Summary
The antigenic reactivity of constituents of Schistosoma mansoni and peanut (Arachis hypogaea) was investigated to determine whether identical antigenic epitopes possessed by both organisms provided a possible explanation for the negative correlation between chronic schistosome infection and atopy to allergens. Aqueous extracts of peanuts were probed in Western immunoblots with rabbit IgG antibodies raised against the egg, cercarial and adult worm stages of S. mansoni. Several molecules in the peanut extract were antigenically reactive with antibodies from the various rabbit anti‐schistosome sera. A pair of cross‐reactive peanut molecules at ~30 000–33 000 molecular weight was purified and both proteins were identified by mass spectrometric analysis as the peanut allergen Ara h 1. Anti‐S. mansoni soluble egg antigen antibodies that were eluted off the peanut molecules reacted with two S. mansoni egg antigens identified by mass spectrometry as IPSE/α‐1 and κ‐5. Alignments of the amino acid sequences of Ara h 1 and either IPSE/α‐1 or κ‐5 revealed a low level of peptide sequence identity. Incubation of nitrocellulose paper carrying electrophoresed peanut molecules, six constituents of other allergic plants and S. mansoni egg antigens in a mild solution of sodium metaperiodate before probing with antibodies, inhibited most of the cross‐reactivities. The results are consistent with the antigenic cross‐reactive epitopes of S. mansoni egg antigens, peanut and other allergic plants being cross‐reactive carbohydrate determinants (CCDs). These findings are novel and an explanation based on ‘blocking antibodies’ could provide an insight for the inverse relationship observed between schistosome infection and allergies.
Keywords: antigenic cross‐reactivity, cross‐reactive carbohydrate determinants, hygiene hypothesis, IgG antibodies, peanut Ara h 1, Schistosoma mansoni
Abbreviations
- CCDs
cross‐reactive carbohydrate determinants
- CFA
complete Freund's adjuvant
- IL‐4
interleukin‐4
- MS
mass spectrometry
- MW
molecular weight
- NMS
normal mouse serum
- SmCh
Schistosoma mansoni cercariae homogenate
- SmSEA
Schistosoma mansoni soluble egg antigens
- SmWh
Schistosoma mansoni adult worm homogenate
- Th1
T helper type 1
Introduction
Schistosomes are parasites belonging to the broad class of invertebrates known as flatworms and responsible for the human disease schistosomiasis, a parasitic disease of public health importance that has chronic, debilitating consequences.1, 2 Eight species in the genus Schistosoma distributed in many tropical countries are known to infect humans,3 though three species, namely Schistosoma mansoni, Schistosoma haematobium and Schistosoma japonicum are medically the most important.
Early schistosome infections, before patency, induce host immunity of the T helper type 1 (Th1) type involving interferon‐γ and tumour necrosis factor‐α.4 However, both acute S. mansoni infection and sensitization by allergens evoke similar immune responses that are Th2‐biased and characterized by the release of cytokines such as interleukin‐4 (IL‐4), IL‐5 and IL‐13, eosinophilia, and production of IgE antibodies.5, 6 Chronic and advanced stages of infection are characterized also by Th2 immunity, but in a form that is ‘modified or ‘modulated’ and that evokes regulatory T‐cell activity and production of cytokines such as IL‐10 and transforming growth factor‐β, which curb excessive inflammatory responses and so enhance survival of the parasite and perhaps that of the host also.4, 7, 8 Incidentally, the characteristics of immunomodulated anti‐parasite Th2 immunity are to some extent similar to those that pertain after successful immunotherapy of allergic diseases.9, 10, 11
Several studies indicate that schistosome‐infected individuals show lower skin‐prick test positivity to allergens,12, 13, 14 including some plant food allergens.15 An inverse relationship has been reported between S. mansoni infection and allergic sensitization in infected individuals.7, 16, 17 The accumulated evidence is consistent with the hygiene hypothesis, which in summary holds that a reduction in the prevalence of microbial and parasitic infections in people in developed or urban areas and/or improved hygiene and increased rates of vaccination, have resulted in immune responsiveness becoming misdirected and reacting against otherwise innocuous molecules in pollens, food, water and venoms, or indeed against the body's own molecules to give rise to autoimmunity.12, 18, 19, 20
Chronic infection with schistosomes has been surmised to have an immunoregulatory effect on allergic sensitization and some of the regulatory mechanisms by which this occurs have been discussed.11, 14, 21 One explanation could be induction of immunomodulated responses by chronic parasitic infections, during which regulatory B and T cells are activated to produce anti‐inflammatory cytokines such as IL‐10 and transforming growth factor‐β and the synthesis of IgG4 is initiated.14, 21 An alternative is the blocking antibody hypothesis: IgG, particularly IgG4, may compete with IgE for common epitopes on allergens.9, 22, 23, 24, 25, 26 IgG‐blocking antibody has been reported in patients undergoing allergen‐specific immunotherapy, for example after allergen‐specific immunotherapy for peanut allergy.27
We have recently used rabbit IgG antibodies to demonstrate antigenic cross‐reactivity between molecules in S. mansoni egg and cercarial extracts and a latex allergen (Hev b 7) and we suggested the possibility that anti‐S. mansoni IgG antibodies could block allergen‐specific IgE reactions.28 The study of antigenic cross‐reactivity between S. mansoni and allergens was therefore continued here with respect to peanut, which is of global medical importance with a relatively high prevalence.29, 30, 31
In this study, peanut constituents were probed for antigenic reactivity with IgG antibodies from rabbits that had been immunized with different stages of S. mansoni. Two peanut molecules that were antigenically cross‐reactive with the parasite were identified as isoforms of Ara h 1. Consistent with this observation, rabbit anti‐S. mansoni IgG antibodies reactive with Ara h 1 reacted with two known S. mansoni egg antigens. Most of the cross‐reactivity was abrogated by prior treatment with sodium metaperiodate, suggesting that it was due to cross‐reactive carbohydrate determinants (CCDs).
Materials and methods
Except when otherwise stated, chemicals, reagents and buffers were bought from Sigma‐Aldrich (Poole, UK) and were all of analytical grade.
Preparation of S. mansoni antigen/soluble extracts
A Puerto‐Rican S. mansoni isolate was maintained by continuous passages through Biomphalaria glabrata and random‐bred CD1 strain mice. Maintenance of the parasite life cycle was performed with strict adherence to the regulations set out in the UK Animals (Scientific Procedures) Act, 1986, (project licence numbers PPL 40/3024 and 40/3595). Animals were killed by administration of a lethal dose of pentobarbitone anaesthetic.
The method of preparation of S. mansoni soluble egg antigens (SmSEA) extracted from the livers and intestines of infected mice harbouring adult worms was as described in ref. 32. SmSEA was lyophilized in 1‐mg aliquots and stored at −80°.
Preparation of allergen extracts
Peanut extract was prepared from fresh peanut seeds bought from a grocery shop. Extraction of protein was performed as described in ref. 28.
Latex extract was prepared from Copydex (Henkel, Winsford, UK), a commercially obtainable product made from natural rubber‐tree latex sap, as described in ref. 28.
All other fruits for preparation of aqueous extracts were bought in ripe form from grocery stores: melon (Cucumis melo spp.), banana (Musa spp.), avocado (Persea americana), tomato (Lycopersicon esculatum) and kiwi (Actinidia deliciosa). Fruit extracts were prepared as described in ref. 28. Frozen extracts were then lyophilized overnight and stored at −20°.
Preparation of extracts from mouse blood, kidney and spleen
Mouse blood was prepared as described in ref. 33. Extracts of mouse kidney and spleen were prepared by homogenization, sonication and centrifugation of the tissues using the same extraction methods as for the fruits above.
Protein estimation in different extracts
The estimation of protein concentration in all extracts was done using the Bio‐Rad DC Protein assay (BioRad, Hemel Hempstead, UK) adapted from ref. 34 using bovine serum albumin as the standard.
Preparation of rabbit polyspecific anti‐S. mansoni egg, anti‐cercariae and anti‐whole adult worm antisera and antisera monospecific for S. mansoni egg antigens
Polyspecific anti‐S. mansoni antisera were raised against homogenates of S. mansoni eggs (anti‐SmSEA), cercariae (anti‐SmCh) and adult worm (anti‐SmWh) by immunization of rabbits with the respective homogenates as in ref. 35 and 36.
Rabbit antisera against two egg antigens, interleukin‐4‐inducing principle from schistosome eggs IPSE/α‐1 and κ‐5, were prepared as described in ref. 36.
Rabbit anti‐mouse serum and anti‐complete Freund's adjuvant antisera
Polyspecific rabbit anti‐normal mouse serum (anti‐NMS) and anti‐complete Freund's adjuvant (anti‐CFA) sera were prepared as described in ref. 33 by injecting rabbits with repeated weekly doses of a 1‐ml emulsion containing CFA with an equal volume of NMS or isotonic saline.
One‐dimensional SDS–PAGE and Western immunoblotting
SDS–PAGE was performed as described in ref. 37, adapted from ref. 38, using 12% acrylamide for the resolving gel and 4·5% acrylamide for the stacking gel. Tris–HCl buffer was used in the preparation of the resolving and stacking buffer solutions and adjusted to pHs of 8·8 and 6·8, respectively.
Western immunoblotting was done as described in ref. 39, adapted as in ref. 36 and 28.
Staining and purification of electrophoresed proteins in SDS–PAGE
SDS–PAGE gels containing electrophoresed proteins were rinsed in distilled water at room temperature three times for 10 min each time. The gels were subsequently covered with SimplyBlue SafeStain (Invitrogen, Carlsbad, CA) for 2 hr for protein staining. Gel de‐staining was performed for an hour in several changes of distilled water to allow visualization of protein bands.
Purification of proteins was by excision of the bands of interest from the array of molecules that were present on a de‐stained gel, elution of protein from the excised gel band by incubation in buffer (0·06 m Tris–HCl + 10% SDS, pH 7·4) overnight at 37°, centrifugation and re‐electrophoresing of the eluate in a second PAGE, as described in ref. 40.
Mass spectrometry and glycosylation analyses of protein samples
Tandem mass spectrometry (MS) was performed on a Waters Corporation Q‐TOF 2 instrument41, 42 according to methods described in ref. 28.
Potential glycosylation sites on amino acid sequences of an MS‐identified allergen and two parasite antigens were predicted using the glycoEP software43 and the ExPASy Bioinformatics Resource Portal (GlycoMod) tool (http://web.expasy.org/glycomod/). The amino acid sequence of the protein was pasted into the software and the prediction based on binary profile of patterns was highlighted while leaving other settings as default and set to run, thereby revealing the number(s) of potential N‐linked or O‐linked glycosylation site(s) on the amino acid sequence of the protein.
Purification of cross‐reactive antibodies by acid elution
Specific anti‐SmSEA antibodies that cross‐reacted with two peanut molecules were purified by acid elution, a method adapted from ref. 44 and modified as in ref. 28.
Treatment of nitrocellulose paper with sodium metaperiodate
The technique was adapted from ref. 45 and 46 and performed as described in ref. 28.
Results
Cross‐reactivity between IgG antibodies in polyspecific anti‐S. mansoni sera and peanut antigens
The constituents of an aqueous extract of raw peanuts were investigated in Western immunoblots for their cross‐reactivity with five polyspecific rabbit antisera raised against homogenates of three different stages of S. mansoni; two anti‐SmSEA antisera, two anti‐SmCh antisera and an antiserum raised against adult worm antigens (anti‐SmWh). Two rabbit antisera, an anti‐CFA and an anti‐NMS were used as controls in the same immunoblot.
Figure 1(a) shows that all five rabbit antisera raised against S. mansoni antigens contained antibodies that cross‐reacted with many different molecules in the peanut extract with molecular weights (MW) ranging from ~7000 to ~200 000 (lanes 1–5). Neither the anti‐NMS nor an anti‐CFA sera used as controls in lanes 6 and 7 reacted against any molecules in the peanut extract.
Figure 1.
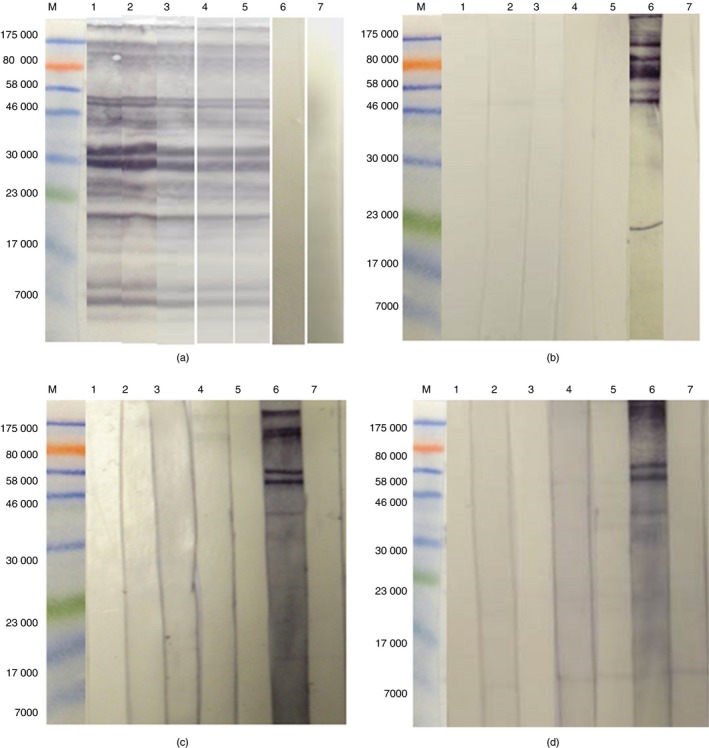
Western immunoblots of electrophoresed peanut and mouse tissue extracts probed with rabbit antisera. (a) Peanut, (b) mouse blood, (c) mouse kidney, (d) mouse spleen. (M) Molecular weight markers, (1) anti‐Schistosoma mansoni soluble egg antigens (anti‐SmSEA) serum, (2) anti‐SmSEA serum, (3) anti‐S. mansoni cercariae homogenate (anti‐SmCH), (4) anti‐SmCH, (5) anti‐S. mansoni adult worm (anti‐SmWh), (6) anti‐normal mouse serum (anti‐NMS), (7) anti‐complete Freund's adjuvant (anti‐CFA). Amounts of protein loaded per lane: 0·1 mg peanut extract; 0·003 mg NMS; 0·021 mg kidney extract; 0·214 mg spleen extract. [Colour figure can be viewed at wileyonlinelibrary.com]
As a control, all five polyspecific anti‐S. mansoni sera and the two control antisera (anti‐NMS and anti‐CFA) were applied separately on immunoblotted constituents of aqueous extracts of mouse tissues (blood, kidney and liver, Fig. 1b–d, respectively). Results showed that none of the five anti‐S. mansoni sera nor the anti‐CFA serum demonstrated any reactivity with antigens in all three extracts from mouse (lanes 1, 2, 3, 4, 5, 7 in Fig. 1b–d). Some antigens in each of the three extracts from mouse showed reactivity with antibodies in a rabbit anti‐NMS that was used as a further control (lane 6 in Fig. 1b–d).
The anti‐SmSEA antiserum used in lane 1, Fig. 1(a) was selected for use in subsequent experiments.
Two peanut antigens that reacted with the rabbit antibodies more conspicuously at ~30 000–33 000 MW in Fig. 1(a) were investigated further. Hence, Fig. 2(a) is a Western blot of peanut extract probed with the rabbit anti‐SmSEA used in lane 1 in Fig. 1(a). Two arrows against the image of the Coomassie‐stained SDS–PAGE gel in Fig. 2(b) point to two peanut molecules of ~30 000 and ~33 000 MW that were deemed to correspond to the most intensely reactive molecules in Fig. 2(a). Both of the peanut antigens of ~30 000–33 000 MW were purified by excision from the gel and re‐electrophoresis in SDS–PAGE to yield two seemingly distinct molecules (Fig. 2c).
Figure 2.
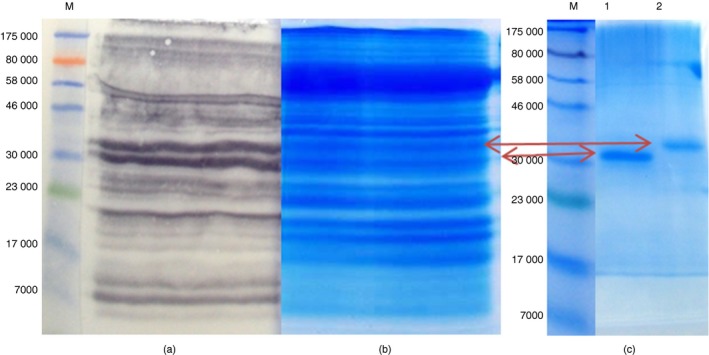
Steps showing the identification and purification of two cross‐reactive peanut antigens. (a) Western immunoblot showing the identification of peanut antigens that are cross‐reactive with IgG antibodies in an anti‐Schistosoma mansoni soluble egg antigen (anti‐SmSEA) serum, (b) a portion of gel “a” stained in Coomassie blue for identification and excision of a pair of cross‐reactive peanut gel bands at ~30 000–33 000 MW intended for purification, (c) Coomasie‐stained SDS–PAGE showing further resolution of the pair of peanut bands into a lower band (lane 1) and a top band (lane 2) for mass spectrometry analysis. [Colour figure can be viewed at wileyonlinelibrary.com]
The two purified peanut bands were subjected to MS analysis separately and data from each of the bands was searched against the NCBInr database using the Mascot ‘MSMS ions search’ tool for peptide matching and protein identification. The results showed that each band had significant matches for two isoforms of the peanut allergen Ara h 1, namely clones P41B and P17, respectively. The MS data from both bands were therefore combined into a single Mascot search, which revealed a more significant match for Ara h 1, clones P41B (GI: 1168391; P43238.1) (see Supplementary material, Table S1) and P17 (GI: 1168390; P43237.1) (see Supplementary material, Table S2), respectively.
Both Ara h 1 molecules, i.e. the ~30 000–33 000 MW pair of peanut antigens, were purified, electrophoresed in SDS–PAGE and electro‐transferred onto nitrocellulose paper (NCP). The NCP was probed in succession with a rabbit anti‐SmSEA antiserum [the same as that used in Fig. 1(a), lane 1] and goat anti‐rabbit IgG as the primary and secondary antibodies, respectively. A horizontal thin strip of NCP, corresponding to the area where the cross‐reactive anti‐SmSEA IgG antibodies bound to the purified ~30 000–33 000 MW peanut doublet antigens (i.e. between the two horizontal arrows in the Supplementary material, Fig. S1), was removed and the primary rabbit IgG antibodies were eluted from the NCP by treatment with low pH buffer.
Investigating the cross‐reactivity of the eluted anti‐SmSEA IgG antibodies
The rabbit anti‐SmSEA IgG antibodies that were cross‐reactive with Ara h 1 and that had been purified from Western immunoblots by acid‐elution were tested for reactivity on the constituents of unfractionated peanut extract, as well as on extracts of three mouse tissues, namely blood, kidney and spleen.
The results in Fig. 3(a) show that the eluted antibodies were, as expected, reactive with the peanut antigens from which they had been eluted, but also with many other antigens in the peanut extract (Fig. 3a, lane 1). A replicate NCP carrying peanut antigens, one incubated with antibody–acid‐elution buffer alone and another with a rabbit anti‐NMS, showed no reactivity (Fig. 3a, lanes 2 and 3, respectively). Furthermore, the eluted antibodies showed no reactivity against any of the three extracts from mouse tissue (lane 1 on Fig. 3b–d) and there was no reactivity after incubation in acid‐elution buffer alone (lane 2, Fig. 3b–d). In contrast, some antigens of mainly high molecular weights in each of the three extracts from mouse showed reactivity with a rabbit anti‐NMS serum (lane 3, Fig. 3b–d).
Figure 3.
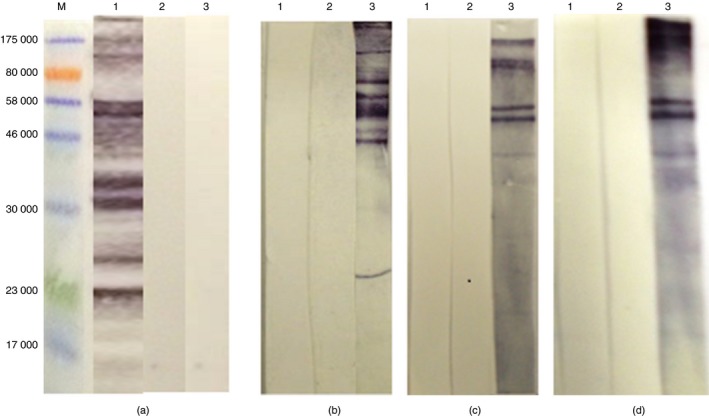
Western immunoblots probing extracts of peanut and mouse tissues with anti‐Schistosoma mansoni soluble egg antigen (anti‐SmSEA) IgG antibodies eluted by low pH buffer from a ~30 000–33 000 MW pair of peanut antigens. (M) Molecular weight marker. (a) Peanut, (b) normal mouse serum (NMS), (c) kidney, (d) spleen. Each strip was probed with: (1) eluted anti‐SmSEA IgG antibodies, (2) incubated only in 1 ml acid‐elution buffer, (3) rabbit anti‐NMS. [Colour figure can be viewed at wileyonlinelibrary.com]
Tentative identification of S. mansoni egg antigens that induced the IgG antibodies in anti‐SmSEA that cross‐reacted with Ara h 1 and other peanut antigens
To identify the S. mansoni egg antigen(s) that potentially induced the cross‐reactive IgG antibodies in anti‐SmSEA, the antibodies eluted from the ~30 000–33 000 MW peanut molecules were used to probe NCP carrying electro‐transferred SmSEA in a Western immunoblot. Results in Fig. 4(a) show that the eluted IgG antibodies were reactive with two egg antigens; a broad band of ~36 000 and a band at ~100 000 MW. Based on previous experience of Western blot reactivity of the constituents of SmSEA, these two antigens were deemed likely to be IPSE/α‐1 and κ‐5, respectively. Support for this conclusion was obtained by showing that the purified antibodies reacted seemingly with the same two antigens in SmSEA (Fig. 4a, lane 1) as did two sera from rabbits immunized respectively with IPSE/α‐1 and κ‐5 (Fig. 4a, lanes 2 and 3). Acid‐elution buffer alone gave no reactivity against SmSEA (Fig. 4a, lane 4).
Figure 4.
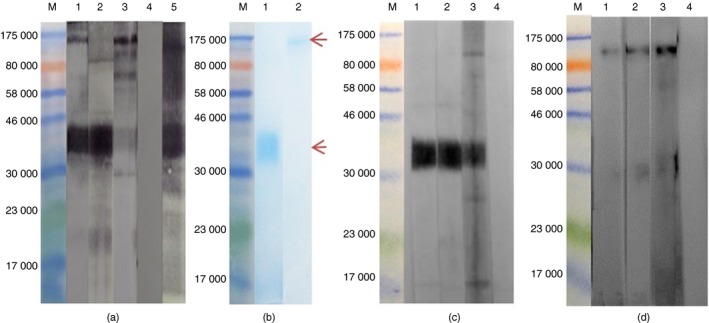
Western immunoblot probing Schistosoma mansoni soluble egg antigens (SmSEA) and two purified egg antigens with eluted anti‐SmSEA IgG antibody to identify cross‐reactive egg antigens. (M) Molecular weight markers. (a) SmSEA probed with; (1) eluted anti‐SmSEA IgG antibodies, (2) rabbit anti‐IPSE/α‐1, (3) rabbit anti‐κ‐5, (4) incubated only in elution buffer, (5) rabbit anti‐SmSEA. (b) SDS–PAGE showing the purified egg antigens after staining in SimplyBlue SafeStain; (1) IPSE/α‐1, (2) κ‐5. (c) Purified IPSE/α‐1 probed with; (1) eluted anti‐SmSEA IgG antibodies, (2) rabbit anti‐IPSE/α‐1, (3) rabbit anti‐SmSEA, (4) incubated only in elution buffer. (d) purified κ‐5 probed with; (1) eluted anti‐SmSEA IgG antibodies, (2) rabbit anti‐κ‐5, (3) rabbit anti‐SmSEA, (4) incubated only in elution buffer. [Colour figure can be viewed at wileyonlinelibrary.com]
To confirm the identity of the two S. mansoni egg antigens putatively identified as IPSE/α‐1 and κ‐5 respectively in Fig. 4(a), purification of the ~36 000 MW and the ~100 000 MW antigenic bands was undertaken by excision of bands corresponding to each separately from SDS–PAGE gels that had been loaded with SmSEA and elution of protein therefrom. Figure 4(b) shows a stained SDS–PAGE gel revealing the two purified egg antigens, of which the ~36 000 band in lane 1 was analysed in tandem MS analysis and its identity was confirmed as the IL‐4‐inducing protein precursor in S. mansoni (GI: 288948570), also known as IPSE/α‐1 (see Supplementary material, Table S3). Thereafter, the two purified antigens were electrophoresed separately and probed with the anti‐SmSEA IgG antibodies eluted from the ~30 000–33 000 MW peanut moiety, two antisera monospecific for each of the respective egg antigens as well as a crude rabbit anti‐SmSEA antiserum. Results in Fig. 4(c) show that the acid‐eluted antibodies and monospecific rabbit anti‐IPSE/α‐1 antibodies were reactive with purified ~36 000 MW egg antigen and likewise, the eluted antibodies also reacted with the purified ~100 000 MW egg antigen as did the monospecific anti‐κ‐5 (Fig. 4d). Untreated rabbit anti‐SmSEA antiserum had antibodies that reacted against both antigens, seemingly IPSE/α‐1 and κ‐5 (lanes 3 in Fig. 4c,d).
Investigating peptide identity between cross‐reactive S. mansoni antigens and Ara h 1
Following the identification of IPSE/α‐1 and κ‐5 as potential inducers of anti‐SmSEA IgG antibodies that are cross‐reactive with Ara h 1, the amino acid sequences of the two egg antigens; [IPSE/α‐1 (GI: 28894857, AAK26170.1) and κ‐5 (GI: 62462031, AAX83114.1)], respectively, were aligned with that of Ara h 1 (GI: 1168391; P43238.1) using the pairwise sequence alignment tool from the EMBL‐EBI database to ascertain the level of peptide identity. Results showed a peptide identity of 3·5% between Ara h 1 and IPSE/α‐1 (se Supplementary material, Table S4), while the alignment of Ara h 1 and κ‐5 revealed an identity of 9·8% (see Supplementary material, Table S5). All these results reflect a low level of protein sequence identity.
The cross‐reactivity of the eluted anti‐SmSEA IgG antibodies with molecules in other plant extracts and the possible involvement of CCDs
Due to the observed cross‐reactivity of the eluted anti‐SmSEA IgG antibodies with Ara h 1 as well as with other peanut molecules, their reactivity with constituents of extracts of six other plants with the potential to cause allergy in humans; latex, banana, tomato, melon, avocado, and kiwi, was investigated by Western blotting. Results in Fig. 5(a) showed that the purified antibodies were cross‐reactive with an array of molecules in extracts of the six constituents of plants as well as the two egg antigens in SmSEA already identified above as IPSE/α‐1 and κ‐5, respectively.
Figure 5.

Constituents of different plants probed with the acid‐eluted anti‐Schistosoma mansoni soluble egg antigen (anti‐SmSEA) IgG antibodies and the effect of treatment with periodate. (a) Incubated in 50 mm sodium acetate buffer before probing with eluted anti‐SmSEA IgG antibodies, (b) Incubated in 20 mm sodium metaperiodate dissolved in 50 mm sodium acetate buffer before probing with the same primary antibody as ‘a’. (M) Molecular weight marker, (1) unfractionated peanut, (2) purified pair of peanut antigens at ~30 000–33 000 MW, (3) crude latex (0·2 mg), (4) banana (0·16 mg), (5) tomato (0·27 mg), (6) melon (0·24 mg), (7) avocado (0·22 mg), (8) kiwi (0·19 mg), (9) SmSEA (0·010 mg). [Colour figure can be viewed at wileyonlinelibrary.com]
As only a low level of amino acid‐sequence identity was found between IPSE/α‐1 and κ‐5, and Ara h 1 (see Supplementary material, Tables S4 and S5 respectively), an alternative possible explanation for the antigenic cross‐reactivity was investigated in terms of shared carbohydrate determinants (CCDs) by means of treatment with mild sodium metaperiodate. Treatment of the replicate NCP in Fig. 5(a) carrying electroblotted proteins with a solution of 20 mm sodium metaperiodate dissolved in sodium acetate buffer, pH 4·5, before probing with the purified antibodies, resulted in disappearance of all antigenic reactivity in lanes 1–8 of Fig. 5(b). Only the broad ~36 000 MW band in SmSEA, identified above as IPSE/α‐1, retained antigenic reactivity (Fig. 5b, lane 9).
This observation is an indication that the antigenic cross‐reactivity demonstrated here is due to a widespread distribution of CCDs on the antigenically cross‐reactive molecules in these extracts.
Predicting potential glycosylation sites on cross‐reactive S. mansoni antigens and Ara h 1
Consequent upon the observations in Fig. 5 and the Supplementary material (Tables S4 and S5, respectively), the amino acid sequence of Ara h 1 (GI: 1168391; P43238.1) was searched against the GlycoEP software for the prediction of potential N‐ and/or O‐linked glycosylation sites.43 Results revealed that Ara h 1 has one potential N‐linked glycosylation site at position N‐521 (shown in underlined and italicized font in the Supplementary material, Table S6), and eight potential O‐linked glycosylation sites (at positions T‐23, 34, 81, 82, 184 and 246 and S‐170 and 523, shown in underlined and italicized font in the Supplementary material, Table S7). Also, a search of Ara h 1 against the expasy software (Bioinformatics Resource Portal) using the glycomod prediction tool confirmed that it has one N‐linked potential glycosylation site at the same position, N‐521, as indicated by the glycoep software (result not shown).
Discussion
We have previously demonstrated that rabbit anti‐schistosome IgG antibodies cross‐react with many different constituents of plants and invertebrates that are allergic to humans.28 The cross‐reactivity between S. mansoni egg and cercarial antigens and a 43 000 MW molecule in natural rubber latex was investigated in detail and the latter was found to be latex allergen Hev b 7.
Allergy to latex is now less important so the methods used in that study were here extended to investigate the extent to which they might be applied to another allergen, namely peanut, allergy to which is currently of more significance than that to latex and the constituents of which were also shown to be antigenically cross‐reactive with S. mansoni.28
Human allergic sensitization to peanut is widespread in the western/developed world.31 Conversely, in developing and underdeveloped countries where the prevalence of S. mansoni and other helminths is higher, incidence of peanut and other food allergies is relatively low and a low prevalence of skin‐prick positivity to peanut has been observed in persons with schistosome infections who are resident in a parasite‐endemic region of Ghana, despite their having anti‐peanut IgE antibodies.15 This observation is consistent with the tenets of the hygiene hypothesis. Results in Fig. 1 confirm the earlier indications28 that extensive IgG cross‐reactivity occurs between S. mansoni and peanut antigens/allergens. The non‐reactivity of the anti‐SmSEA antibodies against any of the antigens/molecules in the three tissue extracts from mouse is an indication that the cross‐reactivity of the purified antibodies with antigens in extracts of peanut and those in other plants with potential to cause allergy in humans is not a ubiquitous property of rabbit sera in general.
The pattern of cross‐reactivity that drew our particular attention was that against a pair of bands at ~30 000–33 000 MW in the peanut extract, which reacted somewhat more intensely than other peanut antigens with antibodies from the two rabbit anti‐S. mansoni egg antisera. Subsequent investigations used one of these rabbit anti‐SmSEA sera as it had previously been observed to be cross‐reactive with antigens in various plant and invertebrate extracts.28
The two peanut proteins that reacted with the rabbit anti‐SmSEA antibodies were purified and MS analysis identified both as the peanut allergen, Ara h 1. Ara h 1 is a glycoprotein monomer usually found to have an approximate molecular mass of 63 000 in SDS–PAGE and IgE immunoblots.47, 48, 49 However, in this study, two lower molecular weight bands, both containing Ara h 1 peptides, were observed at ~30 000–33 000 MW. It is possible that the initial protein mass of ~63 000 was broken down/reduced during sample extraction and processing by SDS, as the 30 000 and 33 000 ‘bands’ observed herein together would add up to about the correct mass of Ara h 1. Hence, the discrepancy with conventional estimates of the molecular mass of Ara h 1 could be due to the existence of various isoforms of the allergen, with a minor form having been previously reported to occur as a 33 000 MW band.50
Ara h 1 is one of the most important peanut allergens,48 as it is recognized by IgE antibodies in approximately 94% of sera from patients with peanut allergy.51
Purified rabbit anti‐SmSEA IgG antibodies that bound to the purified ~30 000–33 000 peanut doublet containing Ara h 1 on NCP and that were eluted by low pH treatment therefrom helped identify two S. mansoni egg antigens that may have induced these cross‐reactive antibodies, namely IPSE/α‐152 and κ‐5,53 respectively. This conclusion is based on the observations in Fig. 4(a, c, d) that the anti‐SmSEA antibodies eluted from the ~30 000–33 000 peanut antigens reacted with the two respective antigens in SmSEA, similarly to two rabbit antisera known to contain antibodies monospecific for IPSE/α‐1 and κ‐5, respectively. Moreover, MS analysis of the purified ~36 000 antigen in SmSEA confirmed its identity as IPSE/α‐1.
IPSE/α‐1 has been identified as a factor in SmSEA capable of inducing the release of IL‐4 from basophils by interacting with IgE on the surface of the cells,54 albeit not in an antigenically specific manner.55, 56 Further reports on IPSE/α‐1 indicate that a nuclear localization signal is present in its amino acid sequence.57 IPSE/α‐1 expression has been reported to be restricted to the egg stage of S. mansoni 52 and the molecule has recently been identified as belonging to the protein superfamily of βγ crystallins.55 The molecule also binds IgG to some extent, also in a ‘non‐specific’ manner (i.e. by a mechanism that does not involve the immunoglobulin's antigen‐binding site), but with a fourfold lower affinity than IgE.55 This immunologically non‐specific interaction has previously been shown not to account for the interaction of the rabbit IgG antibodies with IPSE/α‐1,28 and in this study also it can be seen that IgG in the anti‐κ‐5 antiserum used in Fig. 4a, lane 3, reacted much less intensely with IPSE/α‐1 than anti‐IPSE/α‐1 IgG antibodies.
κ‐5 is another immunogenic glycoprotein in SmSEA and it reacts with both IgG and IgE isotype antibodies in sera from individuals with S. mansoni infection.53 Furthermore, the κ‐5‐specific IgE antibodies in sera of S. mansoni‐infected individuals were directed against the core region of the κ‐5 glycans.58 The biological role of κ‐5 is not yet known.
Attempts to detect peptide similarity between Ara h 1 and the two cross‐reactive S. mansoni egg antigens (IPSE/α‐1 and κ‐5) revealed a low level of identity that is probably insufficient for the cross‐reactivity to be due solely to peptide epitopes (see Supplementary material, Tables S4 and S5). The findings herein are further corroborated by a report53 that the sequence of κ‐5 shows significant similarity only with an uncharacterized mRNA in S. japonicum. Neither IPSE/α‐1 nor κ‐5 have been shown to have allergenic properties, though Ara h 1, a glycoprotein belonging to the vicilin‐seed storage protein family of the 7S globulins,59, 60, 61, 62 has well‐defined allergenicity.
The proteinaceous properties of the three molecules therefore seem unlikely to be responsible for the antigenic S. mansoni/peanut cross‐reactivity, reports on similarities in protein families between allergens and some helminth proteins notwithstanding.63, 64, 65
Anti‐SmSEA IgG antibodies eluted from peanut allergen Ara h 1 cross‐reacted not only with peanut Ara h 1, but also with the constituents of six other plants (Fig. 5a). It is also noteworthy that the anti‐SmSEA antibodies that had been eluted from Ara h 1 cross‐reacted with many other constituents of the peanut extract (Figs 4 and 5a). These observations are remarkable and may be due to the presence of shared epitopes on the cross‐reactive molecules in peanut as well as on constituents of the six other plant extracts.
Previously, cross‐reactivity among plant allergens and with some molecules in invertebrates including helminths, have been reported and suspected to be due to shared CCDs.66, 67, 68 N‐linked glycans containing core fucose and xylose are known to be commonly distributed among different organisms and have been implicated in IgE cross‐reactivity between plants and invertebrates.69, 70 Hence, based on the limited evidence for any involvement of shared peptide epitopes outlined above, investigations on the possible involvement of CCDs in the antigenic cross‐reactivity were initiated. NCP carrying constituents from six different plants were treated with mild sodium metaperiodate before probing with the acid‐eluted anti‐SmSEA IgG antibodies. The treatment abrogated virtually all of the cross‐reactivity against constituents of the plant extracts (Fig. 5b), indicating an involvement of glycan epitopes that are common to both the S. mansoni egg antigens and the peanut allergen Ara h 1, though reactivity against IPSE/α‐1 survived the chemical treatment (Fig. 5b, lane 9).
The reason for the inability of sodium metaperiodate to destroy the reactivity of IPSE/α‐1 with the acid‐eluted anti‐Ara h 1 antibodies remains an enigma, although similar findings with respect to this molecule have been previously reported.28, 45 Anti‐CCD antibodies induced by horseradish peroxidase in mice and cross‐reactive with phytohaemagglutinin also showed resistance to periodate treatment and it was suggested that horseradish peroxidase‐induced anti‐CCD antibodies were reacting with the trisaccharide backbone of the molecule, rather than the periodate‐degradable glycan residues containing xylose, fucose or mannose.71
IPSE/α‐1 and κ‐5 are both glycosylated egg antigens with well‐characterized glycan structures.58, 72, 73 Each peptide of the IPSE/α‐1 dimer contains two N‐linked glycosylation sites, which are fucosylated, in addition to an immunogenic Lewis‐X terminus,72 while κ‐5 has four occupied N‐linked glycans with attached fucose and xylose residues in addition to an immunogenic LDN (GalNAcβ1‐4GlcNAc) residue.58 Previous reports on Ara h 1 showed that it possesses one N‐linked glycan with attached xylose and mannose residues.74 Prediction of potential glycosylation sites on the amino acid sequence of Ara h 1 using recently published software43 also revealed it to possess one potentially N‐linked and eight O‐linked glycosylation sites (see Supplementary material, Tables S6 and S7, respectively) and prediction using the expasy software (GlycoMod) concurred with the above findings. The presence of identical immunogenic glycan structures on the two S. mansoni egg antigens and Ara h 1 could account for the observed cross‐reactivity of the rabbit anti‐SmSEA IgG antibodies.
To summarize, this study has shown antigenic cross‐reactivity between the peanut allergen Ara h 1 and, putatively, two S. mansoni egg antigens; IPSE/α‐1 and κ‐5, as well as with some other antigenic constituents of six plants associated with human allergies. Peptide identities between the two egg antigens and Ara h 1 were not significant and involvement of CCDs has been implicated on the basis that most of the cross‐reactivity was periodate‐sensitive, the antigenicity of IPSE/α‐1 being an exception to this sensitivity. A recent report has indicated that ‘schistosome infection‐induced IgE against CCDs might account largely for high IgE levels to peanut in a population of Ghanaian schoolchildren…(though)… no evidence of IgE‐mediated peanut allergy was found’.15
Findings herein are novel and could offer a possible explanation for the hygiene hypothesis. Glycans in schistosomes and allergens have been shown to drive Th2 immune responsiveness and through the induction of regulatory T cells have been implicated in subsequent immunomodulation of this response in hosts infected with the parasite.8 The consequences of the immunoregulation in turn may have an effect dampening Th2‐driven allergic responses.11 Alternatively, IgG antibodies induced by the parasitic infection and which cross‐react with plant‐ and invertebrate‐derived CCDs may inhibit the reactivity of anti‐allergen IgE antibodies;75 i.e. in schistosome‐endemic areas, the inverse association observed between infection with the parasite and allergies could be due to the anti‐schistosome CCD IgG in some way blocking the activity of allergen‐specific IgE, thereby preventing allergic reactivity. In this context, allergen‐specific immunotherapy is commonly found to induce production of anti‐allergen IgG antibodies, particularly of the IgG4 subclass, and these have been shown to block in vitro reactions mediated by allergen‐specific IgE.22, 76, 77 IgG antibodies induced by helminth infection may have a similar effect.
Thus, schistosome‐induced IgG antibodies that are cross‐reactive with allergens such as Ara h 1 may block allergic hypersensitivity reactions, and so offer an explanation for the relative absence of peanut allergy in schistosome‐infected children in Ghana.15 Allergen‐specific immunotherapy for peanut allergy is having some, but not great, success31 and further work to investigate whether IgG antibodies induced by schistosome infection can more effectively block the reactivity of IgE antibodies reactive against peanut and other allergens, which have CCDs in common is warranted.
Funding
The research was partly funded by a University of Nottingham International Research Excellence Scholarship 2011 (to J.I.).
Disclosures
The authors declare no conflict of interest.
Supporting information
Figure S1. Steps illustrating the methods of purification by acid‐elution of anti‐Schistosoma mansoni soluble egg antigens (anti‐SmSEA) IgG antibodies that are cross‐reactive with the pair of peanut Ara h 1 bands at ~30 000–33 000 MW. Cross‐reactive anti‐SmSEA IgG antibodies were eluted from cut‐out nitrocellulose paper strip as shown in the marked area between two horizontal arrows using elution buffer.
Table S1. MASCOT search output of tandem mass spectrometry data from the purified pair of peanut gel bands at ~30 000–33 000 MW and matching allergen Ara h 1, clone P41B.
Table S2. Mass spectrometry‐derived data matching Ara h 1, clone P17.
Table S3. MASCOT search output of tandem MS data from the purified ~36 000 MW gel band in Schistosoma mansoni soluble egg antigens.
Table S4. Alignment of the amino acid sequences of IPSE/α‐1 and Ara h 1.
Table S5. Alignment of the amino acid sequences of κ‐5 and Ara h 1.
Table S6. Predicting potential N‐linked glycosylation sites for Ara h 1.
Table S7. Predicting potential O‐linked glycosylation sites for Ara h 1.
Acknowledgements
We are very grateful to Beverly Lees of Allergy therapeutics for her kind donation of allergen extracts.
References
- 1. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub‐Saharan Africa. BrazJ Inf Dis 2015; 19:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. www.who.int/iris/bitstream/10665/78074/1/9789241503174_eng.pdf. 2013: pp. 1–81 [accessed on February 25, 2015].
- 3. Standley CJ, Mugisha L, Dobson AP, Stothard JR. Zoonotic schistosomiasis in non‐human primates: past, present and future activities at the human–wildlife interface in Africa. J Helminthol 2012; 86:131–40. [DOI] [PubMed] [Google Scholar]
- 4. Caldas IR, Campi‐Azevedo AC, Oliveira LFA, Silveira AM, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop 2008; 108:109–17. [DOI] [PubMed] [Google Scholar]
- 5. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 2011; 11:375–88. [DOI] [PubMed] [Google Scholar]
- 6. Rujeni N, Taylor DW, Mutapi F. Human schistosome infection and allergic sensitisation. J Parasitol Res 2012; 2012:154743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol 2001; 22:372–7. [DOI] [PubMed] [Google Scholar]
- 8. Klaver EJ, Kuijk LM, Lindhorst TK, Cummings RD, van Die I. Schistosoma mansoni soluble egg antigens induce expression of the negative regulators SOCS1 and SHP1 in human dendritic cells via interaction with the mannose receptor. PLoS ONE 2015; 10:e0124089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy. Clin Transl Allergy 2012; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soyka MB, Holzmann D, Akdis CA. Regulatory cells in allergen‐specific immunotherapy. Immunotherapy 2012; 4:389–96. [DOI] [PubMed] [Google Scholar]
- 11. van Die I, Cummings RD. Glycans modulate immune responses in helminth infections and allergy. Chem Immunol Allergy 2006; 90:91–112. [DOI] [PubMed] [Google Scholar]
- 12. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 2002; 296:490–4. [DOI] [PubMed] [Google Scholar]
- 13. van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG et al Decreased atopy in children infected with Schistosoma haematobium: a role for parasite‐induced interleukin‐10. Lancet 2000; 356:1723–7. [DOI] [PubMed] [Google Scholar]
- 14. Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep 2010; 10:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amoah AS, Obeng BB, Larbi IA, Versteeg SA, Aryeetey Y, Akkerdaas JH et al Peanut‐specific IgE antibodies in asymptomatic Ghanaian children possibly caused by carbohydrate determinant cross‐reactivity. J Allergy Clin Immunol 2013; 132:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa‐Atta L, Sole D et al Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol 2000; 123:145–8. [DOI] [PubMed] [Google Scholar]
- 17. Araujo MI, Hoppe BS, Medeiros M Jr, Carvalho EM. Schistosoma mansoni infection modulates the immune response against allergic and auto‐immune diseases. Mem Inst Oswaldo Cruz 2004; 99:27–32. [DOI] [PubMed] [Google Scholar]
- 18. Strachan DP. Hay fever, hygiene, and household size. BMJ 1989; 299:1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rook GA. The hygiene hypothesis and the increasing prevalence of chronic inflammatory disorders. Trans R Soc Trop Med Hyg 2007; 101:1072–4. [DOI] [PubMed] [Google Scholar]
- 20. Kondrashova A, Seiskari T, Ilonen J, Knip M, Hyoty H. The ‘Hygiene hypothesis’ and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. APMIS 2013; 121:478–93. [DOI] [PubMed] [Google Scholar]
- 21. Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B‐cell induction by helminths: implications for allergic disease. J All Clin Immunol 2011; 128:733–9. [DOI] [PubMed] [Google Scholar]
- 22. Subbarayal B, Schiller D, Mobs C, de Jong NW, Ebner C, Reider N et al Kinetics, cross‐reactivity, and specificity of Bet v 1‐specific IgG4 antibodies induced by immunotherapy with birch pollen. Allergy 2013; 68:1377–86. [DOI] [PubMed] [Google Scholar]
- 23. Nouri‐Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK et al Grass pollen immunotherapy induces mucosal and peripheral IL‐10 responses and blocking IgG activity. J Immunol 2004; 172:3252–9. [DOI] [PubMed] [Google Scholar]
- 24. James LK, Till SJ. Potential mechanisms for IgG4 inhibition of immediate hypersensitivity reactions. Curr Allergy Asth Rep 2016; 16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao D, Lai X, Tian M, Jiang Y, Zheng Y, Gjesing B et al The functional IgE‐blocking factor induced by allergen‐specific immunotherapy correlates with IgG4 antibodies and a decrease of symptoms in house dust mite‐allergic children. Int Arch Allergy Immunol 2016; 169:113–20. [DOI] [PubMed] [Google Scholar]
- 26. Ostojic V. Increased specific immunoglobulin G4 antibodies induced by natural exposure to ambrosia pollen in patients with allergy. Allergy Asthma Proc 2016; 37:115–20. [DOI] [PubMed] [Google Scholar]
- 27. Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM et al Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J All Clin Immunol 2013; 131:128–34 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doenhoff JM, El‐Faham M, Liddell S, Fuller RH, Stanley GR, Schramm G et al Cross‐reactivity between Schistosoma mansoni antigens and the latex allergen Hev b 7: putative implication of Cross‐Reactive Carbohydrate Determinants (CCDs). PLoS ONE 2016; 11:e0159542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ben‐Shoshan M, Kagan RS, Alizadehfar R, Joseph L, Turnbull E, St Pierre Y et al Is the prevalence of peanut allergy increasing? A 5‐year follow‐up study in children in Montreal J Allergy Clin Immunol 2009; 123:783–8. [DOI] [PubMed] [Google Scholar]
- 30. Ben‐Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L, Pierre YS et al Demographic predictors of peanut, tree nut, fish, shellfish, and sesame allergy in Canada. J Allergy (Cairo) vol. 2012, Article ID 858306, 6 pages, 2012. doi:10.1155/2012/858306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Commins SP, Kim EH, Orgel K, Kulis M. Peanut allergy: new developments and clinical implications. Curr Allergy Asth Reports 2016; 16:35. [DOI] [PubMed] [Google Scholar]
- 32. Doenhoff MJ, Modha J, Curtis RH, Adeoye GO. Immunological identification of Schistosoma mansoni peptidases. Mol Biochem Parasitol 1988; 31:233–40. [DOI] [PubMed] [Google Scholar]
- 33. Darani HY, Doenhoff MJ. An association between Schistosoma mansoni worms and an enzymatically active protease/peptidase in mouse blood. Parasitology 2008; 135:467–72. [DOI] [PubMed] [Google Scholar]
- 34. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265–75. [PubMed] [Google Scholar]
- 35. Modha J, Parikh V, Gauldie J, Doenhoff MJ. An association between schistosomes and contrapsin, a mouse serine protease inhibitor (serpin). Parasitology 1988; 96(Pt 1):99–109. [DOI] [PubMed] [Google Scholar]
- 36. Dunne DW, Agnew AM, Modha J, Doenhoff MJ. Schistosoma mansoni egg antigens: preparation of rabbit antisera with monospecific immunoprecipitating activity, and their use in antigen characterization. Parasite Immunol 1986; 8:575–86. [DOI] [PubMed] [Google Scholar]
- 37. Studier FW. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol 1973; 79:237–48. [DOI] [PubMed] [Google Scholar]
- 38. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680–5. [DOI] [PubMed] [Google Scholar]
- 39. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 1979; 76:4350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Igetei JE, Liddell S, El‐Faham M, Doenhoff MJ. Purification of a chymotrypsin‐like enzyme present on adult Schistosoma mansoni worms from infected mice and its characterization as a host carboxylesterase. Parasitology 2016; 143:646–57. [DOI] [PubMed] [Google Scholar]
- 41. Steen H, Mann M. The ABC's (and XYZ's) of peptide sequencing. Nat Rev Mol Cell Biol 2004; 5:699–711. [DOI] [PubMed] [Google Scholar]
- 42. Papayannopoulos IA. The interpretation of collision‐induced dissociation tandem mass‐spectra of peptides. Mass Spectrom Rev 1995; 14:49–73. [Google Scholar]
- 43. Chauhan JS, Rao A, Raghava GP. In silico platform for prediction of N‐, O‐ and C‐glycosites in eukaryotic protein sequences. PLoS ONE 2013; 8:e67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rybicki EP. Affinity purification of specific antibodies from plant virus capsid protein immobilised on nitrocellulose. J Phytopathol 1986; 116:30–8. [Google Scholar]
- 45. Hamilton JV, Chiodini PL, Fallon PG, Doenhoff MJ. Periodate‐sensitive immunological cross‐reactivity between keyhole limpet haemocyanin (KLH) and serodiagnostic Schistosoma mansoni egg antigens. Parasitology 1999; 118(Pt 1):83–9. [DOI] [PubMed] [Google Scholar]
- 46. Eberl M, Langermans JA, Vervenne RA, Nyame AK, Cummings RD, Thomas AW et al Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J Inf Dis 2001; 183:1238–47. [DOI] [PubMed] [Google Scholar]
- 47. Jenkins JA, Griffiths‐Jones S, Shewry PR, Breiteneder H, Mills EN. Structural relatedness of plant food allergens with specific reference to cross‐reactive allergens: an in silico analysis. J Allergy Clin Immunol 2005; 115:163–70. [DOI] [PubMed] [Google Scholar]
- 48. Shin DS, Compadre CM, Maleki SJ, Kopper RA, Sampson H, Huang SK et al Biochemical and structural analysis of the IgE binding sites on Ara h 1, an abundant and highly allergenic peanut protein. J Biol Chem 1998; 273:13753–9. [DOI] [PubMed] [Google Scholar]
- 49. Breiteneder H, Mills ENC. Molecular properties of food allergens. J Allergy Clin Immunol 2005; 115:14–23. [DOI] [PubMed] [Google Scholar]
- 50. Marsh J, Rigby N, Wellner K, Reese G, Knulst A, Akkerdaas J et al Purification and characterisation of a panel of peanut allergens suitable for use in allergy diagnosis. Mol Nutr Food Res 2008; 52(Suppl 2):S272–85. [DOI] [PubMed] [Google Scholar]
- 51. Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA. Recombinant peanut allergen Ara h I expression and IgE binding in patients with peanut hypersensitivity. J Clin Inv 1995; 96:1715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, Galle J et al IPSE/α‐1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol Biochem Parasitol 2006; 147:9–19. [DOI] [PubMed] [Google Scholar]
- 53. Schramm G, Hamilton JV, Balog CI, Wuhrer M, Gronow A, Beckmann S et al Molecular characterisation of κ‐5, a major antigenic glycoprotein from Schistosoma mansoni eggs. Mol Biochem Parasit 2009; 166:4–14. [DOI] [PubMed] [Google Scholar]
- 54. Schramm G, Falcone FH, Gronow A, Haisch K, Mamat U, Doenhoff MJ et al Molecular characterization of an interleukin‐4‐inducing factor from Schistosoma mansoni eggs. J Biol Chem 2003; 278:18384–92. [DOI] [PubMed] [Google Scholar]
- 55. Meyer NH, Mayerhofer H, Tripsianes K, Blindow S, Barths D, Mewes A et al A crystallin fold in the interleukin‐4‐inducing principle of Schistosoma mansoni eggs (IPSE/α‐1) mediates IgE binding for antigen‐independent basophil activation. J Biol Chem 2015; 290:22111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schramm G, Mohrs K, Wodrich M, Doenhoff MJ, Pearce EJ, Haas H et al Cutting edge: IPSE/α‐1, a glycoprotein from Schistosoma mansoni eggs, induces IgE‐dependent, antigen‐independent IL‐4 production by murine basophils in vivo . J Immunol 2007; 178:6023–7. [DOI] [PubMed] [Google Scholar]
- 57. Kaur I, Schramm G, Everts B, Scholzen T, Kindle KB, Beetz C et al Interleukin‐4‐inducing principle from Schistosoma mansoni eggs contains a functional C‐terminal nuclear localization signal necessary for nuclear translocation in mammalian cells but not for its uptake. Infect Immun 2011; 79:1779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meevissen MH, Balog CI, Koeleman CA, Doenhoff MJ, Schramm G, Haas H et al Targeted glycoproteomic analysis reveals that κ‐5 is a major, uniquely glycosylated component of Schistosoma mansoni egg antigens. Mol Cell Proteomics 2011; 10:1–14:M110.005710 , 1–14, doi: 10.1074/mcp.M110.005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h 1, Ara h 2 and Ara h 3 in peanut‐allergic patients, as determined by immunoglobulin E Western blotting, basophil‐histamine release and intracutaneous testing: Ara h 2 is the most important peanut allergen. Clin Exp Allergy 2004; 34:583–90. [DOI] [PubMed] [Google Scholar]
- 60. de Leon MP, Rolland JM, O'Hehir RE. The peanut allergy epidemic: allergen molecular characterisation and prospects for specific therapy. Expert Rev Mol Med 2007; 9:1–18. [DOI] [PubMed] [Google Scholar]
- 61. Burks AW, Williams LW, Helm RM, Connaughton C, Cockrell G, O'Brien T. Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges. J Allergy Clin Immunol 1991; 88:172–9. [DOI] [PubMed] [Google Scholar]
- 62. Mueller GA, Maleki SJ, Pedersen LC. The molecular basis of peanut allergy. Cur Allergy Asth Rep 2014; 14:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Farnell EJ, Tyagi N, Ryan S, Chalmers IW, Pinot de Moira A, Jones FM et al Known allergen structures predict Schistosoma mansoni IgE‐binding antigens in human infection. Front Immunol 2015; 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tyagi N, Farnell EJ, Fitzsimmons CM, Ryan S, Tukahebwa E, Maizels RM et al Comparisons of allergenic and metazoan parasite proteins: allergy the price of immunity. PLoS Comput Biol 2015; 11:e1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Santiago HC, LeeVan E, Bennuru S, Ribeiro‐Gomes F, Mueller E, Wilson M et al Molecular mimicry between cockroach and helminth glutathione S‐transferases promotes cross‐reactivity and cross‐sensitization. J Allergy Clin Immunol 2012; 130:248–56 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aalberse RC. Food allergens. Env Toxicol Pharmacol 1997; 4:55–60. [DOI] [PubMed] [Google Scholar]
- 67. Malandain H. IgE‐reactive carbohydrate epitopes – classification, cross‐reactivity, and clinical impact. Eur Ann Allergy Clin Immunol 2005; 37:122–8. [PubMed] [Google Scholar]
- 68. Faye L, Chrispeels MJ. Common antigenic determinants in the glycoproteins of plants, mollusks and insects. Glycoconjugate J 1988; 5:245–56. [Google Scholar]
- 69. Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F. Core α1,3‐fucose is a key part of the epitope recognized by antibodies reacting against plant N‐linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology 1998; 8:651–61. [DOI] [PubMed] [Google Scholar]
- 70. van Kuik JA, van Halbeek H, Kamerling JP, Vliegenthart JF. Primary structure of the low‐molecular‐weight carbohydrate chains of Helix pomatia α‐hemocyanin. Xylose as a constituent of N‐linked oligosaccharides in an animal glycoprotein. J Biol Chem 1985; 260:13984–8. [PubMed] [Google Scholar]
- 71. Hino S, Matsubara T, Urisu A, Aoki N, Sato C, Okajima T et al Periodate‐resistant carbohydrate epitopes recognized by IgG and IgE antibodies from some of the immunized mice and patients with allergy. Biochem Biophys Res Commun 2009; 380:632–7. [DOI] [PubMed] [Google Scholar]
- 72. Wuhrer M, Balog CI, Catalina MI, Jones FM, Schramm G, Haas H et al IPSE/α‐1, a major secretory glycoprotein antigen from schistosome eggs, expresses the Lewis X motif on core‐difucosylated N‐glycans. FEBS J 2006; 273:2276–92. [DOI] [PubMed] [Google Scholar]
- 73. Meevissen MH, Wuhrer M, Doenhoff MJ, Schramm G, Haas H, Deelder AM et al Structural characterization of glycans on ω‐1, a major Schistosoma mansoni egg glycoprotein that drives Th2 responses. J Proteome Res 2010; 9:2630–42. [DOI] [PubMed] [Google Scholar]
- 74. Kolarich D, Altmann F. N‐Glycan analysis by matrix‐assisted laser desorption/ionization mass spectrometry of electrophoretically separated nonmammalian proteins: application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal Biochem 2000; 285:64–75. [DOI] [PubMed] [Google Scholar]
- 75. Jin C, Hantusch B, Hemmer W, Stadlmann J, Altmann F. Affinity of IgE and IgG against cross‐reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol 2008; 121:185–90 e2. [DOI] [PubMed] [Google Scholar]
- 76. Siman IL, de Aquino LM, Ynoue LH, Miranda JS, Pajuaba AC, Cunha‐Junior JP et al Allergen‐specific IgG antibodies purified from mite‐allergic patients sera block the IgE recognition of Dermatophagoides pteronyssinus antigens: an in vitro study. Clin Dev Immunol 2013; 2013:657424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marth K, Breyer I, Focke‐Tejkl M, Blatt K, Shamji MH, Layhadi J et al Nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS‐fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol 2013; 190:3068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Steps illustrating the methods of purification by acid‐elution of anti‐Schistosoma mansoni soluble egg antigens (anti‐SmSEA) IgG antibodies that are cross‐reactive with the pair of peanut Ara h 1 bands at ~30 000–33 000 MW. Cross‐reactive anti‐SmSEA IgG antibodies were eluted from cut‐out nitrocellulose paper strip as shown in the marked area between two horizontal arrows using elution buffer.
Table S1. MASCOT search output of tandem mass spectrometry data from the purified pair of peanut gel bands at ~30 000–33 000 MW and matching allergen Ara h 1, clone P41B.
Table S2. Mass spectrometry‐derived data matching Ara h 1, clone P17.
Table S3. MASCOT search output of tandem MS data from the purified ~36 000 MW gel band in Schistosoma mansoni soluble egg antigens.
Table S4. Alignment of the amino acid sequences of IPSE/α‐1 and Ara h 1.
Table S5. Alignment of the amino acid sequences of κ‐5 and Ara h 1.
Table S6. Predicting potential N‐linked glycosylation sites for Ara h 1.
Table S7. Predicting potential O‐linked glycosylation sites for Ara h 1.


