO to the plasma membrane of human immune cells. Human peripheral blood mononuclear cells (PBMCs) were pulsed with dtLLO (red line) or wild‐type LLO (wtLLO) (blue line) for 10 or 20 min. Non‐pulsed cells were included (black line) as a negative control. The pulsed cells were fixed and stained as described previously in the Materials and methods section and analysed by flow cytometry (a,c). Bars (b,d) represent the mean fluorescence intensity for each variable in (a) and (c). To evaluate the role of cholesterol in the binding of the toxoid to the plasma membranes, PBMCs were treated (pink line) or not (red line) with methyl‐β‐cyclodextrin (mβCD) for 30 min at 37ºC, washed and then pulsed with dtLLO for 10 or 20 min, then the cells were fixed, stained and analysed by flow cytometry (e,g). Non‐pulsed cells were treated (blue line) or not (black line), with mβCD as controls. Bars (f,h) represent the mean fluorescence intensity for each variable in (e) and (g). One representative experiment of three independent experiments is shown. [Colour figure can be viewed at wileyonlinelibrary.com]
Cholesterol depletion abrogates cytokine production in dtLLO‐activated human PBMCs
A previous report on dtLLO biological activity has shown that the toxoid induces up‐regulation of proinflammatory cytokine mRNAs and over‐expression of co‐stimulatory molecules in dtLLO‐stimulated bone marrow‐derived DCs (BMDCs) from both wild‐type (C57BL6) and TLR‐4−/−‐deficient mice 23, discarding a TLR‐4‐dependent mechanism for the immunomodulatory activity of dtLLO. Also, the adjuvant activity of dtLLO cannot be explained by cell activation by the pore‐forming property. Because the toxoid preserves its binding capacity to cell membranes, we decided to evaluate the biological role of cholesterol for the proinflammatory activity of dtLLO on human PBMCs. We know that the integrity of cholesterol‐enriched microdomains, called lipid rafts, of eukaryotic cells is required for both activation and cell signalling. Therefore, we explored whether cholesterol depletion and raft disruption in PBMCs could influence the proinflammatory cytokine production induced by dtLLO. Pretreatment of PBMCs (1 × 106) with mβCD and subsequent stimulation with dtLLO abrogated completely the IL‐6 and TNF‐α production in activated human PBMCs in an mβCD dose‐dependent manner (Fig. 9a,b). The concentrations of mβCD were titrated in initial experiments without adverse effects on viability for the tested times. In summary, our results suggest that the immunomodulatory effects of dtLLO on human cells are dependent upon binding to cholesterol and the integrity of lipid rafts.
Figure 9.
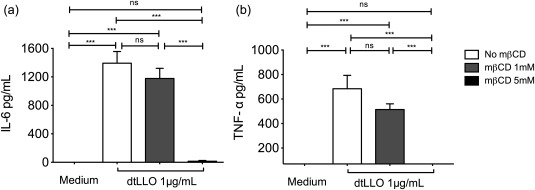
Cholesterol depletion abrogates the proinflammatory effect induced by detoxified listeriolysin O (dtLLO). Human peripheral blood mononuclear cells (PBMCs) were treated with mβCD 1 mM or 5 mM for 30 min at 37ºC to deplete cholesterol from the plasma membrane. Then, cells were stimulated with dtLLO (1 μg/ml) for 4 h at 37ºC and the supernatants were collected and analysed for production of (a) interleukin (IL)‐6 and (b) tumour necrosis factor (TNF)‐α. Significant P‐values for all comparisons are shown as follows: *P < 0·05; **P < 0·01; ***P < 0·001. Non‐significant P‐values are shown as n.s. One representative experiment of three independent experiments is shown.
Fisetin abrogates cytokine production in dtLLO‐activated human PBMCs
In order to address the mechanism and signalling pathway involved in the activation of human cells mediated by dtLLO we used the flavonoid fisetin (3, 7, 3′, 4′‐tetrahydroxyflavone), which has been described as a suppressor of the nuclear factor (NF)‐κB signalling pathway 33. Fisetin blocks the phosphorylation and degradation of IκBα, which leads in turn to suppression of the phosphorylation and nuclear translocation of the p65 NF‐κB subunit 33. Treatment of PBMCs with dtLLO in the presence of the highest concentration of fisetin tested abrogated the production of TNF‐α, IL‐6, IL‐12 and IL‐10 significantly (Fig. 10a–d). Cell viability was tested before and after stimulation using trypan blue, with no cytotoxic effects observed (data not shown). These results suggest that the NF‐κB signalling pathway is involved in the inflammatory process induced by dtLLO.
Figure 10.
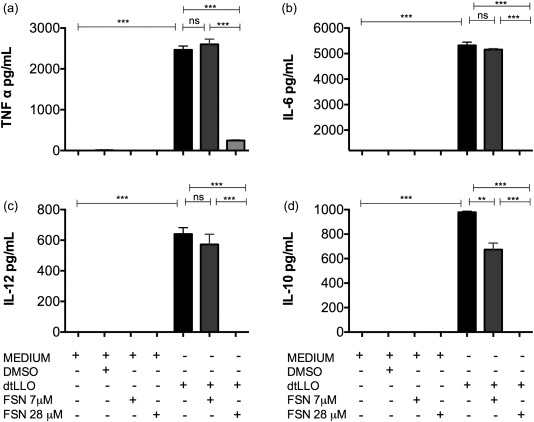
Involvement of the nuclear factor (NF)‐κB signalling in detoxified listeriolysin O (dtLLO) proinflammatory activity. Human peripheral blood mononuclear cells (PBMCs) were treated with dtLLO in the presence or absence of fisetin at 7 and 28 μM concentrations. After 24 h, the supernatants were harvested and the production of (a) tumour necrosis factor (TNF)‐α, (b) interleukin (IL)‐6, (c) IL‐12 and (d) IL‐10 were measured by enzyme‐linked immunosorbent assay (ELISA). Fisetin abrogates the production of all the cytokines evaluated in a dose‐dependent manner. Significant P‐values for all comparisons are shown as follows: **P < 0·01; ***P < 0·001. Non‐significant P‐values are shown as n.s. One representative experiment of three independent experiments is shown.
Discussion
Adjuvants constitute an essential element to improve and modulate the immune response against antigens included in vaccine formulations. Characterization of the safety and efficiency of these molecules in the vaccination field is the first, and one of the most important points to consider, before their clinical use. Here, we investigated the in‐vivo safety and adjuvant properties of dtLLO, a non‐cytolytic LLO mutant.
First, the in‐vitro toxicity of the wtLLO and dtLLO was assessed using RBCs from human donors. As described previously for sheep RBCs 23, dtLLO did not show detectable haemolytic activity, even at the highest concentration of protein that we tested. These results confirm that the mutations introduced into the undecapeptide sequence abrogates completely the haemolytic effects, regardless of the species of RBCs used. In order to evaluate a potential systemic toxicity, the lethality rate after intravenous administration of dtLLO or wtLLO was determined. The results observed after i.v. challenge with the toxoid confirmed the findings of Wallecha et al. 23, that dtLLO is also a non‐toxic molecule in vivo. The high survival rate of mice after i.v. challenge with dtLLO is in agreement with the survival rate observed after immunization using dtLLO as an adjuvant i.p., with a similar dose to that administered i.v.
To the best of our knowledge, our study has performed the first histopathological description of the toxic effects of recombinant wtLLO and dtLLO on target organs such as brain, lung, heart, spleen, liver and kidney. Mice challenged with wtLLO exhibited multiple focal haemorrhages in heart, lung, liver and brain. The hypercontracted fibres observed in the heart caused by wtLLO toxicity explained previous findings on functional damage to heart muscle in LLO‐challenged mice 18. Systemic wtLLO administration induced lung oedema, intra‐alveolar haemorrhage and focal subpleural haemorrhage, suggesting that all these injury signals may lead to respiratory failure associated with cardiotoxic effects. In kidney, wtLLO induced epithelial vacuolization, which suggests a direct toxic effect of this toxin on the renal tubule. Moreover, neurotoxicity signals after challenge with wtLLO were evident after a few minutes of toxin administration. The main anatomical disorders in brain included severe congestion, focal subarachnoid haemorrhage, extravasation of proteinaceous material, ischaemic neurones in the cerebellum (Purkinje cells, dentate nucleus) and hippocampus neuropil oedema, vacuolation of neurones and focal haemorrhage (brain stem). Thus, the central nervous system is another primary target organ of wtLLO. The rapid neurotoxicity observed after a few minutes of wtLLO administration suggests a direct effect of the toxin on the central nervous system. In contrast with the detrimental systemic effects of wtLLO, histopathological analysis revealed no significant abnormalities in tissue of brain, lung, heart, kidney, liver and spleen in the mice group challenged with dtLLO. Based on previous in‐vitro scientific evidence concerning the rapid pro‐apoptotic effects of wtLLO on lymphocytes, which is characterized by activation of caspases as quickly as 30 min 19, and the previous results on the toxic effects on the C3.F6 cell line after only 60 min incubation with wtLLO 30, we examined the tissue sections of mice challenged with dtLLO for apoptotic cell death. Our in‐vivo results are in agreement with previous in‐vitro results with other LLO mutants, showing that mutation of the tryptophans to alanines at both residues 491 and 492 or at only residue 492 in the wtLLO amino acid sequence led to a significant reduction in the pro‐apoptotic activity of LLO 30. Taken together, the immunohistochemistry results and TUNEL assay confirmed that dtLLO toxoid is a safe molecule without significant systemic toxic effects, such as those observed with wtLLO.
Other important findings in our study included evaluation of the proinflammatory potential of dtLLO in vivo after i.p. challenge of mice with the toxoid. It is well known that not all inflammatory molecules can be considered as safe adjuvants. The extreme toxicity of some microbial molecules such as LPS is a major limitation for this purpose. Previously, dtLLO has been tested for its potential to induce the expression of inflammatory genes such as TNF‐α or IL‐6 using an in‐vitro approach 23; however, no evidence was provided about the magnitude of the inflammatory response induced by the toxoid in vivo. In order to test the immunostimulatory properties of dtLLO, in our study we evaluated the production of two typical inflammatory mediators: IL‐6, a non‐specific cytokine, and IL‐12, a polarizing cytokine, compared against a very toxic and highly inflammatory molecule, LPS. Our results showed that dtLLO induced low and tolerable levels of inflammation compared to the high levels of inflammation induced by LPS. We know from previous studies that low levels of inflammatory cytokines are sufficient to orchestrate specific immune responses effectively 34. Low levels of inflammatory cytokines induced by the toxoid can represent an advantage in the use of this molecule in vivo, such that it is not a toxic or highly inflammatory molecule with adverse effects for the host, but is effective in stimulating and modulating the innate immune system in vivo. In this context, our observations are extremely important in predicting the biological behaviour of dtLLO as a potential safe adjuvant in human vaccination compared with the traditional toxic and highly inflammatory effects of different adjuvants already licensed, such as alum or adjuvant candidates that are currently under clinical investigation, such as Toll‐like receptor ligands 35 or oil‐in‐water emulsions 36. Some of these adjuvants have the disadvantage of promoting antigen deposition, which can induce adverse effects such as granuloma formation, subcutaneous nodules and contact hypersensitivity triggered by T cells that infiltrate the injection site 37.
In addition to the safety evaluation, we tested here a new application for dtLLO as an adjuvant molecule to enhance the humoral‐specific immune response against DENV antigens. Our results showed that specific IgG antibodies against E protein from DENV4 were induced successfully at high levels for the experimental vaccine containing dtLLO as adjuvant. Interestingly, when dtLLO was used in combination with E protein we detected a diversified profile of IgG1 and IgG2a isotypes, in contrast to the predominance of IgG1 isotype induced by the formulation containing alum. While the IgG1 isotype is associated with a Th2 profile, production of IgG2a is associated with a Th1 immune response 38. The in‐vivo production of IL‐12 in mice after immunization with dtLLO can explain the humoral profile for E protein from DENV that we found, particularly the induction of specific anti‐E‐protein IgG2a antibodies. Considering dtLLO as an effective immunomodulatory molecule with the advantage of promoting IgG isotype diversification is a remarkable result, and is consistent with a study using a B cell receptor idiotype (Id) conjugated with a non‐haemolytic form of LLO, compared with the Id conjugated with keyhole limpet haemocyanin (KLH) as immunotherapy for non‐Hodgkins lymphoma. The Id‐LLO induced a more powerful Th1 response, characterized by high‐titre IgG2a anti‐Id antibodies and the presence of CD4+ cells secreting IFN‐γ 39. This evidence supports a potential advantage in the use of dtLLO with respect to the alum as an effective adjuvant to induce Th1 responses. Modulation towards Th1 responses is required to gain protection mainly against intracellular pathogens, a clear problem with the current use of alum as an adjuvant in human vaccination.
Antibody isotype diversification is essential for the immune system to mount protective humoral responses using different biological and effector functions. Previously, it has been described for other experimental dengue vaccines that neutralizing activity can be associated with either IgG1 or IgG2a antibodies 40. In addition to the neutralizing property, IgG2a antibodies can mediate complement activation and ADCC mechanism, which together are essential biological functions for viral immunity.
It remains to be evaluated in future studies if dtLLO can enhance the cellular immune response against microbial antigens from DENV CD4+ and CD8+‐specific T cells, in the same way as shown previously against tumour antigens 23.
In order to explore fully the potential use of dtLLO as an adjuvant in human vaccination, we analysed the proinflammatory properties of the toxoid on human immune cells. As reported previously using murine cells using an in‐vitro approach 23, or our own in‐vivo findings in the mouse model, we found that dtLLO preserves the proinflammatory property on human immune cells. A robust production of inflammatory mediators such as IL‐6 and TNF‐α was induced after stimulation of human PBMCs with dtLLO after 24 h of stimulation. Interestingly, medium levels of IL‐12, a Th1 polarizing cytokine, and IL‐10, a key cytokine to promote humoral responses, were detected in the supernatant of PBMCs stimulated with dtLLO. These results were reproducible using DCs, confirming the immunostimulatory effect of dtLLO on immune cells from human origin.
Our experiments using a pretreatment of dtLLO with polymyxin B were unable to inhibit cytokine secretion in human cells, confirming that it is dtLLO itself, and not a potential effect mediated by LPS contamination, that is stimulating cytokine production in the assays.
Finally, we decided to explore the mechanism involved in both activation and inflammation induced by dtLLO in human immune cells. A clearer understanding of the immunostimulatory property of dtLLO is a key issue in order to harness the potential of this toxoid for human vaccination. A previous report concerning dtLLO has shown that the toxoid induces up‐regulation of co‐stimulatory markers and TNF‐α mRNA expression in BMDCs from TLR‐4−/− mice, confirming there is a TLR‐4‐independent mechanism involved in the proinflammatory activity of dtLLO. Previously, it has been reported that LLO triggers cell signalling in murine J774 cells via spontaneous aggregation of rafts 41. Lipid rafts aggregation by LLO was independent of its cytolytic activity, but depended upon oligomerization of the monomers. A major observation in that study was that a cholesterol pre‐inactivated form of LLO also aggregates lipid rafts independently of pore formation 41.
Here we studied the role of cholesterol and lipid raft disruption in the activation of human immune cells. Lipid rafts are cholesterol‐rich membrane microdomains in the outer leaflet of the plasma membrane. These microdomains regulate membrane key functions in eukaryotic cells, such as signal transduction and vesicular trafficking 42. Our results demonstrate that plasma membrane cholesterol plays a critical role in the binding and the inflammatory response initiated by dtLLO on human immune cells. Treatment with mβCD clearly inhibited the toxoid binding and also the expression of IL‐6 and TNF‐α when PBMCs were stimulated by dtLLO. To the best of our knowledge, ours is the first study demonstrating that a mutated and detoxified LLO version retains its proinflammatory activity through a plasma membrane cholesterol‐binding mechanism. Our data suggest that cholesterol depletion by mβCD in human immune cells could affect dtLLO binding, and also the integrity of lipid rafts, contributing to the inhibition of the cell signalling induced by dtLLO. Taken together, we have added new data concerning the mechanism by which this LLO mutant could be considered an adjuvant molecule, which is different to the activation of TLRs by natural or synthetics ligands, depot effect or antigen delivery vehicle mechanisms.
Finally, the results observed in fisetin‐treated human immune cells confirm that dtLLO induces activation and production of inflammatory mediators using the canonical NF‐κB signalling pathway.
In summary, the results shown here confirm that the dtLLO mutant is a safe and effective adjuvant molecule in a mouse model. We also demonstrated that the toxoid exerts its immunostimulatory properties in a cholesterol‐dependent mechanism on human immune cells. Taken together, these results supply new evidence concerning dtLLO as a promising adjuvant, not only to enhance the cellular 23 immune response against tumours, as described previously, but also to potentiate the robust humoral responses required for prophylactic anti‐microbial vaccines.
Disclosure
Y. P. has a financial interest in Advaxis Inc., a publicly traded immunotherapy company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of L. monocytogenes or listerial products as immunotherapies or vaccines. All other authors have no conflicts of interest to declare.
Author contributions
H. V.‐C. conceived and designed the study. K. G. H.‐F., A. L. C.‐G., G. M.‐S., R. R.‐R., L. A. S.‐V., P. T.‐D., I. Y. I.‐H. and O. L.‐F. performed the experiments. Y. P., L. W., J. T.‐S., J. M.‐B., L. C.‐B. and H. V.‐C. contributed reagents, materials and analysis tools. H. F. K. G. and H. V. C. wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
This study was funded by Fondo Mixto CONACyT‐Gobierno del Estado de Veracruz Project ID 109270, 128001 and Fondo Sectorial SSA/IMSS/ISSSTE SALUD 2015‐2 project 261693. K. G. H.‐F. gratefully acknowledges the scholarship from CONACyT (279262) to pursue postgraduate studies.
References
- 1. Gupta RK, Siber GR. Adjuvants for human vaccines – current status, problems and future prospects. Vaccine 1995; 13:1263–76. [DOI] [PubMed] [Google Scholar]
- 2. Rappuoli R, Mandl CW, Black S, Gregorio ED. Vaccines for the twenty‐first century society. Nat Rev Immunol 2011; 11:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gekara NO, Westphal K, Ma B, Rohde M, Groebe L, Weiss S. The multiple mechanisms of Ca2+ signalling by listeriolysin O, the cholesterol‐dependent cytolysin of Listeria monocytogenes . Cell Microbiol 2007; 9:2008–21. [DOI] [PubMed] [Google Scholar]
- 4. Nishibori T, Xiong H, Kawamura I, Arakawa M, Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun 1996; 64:3188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kayal S, Lilienbaum A, Poyart C, Memet S, Israel A, Berche P. Listeriolysin O‐dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF‐kappa B and upregulation of adhesion molecules and chemokines. Mol Microbiol 1999; 31:1709–22. [DOI] [PubMed] [Google Scholar]
- 6. Tsukada H, Kawamura I, Fujimura T, Igarashi K, Arakawa M, Mitsuyama M. Induction of macrophage interleukin‐1 production by Listeria monocytogenes hemolysin. Cell Immunol 1992; 140:21–30. [DOI] [PubMed] [Google Scholar]
- 7. Park JM. Anthrolysin O and other Gram‐positive cytolysins are Toll‐like receptor 4 agonists. J Exp Med 2004; 200:1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meixenberger K, Pache F, Eitel J et al Listeria monocytogenes‐infected human peripheral blood mononuclear cells produce IL‐1beta, depending on listeriolysin O and NLRP3. J Immunol 2010; 184:922–30. [DOI] [PubMed] [Google Scholar]
- 9. Stier EM, Mandal M, Lee KD. Differential cytosolic delivery and presentation of antigen by listeriolysin O‐liposomes to macrophages and dendritic cells. Mol Pharm 2005; 2:74–82. [DOI] [PubMed] [Google Scholar]
- 10. Mandal M, Kawamura KS, Wherry EJ, Ahmed R, Lee KD. Cytosolic delivery of viral nucleoprotein by listeriolysin O‐liposome induces enhanced specific cytotoxic T lymphocyte response and protective immunity. Mol Pharm 2004; 1:2–8. [DOI] [PubMed] [Google Scholar]
- 11. Carrero J. A, Vivanco‐Cid H, Unanue ER. Granzymes drive a rapid listeriolysin O‐induced T cell apoptosis. J Immunol 2008; 181:1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs T, Darji A, Frahm N et al Listeriolysin O: cholesterol inhibits cytolysis but not binding to cellular membranes. Mol Microbiol 1998; 28:1081–9. [DOI] [PubMed] [Google Scholar]
- 13. Tsuchiya K, Kawamura I, Takahashi A, Nomura T, Kohda C, Mitsuyama M. Listeriolysin O‐induced membrane permeation mediates persistent interleukin‐6 production in Caco‐2 cells during listeria monocytogenes infection in vitro listeriolysin O‐induced membrane permeation mediates persistent interleukin‐6 production in Caco‐2 cells during Listeria monocytogenes infection in vitro . Infect Immun 2005;73:3869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guzmén CA, Domann E, Ronde M et al Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes . Mol Microbiol 1996; 20:119–26. [DOI] [PubMed] [Google Scholar]
- 15. Pillich H, Loose M, Zimmer K‐P, Chakraborty T. Activation of the unfolded protein response by listeria monocytogenes. Cell Microbiol 2012; 14:949–64. [DOI] [PubMed] [Google Scholar]
- 16. Geoffroy C, Gaillard JL, Alouf JE, Berche P. Purification, characterization, and toxicity of the sulfhydryl‐activated hemolysin listeriolysin O from Listeria monocytogenes . Infect Immun 1987; 55:1641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kingdon GC, Sword CP. Biochemical and immunological effects of Listeria monocytogenes hemolysin. Infect Immun 1970; 1:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kingdon GC, Sword CP. Cardiotoxic and lethal effects of Listeria monocytogenes hemolysin. Infect Immun 1970; 1:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carrero JA, Calderon B, Unanue ER. Listeriolysin O from Listeria monocytogenes is a lymphocyte apoptogenic molecule. J Immunol 2004; 172:4866–74. [DOI] [PubMed] [Google Scholar]
- 20. Kohda C, Kawamura I, Baba H et al Dissociated linkage of cytokine‐inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes . Infect Immun 2002; 70:1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michel E, Reich KA, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol 1990; 4:2167–78. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe I, Nomura T, Tominaga T et al Dependence of the lethal effect of pore‐forming haemolysins of Gram‐positive bacteria on cytolytic activity. J Med Microbiol 2006; 55:505–10. [DOI] [PubMed] [Google Scholar]
- 23. Wallecha A, Wood L, Pan ZK, Maciag PC, Shahabi V, Paterson Y. Listeria monocytogenes‐derived listeriolysin O has pathogen‐associated molecular pattern‐like properties independent of its hemolytic ability. Clin Vaccine Immunol 2013; 20:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol 2002; 156:1029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakhon OS, Victor KA, Choy A, Tsuchiya T, Eulgem T, Pedra JHF. NSD1 mitigates caspase‐1 activation by listeriolysin O in macrophages. PLoS One 2013; 8:e75911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vázquez‐Boland JA, Kuhn M, Berche P et al Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 2001; 14:584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Merrick JC, Edelson BT, Bhardwaj V, Swanson PE, Unanue ER. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol 1997; 151:785–92. [PMC free article] [PubMed] [Google Scholar]
- 28. Rogers HW, Callery MP, Deck B, Unanue ER. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol 1996; 156:679–84. [PubMed] [Google Scholar]
- 29. Rosado CJ, Kondos S, Bull TE et al The MACPF/CDC family of pore‐forming toxins. Cell Microbiol 2008; 10:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carrero J. A, Vivanco‐Cid H, Unanue ER. Listeriolysin O is strongly immunogenic independently of its cytotoxic activity. PLOS ONE 2012; 7:e32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferguson NM, Rodríguez‐Barraquer I, Dorigatti I, Mier‐y‐Teran‐Romero L, Laydon DJ, Cummings DAT. Benefits and risks of the Sanofi‐Pasteur dengue vaccine: modeling optimal deployment. Science 2016; 353:1033–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wahala WMPB Kraus AA, Haymore LB, Accavitti‐Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III‐reactive antibody. Virology 2009; 392:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin‐dependent kinase 6, down‐regulates nuclear factor‐κB‐regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK‐1 and receptor‐interacting protein‐regulated IκBα kinase. Mol Pharmacol 2007; 71:1703–14. [DOI] [PubMed] [Google Scholar]
- 34. Buglione‐Corbett R, Pouliot K, Marty‐Roix R et al Serum cytokine profiles associated with specific adjuvants used in a DNA prime‐protein boost vaccination strategy. PLOS ONE 2013; 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huleatt JW, Nakaar V, Desai P et al Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 2008; 26:201–14. [DOI] [PubMed] [Google Scholar]
- 36. Jensen FC, Savary JR, Diveley JP, Chang JC. Adjuvant activity of incomplete Freund's adjuvant. Adv Drug Deliv Rev 1998; 32:173–86. [DOI] [PubMed] [Google Scholar]
- 37. Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol 2004; 82:488–96. [DOI] [PubMed] [Google Scholar]
- 38. Germann T, Bongartz M, Dlugonska H et al Interleukin‐12 profoundly up‐regulates the synthesis of antigen‐specific complement‐fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo . Eur J Immunol 1995; 25:823–9. [DOI] [PubMed] [Google Scholar]
- 39. Neeson P, Pan ZK, Paterson Y. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol Immunother 2008; 57:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smucny JJ, Kelly EP, Macarthy PO, King AD. Murine immunoglobulin G subclass responses following immunization with live Dengue virus or a recombinant dengue envelope protein. Am J Trop Med Hyg 1995; 53:432–7. [DOI] [PubMed] [Google Scholar]
- 41. Gekara NO, Jacobs T, Chakraborty T, Weiss S. The cholesterol‐dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol 2005; 7:1345–56. [DOI] [PubMed] [Google Scholar]
- 42. Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 2003; 44:655–67. [DOI] [PubMed] [Google Scholar]


