Summary
Acinetobacter baumannii is a multi‐drug resistant, Gram‐negative bacteria and infection with this organism is one of the major causes of mortality in intensive care units. Inflammasomes are multiprotein oligomers that include caspase‐1, and their activation is required for maturation of interleukin‐1β (IL‐1β). Inflammasome signalling is involved in host defences against various microbial infections, but the precise mechanism by which A. baumannii activates inflammasomes and the roles of relevant signals in host defence against pulmonary A. baumannii infection are unknown. Our results showed that NLRP3, ASC and caspase‐1, but not NLRC4, are required for A. baumannii‐induced production of IL‐1β in macrophages. An inhibitor assay revealed that various pathways, including P2X7R, K+ efflux, reactive oxygen species production and release of cathepsins, are involved in IL‐1β production in macrophages in response to A. baumannii. Interleukin‐1β production in bronchoalveolar lavage (BAL) fluid was impaired in NLRP3‐deficient and caspase‐1/11‐deficient mice infected with A. baumannii, compared with that in wild‐type (WT) mice. However, the bacterial loads in BAL fluid and lungs were comparable between WT and NLRP3‐deficient or caspase‐1/11‐deficient mice. The severity of lung pathology was reduced in NLRP3‐ deficient, caspase‐1/11‐ deficient and IL‐1‐receptor‐deficient mice, although the recruitment of immune cells and production of inflammatory cytokines and chemokines were not altered in these mice. These findings indicate that A. baumannii leads to the activation of NLRP3 inflammasome, which mediates IL‐1β production and lung pathology.
Keywords: Acinetobacter baumannii, interleukin‐1β, macrophages, NLRP3 inflammasome
Abbreviations
- BAL
bronchoalveolar lavage
- BMDMs
bone‐marrow‐derived macrophages
- CFU
colony‐forming units
- IFN‐γ
interferon‐γ
- IL‐1β
interleukin‐1β
- IL‐1R
interleukin‐1 receptor
- MOI
multiplicity of infection
- NAC
N‐acetyl‐l‐cysteine
- NLRs
Nod‐like receptors
- oxATP
oxidized ATP
- ROS
reactive oxygen species
- TNF‐α
tumour necrosis factor‐α
- WT
wild‐type
Introduction
Acinetobacter baumannii is a ubiquitous Gram‐negative coccobacillus that can survive for long periods in the environment, including in soil and water and on the skin of healthy humans. During the last decade, it has emerged as a major antibiotic‐resistant nosocomial bacterium that causes high morbidity and mortality, especially in patients with weakened immune systems.1, 2 Infection by A. baumannii can cause pneumonia, bacteraemia, bloodstream infection, skin infection, urinary tract infection and meningitis.3 Treating these infections has become increasingly difficult because of the emergence of resistance to multiple antibiotics.4 Innate immune responses play an important role in host defence against various acute bacterial infections. The inflammatory response against A. baumannii is characterized by the recruitment of neutrophils and macrophages,5, 6 principle innate immune cells, to the site of infection. Pattern recognition receptors, including Toll‐like receptors and Nod‐like receptors (NLRs), are critical for initiating host innate immune responses, resulting in the activation of immune cells and production of various inflammatory molecules to remove bacteria. Recent studies have indicated that Toll‐like receptors 2, 4 and 9 are involved in host defences against A. baumannii infection in vitro and in vivo.7, 8, 9
Interleukin‐1β (IL‐1β) is a potent pro‐inflammatory cytokine produced in various types of cells in response to infection, inflammation, injury or immunologic challenge.10 Although IL‐1β exerts a protective effect during infections, overexpression of this molecule can lead to tissue damage, and dysregulated inflammasome activation has been implicated in the pathogenesis of a variety of inflammatory diseases.11, 12 Unlike other cytokines, IL‐1β is produced in an immature form by transcriptional regulation and then is cleaved and activated by the proteolytic enzyme caspase‐1. Inflammasomes are multiple protein complexes that regulate caspase‐1 activation in innate immune responses, and NLRs, NLRP3 and NLRC4, are representative receptors for inflammasomes. NLRP3 can be activated by various stimuli such as extracellular ATP, potassium (K+) efflux, pore‐forming toxins, mitochondrial reactive oxygen species (ROS) and destabilized lysosomes.13, 14 NLRC4 responds to bacterial flagellin and type III secretion system components, with the assistance of neuronal apoptosis inhibitory proteins.13, 14 These receptor proteins mediate the production of IL‐1β and IL‐18 and a distinct cell death called pyroptosis in immune cells, and play important roles in host defence against various bacteria.15
A previous study suggested that various gene polymorphisms of IL‐1 receptor (IL‐1R) antagonist may be associated with the severity of A. baumannii‐induced pneumonia.16 However, the mechanism by which A. baumannii activates inflammasomes and induces IL‐1β production in immune cells and the in vivo roles of inflammasome‐related signalling in pulmonary A. baumannii infections have not been elucidated. We show here that the NLRP3/ASC/caspase‐1 axis is essential for A. baumannii‐induced production of IL‐1β in macrophages and identify the signalling molecules that mediate lung pathology in mice infected with A. baumannii.
Materials and methods
Mice
NLRP3−/−, NLRC4−/−, caspase‐1/11−/− and ASC−/− mice on a C57BL/6 background were gifts from Prof. Gabriel Núñez (University of Michigan) and have been previously described.17, 18 Wild‐type (WT) C57BL/6 mice were purchased from Koatech (Pyeongtaek, Korea). The animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee of Chonnam National University (Gwangju, Korea).
Bacterial preparation
Acinetobacter baumannii strain KCCM 35453 (ATCC 15150) was purchased from the Korean Culture Centre of Microorganisms (Seoul, Korea). To prepare the bacterium, single colonies of the test strain were inoculated into 10 ml of Luria–Bertani broth supplemented with ampicillin (50 μg/ml) and grown overnight at 37° with shaking. A 1 : 5 dilution of the culture suspension was allowed to grow in fresh medium at 37° with shaking for an additional 2 hr. Bacteria were washed and resuspended with sterile PBS to 109 colony‐forming units (CFU)/ml. Bacteria were diluted to the desired concentrations for use in the experiment.
Cell culture and bacterial infection
Bone‐marrow‐derived macrophages (BMDMs) were prepared as previously described.19 Thioglycollate‐elicited neutrophils were isolated from the mouse peritoneal cavity as previously described.20 Briefly, mice were injected intraperitoneally with 2 ml of 4% thioglycollate broth (Sigma Aldrich, St Louis, MO). Four hours later, 5 ml of sterile PBS was injected intraperitoneally and peritoneal lavage was obtained. Red blood cells were lysed with a lysis buffer containing ammonium chloride and total cell numbers were counted with a haemocytometer. The lung epithelial cells murine Lewis lung carcinoma cells and L‐929 murine fibroblast cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Both lung epithelial cells and L‐929 cells were cultured with Dulbecco's modified Eagle's medium (Welgene, Gyeongsan, Gyeongbuk, Korea) containing 10% fetal bovine serum and 1 × penicillin/streptomycin in a 5% CO2 incubator at 37°. To measure cytokine levels, the cells were seeded in 48‐well plates at a concentration of 2 × 105 cells/well and incubated in a 5% CO2 incubator at 37° overnight. Subsequently, cells were either infected or not infected with A. baumannii at the indicated multiplicity of infection (MOI) by exposure for 60 min, and extracellular bacterial growth was inhibited by gentamicin (50 μg/ml) treatment. The culture supernatant was collected 24 hr after infection for cytokine measurement.
Measurement of cytokines
The concentrations of CXCL1, CXCL2, CCL2, IL‐1β, IL‐4, IL‐5, IL‐6, IL‐10, IL‐13, IL‐17, tumour necrosis factor‐α (TNF‐α) and interferon‐γ (IFN‐γ) in culture supernatants from A. baumannii‐infected BMDMs or in bronchoalveolar lavage (BAL) fluid of infected mice were determined by an ELISA kit, used according to the manufacturer's manual (R&D Systems, Minneapolis, MN).
Lactate dehydrogenase measurement
Lactate dehydrogenase (LDH) level in culture supernatant was measured using a commercial kit (CytoTox 96®; Promega, Madison, WI) as a manufacturer's recommendation.
Inhibitor assay
Potassium chloride, N‐acetyl‐l‐cysteine (NAC), oxidized ATP (oxATP) and glyburide were purchased from Sigma (St Louis, MO). CA‐074 methyl ester (CA‐074Me) and Y‐VAD (Caspase‐1 inhibitor) were obtained from Calbiochem (La Jolla, CA). The BMDMs were infected with A. baumannii at an MOI of 10 by exposure for 24 hr with or without pretreatment with each inhibitor for 2 hr at the indicated doses.
Immunoblotting
For immunoblotting, BMDMs were seeded and incubated overnight in six‐well plates at a concentration of 2 × 106 cells/well and infected with A. baumannii at an MOI of 10 by exposure for 24 hr. Culture supernatants and remaining cells were mixed with a lysis buffer containing Nonidet P‐40, complete protease inhibitor cocktail (Roche, Mannheim, Germany) and 2 mm dithiothreitol. To detect pro‐forms of caspase‐1 and IL‐1β, cell lysates were used; protein samples from culture supernatants were used to detect cleaved forms of these proteins. Samples were separated by 12% SDS–PAGE and transferred to nitrocellulose membranes. The membranes were immunoblotted with the primary antibodies anti‐mouse caspase‐1 (Enzo Life Science, Farmingdale, NY), anti‐mouse‐IL‐1β (R&D Systems) and anti‐β‐actin (Santa Cruz Biotechnology, Santa Cruz, CA). After immunoblotting with secondary antibodies, proteins were detected using an enhanced chemiluminescence reagent (BioRad, Hercules, CA).
In vivo experiments
Mice were anaesthetized by intraperitoneal injection of 10 mg/kg Rompun (Bayer, Seoul, Korea) and 50 mg/kg Zoletil (Virbac, Seoul, Korea), and then inoculated intranasally with 30 μl of A. baumannii (3 × 107 CFU) suspension in PBS. BAL fluids were collected at 6 hr, 1 day, or 3 days after infection and used to quantify immune cell populations, cytokines and chemokines produced, and bacterial loads. In a separate experiment using the same protocol, the right lobe of the lungs was collected from each mouse, and a lysate was prepared by homogenizing the tissue in PBS, to determine bacterial growth. The left lung lobe was used to prepare a slide for histopathological examination.
Bacterial counts in BAL fluid and lungs
Fifty microlitres of serially diluted BAL fluid or lung homogenates was spread onto Luria–Bertani agar plates. Following overnight culture in a 37° incubator, bacterial colonies were counted, and the number of bacteria was expressed as CFU/ml in BAL fluid or CFU/g in lung tissue.
Histopathological examination
The left lobes of lungs were harvested and fixed in 10% neutral formalin for histopathological observation. The tissues were routinely processed in an alcohol and xylene series and embedded in paraffin. Three‐micrometre sections were prepared, stained with haematoxylin & eosin, and examined by microscopy. Histopathology of the lung was blindly evaluated, with an arbitrary scoring system of 0–6 applied (0 = normal; 1 = mild lesions; 2 = mild to moderate lesions; 3 = moderate lesions; 4 = moderate to severe lesions; 5 = severe lesions; and 6 = very severe lesions throughout the lobe).
Cell counts in BAL fluid
The total numbers of cells in BAL fluids were counted using a haemocytometer, and a differential cell count based on morphological criteria was performed using Diff‐Quick staining.
Statistical analysis
The statistical significance of differences between groups was determined by a two‐tailed Student's t‐test or one‐way analysis of variance followed by post hoc analysis (Newman–Keuls multiple comparison test) (GraphPad Prism 5; GraphPad Software Inc., La Jolla, CA, USA). Values of P that were < 0·05 were considered significant.
Results
NLRP3 inflammasome contributes to IL‐1β production in A. baumannii‐infected macrophages
Interleukin‐1β maturation and secretion are tightly regulated by inflammasome activation.21 To determine the core inflammasome component that regulates IL‐1β production in response to A. baumannii, BMDMs from WT and NLRP3‐deficient and NLRC4‐deficient mice were infected with A. baumannii at various MOIs, and the IL‐1β levels in culture supernatants were measured. Infection with A. baumannii led to IL‐1β production in BMDMs from WT and NLRC4‐deficient mice in a dose‐dependent manner, but IL‐1β production was abolished in NLRP3‐deficient cells (Fig. 1a). In contrast, the levels of IL‐6 and TNF‐α induced by A. baumannii were comparable between macrophages from WT and NLRP3‐deficient and NLRC4‐deficient mice (Fig. 1b,c). We confirmed the involvement of NLRP3 in A. baumannii‐induced IL‐1β production using an inhibitor assay. An NLRP3 inflammasome inhibitor, glyburide, reduced IL‐1β production in BMDMs in response to A. baumannii in a dose‐dependent manner (Fig. 1d). Furthermore, NLRP3 inhibition led to impaired production of IL‐1β in murine alveolar macrophage MH‐S cells (Fig. 1e). NLRP3 associates with the adaptor molecule ASC and subsequently oligomerizes with caspase‐1 to form an active inflammasome complex.22 Therefore, we investigated whether ASC and caspase‐1 were also required for A. baumannii‐induced production of IL‐1β in macrophages. Results showed that IL‐1β production in response to A. baumannii infection was absolutely impaired in ASC‐ and caspase‐1/11‐deficient BMDMs (Fig. 1f). As expected, IL‐6 and TNF‐α levels were not different between cells from WT and ACS‐deficient and caspase‐1/11‐deficient mice (Fig. 1g,h). These findings suggest that the NLRP3/ASC/caspase‐1 complex is essential for IL‐1β production by macrophages in response to A. baumannii.
Figure 1.
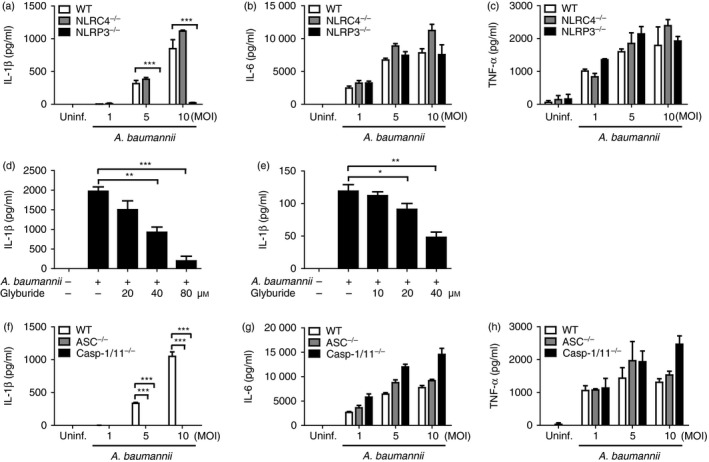
NLRP3, ASC and caspase‐1 are required for interleukin‐1β (IL‐1β) production in bone‐marrow‐derived macrophages (BMDMs) in response to Acinetobacter baumannii. BMDMs from wild‐type and NLRC4‐, NLRP3‐, ASC‐ and caspase‐1/11‐deficient mice were infected with A. baumannii at the indicated multiplicities of infection (MOIs) (a–c and f–h). For an inhibitor assay, BMDMs (d) and murine bronchoepithelial MH‐S cells (e) were pretreated with various doses of glyburide (an NLRP3 inhibitor) for 2 hr and subsequently infected with A. baumannii at an MOI of 10. One hour after infection, the cells were treated with gentamicin to inhibit extracellular bacterial growth and further incubated for 24 hr. The concentrations of IL‐1β, IL‐6 and tumour necrosis factor‐α (TNF‐α) in culture supernatants were determined by ELISA. The results are from one experiment that is representative of three independent experiments and are expressed as means ± SD. *P < 0·05, **P < 0·01 and ***P < 0·001.
Caspase‐1 inflammasome is also involved in a process of distinct cell death called pyroptosis.23 To determine whether the NLRP3 inflammasome mediates A. baumannii‐induced pyroptosis, LDH release in culture supernatant was measured. A comparable level of LDH was detected in NLRP3‐deficient and ASC‐deficient BMDMs as well as WT cells in response to A. baumannii (see Supplementary material, Fig. S1a,b). The LDH level was significantly lower in caspase‐1/11‐deficient BMDMs compared with WT cells, although caspase‐1/11‐deficient cells could release substantial levels of LDH at high MOI (1/10) (see Supplementary material, Fig. S1b). To clarify whether caspase‐1 is involved in A. baumannii‐induced pyroptosis in macrophages, an inhibitor assay was performed. Although treatment with a caspase‐1‐specific inhibitor, Y‐VAD, decreased A. baumannii‐induced production of IL‐1β in a dose‐dependent manner, it did not influence the LDH release in BMDMs (see Supplementary material, Fig. S1c,d), suggesting that caspase‐11‐mediated signalling, but not caspase‐1, may play an important role in A. baumannii‐induced pyroptotic cell death in macrophages.
NLRP3 and ASC are essential for caspase‐1 activation and IL‐1β maturation in macrophages during A. baumannii infection
We next sought to determine whether NLRP3 inflammasome mediates cleavage of caspase‐1 and IL‐1β in A. baumannii‐infected macrophages. Infection with A. baumannii induced the cleavage of caspase‐1 and IL‐1β in BMDMs from WT and NLRC4‐deficient mice, but not in those from NLRP3‐deficient mice (Fig. 2a). The protein levels of pro‐caspase‐1 and pro‐IL‐1β were comparable between cells from the different mice, regardless of A. baumannii infection (Fig. 2a). In addition, cleavage of caspase‐1 and IL‐1β was abolished in ASC‐deficient BMDMs during A. baumannii infection (Fig. 2b). Caspase‐1 deficiency also inhibited IL‐1β cleavage in BMDMs (Fig. 2b). Taken together, NLRP3 and ASC are required for A. baumannii‐induced activation of caspase‐1, which results in IL‐1β maturation in macrophages.
Figure 2.
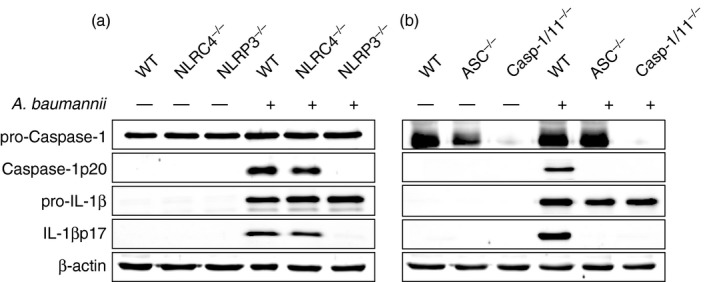
NLRP3/ASC inflammasomes contribute to caspase‐1 activation and interleukin‐1β (IL‐1β) maturation during Acinetobacter baumannii infection in bone‐marrow‐derived macrophages (BMDMs). BMDMs from wild‐type and NLRC4‐, NLRP3‐, ASC‐ and caspase‐1/11‐deficient mice were infected with A. baumannii at a multiplicity of infection (MOI) of 10 for 24 hr with gentamycin treatment as previously described (a and b). Culture supernatants and cell lysates were separately prepared and used for a Western blot analysis to detect cleaved and immature forms of caspase‐1 and IL‐1β. Antibody to β‐actin was used as a loading control.
P2X7R signalling, K+ efflux, ROS production and lysosomal destabilization are involved in A. baumannii‐induced IL‐1β production in macrophages
NLRP3 activation is mediated by various danger signals such as K+ efflux, extracellular ATP, ROS generation and cathepsin B release from lysosomes.24 To determine whether any of these factors are involved in A. baumannii‐induced inflammasome activation, we performed signal inhibition assays. Pretreatment with oxATP, an inhibitor of P2X7R, and extracellular KCl reduced A. baumannii‐induced production of IL‐1β in BMDMs in a dose‐dependent manner (Fig. 3a,b). Although IL‐6 and TNF‐α production was slightly reduced by oxATP, the amounts were still substantial (see Supplementary material, Fig. S2a,b). In addition, extracellular KCl did not influence IL‐6 and TNF‐α production in response to A. baumannii (see Supplementary material, Fig. S2c,d). Acinetobacter baumannii can induce ROS generation in immune cells,25 we therefore examined the effect of ROS on IL‐1β production. Addition of a high concentration of NAC (20 mm), a ROS inhibitor, inhibited IL‐1β production (Fig. 3c), at a concentration that MTT assay revealed was not cytotoxic (data not shown). Moreover, the IL‐6 level was not influenced by NAC, whereas TNF‐α production was inhibited by NAC in a dose‐dependent manner (see Supplementary material, Fig. S2e,f). The level of IL‐1β, but not IL‐6 and TNF‐α, was also dose‐dependently down‐regulated by a cathepsin B inhibitor (CA‐074‐Me) (Fig. 3d, and see Supplementary material, Fig. S2g,h). These findings indicate that many signals, including K+ efflux, ATP/P2X7R, ROS and cathepsin B, may participate in A. baumannii‐induced caspase‐1 activation and IL‐1β maturation in macrophages, although it still remains to be clarified whether P2X7R or ROS signalling are directly involved in A. baumannii‐induced activation of NLRP3 inflammasome.
Figure 3.
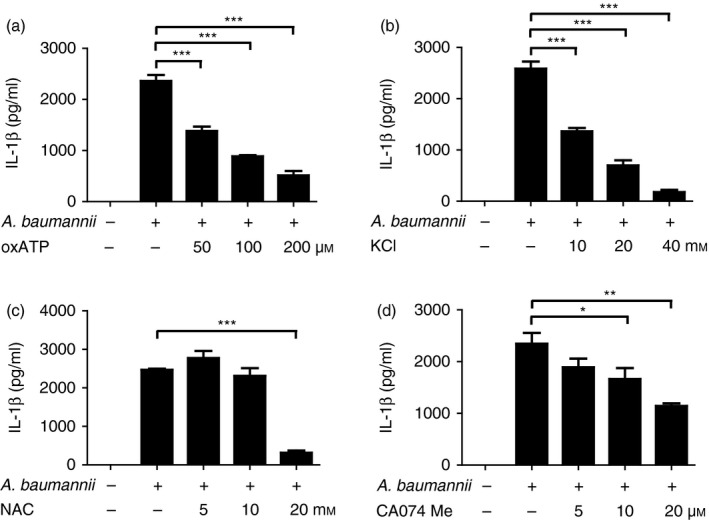
Extracellular ATP, K+ efflux, reactive oxygen species (ROS) generation and lysosomal destabilization are involved in Acinetobacter baumannii‐induced interleukin‐1β (IL‐1β) production in macrophages. Bone‐marrow‐derived macrophages (BMDMs) were pretreated with various doses of oxATP (a), KCl (b), NAC (c) and CA‐074Me (d) for 2 hr before infection. The cells were then infected with A. baumannii at a multiplicity of infection (MOI) of 10 in the presence of each inhibitor. Twenty‐four hours after infection, the levels of IL‐1β in culture supernatant were determined by ELISA. The results are from one experiment that is representative of three independent experiments and are expressed as means ± SD. *P < 0·05, **P < 0·01 and ***P < 0·001.
NLRP3 and caspase‐1 deficiencies lead to impaired production of IL‐1β in BAL fluids of A. baumannii‐infected mice
We next sought to determine the in vivo role of NLRP3 inflammasome in a mouse model of pulmonary A. baumannii infection. Mice were intranasally infected with the bacterium, and levels of various cytokines and chemokines in BAL fluid were determined at the indicated time‐points. Levels of IL‐1β were significantly lower in BAL fluids from NLRP3‐deficient mice, compared with those in fluids from WT mice (Fig. 4a). Similarly, IL‐1β production by A. baumannii was impaired in BAL fluids from caspase‐1/11‐deficient mice (Fig. 4b). NLRP3 deficiency did not affect IL‐6 production at 6 hr and 1 day after A. baumannii infection, whereas IL‐6 levels were lower in caspase‐1/11‐deficient mice than in WT mice at 1 day after infection (Fig. 4c,d). Levels of TNF‐α, IL‐10, CXCL1, CXCL2 and CCL2 were not different in the BAL fluids of WT and NLRP3‐deficient or caspase‐1/11‐deficient mice (Fig. 4e–h and see Supplementary material, Fig. S3a–f). These results suggest that NLRP3 inflammasome regulates IL‐1β production in airways in response to A. baumannii infection. In addition, we measured helper T cells producing cytokines including IFN‐γ, IL‐4, IL‐5, IL‐13 and IL‐17 in BAL fluids to determine involvement of T cells on A. baumannii‐induced inflammation. However, values of all tested cytokines were below the minimum detectable level even in BAL fluids of WT mice as well as NLRP3‐, caspase‐1/11‐ and ASC‐deficient mice (data not shown), indicating that T cells may play little role in A. baumannii‐induced lung inflammation.
Figure 4.
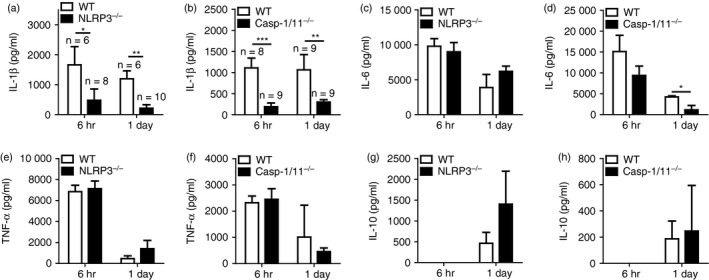
Production of interleukin‐1β (IL‐1β) in bronchoalveolar lavage (BAL) fluid is impaired in NLRP3‐ and caspase‐1/11‐deficient mice infected with Acinetobacter baumannii. Wild‐type and NLRP3‐ or caspase‐1/11‐deficient mice were intranasally infected with A. baumannii (3 × 107 colony‐forming units in PBS) and killed at 6 hr and 1 day after infection. BAL fluid was collected from each mouse, and the levels of IL‐1β (a and b), IL‐6 (c and d), tumour necrosis factor‐α (TNF‐α) (e and f) and IL‐10 (g and h) were measured by ELISA. The results were expressed as means ± SD. *P < 0·05, **P < 0·01 and ***P < 0·001.
NLRP3 inflammasome does not control bacterial growth but contributes to lung pathology in mice infected with A. baumannii
Inflammasome activation plays an important role in host defence and restriction of bacterial growth.15, 26 Therefore, we evaluated whether NLRP3 inflammasome contributes to restricting bacterial growth and promoting lung pathology in A. baumannii‐infected mice. As shown in Fig. 5(a,b), bacterial loads in BAL fluids were not different between WT and NLRP3‐deficient or caspase‐1/11‐deficient mice at 6 hr and 1 day after infection. On day 3, bacteria were not detected in BAL fluid from any mouse (data not shown). Results from a separate experiment also showed that NLRP3 and caspase‐1 were dispensable for controlling bacterial growth in the lungs of A. baumannii‐infected mice at day 1 and 3 after infection (Fig. 5c,d). Pulmonary infection with A. baumannii induced lung lesions that were characterized by infiltration of inflammatory cells (primarily neutrophils and monocytes) and oedema in perivascular and peribronchial spaces. Lung pathology was ameliorated in NLRP3‐deficient and caspase‐1/11‐deficient mice compared with that in WT mice on days 1 and 3 after infection (Fig. 5e,f), although there were no differences in total cell counts and the ratio of inflammatory cells between BAL fluids from WT and NLRP3‐deficient or caspase‐1/11‐deficient mice (see Supplementary material, Fig. S4a–h).
Figure 5.
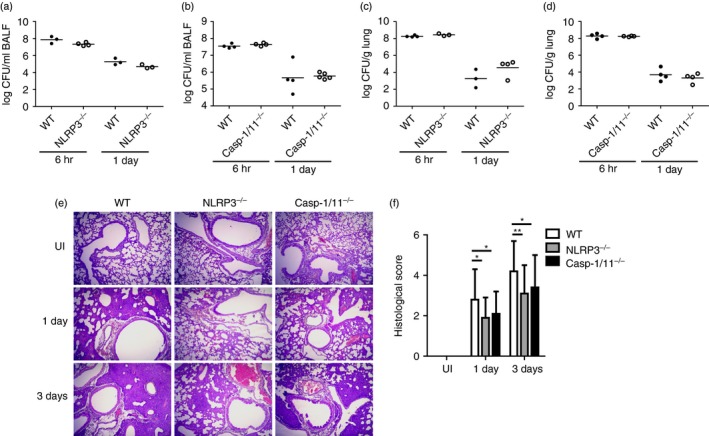
NLRP3 and caspase‐1 deficiency reduces lung pathology in Acinetobacter baumannii‐infected mice, although it does not affect bacterial clearance. Wild‐type and NLRP3‐ or caspase‐1/11‐deficient mice were intranasally infected with A. baumannii (3 × 107 colony‐forming units in PBS) and killed at 6 hr, 1 day or 3 days after infection. Bacterial loads in bronchoalveolar lavage (BAL) fluid (a and b) or lung homogenates (c and d) were counted by plating assay, and lung histopathology was evaluated (e; × 100 magnification) as described in the Materials and methods. Histological scores are shown as means ± SD (f). These results were from one experiment that is representative of two independent experiments. *P < 0·05 and **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary. com]
Neutrophils can produce IL‐1β in response to A. baumannii via NLRP3‐dependent and NLRP3‐independent pathways
Although A. baumannii‐induced IL‐1β production was absolutely dependent on NLRP3 inflammasome signalling in BMDMs (Fig. 1a,f), IL‐1β level in BAL fluids was still detectable in NLRP3‐deficient or caspase‐1/11‐deficient mice (Fig. 4a,b). We therefore investigated whether other cell types are involved in IL‐1β production in response to A. baumannii. Results showed that neutrophils, but not lung epithelial cells and fibroblasts (L929), could produce huge amounts of IL‐1β at a MOI 1/10 (Fig. 6a). As neutrophils are known to process IL‐1β maturation through several mechanisms including caspase‐1 and serine proteases,27, 28 we sought to determine whether NLRP3 inflammasome is involved in IL‐1β production in neutrophils in response to A. baumannii. At low MOIs (1/0·1 and 1/1), the production of IL‐1β was totally impaired in NLRP3‐deficient and caspase‐1/11‐deficient neutrophils (Fig. 6b). At a high MOI (1/10), NLRP3‐deficient and caspase‐1/11‐deficient neutrophils could produce detectable levels of IL‐1β in response to A. baumannii, although the level was still lower than in WT cells (Fig. 6b). Levels of IL‐6 and TNF‐α were comparable between WT and NLRP3‐deficient or caspase‐1/11‐deficient neutrophils (Fig. 6c,d). These findings suggest that neutrophils are also responsible for A. baumannii‐induced IL‐1β production through NLRP3‐dependent and ‐independent pathways and can be a major cell type for in vivo IL‐1β production in response to A. baumannii via NLRP3 inflammasome‐independent signalling.
Figure 6.
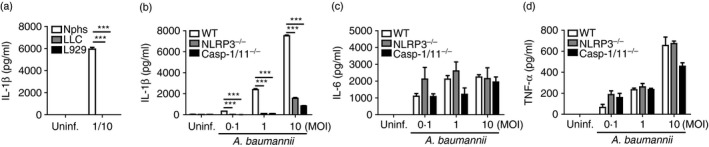
NLRP3 and caspase‐1 are involved in interleukin‐1β (IL‐1β) production in neutrophils in response to Acinetobacter baumannii. Thioglycollate‐elicited neutrophils (Nphs), lung epithelial cells and L‐929 cells were seeded in a 48‐well plate (2 × 105 cells/well) and infected with A. baumannii at a multiplicity of infection (MOI) of 1/10 for 24 hr (a). In addition, neutrophils from wild‐type, NLRP3‐ and caspase‐1/11‐deficient mice were infected with different doses of A. baumannii for 24 hr (b–d). Levels of IL‐1β, IL‐6 and tumour necrosis factor‐α (TNF‐α) in culture supernatants were measured by ELISA. The results were expressed as means ± SD. ***P < 0·001.
IL‐1R‐deficient mice display decreased lung pathology in response to A. baumannii infection
We finally tried to clarify whether IL‐1β signalling mediates A. baumannii‐induced lung pathology in mice. Wild‐type and IL‐1R‐deficient mice were infected intranasally with A. baumannii, and bacterial loads, cytokine production and histopathology in BAL fluids or lung were examined. Consistent with the results presented above, bacterial loads in BAL fluids and lung homogenates were similar between WT and IL‐1R‐deficient mice (Fig. 7a,b). Levels of most cytokines, including IL‐1β, in the BAL fluids of IL‐1R‐deficient mice were comparable to those in WT mice, although IL‐6 levels were lower in IL‐1R‐deficient than in WT mice at day 1 after infection (Fig. 7c–f). However, histopathological analysis revealed that the severity of A. baumannii‐induced lung pathology was still less in IL‐1R‐deficient mice than in WT mice (Fig. 7g,h). Taken together, NLRP3 inflammasome‐mediated IL‐1β production is likely to play a role in the development of lung pathology during A. baumannii infection.
Figure 7.
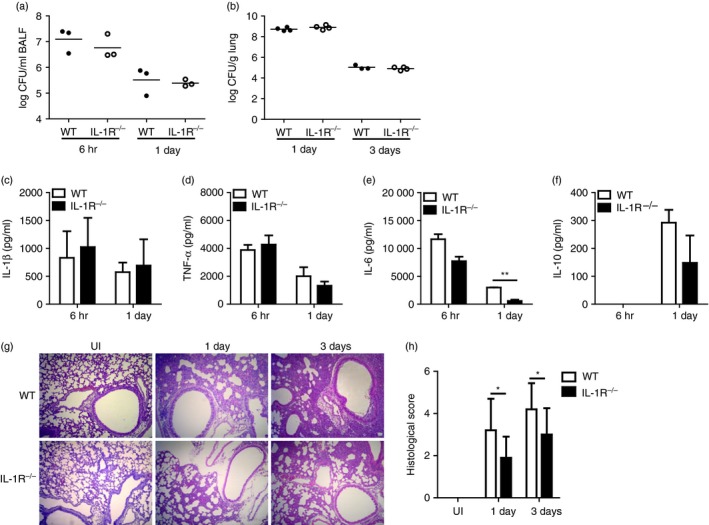
Lung pathology in response to Acinetobacter baumannii infection is reduced in interleukin‐1 receptor (IL‐1R) ‐deficient mice. Wild‐type and IL‐1R‐deficient mice were intranasally infected with A. baumannii (3 × 107 colony‐forming units in PBS) and killed at 6 hr, 1 day or 3 days after infection. Bacterial loads in bronchoalveolar lavage (BAL) fluid (a) or lung homogenates (b) were counted by plating assay. Cytokine production in BAL fluid was measured by ELISA (c–f). Lungs were histopathologically examined (g; × 100 magnification), and histological scores are shown as means ± SD (h). *P < 0·05 and **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In the present study, we revealed that A. baumannii infection leads to activation of NLRP3 inflammasome, which is essential for IL‐1β processing and secretion from macrophages, although it may be dispensable for A. baumannii‐induced pyroptosis. Moreover, NLRP3 inflammasome was required for optimal in vivo production of IL‐1β in mice with pulmonary A. baumannii infection. In contrast, NLRC4 seemed to be dispensable for caspase‐1 activation and IL‐1β processing in macrophages in response to A. baumannii infection. NLRC4 can detect bacterial flagellin and needle and rod proteins of the type III secretion system, which results in activation of caspase‐1, as well as IL‐1β and IL‐18 maturation and pyroptosis.29 NLRC4 inflammasome also contributes to restricting bacterial growth in macrophages or various pathogens, including Legionella pneumophila and Salmonella typhimurium, in vivo.29 Acinetobacter baumannii is non‐motile because flagellar genes are absent,30, 31 and it uses a type VI secretion system for bacterial competition.32 These reasons may explain why NLRC4 signalling is dispensable for A. baumannii‐induced IL‐1β maturation and caspase‐1 activation in macrophages.
Several cellular events, including K+ efflux, mitochondrial ROS generation and cathepsin release from damaged lysosomes, have been proposed as mechanisms underlying the activation of NLRP3 inflammasome.14, 33 In the present study, high concentrations of extracellular K+ and inhibitors of P2X7R, ROS and cathepsin B reduced A. baumannii‐induced production of IL‐1β in macrophages. Bacteria seem to activate inflammasomes through diverse mechanisms at various times.15 Signals such as oxidized mitochondrial DNA, ATP, K+ and cathepsin B are involved in the activation of NLRP3 inflammasome and production of IL‐1β in macrophages in response to Porphyromonas gingivalis or Chlamydia pneumoniae infections.15, 34, 35 The precise mechanism by which A. baumannii activates NLRP3 inflammasome is still not understood. It is difficult to determine the precise cellular events that result in activation of NLRP3 inflammasome, because NLRP3 activators trigger multiple and interrelated cellular signals. A recent study by Muñoz‐Planillo et al.36 showed that bacterial toxins and phagocytosis of particulate materials trigger K+ efflux from macrophages, which is the minimal signal to activate NLRP3 inflammasome. Indeed, inflammasomes in various bacteria have been reported to be activated by K+ efflux.15 In the same study, the authors also claimed that ROS production and the formation of a large pore are not necessary to activate NLRP3 inflammasome.36 Acinetobacter baumannii possesses various virulence factors, including pore‐forming toxin,37 and its outer membrane protein A (AbompA) induces ROS production in dendritic cells.25 Therefore, further study using mutant strains is recommended to identify the bacterial factors that activate inflammasomes in host cells.
The role of inflammasome activation in host defence depends on the infecting microbes. In mice infected with Pseudomonas aeruginosa, which uses NLRC4 and the type III secretion system to activate inflammasome, NLRC4 deficiency and caspase‐1 inhibition led to increased bacterial clearance and a lower level of IL‐1β production in BAL fluids.38 Caspase‐1 inhibition also reduced tissue damage and mortality in P. aeruginosa‐infected mice.38 In contrast, caspase‐1 and IL‐1β are critical for bacterial clearance and survival in mice infected with C. pneumoniae.39 In pneumococcal infections, bacterial clearance from lungs is impaired in NLRP3‐deficient and ASC‐deficient mice, but only ASC is critical for mouse survival.40 In that study, NLRP3 and ASC did not affect lung pathology early in infection (6 hr), although levels of inflammatory cytokines were lower in the lungs of NLRP3‐deficient and ASC‐deficient mice than in WT mice.40 Late in infection (48 hr), inflammatory cytokine production was greater in the lungs of NLRP3‐deficient and ASC‐deficient mice, but lung pathology was reduced only in ASC‐deficient mice.40 In the present study, NLRP3 and caspase‐1 were required for optimal production of IL‐1β in the lungs of mice infected with A. baumannii. However, NLRP3 and caspase‐1 seem to be dispensable for bacterial clearance and lung and inflammatory cell recruitment in the airways of mice infected with A. baumannii. Remarkably, lung pathology was improved in NLRP3‐deficient and caspase‐1/11‐deficient mice. Our results were very similar to those of a study by Kebaier et al.,41 which showed the role of NLRP3 inflammasome in host defence against Staphylococcus aureus infection. In that study, the bacterial burden in BAL fluid and lungs was similar between WT and NLRP3‐deficient mice.41 Interleukin‐1β levels in BAL fluid and lungs were lower in NLRP3‐deficient mice than in WT mice, whereas TNF‐α production was comparable between the two.41 Lung pathology was also improved in NLRP3‐deficient mice.41 In the present study, we found no additional evidence to support the effect of NLRP3 on A. baumannii‐induced lung pathology, because the number of total recruited cells and ratio of immune cell types in BAL fluids were not different between WT and NLRP3‐deficient or caspase‐1/‐11‐deficient mice. Additional study is recommended to clarify the precise mechanism by which NLRP3 mediates lung pathology in A. baumannii infection. We also found that IL‐1R‐deficient mice displayed reduced lung pathology, although in vivo production of IL‐1β was not impaired. These findings suggest that NLRP3/caspase‐1/IL‐1β may contribute to lung pathology in mice infected with A. baumannii.
In conclusion, A. baumannii induces IL‐1β production in macrophages through NLRP3/ASC/caspase‐1. In addition, various signals such as K+ efflux, P2X7R, ROS and cathepsins may be involved in IL‐1β production and NLRP3 inflammasome activation. The in vivo role of NLRP3 inflammasome in host defence against A. baumannii infection seems to be very limited, although it does mediate lung pathology. Because pulmonary infection with A. baumannii causes severe disease in immunocompromised patients rather than in healthy persons, especially in intensive care units, defining the role of NLRP3 inflammasome and IL‐1β in an animal model of A. baumannii infection that mimics patients in intensive care units will be necessary.
Disclosures
The authors have no financial conflicts of interest.
Supporting information
Figure S1. NLRP3 inflammasome is dispensable for Acinetobacter baumannii‐induced pyroptotic cell death in bone‐marrow‐derived macrophages.
Figure S2. Measurement of interleukin‐6 and tumour necrosis factor‐α in culture supernatant of Acinetobacter baumannii‐infected bone‐marrow‐derived macrophages in the absence or presence of oxATP, extracellular KCl, NAC and CA074 Me.
Figure S3. Chemokine production in bronchoalveolar lavage fluid of wild‐type (WT) and NLRP3‐ and caspase‐1/11‐deficient mice infected with Acinetobacter baumannii.
Figure S4. Inflammatory cell recruitment into bronchoalveolar lavage (BAL) fluid of wild‐type (WT) and NLRP3‐ and caspase‐1/11‐deficient mice with Acinetobacter baumannii infection.
Acknowledgements
This study was supported by the Basic Research in Science and Engineering programme, funded by the National Research Foundation of Korea (NRF) in the Ministry of Science, ICT and Future Planning of Korea (MSIP) (grant no. NRF‐2015R1A2A2A01002360).
References
- 1. Doughari HJ, Ndakidemi PA, Human IS, Benade S. The ecology, biology and pathogenesis of Acinetobacter spp.: an overview. Microbes Environ 2011; 26:101–12. [DOI] [PubMed] [Google Scholar]
- 2. McConnell MJ, Actis L, Pachon J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 2013; 37:130–55. [DOI] [PubMed] [Google Scholar]
- 3. Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011; 63:1055–60. [DOI] [PubMed] [Google Scholar]
- 4. Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006; 42:692–9. [DOI] [PubMed] [Google Scholar]
- 5. van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun 2007; 75:5597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One 2012; 7:e40019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim CH, Jeong YJ, Lee J, Jeon SJ, Park SR, Kang MJ et al Essential role of toll‐like receptor 4 in Acinetobacter baumannii‐induced immune responses in immune cells. Microb Pathog 2013; 54:20–5. [DOI] [PubMed] [Google Scholar]
- 8. Kim CH, Kim DJ, Lee SJ, Jeong YJ, Kang MJ, Lee JY et al Toll‐like receptor 2 promotes bacterial clearance during the initial stage of pulmonary infection with Acinetobacter baumannii . Mol Med Rep 2014; 9:1410–4. [DOI] [PubMed] [Google Scholar]
- 9. Noto MJ, Boyd KL, Burns WJ, Varga MG, Peek RM Jr, Skaar EP. Toll‐like receptor 9 contributes to defense against Acinetobacter baumannii infection. Infect Immun 2015; 83:4134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1β in inflammatory disorders. Nat Clin Pract Rheumatol 2008; 4:34–42. [DOI] [PubMed] [Google Scholar]
- 11. Park E, Na HS, Song YR, Shin SY, Kim YM, Chung J. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun 2014; 82:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahoo M, Ceballos‐Olvera I, del Barrio L, Re F. Role of the inflammasome, IL‐1β, and IL‐18 in bacterial infections. ScientificWorldJournal 2011; 11:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franchi L, Munoz‐Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012; 13:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vladimer GI, Marty‐Roix R, Ghosh S, Weng D, Lien E. Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol 2013; 16:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu MJ, Lu YC, Hsu YC, Liu WS, Wu WT. Interleukin‐1 receptor antagonist gene polymorphism in patients with multidrug‐resistant Acinetobacter baumannii‐associated pneumonia. Ann Thorac Med 2012; 7:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanneganti TD, Ozoren N, Body‐Malapel M, Amer A, Park JH, Franchi L et al Bacterial RNA and small antiviral compounds activate caspase‐1 through cryopyrin/Nalp3. Nature 2006; 440:233–6. [DOI] [PubMed] [Google Scholar]
- 18. Franchi L, Amer A, Body‐Malapel M, Kanneganti TD, Ozoren N, Jagirdar R et al Cytosolic flagellin requires Ipaf for activation of caspase‐1 and interleukin 1β in salmonella‐infected macrophages. Nat Immunol 2006; 7:576–82. [DOI] [PubMed] [Google Scholar]
- 19. Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a γ‐interferon receptor that regulates macrophage tumoricidal activity. J Exp Med 1984; 160:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong YJ, Kang MJ, Lee SJ, Kim CH, Kim JC, Kim TH et al Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils. Immunology 2014; 143:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL‐1β and IL‐18 processing during infection. Trends Immunol 2011; 32:110–6. [DOI] [PubMed] [Google Scholar]
- 22. Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev 2011; 243:136–51. [DOI] [PubMed] [Google Scholar]
- 23. Miao EA, Rajan JV, Aderem A. Caspase‐1‐induced pyroptotic cell death. Immunol Rev 2011; 243:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franchi L, Eigenbrod T, Munoz‐Planillo R, Nunez G. The inflammasome: a caspase‐1‐activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JS, Choi CH, Kim JW, Lee JC. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J Microbiol 2010; 48:387–92. [DOI] [PubMed] [Google Scholar]
- 26. Kim JJ, Jo EK. NLRP3 inflammasome and host protection against bacterial infection. J Korean Med Sci 2013; 28:1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG et al Neutrophil IL‐1β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase‐1 activation and is dependent on K+ efflux. J Immunol 2015; 194:1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guma M, Ronacher L, Liu‐Bryan R, Takai S, Karin M, Corr M. Caspase 1‐independent activation of interleukin‐1β in neutrophil‐predominant inflammation. Arthritis Rheum 2009; 60:3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao Y, Shao F. The NAIP‐NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev 2015; 265:85–102. [DOI] [PubMed] [Google Scholar]
- 30. McBride MJ. Shining a light on an opportunistic pathogen. J Bacteriol 2010; 192:6325–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clemmer KM, Bonomo RA, Rather PN. Genetic analysis of surface motility in Acinetobacter baumannii . Microbiology 2011; 157:2534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carruthers MD, Nicholson PA, Tracy EN, Munson RS Jr. Acinetobacter baumannii utilizes a type VI secretion system for bacterial competition. PLoS One 2013; 8:e59388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014; 157:1013–22. [DOI] [PubMed] [Google Scholar]
- 34. Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S et al Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012; 36:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL‐1 and the NLRP3/ASC inflammasome. J Immunol 2010; 184:5743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munoz‐Planillo R, Kuffa P, Martinez‐Colon G, Smith BL, Rajendiran TM, Nunez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013; 38:1142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Acosta J, Merino M, Viedma E, Poza M, Sanz F, Otero JR et al Multidrug‐resistant Acinetobacter baumannii harboring OXA‐24 carbapenemase, Spain. Emerg Infect Dis 2011; 17:1064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 2013; 123:1630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK et al Caspase‐1 dependent IL‐1β secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS ONE 2011; 6:e21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Lieshout MH, Scicluna BP, Florquin S, der van Poll T. NLRP3 and ASC differentially affect the lung transcriptome during pneumococcal pneumonia. Am J Respir Cell Mol Biol 2014; 50:699–712. [DOI] [PubMed] [Google Scholar]
- 41. Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD et al Staphylococcus aureus α‐hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 2012; 205:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. NLRP3 inflammasome is dispensable for Acinetobacter baumannii‐induced pyroptotic cell death in bone‐marrow‐derived macrophages.
Figure S2. Measurement of interleukin‐6 and tumour necrosis factor‐α in culture supernatant of Acinetobacter baumannii‐infected bone‐marrow‐derived macrophages in the absence or presence of oxATP, extracellular KCl, NAC and CA074 Me.
Figure S3. Chemokine production in bronchoalveolar lavage fluid of wild‐type (WT) and NLRP3‐ and caspase‐1/11‐deficient mice infected with Acinetobacter baumannii.
Figure S4. Inflammatory cell recruitment into bronchoalveolar lavage (BAL) fluid of wild‐type (WT) and NLRP3‐ and caspase‐1/11‐deficient mice with Acinetobacter baumannii infection.


