Summary
The peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) is known to have multiple anti‐inflammatory effects, typically observed in endothelial cells, macrophages, T cells and B cells. Despite the fact that mast cells are important mediators of inflammation, to date, the role of PPAR β/δ in mast cells has not been examined. Hence, the present study examined the hypothesis that PPAR β/δ modulates mast cell phenotype. Bone‐marrow‐derived mast cells (BMMCs) and peritoneal mast cells from Pparβ/δ +/+ mice expressed higher levels of high‐affinity IgE receptor (Fcε RI) compared with Pparβ/δ −/− mice. BMMCs from Pparβ/δ +/+ mice also exhibited dense granules, associated with higher expression of enzymes and proteases compared with Pparβ/δ −/− mice. Resting BMMCs from Pparβ/δ +/+ mice secreted lower levels of inflammatory cytokines, associated with the altered activation of phospholipase Cγ1 and extracellular signal‐regulated kinases compared with Pparβ/δ −/− mice. Moreover, the production of cytokines by mast cells induced by various stimuli was highly dependent on PPAR β/δ expression. This study demonstrates that PPAR β/δ is an important regulator of mast cell phenotype.
Keywords: bone marrow‐derived mast cells, cytokine, inflammation, peroxisome proliferator‐activated receptor‐β/δ
Abbreviations
- APC
allophycocyanin
- BMMC
bone‐marrow‐derived mast cell
- DNP‐HSA
dinitrophenyl‐human serum albumin
- ERK
extracellular signal‐regulated kinases
- HEPES
4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid
- IL
interleukin
- JNK
Jun N‐terminal kinases
- LPS
lipopolysaccharide
- PE
phycoerythrin
- PLC
phospholipase C
- PMC
peritoneal mast cells
- PPAR
peroxisome proliferator‐activated receptor
- TNF‐α
tumour necrosis factor‐α
- TPA
12‐O‐tetradecanoylphorbol‐13‐acetate
Introduction
Mast cells derived from bone marrow progenitors are found in many types of tissues where their differentiation is influenced by the tissue microenvironment.1, 2 In addition to expression of the high‐affinity receptor for IgE (FcεRI), mature mast cells contain a large amount of secretory granules filled with preformed and actively synthesized molecules, including amines, proteases, enzymes, peptides and cytokines.3 These mast cell molecules can be released into the extracellular environment following mast cell activation and/or degranulation and mediate different biological effects.3 Hence, mast cells not only contribute to allergic inflammation through the antigen‐dependent secretion of histamine and inflammatory factors, but also have an important role in regulating infectious agent‐mediated innate immunity.4
Peroxisome proliferator activated receptor‐β/δ (PPARβ/δ) regulates diverse cellular activities, including cell proliferation, differentiation, inflammation, and lipid and glucose homeostasis.5, 6 Although PPARβ/δ is expressed in both human and mouse mast cells,7, 8 the intrinsic role of PPARβ/δ in the function of mast cells has yet to be extensively evaluated. There is a large body of evidence indicating that PPARβ/δ promotes anti‐inflammatory activities in many cell types.9 For example, it was shown that PPARβ/δ protects against experimentally induced colitis.10 Additionally, PPARβ/δ attenuates immune cell infiltration in the dermis and subcutaneous tissue in response to topical application of 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA).11 TPA is known to induce mast cell activation,12 which is important for the recruitment of other immune cell types, such as neutrophils, T cells and monocytes.3 Collectively, these observations support the hypothesis that PPARβ/δ regulates mast cell activity, which was examined in the present study.
Materials and methods
Mice
Wild‐type (Pparβ/δ +/+) and Pparβ/δ‐null (Pparβ/δ −/−) female mice on a C57BL/6 genetic background11 were housed in a vivarium as previously described.13 All studies performed were approved by The Pennsylvania State University Institutional Animal Care and Use Committee.
Culture of primary bone marrow‐derived mast cells
Bone marrow cells were isolated from femurs and tibias of female Pparβ/δ +/+ and Pparβ/δ −/− mice (6–8 weeks of age) as previously described14 and cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 1% penicillin–streptomycin, 10 mm HEPES, 2 mm l‐glutamine (Gibco), 50 μm β‐mercaptoethanol (Sigma‐Aldrich, St Louis, MO) and 10 ng/ml mouse interleukin‐3 (IL‐3) (PeproTech, Rocky Hill, NJ) at 37° with 5% carbon dioxide. Non‐adherent cells were transferred to complete medium with fresh IL‐3 every 2 days. After 4 weeks of culture, immature mast cells were cultured in complete medium with both 10 ng/ml IL‐3 and 50 ng/ml stem cell factor (SCF) for another 4 weeks to stimulate maturation. Expression of c‐KIT and FcεRI in bone‐marrow‐derived mast cells (BMMCs) were analysed with a Cytomic FC500 flow cytometer (Beckman Coulter, Brea, CA) using antibodies against CD117 (c‐KIT) conjugated with FITC and FcεRIα conjugated with phycoerythrin (eBioscience, San Diego, CA) to quantify mast cell maturation.14 Cytospun cells were fixed and stained with toluidine blue or alcian blue/safranin O (Sigma‐Aldrich) as previously described.14, 15 A cell counter (Beckman Coulter, Brea, CA) was used to determine the cell number over time, and the relative growth rates were normalized to initial cell number on day 0. BMMCs cultured in media containing IL‐3 for 4 weeks followed by IL‐3/SCF co‐stimulation for 2–4 weeks were used for further analyses as described below.
Transmission electron microscope and qualitative assessment of BMMC maturation
After culturing with IL‐3 and IL‐3/SCF for 6–8 weeks, BMMCs derived from Pparβ/δ +/+ and Pparβ/δ −/− mice were fixed in 2% glutaraldehyde in 0·1 m sodium cacodylate buffer (pH 7·4), post‐fixed in 1% osmium tetroxide, dehydrated with serial ethanol dilutions and embedded in Eponate. Ultrathin sections (80 nm) were stained with uranyl acetate and lead citrate, and analysed with a transmission electron microscope (JEOL JEM 1200 EXII, Peabody, MA). Stages of mast cell maturation were determined by the granule number and structure as previously described.16 Resting mast cells were then classified into five groups, indicating their maturity: stage A, fully mature BMMCs filled with dense granules and no empty chamber; stage B, mature BMMCs with a few empty chambers and a few chambers filled with dense granules; stage C, incompletely mature BMMCs containing a large number of chambers with dense but small granules; stage D, immature BMMCs containing large chambers with diffuse granules; stage E, immature BMMCs with large empty chambers. To induce degranulation, BMMCs were also sensitized with 1 μg/ml mouse anti‐dinitrophenyl‐IgE (clone SPE‐7; Sigma‐Aldrich) and then activated by 100 ng/ml dinitrophenyl‐human serum albumin (DNP‐HSA) (Sigma‐Aldrich) for 4 hr. Five mice per genotype and 30–40 mast cells per mouse were assessed.
Cell proliferation assay
Cell proliferation was determined by flow cytometry using a carboxyfluorescein succinimidyl ester (CFSE) proliferation kit (Life Technologies, Carlsbad, CA) following the manufacturer's recommended procedure. BMMCs (2 × 106) derived from Pparβ/δ +/+ and Pparβ/δ −/− mice were initially labelled with 5 μm CFSE, and cell proliferation was monitored by flow cytometry up to 5 days. Cells treated with 10 μg/ml mitomycin C (Sigma‐Aldrich) to induce cell cycle arrest were used as a control.
Cell survival and apoptosis
Growth factor withdrawal has been shown to induce apoptosis in many types of cells, including mast cells.17 BMMCs (2 × 107) derived from Pparβ/δ +/+ and Pparβ/δ −/− mice were pre‐treated with or without GW0742 (1 μm), a specific PPARβ/δ agonist, for 1 hr and then incubated in media without IL‐3 and SCF in the presence or absence of GW0742 for up to 6 days. Cell numbers were counted daily with a Coulter counter and survival rates in response to IL‐3 and SCF withdrawal were determined. At the end of the experiment, cells were stained with 7‐aminoactinomycin D and annexin V‐FITC (eBioscience) following the manufacturer's recommended procedures. Apoptosis was measured with a Cytomic FC500 flow cytometer (Beckman Coulter).
Antigen‐dependent and antigen‐independent activation of BMMCs
To induce antigen‐mediated activation, BMMCs (2 × 106) were sensitized overnight with 1 μg/ml mouse anti‐DNP‐IgE (IgE) and then activated by 100 ng/ml DNP‐HSA for 4 hr. To induce an antigen‐independent response, BMMCs (2 × 106) were treated with 100 ng/ml lipopolysaccharide (LPS) or 500 nm TPA for 24 hr. Both LPS and TPA were purchased from Sigma‐Aldrich. BMMCs (2 × 106) were also exposed to UVB (280–315 nm, 50 mJ/cm2) using a CL‐1000 Ultraviolet Crosslinker (Ultra‐Violet Products, Upland, CA) and then cultured for 24 hr post‐UVB exposure.
Preparation of peritoneal mast cells
Peritoneal cells were collected from Pparβ/δ +/+ and Pparβ/δ −/− mice as previously described.18 Peritoneal cells (2 × 104) were cytospun onto glass slides and stained with toluidine blue. Peritoneal mast cells (PMCs) (c‐KIT+/FcεRI+) were identified by Cytomic FC500 flow cytometer (Beckman Coulter) using anti‐CD117 (c‐KIT)‐FITC and anti‐FcεRIα‐PE antibodies (eBioscience, San Diego, CA), and the number of PMCs was then quantified. The total number of peritoneal cells was normalized by body weight, and the percentage of PMCs was calculated by dividing the number of PMCs with total number of peritoneal cells.
To induce cytokine production, PMCs were cultured in medium containing LPS (100 ng/ml) or TPA (500 nm) followed by the addition of a protein transport inhibitor, brefeldin A (eBioscience, San Diego, CA). PMCs were then fixed and stained with anti‐IL‐10‐APC antibody (eBioscience, San Diego, CA) following the manufacturer's recommended protocol. Intracellular expression of IL‐10 in c‐KIT+/FcεRI+ PMC populations was determined with a Cytomic FC500 flow cytometer.
Western blot analysis
Quantitative Western blot analysis using a radioactive detection method was performed as previously described.19 To analyse the expression of phosphoproteins, BMMCs (2 × 106) were homogenized in RIPA buffer supplemented with a protease inhibitor cocktail, 5·4 mm sodium pyrophosphate, 50 mm sodium fluoride and 1 mm sodium orthovanadate (Sigma‐Aldrich). Membranes were blocked with either 5% non‐fat milk or phosphoprotein blocker (Millipore, Billerica, MA). Primary antibodies included carboxypeptidase 3 (CPA3; ABBIOTEC, LLC, San Diego, CA), IκB, phospho‐IκB, ACTIN (Santa Cruz Biotechnology, Santa Cruz, CA), phospholipase Cγ1 (PLCγ1), phosho‐PLCγ1, PLCγ2, phosho‐PLCγ2, p65, phospho‐p65, AKT, phospho‐AKT, extracellular signal‐regulated kinases (ERK), phospho‐ERK, Jun N‐terminal kinase (JNK), phospho‐JNK (Cell Signaling Technology, Danvers, MA) and lactate dehydrogenase (Rockland Immunochemicals, Inc., Limerick, PA). The expression level of each protein was normalized to ACTIN or lactate dehydrogenase, and the normalized expression of the phosphoproteins was normalized to the non‐phosphorylated protein of interest.
Quantitative real‐time PCR
Expression of mast cell mediator genes in BMMCs in response to DNP‐HSA, UVB, LPS and TPA treatment was determined by quantitative PCR analysis as previously described.19 Mast cell mediator genes included carboxypeptidase A3 (Cpa3), β‐hexosaminidase A (Hexa), β‐hexosaminidase B (Hexb), mast cell protease 4 (Mcpt4), chymase 1 (Cma1, also known as Mcpt5), tryptase β‐2 (Tpsb2, also known as Mcpt6), Il6, Il10, tumour necrosis factor‐α (Tnfa) and vascular endothelial growth factor (Vegf). Each assay included a standard curve and a non‐template control that were performed in triplicate. Relative mRNA levels of target genes were normalized to the mRNA level of glyceraldehyde‐3‐phosphate dehydrogenase (Gapdh).
Cytokine assay
To determine secretory cytokine levels in mast cells, MILLIPLEX MAP Multiplex cytokine assays (EMD Millipore, Billerica, MA) were used following the manufacturer's recommended procedures. Basal levels of 17 mouse cytokines, including granulocyte‐colony stimulating factor, granulocyte–macrophage colony‐stimulating factor, interferon‐γ, IL‐1β, IL‐2, IL‐3, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, IL‐12, IL‐13, monocyte chemo‐attractant protein‐1, tumour necrosis factor‐α (TNF‐α), vascular endothelial growth factor and macrophage inflammatory protein‐2, in supernatants of BMMCs (2 × 106) derived from Pparβ/δ +/+ and Pparβ/δ −/− mice were quantified 24 hr after culture using a Bio‐Plex multiplex system (Bio‐Rad, Hercules, CA).
Statistical analysis
The data were subjected to either Student's t‐test or a parametric one‐way analysis of variance followed by Tukey test for post hoc comparisons (prism 5.0, GraphPad Software Inc., La Jolla, CA).
Results
PPARβ/δ modulates BMMC phenotype
The BMMCs were isolated from Pparβ/δ +/+ and Pparβ/δ −/− mice and initially cultured with IL‐3 for 28 days to provide sufficient numbers of BMMCs to analyse. After this 28‐day culture period, BMMCs were cultured with both IL‐3 and SCF for 2–4 weeks to induce maturation. As they mature, BMMCs increase cell surface expression of FcεRI and c‐KIT. After 6 days of culture with IL‐3, a greater percentage of BMMCs from Pparβ/δ +/+ mice exhibited higher cell surface expression of FcεRI and c‐KIT as assessed by flow cytometry, whereas the percentage of BMMCs from Pparβ/δ −/− mice with cell surface expression of FcεRI and c‐KIT was markedly lower (Fig. 1a). This difference between genotypes persisted until 21 days of culture, when the relative population of BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice both expressed a relatively high level of cell surface FcεRI and c‐KIT (Fig. 1a). After 24 days of culture with IL‐3 there were no differences in the percentage of mature BMMCs, basophils, or other cell types between genotypes, however, ~3·3% of BMMCs from Pparβ/δ −/− mice exhibited relatively lower expression of FcεRI, compared with Pparβ/δ +/+ mice (Figs 1a,b). BMMCs from Pparβ/δ +/+ mice that were induced to more complete maturation by co‐treatment with IL‐3 and SCF (from day 28 onward) exhibited high cell surface expression of FcεRI and c‐KIT, whereas a significant percentage of BMMCs from Pparβ/δ −/− mice exhibited lower expression of FcεRI and c‐KIT (Fig. 1a). Interestingly, the mean fluorescent intensity of cell surface expression of FcεRI was higher in Pparβ/δ +/+ BMMCs compared with BMMCs from Pparβ/δ −/− mice over time (Fig. 1c). By contrast, the mean fluorescent intensity of cell surface expression of c‐KIT was not different between BMMCs in either genotype over time (Fig. 1d).
Figure 1.
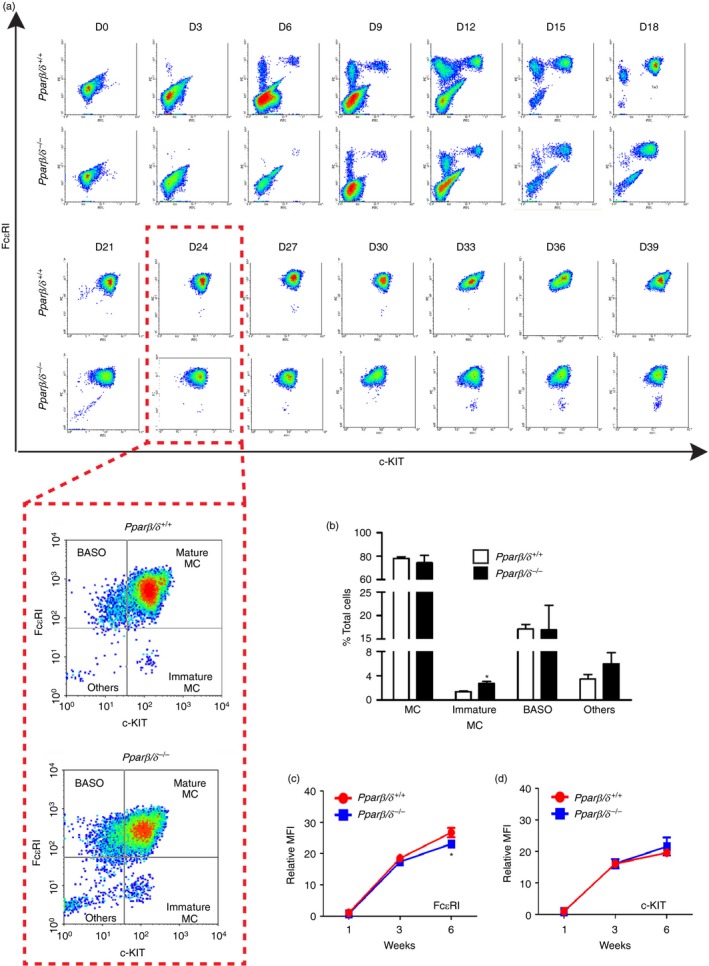
Peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) promotes maturation and differentiation of bone‐marrow‐derived mast cells (BMMCs). BMMCs were isolated from adult female Pparβ/δ +/+ and Pparβ/δ −/− mice, and cultured in media containing interleukin‐3 (IL‐3) for 4 weeks followed by co‐stimulation with IL‐3 and stem cell factor (SCF) for 2 weeks. (a) Representative flow cytometric analyses of BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice showing changes in expression of Fcε RI and c‐KIT from day 0 (D0) to day 39 (D39) of culture. The red dashed lines surrounding the two boxes illustrate a closer view of flow cytometric data on day 24 (D24). (b) After culturing for 24 days, the percentages of mature mast cells (MC), immature mast cells, basophils (BASO) and other cells (others) in total cell suspension were determined by flow cytometry. The relative mean fluorescent intensities (MFI) of (c) Fcε RI and (d) c‐KIT of BMMCs over time were determined by flow cytometry. Values represent the mean ± SEM. *Significantly different than Pparβ/δ +/+ mice, P ≤ 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
The BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice were stained with toluidine blue or alcian blue/safranin O to determine whether PPARβ/δ influenced granule content of mast cells. BMMCs from Pparβ/δ +/+ mice exhibited a high content of granules as shown by relatively intense staining with toluidine blue compared with BMMCs from Pparβ/δ −/− mice, which exhibited relatively lower staining with toluidine blue (Fig. 2a). Interestingly, the majority of BMMCs from Pparβ/δ +/+ mice also exhibited red granules, indicating strong safranin O staining with minimal alcian blue staining (Fig. 2b). In contrast, BMMCs from Pparβ/δ −/− mice exhibited blue granules or mixed colours, indicating moderate safranin O staining and strong alcian blue staining (Fig. 2b). Since the safranin‐positive staining can correlate with the progressive stages of maturation,20 these observations suggest that PPARβ/δ may influence the content of granules in mast cells. In addition, transmission electron microscopy was performed to examine control, resting phase BMMCs and BMMCs induced to degranulate using IgE + DNP‐HSA. Resting BMMCs from Pparβ/δ +/+ mice contained dark and more compact granules, whereas BMMCs from Pparβ/δ −/− mice exhibited lighter and markedly less dense granules (Fig. 2c). It is worth noting that the percentage of resting BMMCs from Pparβ/δ −/− mice with empty granules was greater than resting BMMCs from Pparβ/δ +/+ mice (Fig. 2d). Degranulation was induced in BMMCs from both Pparβ/δ +/+ and Pparβ/δ −/− mice in response to IgE and DNP‐HSA stimulation, but this effect was greater in BMMCs from Pparβ/δ −/− mice (Fig. 2d) and may reflect the difference in degranulation observed in the resting phase between genotypes (Fig. 2c). Although these data suggest that PPARβ/δ may influence degranulation of mast cells, more definitive biochemical quantification of granule content, such as the level and activity of β‐hexosaminidase or histamine, should be addressed in future studies.
Figure 2.
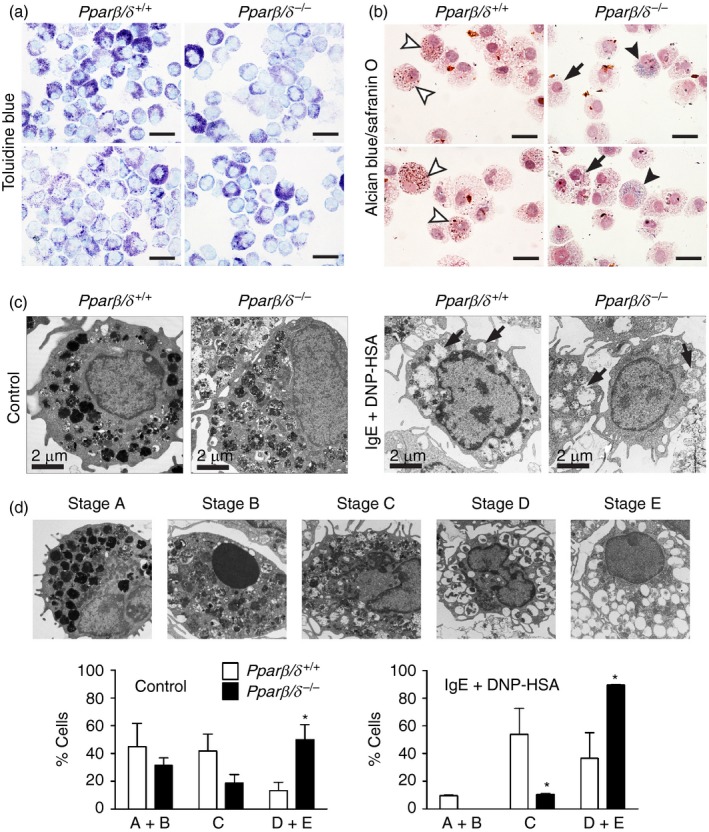
Peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) regulates maturation and granule formation of bone‐marrow‐derived mast cells (BMMCs). BMMCs were isolated from adult female Pparβ/δ +/+ and Pparβ/δ −/− mice, and cultured in media containing interleukin‐3 (IL‐3) for 4 weeks followed by co‐stimulation with IL‐3 and stem cell factor (SCF) for 2 weeks. (a) Representative photomicrographs of cytospun BMMCs from adult Pparβ/δ +/+ and Pparβ/δ −/− mice, showing a denser granules distribution in BMMCs from Pparβ/δ +/+ mice by toluidine blue staining. (b) Representative photomicrographs of cytospun BMMCs from adult Pparβ/δ +/+ and Pparβ/δ −/− mice, illustrating a heterogeneous distribution of granules in BMMCs by Alcian blue/safranin O double staining. BMMCs from Pparβ/δ +/+ mice exhibited higher content of red granules (white arrowheads), consistent with a mature phenotype. However, BMMCs from Pparβ/δ −/− mice showed a higher content of blue granules (black arrowheads) or mild to moderate level of blue granules (black arrow), consistent with a less mature phenotype. Magnification × 100. Bar = 50 μm. (c) Representative photomicrographs of BMMCs from adult Pparβ/δ +/+ and Pparβ/δ −/− mice as assessed by transmission electron microscopy. BMMCs from Pparβ/δ +/+ mice exhibited darker and denser granules than BMMCs from Pparβ/δ −/− mice. BMMCs were also sensitized by IgE/DNP‐HSA activation to initiate degranulation, represented as empty granules (arrows). Magnification = 4000×. Bar = 2 μm. (d) Upper panel: Representative photomicrographs of BMMCs showing the five different stages of mast cell maturation as defined by others.16 Lower panels: The average percentages of each classification is shown for each genotype in resting and IgE/DNP‐HSA treated mast cells. For Pparβ/δ +/+ control mast cells, the absolute number of cells in stages A+B, C, and D+E were 15·7 ± 5·9, 14·6 ± 4·2 and 4·7 ± 2·1. For Pparβ/δ +/+ mast cells stimulated with IgE/dinitrophenyl‐human serum albumin (DNP‐HSA), the absolute number of cells in stages A+B, C, and D+E were 3·3 ± 0·2, 18·9 ± 6·6 and 12·8 ± 6·4. For Pparβ/δ −/− control mast cells, the absolute numbers of cells in stages A+B, C and D+E were 11·0 ± 1·9, 6·5 ± 2·2 and 17·5 ± 3·8. For Pparβ/δ −/− mast cells stimulated with IgE/DNP‐HSA, the absolute numbers of cells in stages A+B, C and D+E were 0·0 ± 0·0, 3·7 ± 0·2 and 31·3 ± 0·2. These values are consistent with the range in BMMCs examined per genotype and treatment from a total of five mice per treatment group (control and stimulated; 30–40 BMMCs). Values represent the mean ± SEM. *Significantly different than Pparβ/δ +/+, P ≤ 0·05. [Colour figure can be viewed at wileyonlinelibrary. com]
PPARβ/δ suppresses proliferation of BMMCs independent of cytokine‐withdrawal induced cell death
As PPARβ/δ regulates cell proliferation and differentiation in various cell types,5, 13 proliferation of BMMCs derived from Pparβ/δ +/+ and Pparβ/δ −/− mice was examined. BMMCs from Pparβ/δ +/+ mice exhibited slower growth compared with BMMCs from Pparβ/δ −/− mice (Fig. 3a). Similarly, flow cytometric analyses revealed that proliferation of BMMCs from Pparβ/δ +/+ mice exhibited slower growth compared with BMMCs from Pparβ/δ −/− mice (Fig. 3b). Additionally, BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice were cultured for 6 weeks in the presence of IL‐3 and SCF, and then cultured in media without IL‐3 and SCF for up to 6 days. Cytokine deprivation caused a gradual decrease in survival rates of BMMCs from both genotypes (Fig. 3c). Ligand activation of PPARβ/δ with the specific agonist GW0742 had no effect on survival rates in either genotype in response to cytokine withdrawal (Fig. 3c). No significant differences in apoptosis or late apoptosis/necrosis of BMMCs were observed between genotypes in response to cytokine depletion (Fig. 3d).
Figure 3.
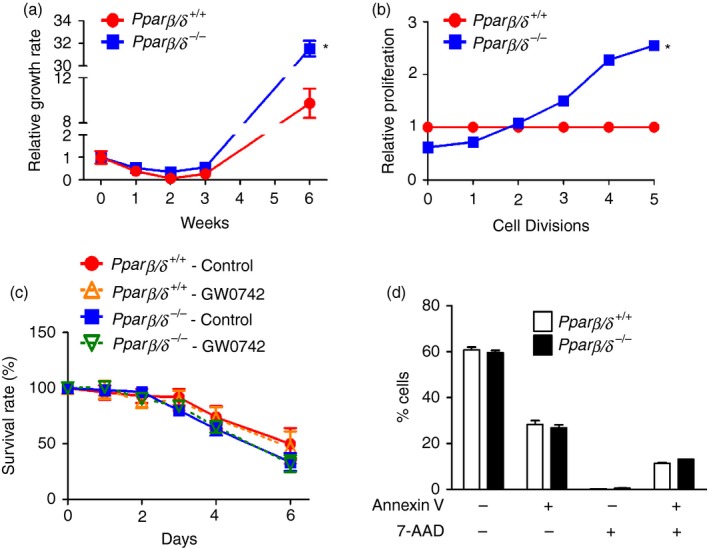
Peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) suppresses proliferation of bone marrow‐derived mast cells (BMMCs) independent of cytokine‐withdrawal induced cell death. BMMCs were isolated from adult female Pparβ/δ +/+ and Pparβ/δ −/− mice, and cultured in media containing interleukin‐3 (IL‐3) for 4 weeks followed by co‐stimulation with IL‐3 and stem cell factor (SCF) for 2 weeks. (a) Relative growth rates of BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice over time. (b) Relative proliferation of BMMCs over five cell divisions in Pparβ/δ +/+ and Pparβ/δ −/− mice as assessed by flow cytometry. Values were normalized to that of Pparβ/δ +/+ BMMCs. (c) The changes in survival rates of BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice cultured in media without IL‐3 and SCF for up to 6 days, in the presence or absence of the PPAR β/δ specific agonist GW0742 (1 μm). (d) Quantification of BMMCs labelled with annexin V and 7‐aminoactinomycin D in response to depletion of IL‐3 and SCF for 6 days as assessed by flow cytometry. Values represent the mean ± SEM. *Significantly different than Pparβ/δ +/+, P ≤ 0·05. [Colour figure can be viewed at wileyonlinelibrary. com]
PPARβ/δ influences the number of PMCs and their IL‐10 production
To determine whether PPARβ/δ has a direct influence on the development of tissue mast cells, peritoneal cells freshly isolated from peritoneal lavage of Pparβ/δ +/+ and Pparβ/δ −/− mice were examined. Peritoneal mast cells were stained with toluidine blue (Fig. 4a). The number of total peritoneal cells between genotypes was not different (Fig. 4a,b). However, Pparβ/δ +/+ mice exhibited a greater percentage of mature PMCs (c‐KIT+/FcεRI+) compared with Pparβ/δ −/− mice as assessed by flow cytometry (Fig. 4c). This contributed to a greater percentage of PMCs in total peritoneal cells in Pparβ/δ +/+ mice compared with Pparβ/δ −/− mice (Fig. 4d). Moreover, flow cytometric analysis indicated that PMCs from Pparβ/δ +/+ mice have a relatively higher level of FcεRI and c‐KIT expression as the intensity was higher in Pparβ/δ +/+ mice compared with Pparβ/δ −/− mice (Fig. 4e,f). Additionally, mature PMCs (c‐KIT+/FcεRI+) from Pparβ/δ +/+ mice exhibited a significantly lower level of IL‐10 expression compared with Pparβ/δ −/− mice based on flow cytometry (Fig. 4 g). Similar results were observed between genotypes in spleen mast cells, which showed a more mature phenotype in Pparβ/δ +/+ mice that exhibited higher expression of FcεRI (see Supplementary material, Fig. S1). TPA treatment caused an induction of IL‐10 expression in mature PMCs (c‐KIT+/FcεRI+) from both Pparβ/δ +/+ and Pparβ/δ −/− mice (Fig. 4 g). LPS treatment had no effect on IL‐10 expression in either genotype (Fig. 4 g), however, basal expression of IL‐10 was higher in mature PMCs from Pparβ/δ −/− mice compared with mature PMCs from Pparβ/δ +/+ mice.
Figure 4.
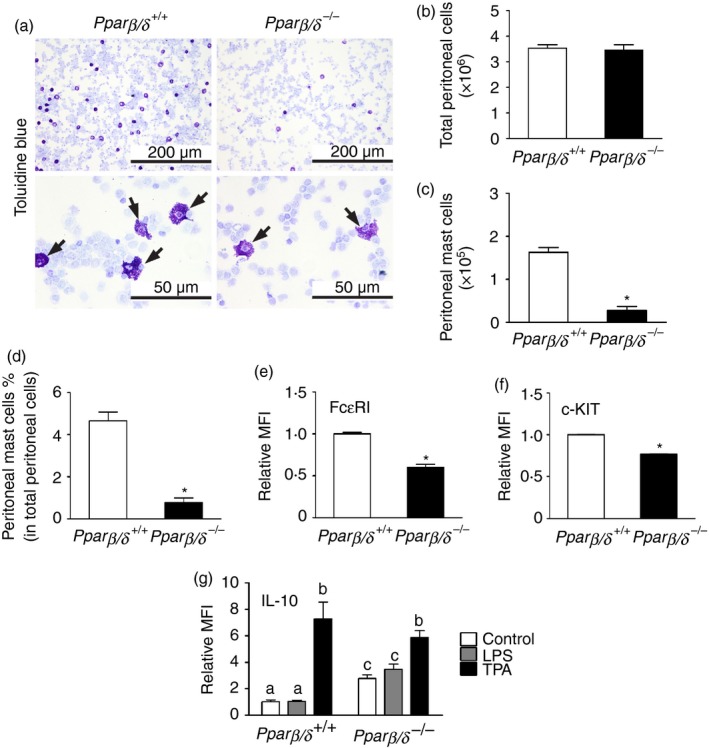
Peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) is required for maturation of peritoneal mast cells (PMCs). (a) Representative photomicrographs of cytospun peritoneal cells from adult Pparβ/δ +/+ and Pparβ/δ −/− mice stained by toluidine blue. Arrowheads indicated toluidine blue‐positive mast cells. Upper panels: Magnification × 20. Bar = 200 μm. Lower panels: Magnification × 40. Bar = 50 μm. The numbers of (b) total peritoneal cells and (c) PMCs were counted, and (d) the average percentage of mast cells in total peritoneal cells was determined. Relative mean fluorescent intensities (MFI) of (e) Fcε RI and (f) c‐KIT of PMCs were determined by flow cytometry. Values represent the mean ± SEM. *Significantly different from Pparβ/δ +/+, P ≤ 0·05. (g) Peritoneal cells from adult Pparβ/δ +/+ and Pparβ/δ −/− mice were treated with lipopolysaccharide (LPS) or 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) for 24 hr, and expression of interleukin‐10 was determined by flow cytometry. Values represent the mean ± SEM. Values with different superscript letters are significantly different at P ≤ 0·05. [Colour figure can be viewed at wileyonlinelibrary. com]
PPARβ/δ regulates mast cell mediators associated with activation of PLCγ1 and ERK
Mast cells produce a variety of chemical mediators including proteases and cytokines responsible for modulating inflammation and allergic reactions. These mediators are released upon activation and/or degranulation of mast cells.3 BMMCs from Pparβ/δ +/+ mice expressed significantly higher mRNA levels of preformed proteases, including Cpa3, Mcp4, Cma1, Tspb2, Hexa and Hexb, than BMMCs from Pparβ/δ −/− mice (Fig. 5a). The change in relative expression of CPA3 was confirmed at the protein level as well (data not shown). Moreover, compared with BMMCs from Pparβ/δ −/− mice, BMMCs from Pparβ/δ +/+ mice secreted lower levels of cytokines, including granulocyte–macrophage colony‐stimulating factor, interferon‐γ, IL‐2, IL‐3, IL‐4, IL‐6, IL‐10, TNF‐α and macrophage inflammatory protein‐2 (Fig. 5b). Mast cell mediators released to the extracellular environment are controlled by a complicated kinase and enzyme network,21, 22 so the activation of various kinases and enzymes was assessed by quantitative Western blot analyses. The level of pPLCγ1 was higher in BMMCs from Pparβ/δ +/+ mice than BMMCs from Pparβ/δ −/− mice (Fig. 5c). By contrast, the level of pERK in BMMCs from Pparβ/δ +/+ mice was lower compared with BMMCs from Pparβ/δ −/− mice (Fig. 5c). Expression and/or activation of PLCγ2, AKT, p65, IκB and p38 of BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice were comparable (Fig. 5c). It is worth noting that the expression of p38 in BMMCs from Pparβ/δ +/+ mice was lower compared with BMMCS from Pparβ/δ −/− mice (Fig. 5c).
Figure 5.
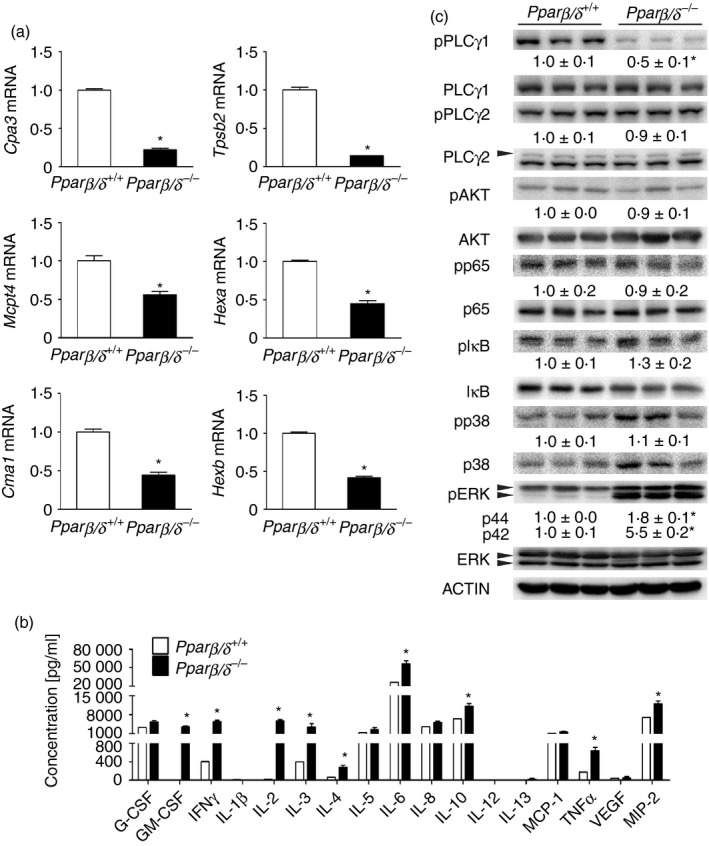
Peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) regulates mast cell mediators associated with activation of phospholipase Cγ1 (PLC γ1)and extracellular signal regulated kinase (ERK). (a) mRNA expression in resting bone‐marrow‐derived mast cells (BMMCs) of Cpa3, Mcp4, Cma1, Tspb2, Hexa and Hexb were determined by quantitative PCR. (b) The average concentrations of indicated inflammatory cytokines released from resting BMMCs were determined. (c) Quantitative Western blot analysis of pPLC γ1, PLC γ1, pPLC γ2, PLC γ2, pAKT, AKT, pp65, p65, pI κB, IκB, pp38, p38, pERK and ERK expression in resting BMMCs. Relative expression level of target protein was normalized to ACTIN. Values represent the mean ± SEM. *Significantly different than Pparβ/δ +/+, P ≤ 0·05. [Colour figure can be viewed at wileyonlinelibrary. com]
PPARβ/δ differentially regulates cytokine production in BMMCs in response to antigen‐dependent and antigen‐independent activation
To investigate the role of PPARβ/δ in antigen‐dependent and ‐independent activation of mast cells, mRNA levels of Il6, Il10, Tnfa and Vegf in BMMCs from Pparβ/δ +/+ and Pparβ/δ −/− mice were examined. The mRNA expression of Il6, Il10, Tnfa and Vegf in BMMCs from Pparβ/δ +/+ mice were significantly lower than BMMCs from Pparβ/δ −/− mice (Fig. 6), consistent with the finding that BMMCs from Pparβ/δ +/+ mice released lower levels of IL‐6, IL‐10 and TNF‐α compared with those from Pparβ/δ −/− mice (Fig. 5b). To induce antigen‐dependent activation, BMMCs were treated with IgE and DNP‐HSA. Antigen stimulation had no effect on Il6, Il10 and Vegf mRNA expression in BMMCs from Pparβ/δ +/+ mice (Fig. 6a), but caused increased Tnfa expression (Fig. 6a). The lack of change in Il6 mRNA expression in Pparβ/δ +/+ BMMCs may reflect the timing of analysis as this was measured 4 hr post‐activation, and peak Il6 mRNA expression occurs 1 hr post‐activation.23 By contrast, BMMCs from Pparβ/δ −/− mice exhibited higher basal levels of Il6, Il10, Tnfa and Vegf mRNA compared with Pparβ/δ +/+ BMMCs, but lower expression of Il6 and Il10 mRNA and a greater induction of Tnfa and Vegf expression in response to IgE and DNP‐HSA treatment (Fig. 6a). Secretion of IL‐6 was increased in response to IgE and DNP‐HSA in Pparβ/δ +/+ BMMCs, whereas basal secretion of IL‐6 was markedly higher in control BMMCs from Pparβ/δ −/− mice, but was not induced by IgE and DNP‐HSA (see Supplementary material, Fig. S2a).
Figure 6.
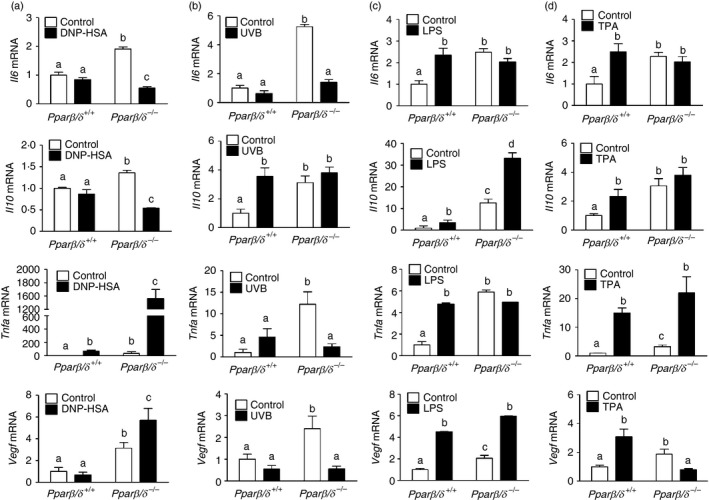
Peroxisome proliferator‐activated receptor‐β/δ (PPAR β/δ) differentially regulates cytokine production in bone‐marrow‐derived mast cells (BMMCs) in response to antigen‐dependent and antigen‐independent activation. (a) BMMCs were sensitized by IgE/dinitrophenyl‐human serum albumin (DNP‐HSA) treatment. The mRNA expression of Il6, Il10, Tnfa and Vegf in BMMCs were determined by quantitative PCR. BMMCs were exposed to (b) UVB irradiation, or treated with either (c) lipopolysaccharide (LPS) or (d) 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) for 24 hr. The mRNA expression of Il6, Il10, Tnfa and Vegf in BMMCs was determined by quantitative PCR. Values represent the mean ± SEM. Values with different superscript letters are significantly different at P ≤ 0·05.
Exposure to UVB induced antigen‐independent mast cell activation and caused no further changes in Il6, Tnfa and Vegf mRNA expression in BMMCs from Pparβ/δ +/+ mice (Fig. 6b). However, Il10 mRNA expression was significantly increased in BMMCs from Pparβ/δ +/+ mice compared with control (Fig. 6b). In contrast, UVB irradiation decreased Il6, Tnfa and Vegf mRNA expression in BMMCs from Pparβ/δ −/− mice (Fig. 6b), but showed no effect on Il10 mRNA expression (Fig. 6b). Secretion of IL‐6 was increased in response to UVB exposure in both Pparβ/δ +/+ and Pparβ/δ −/− BMMCs, and basal secretion of IL‐6 was markedly higher in control BMMCs from Pparβ/δ −/− mice compared with control BMMCs from Pparβ/δ +/+ mice (see Supplementary material, Fig. S2b).
The BMMCs were also treated with LPS or TPA to initiate antigen‐independent activation. Interestingly, LPS enhanced expression of Il6, Il10, Tnfa and Vegf mRNA in BMMCs from Pparβ/δ +/+ mice (Fig. 6c), However, LPS‐induced cytokine production was only observed in Il10 and Vegf mRNA expression in BMMCs from Pparβ/δ −/− mice (Fig. 6c). Similarly, TPA caused increased expression of Il6, Il10, Tnfa and Vegf mRNA in BMMCs from Pparβ/δ +/+ mice (Fig. 6d). No significant difference was observed in Il6 and Il10 mRNA levels in in BMMCs from Pparβ/δ −/− mice following TPA treatment (Fig. 6d). TPA‐treated BMMCs from Pparβ/δ −/− mice exhibited higher Tnfa and lower Vegf expression compared with controls (Fig. 6d).
Discussion
As mast cells are found in Pparβ/δ −/− mice, PPARβ/δ is clearly not essential for the development of mast cells. However, the present studies are the first to demonstrate an essential role for PPARβ/δ in the distribution and phenotype of mast cells in mice. Genetic disruption of PPARβ/δ caused lower expression of key mast cell signalling molecules (i.e. FcεRI and c‐KIT), which may indicate a role for PPARβ/δ in maturation, ultrastructure, as well as the expression levels of critical signalling proteins (i.e. enzymes and cytokines) in mast cells. Moreover, BMMCs from Pparβ/δ −/− mice exhibited marked increases in ERK activity and cell proliferation compared with BMMCs from Pparβ/δ +/+ mice. These observations are consistent with previous work showing a PPARβ/δ‐dependent of ERK activity as a key signalling protein mediating somatic cell maturation and modulation of cell proliferation.13, 24, 25 Interestingly, no significant differences in apoptosis of BMMCs from Pparβ/δ +/+ or Pparβ/δ −/− mice were observed. To date, whether PPARβ/δ is pro‐apoptotic or anti‐apoptotic remains controversial (reviewed in refs 5, 6); however, results from the present study support previous work showing that PPARβ/δ has no direct effect on apoptosis.19, 26
There are two primary subtypes of mast cells: connective tissue mast cells residing in the skin, peritoneal cavity and submucosa of the intestinal tract, and mucosal mast cells located at mucosal surfaces.27 These two types of mast cells differ in their histochemical properties, mediator contents and the responses to mast cell activation.27, 28 In the present study, BMMCs from Pparβ/δ +/+ mice cultured with IL‐3 and SCF to induce maturation, exhibited positive staining for safranin O in cytoplasmic granules and expressed specific chymases and tryptases, similar to a connective tissue mast cell‐like phenotype. By contrast, BMMCs from Pparβ/δ −/− mice did not exhibit these characteristics indicated by the reduced protease production and histochemical staining. This suggests that PPARβ/δ may modulate maturation and synthesis of essential components in connective tissue mast cells that ultimately influence their function. However, further characterization should be performed to more definitively demonstrate a functional role for PPARβ/δ in regulating mast cells. Interestingly, others have shown that ligand activation of PPARβ/δ with carbaprostacyclin represses pro‐inflammatory cytokines in human mast cells,7 which is consistent with the phenotype observed in the present studies. However, since the affinity of carbaprostacyclin for the cell surface prostaglandin I2 receptor, compared with the nuclear receptor PPARβ/δ, is not known, it is uncertain whether this anti‐inflammatory effect is indeed mediated by PPARβ/δ. Nevertheless, there is evidence indicating that endogenous ligands are present in cells capable of modulating PPARβ/δ‐dependent gene expression,29 hence, the effects observed in the present studies may support this hypothesis.
The N‐MYC downstream‐regulated gene 1 (NDRG1) is a key regulator of maturation of connective tissue mast cells.28 Similar to the present study, genetic disruption of the Ndrg1 gene in mice results in a reduction of basal and inducible degranulation capacity of mast cells associated with decreased PLCγ1 activity.14 Although the ability of PPARβ/δ to regulate degranulation requires further characterization, this is similar to the phenotype observed with BMMCS from Pparβ/δ −/− mice. Hence, based on the present studies and those of others,14 PPARβ/δ and NDRG1 may have interrelated biological functions. In contrast to BMMCs from Pparβ/δ −/− mice, cytokine expression and mRNA expression of four major proteases in connective tissue mast cells (Mcpt4, Cma1, Tpsb2 and Cpa3) are not different between Ndrg1 +/+ and Ndrg1 −/− mice.14 PPARβ/δ may regulate the development of connective tissue mast cells in part through a mechanism that may require NDRG1, and this possibility should be examined in greater detail.
Surprisingly, ~ 50% of BMMCs from Pparβ/δ −/− mice exhibited empty chambers or large chambers with diffuse granules in the cytoplasm even without antigen stimulation. This demonstrates that PPARβ/δ modulates the basal phenotype of BMMCs. The diffuse granules in BMMCs may reflect decreased production of mast cell enzymes and apparently less mature phenotype of BMMCs in Pparβ/δ −/− compared with Pparβ/δ +/+ mice. The mechanisms mediating PPARβ/δ‐dependent regulation of mast cell degranulation cannot be determined from the present study. One possibility is that the BMMCs spontaneously degranulate and release mast cell mediators through a mechanism involving repression by PPARβ/δ. Alternatively, as PPARβ/δ can prevent oxidative stress, it remains possible that the absence of PPARβ/δ and its activity induced by endogenous ligands, can prevent membrane damage caused by increased oxidative stress,30 which could decrease the stability of the mast cell membrane and impact mast cell function.31, 32. Hence, PPARβ/δ could influence the release of BMMC granule contents through multiple mechanisms. Interestingly, Pparβ/δ −/− mice do not exhibit an IgE class switching in B cells (data not shown); hence the spontaneous degranulation and the release of inflammatory cytokines in BMMCs from Pparβ/δ −/− mice is probably not the result of altered IgE production. Further studies are needed to determine the functional significance of the observed differences in mast cell chamber phenotype.
Immunoglobulin E and DNP‐HSA stimulation effectively induced degranulation in BMMCs from Pparβ/δ +/+ mice based on transmission electron microscope analysis. Almost all of the BMMCs from Pparβ/δ −/− mice also contained empty chambers, consistent with degranulation, in response to antigen stimulation based on transmission electron microscope analysis. Further biochemical analyses are needed to delineate the specific effector molecules that reflect this phenotype, and the mechanisms mediated by PPARβ/δ underlying this grossly apparent phenotypic difference between genotypes. Mast cell secretory granules are best known to function as inflammatory mediators.3, 33 The anti‐inflammatory role of PPARβ/δ has also been examined in several models, including monocytes, macrophages, dendrite cells and endothelial cells, where it is also important for cell quiescence.9 As noted above, earlier work suggested that ligand activation of PPARβ/δ inhibits cytokine production.7 Numerous studies have established that PPARβ/δ down‐regulates expression of pro‐inflammatory mediators by interfering with the p65 subunit of the nuclear factor‐κB complex.9 Further experimentation is needed to determine whether this mediates the similar effects observed in BMMCs in the present study. There is also evidence that ligand activation can repress expression of IL‐10.34 Consistent with this observation, enhanced expression of IL‐10 expression was noted in BMMCs from Pparβ/δ −/− mice. As IL‐10 is known to have anti‐inflammatory activities, further studies are needed to determine how PPARβ/δ modulates the balance of pro‐inflammatory and anti‐inflammatory cytokines and how this balance influences mast cell function.
Mast cells are also capable of presenting antigens and effectively modulating innate immunity against pathogens,35 which is known to be influenced by FcεRI expression.36 In the present study, BMMCs from Pparβ/δ +/+ mice exhibited higher FcεRI expression with lower levels of inflammatory cytokines compared with mast cells from Pparβ/δ −/− mice. This suggests that PPARβ/δ may be required for modulating innate and adaptive immunity against parasitic pathogens and allergens, possibly through regulating the process of immunosuppression. Both LPS and TPA treatments caused marked induction of inflammatory cytokines in BMMCs from Pparβ/δ +/+ mice. BMMCs from Pparβ/δ −/− mice exhibited increased sensitivity to chemically induced inflammation and were not capable of maintaining cytokine production in mast cells. It is well known that LPS induces cytokine production in mast cells without degranulation.37, 38 The present study further demonstrated that the LPS‐induced IL‐6 and TNF‐α expression are PPARβ/δ‐dependent, whereas LPS‐induced IL‐10 and vascular endothelial growth factor expression are not. These observations support the view that PPARβ/δ is critical for modulating host defence against pathogens by modulating the inflammatory process. PPARβ/δ was reported to mediate FcεRI expression associated with a decreased histamine release from human basophils.39 However, neither FcεRI nor c‐KIT expression in mast cells was altered by the specific PPARβ/δ agonist GW0742 in the present study (data not shown). The different results between the previous study and the present study may be due to the fact that the relatively high concentration of PPARβ/δ ligands used in previous report may have caused off‐target effects.
The mechanisms by which PPARβ/δ regulates inflammation involve complex networks of tissues and immunological components. The present study demonstrates PPARβ/δ‐dependent effects in mast cell responses to various stimuli, establishing that PPARβ/δ may be important to orchestrate inflammatory responses in mast cells. How PPARβ/δ could be a viable cellular target for the therapeutic intervention of mast cell‐mediated disorders remains to be further investigated.
Disclosures
The authors declare that they have no financial or commercial conflicts of interests.
Supporting information
Figure S1. Peroxisome proliferator‐activated receptor‐β/δ regulates maturation of spleen mast cells.
Figure S2. Role of peroxisome proliferator‐activated receptor‐β/δ in antigen‐dependent and antigen‐independent activation of bone‐marrow‐derived mast cells.
Acknowledgements
The authors gratefully thank the Microscopy and Cytometry Facility at the Huck Institute of Life Sciences of The Pennsylvania State University for providing technique support with flow cytometry, histology equipment and transmission electron microscopy. This study was supported by the National Institutes of Health and National Cancer Institute grants R01‐CA124533 (J.M. Peters), R01‐CA141029 (J.M. Peters), 1ZIABC005561 (F.J. Gonzalez), 1ZIABC005562 (F.J. Gonzalez), and 1ZIABC005708 (F.J. Gonzalez).
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
References
- 1. Hallgren J, Gurish MF. Mast cell progenitor trafficking and maturation. Adv Exp Med Biol 2011; 716:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dahlin JS, Hallgren J. Mast cell progenitors: origin, development and migration to tissues. Mol Immunol 2015; 63:9–17. [DOI] [PubMed] [Google Scholar]
- 3. Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol 2014; 14:478–94. [DOI] [PubMed] [Google Scholar]
- 4. Bischoff SC. Role of mast cells in allergic and non‐allergic immune responses: comparison of human and murine data. Nat Rev Immunol 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 5. Peters JM, Gonzalez FJ, Muller R. Establishing the role of PPARβ/δ in carcinogenesis. Trends Endocrinol Metab 2015; 26:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator‐activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer 2012; 12:181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. Peroxisome proliferator‐activated receptors are expressed in human cultured mast cells: a possible role of these receptors in negative regulation of mast cell activation. Eur J Immunol 2000; 30:3363–70. [DOI] [PubMed] [Google Scholar]
- 8. Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. Peroxisome proliferator‐activated receptors are expressed in mouse bone marrow‐derived mast cells. FEBS Lett 2000; 467:259–62. [DOI] [PubMed] [Google Scholar]
- 9. Kilgore KS, Billin AN. PPARβ/δ ligands as modulators of the inflammatory response. Curr Opin Investig Drugs 2008; 9:463–9. [PubMed] [Google Scholar]
- 10. Hollingshead HE, Morimura K, Adachi M, Kennett MJ, Billin AN, Willson TM, et al PPARβ/δ protects against experimental colitis through a ligand‐independent mechanism. Dig Dis Sci 2007; 52:2912–9. [DOI] [PubMed] [Google Scholar]
- 11. Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, et al Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator‐activated receptor β/δ . Mol Cell Biol 2000; 20:5119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sagi‐Eisenberg R, Foreman JC, Shelly R. Histamine release induced by histone and phorbol ester from rat peritoneal mast cells. Eur J Pharmacol 1985; 113:11–7. [DOI] [PubMed] [Google Scholar]
- 13. Yao PL, Chen L, Hess RA, Muller R, Gonzalez FJ, Peters JM. Peroxisome proliferator‐activated receptor‐D (PPARD) coordinates mouse spermatogenesis by modulating extracellular signal‐regulated kinase (ERK)‐dependent signaling. J Biol Chem 2015; 290:23416–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taketomi Y, Sunaga K, Tanaka S, Nakamura M, Arata S, Okuda T, et al Impaired mast cell maturation and degranulation and attenuated allergic responses in Ndrg1‐deficient mice. J Immunol 2007; 178:7042–53. [DOI] [PubMed] [Google Scholar]
- 15. Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, et al Stem cell factor programs the mast cell activation phenotype. J Immunol 2012; 188:5428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berent‐Maoz B, Gur C, Vita F, Soranzo MR, Zabucchi G, Levi‐Schaffer F. Influence of FAS on murine mast cell maturation. Ann Allergy Asthma Immunol 2011; 106:239–44. [DOI] [PubMed] [Google Scholar]
- 17. Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl‐2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl‐XL upon IgE‐receptor activation. Cell Death Differ 2005; 12:136–44. [DOI] [PubMed] [Google Scholar]
- 18. Jippo T, Morii E, Ito A, Kitamura Y. Effect of anatomical distribution of mast cells on their defense function against bacterial infections: demonstration using partially mast cell‐deficient tg/tg mice. J Exp Med 2003; 197:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao PL, Chen LP, Dobrzanski TP, Phillips DA, Zhu B, Kang BH, et al Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator‐activated receptor‐β/δ‐ and retinoic acid receptor‐dependent mechanisms. Oncotarget 2015; 6:36319–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yong LC, Watkins S, Wilhelm DL. The mast cell: distribution and maturation in the peritoneal cavity of the adult rat. Pathology 1975; 7:307–18. [DOI] [PubMed] [Google Scholar]
- 21. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast‐cell activation. Nat Rev Immunol 2006; 6:218–30. [DOI] [PubMed] [Google Scholar]
- 22. Koranteng RD, Swindle EJ, Davis BJ, Dearman RJ, Kimber I, Flanagan BF, et al Differential regulation of mast cell cytokines by both dexamethasone and the p38 mitogen‐activated protein kinase (MAPK) inhibitor SB203580. Clin Exp Immunol 2004; 137:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gurish MF, Ghildyal N, Arm J, Austen KF, Avraham S, Reynolds D, et al Cytokine mRNA are preferentially increased relative to secretory granule protein mRNA in mouse bone marrow‐derived mast cells that have undergone IgE‐mediated activation and degranulation. J Immunol 1991; 146:1527–33. [PubMed] [Google Scholar]
- 24. Zhu B, Ferry CH, Blazanin N, Bility MT, Khozoie C, Kang BH, et al PPARβ/δ promotes HRAS‐induced senescence and tumor suppression by potentiating p‐ERK and repressing p‐AKT signaling. Oncogene 2014; 33:5348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu B, Ferry CH, Markell LK, Blazanin N, Glick AB, Gonzalez FJ, et al The nuclear receptor peroxisome proliferator‐activated receptor‐β/δ (PPARβ/δ) promotes oncogene‐induced cellular senescence through repression of endoplasmic reticulum stress. J Biol Chem 2014; 289:20102–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yao PL, Morales JL, Zhu B, Kang BH, Gonzalez FJ, Peters JM. Activation of peroxisome proliferator‐activated receptor‐β/δ (PPAR‐β/δ) inhibits human breast cancer cell line tumorigenicity. Mol Cancer Ther 2014; 13:1008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 2011; 12:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taketomi Y, Sugiki T, Saito T, Ishii S, Hisada M, Suzuki‐Nishimura T, et al Identification of NDRG1 as an early inducible gene during in vitro maturation of cultured mast cells. Biochem Biophys Res Commun 2003; 306:339–46. [DOI] [PubMed] [Google Scholar]
- 29. Khozoie C, Borland MG, Zhu B, Baek S, John S, Hager GL, et al Analysis of the peroxisome proliferator‐activated receptor‐β/δ (PPARβ/δ) cistrome reveals novel co‐regulatory role of ATF4. BMC Genom 2012; 13:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao GF, Xu SH, Chao Y, Xie LD, Xu CS, Wang HJ. PPARδ activation inhibits homocysteine‐induced p22(phox) expression in EA.hy926 cells through reactive oxygen species/p38MAPK pathway. Eur J Pharmacol 2014; 727:29–34. [DOI] [PubMed] [Google Scholar]
- 31. Puri N, Kruhlak MJ, Whiteheart SW, Roche PA. Mast cell degranulation requires N‐ethylmaleimide‐sensitive factor‐mediated SNARE disassembly. J Immunol 2003; 171:5345–52. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki K, Verma IM. Phosphorylation of SNAP‐23 by IκB kinase 2 regulates mast cell degranulation. Cell 2008; 134:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pejler G, Ronnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood 2010; 115:4981–90. [DOI] [PubMed] [Google Scholar]
- 34. Kanakasabai S, Chearwae W, Walline CC, Iams W, Adams SM, Bright JJ. Peroxisome proliferator‐activated receptor δ agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology 2010; 130:572–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abraham SN. St John AL. Mast cell‐orchestrated immunity to pathogens. Nat Rev Immunol 2010; 10:440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gong J, Yang NS, Croft M, Weng IC, Sun L, Liu FT, et al The antigen presentation function of bone marrow‐derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcεRI and MHC II. BMC Immunol 2010; 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marshall JS. Mast‐cell responses to pathogens. Nat Rev Immunol 2004; 4:787–99. [DOI] [PubMed] [Google Scholar]
- 38. Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol 2004; 114:21–7. [DOI] [PubMed] [Google Scholar]
- 39. Fujimura Y, Tachibana H, Yamada K. Peroxisome proliferator‐activated receptor ligands negatively regulate the expression of the high‐affinity IgE receptor FcεRI in human basophilic KU812 cells. Biochem Biophys Res Commun 2002; 297:193–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Peroxisome proliferator‐activated receptor‐β/δ regulates maturation of spleen mast cells.
Figure S2. Role of peroxisome proliferator‐activated receptor‐β/δ in antigen‐dependent and antigen‐independent activation of bone‐marrow‐derived mast cells.


