Summary
Since the discovery of the lectin pathway of complement activation, numerous clinical cohorts have been examined for one or more proteins, with the intention of uncovering the functions of the proteins or with the aim of discovering new biomarkers or diagnostic tools. To unveil the abnormal, it is pivotal to know the normal. Our aim was to describe the concentrations of the 11 known proteins of the lectin pathway in serum and plasma and to uncover possible gender differences, age and diurnal variations, which must be taken into account for investigation in different cohorts. We examined the concentrations of all lectin pathway proteins mannan‐binding lectin (MBL), H‐ficolin, L‐ficolin, M‐ficolin, collectin‐K1, collectin‐L1, MBL‐associated serine protease 2 (MASP‐2), MASP‐3, MBL‐associated protein of 44 kDa (MAp44) and MAp19 in 300 Danish blood donors in serum and ethylenediamine tetraacetic acid (EDTA) plasma in established assays, and we further developed a new assay to measure MASP‐1 in the same samples. We found significant differences in concentrations between serum and plasma for all proteins except for MBL and MASP‐3. H‐ficolin, M‐ficolin and MAp19 displayed convincing diurnal variation. H‐ficolin, in particular, halved from morning to the middle of the night. There were gender differences for most proteins, whereas age did not seem to influence concentration. The present study underlines the necessity of considering which material to use, correct matching and a trial design that takes the nature of the protein into account in order for the outcome of cohort studies to have significant relevance.
Keywords: complement system, diurnal variation, innate immunity, lectin pathway
Introduction
The complement system is an important part of the innate immune system. Activation of the complement system sets off a proteolytic cascade leading to a number of immunological effector functions, including recruitment of inflammatory cells, augmentation of phagocytosis and formation of the membrane attack complex (MAC) in cell membranes and aids in directing the subsequent adaptive immune response 1. The lectin pathway (LP) of the complement system is one of three pathways that result in complement activation 2.
The LP is initiated by pattern recognition 3. The pattern recognition molecules (PRM) of the LP are M‐ficolin (or ficolin‐1), L‐ficolin (or ficolin‐2), H‐ficolin (or ficolin‐3), mannan‐binding lectin (MBL), collectin liver‐1 (CL‐L1, also called CL‐10) and collectin kidney‐1 (CL‐K1, also called CL‐11). CL‐L1 and CL‐K1 are in serum and plasma found primarily in heteromeric complexes called CL‐LK 4. The PRMs bind to patterns of ligands, e.g. surface‐linked carbohydrates or acetyl groups on pathogens or damaged self‐tissue 5. The PRMs are associated with three enzymes named MBL‐associated serine proteases (MASP‐1, MASP‐2 and MASP‐3) and with two proteins without enzymatic activity, MBL‐associated protein of 44 kDA (MAp44, also termed MAP1) and MAp19 (also termed sMAP) 6.
Although several projects have described binding of the pattern recognition molecules to different microorganisms and surfaces and subsequent activation of the complement system 3, much is still unknown about the function of the LP. The LP is highly preserved in different species 7, indicating its importance, and deficiencies have been described with regard to several diseases 8. As an example, the discovery of polymorphisms in the MASP1 gene (encoding MASP‐1, MASP‐3 and MAp44) and the COLEC11 (encoding CL‐K1) gene leading to developmental anomaly (the so‐called 3MC syndrome) have raised the idea of potentially new functions of the complement system in embryonic development 9. A number of reports exist on MASPs and coagulation cross‐talks 10, 11, without definite evidence of physiological relevance. Further, it has been demonstrated recently that MASP‐3 is involved in activation of the alternative pathway of complement activation 12.
Since the discovery of the LP, a growing number of cohort studies have been published in the search for biomarkers and potential diagnostic properties of LP proteins. In order for such studies to be both conclusive and comparable with each other, it is pivotal to know what is ‘normal’. Very little has been published concerning LP proteins with regard to gender variation, age correlation and potential diurnal variation, which may influence the results and conclusion of comparative studies. Age‐ and gender‐matching of controls is often not considered necessary 13. At times, information concerning gender is even omitted 14, and in studies in which patient inclusion happens throughout the day and night the question of diurnal variation is not addressed 15, 16.
Our objectives were to measure and describe the serum and plasma concentrations of the lectin pathway proteins using 10 well‐established assays and a newly established assay to elucidate if or when gender differences, age and diurnal variation could affect results, and thus require consideration with regard to matching when measured in different populations.
Material and methods
Blood samples
Samples from 300 blood donors, 150 men and 150 women, were collected at the blood bank of Aarhus University Hospital, Denmark. Blood was collected in ethylenediamine tetraacetic acid (EDTA) plasma tubes (8 ml) and serum tubes (10 ml) with clotting allowed for 1 h at room temperature (Alere Inc., Waltham, MA, USA; #367525 and #367896, respectively) and centrifuged at 2000 g for 10 min. Plasma and serum were collected, aliquoted and frozen immediately at −80ºC.
Monoclonal antibody for MASP‐1 assay
Monoclonal antibody (mAb) against MASP‐1 was produced by GenScript Inc. (Piscataway, NJ, USA). The general protocol can be found online. In brief, BALB/c mice were immunized with peptide representing the 15 C‐terminal amino acids of MASP‐1 (CHHNKDWIQRVTGVR) conjugated to keyhole limpet haemocyanin. Sera from the mice were tested for reactivity against the peptide coated onto microtitre wells. Mice exhibiting high titres were selected and spleen cells from these mice were fused with myeloma cells, and selection of hybridomas was performed on wells coated with the peptide used for immunization. After expansion, cloning, etc. the antibodies were purified by affinity chromatography on protein G beads. The cell line chosen for production of the coating antibody used in the assay described below was termed 5A6B7.
The previously described mAb 5F5 was used as detection antibody 17, which recognizes the CCP1 domain of the three proteins derived from the MASP1 gene. The mAb was biotinylated with 167 μg of biotinyl‐N‐hydroxysuccinimide (Sigma, St Louis, MO, USA) per mg of antibody 18.
Time‐resolved immunofluorometric assay (TRIFMA)
FluoroNunc MaxiSorp microtitre plates (Nunc, Roskilde, Denmark; # 437958 or # 43791) were incubated with 5 µg of mouse anti‐human MASP‐1 (5A6B7) per ml coating buffer [0·1 M sodium bicarbonate, pH 9·6 with 0·09% (w/v) sodium azide] overnight at room temperature (RT). Residual binding sites were blocked with 1 mg of HSA per ml of Tris‐buffered saline (TBS) (10 mM Tris/base, 145 mM NaCl, pH 7·4). After washing with TBS containing 0·05% Tween 20 (TBS/Tw), 100 µl of test samples and three quality controls (normal plasma chosen to reflect a wide distribution of concentrations) were added to the plate diluted 1/30 in sample buffer [10 mM Tris/base, 145 mM NaCl, 0.05% Tween‐20, 5 mg HSA/ml, heat‐aggregated human immunoglobulin (Ig)G 100 µg/ml, bovine IgG 100 µg/ml, mouse IgG 100 µg/ml, pH 7·4]. All samples were added in duplicate to duplicate wells. The standard curve was constructed from a standard plasma pool with a known MASP‐1 concentration, established by comparison with purified recombinant MASP‐1 19, initially diluted 10‐fold followed by serial twofold dilutions. After incubation overnight at 4°C, the wells were washed with TBS/Tw containing 5 mM CaCl2 (TBS/Tw/Ca2+) and incubated with biotin‐5F5 anti‐MASP‐1 antibody at 0·4 µg per ml TBS/Tw/Ca2+ for 2 h at RT. After washing with TBS/Tw/Ca2+, the wells were incubated with europium‐labelled streptavidin (Perkin Elmer, Waltham, MA, USA; #1244‐360) 1/1000 in TBS/Tw, 25 µM EDTA for 1 h at RT. After washing, quantification of europium was performed by adding 200 µl of enhancement solution (Ampliquon laboratory reagents #Q99800; Ampliqon, Odense, Denmark) per well releasing and encapsulating the bound europium, and the fluorescence was read as time‐resolved flourometry on a Victor 5+ from Perkin Elmer.
Gel‐permeation chromatography (GPC)
Normal human serum was subjected to fractionation by GPC on a Superose 6 HR 10/300 column (GE Healthcare, Cardiff, UK). The column buffer comprised TBS, 5 mM CaCl2 or TBS, 10 mM EDTA and 0·86 M NaCl. The column was run at a flow rate of 1 ml/min, and 250 µl fractions were collected in Tween 20‐treated 96‐well microtitre plates. Fractions were assayed for MASP‐1 by the above‐described TRIFMA. The elution volume of IgM, MBL, H‐ and L‐ficolin, purified IgG and HSA on this column was estimated previously 20.
Western blotting
The reactivity of the mAb 5A6B7 was tested by Western blotting. Plasma (1 µl) was added to ¼‐volume sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) sample buffer [30 mM Tris‐HCl, 10% (v/v) glycerol, 8 M urea, 3% (w/v) SDS, 0·1% (w/v) bromophenol blue, pH 8·9], TBS/Tween was added to reach the desired sample volume (20 µl) and the proteins in the sample were separated by electrophoresis on a 4–15% gradient gel (Bio‐Rad Criterion TGX gels #567‐1083; Bio‐Rad, Hercules, CA, USA). One‐tenth volume of dithiothreitol (DDT), 0·6 M, was added to the samples to be reduced, while iodoacetic acid (IAA), 1·4 M, was added to all samples before loading. Separated proteins were blotted onto nitrocellulose membranes (Bio‐Rad #170‐4159). The membrane was blocked for 30 min at RT in TBS with 0·1% Tween (v/v) and washed three times in TBS/Tw. The membrane was incubated overnight at RT on a rocking table with 1 µg mAb 5A6B7 per ml primary buffer [TBS/Tw, 1 mM EDTA, pH 7·4, 1 mg human serum albumin (CSL Behring, Lyngby, Denmark; #109697) per ml, 100 μg normal human IgG (CSL Behring; #007815) per ml]. After washing three times in TBS/Tw, the membrane was incubated with horseradish peroxidase‐labelled rabbit anti‐mouse antibody (Dako, Glostrup, Denmark; #P0260) diluted 4000‐fold in secondary buffer (TBS/Tween, 100 μg normal human IgG/ml, 1 mM EDTA, pH 7·4) for 2 h on a rocking table followed by washing three times in TBS/Tw and developed with SuperSignal West Pico chemiluminescent substrate (Pierce, ThermoScientific, Waltham, MA, USA; #34080). Emission was recorded by a charge‐coupled device (CCD) camera (Fuji, Tokyo, Japan; LAS‐4000).
Other assays
Plasma and serum concentrations of the other 10 lectin pathway proteins were determined using in‐house‐produced assays for MBL 21, M‐ficolin 20, H‐ficolin 22, CL‐L1 23, CL‐K1 24, MASP‐2 25, MASP‐3 17, MAp44 17 and MAp19 26. The principle in these assays is the same as for the new MASP‐1 TRIFMA described above, i.e. sandwich‐type immune assays using europium‐labelled detecting agents. The concentration of L‐ficolin in plasma was measured using an enzyme‐linked immunosorbent assay (ELISA) (Hycult Biotech, Uden, the Netherlands; #HK336‐02), as instructed by the manufacturer. It has been described previously that L‐ficolin binds to the silica particles in serum tubes 27, yielding lower concentrations in serum than in plasma. As the addition of silica particles is not standardized, it is advisable not to measure L‐ficolin in serum, particularly when conducting comparative studies. We thus only give values for L‐ficolin in plasma.
Effect of freezing–thawing and diurnal variation
To test the storage stability of the LP proteins, a pool of EDTA plasma was aliquoted in 500‐µl samples. The samples were submitted to nine freeze/thaw cycles. Each thawing was for 1 h at RT; the freezing temperature was −80oC. The samples were measured in all the above‐described TRIFMA assays before freezing and after nine freeze/thaw cycles.
The influence of diurnal variation on protein concentration in plasma was investigated measuring plasma concentrations of all the lectin pathway proteins in blood from six healthy individuals drawn at 4‐h intervals during a 24‐h period. The first sample, drawn at 6 a.m., was a fasting sample. Blood (8 ml) was drawn in EDTA tubes and centrifuged at 2000 g for 10 min. Plasma was collected and frozen immediately at −80ºC.
Statistics
Data were checked for normality by Q–Q plots and histograms. Where Gaussian distribution could not be assumed, non‐parametric tests were used for statistical analysis. P‐values < 0·05 were considered statistically significant. Stata version 12 and GraphPad Prism software package (version 6.0) were used for data management and statistical calculations.
Ethics
The Danish Data Protection Agency and the Regional Committee on Health Research Ethics approved the study (case no. 1‐10‐72‐214‐13). Blood was collected after informed consent according to the Declaration of Helsinki.
Results
MAb anti‐MASP‐1 antibody
The mAb MASP‐1‐antibody (5A6B7) recognizes a protein band at approximately 75 kDa on Western blots of plasma analysed under non‐reducing conditions and at approximately 90 kDa under reducing conditions, which is the expected mobility of non‐activated MASP‐1 (Fig. 1a). No other reactivities with serum proteins were observed using this antibody.
Figure 1.
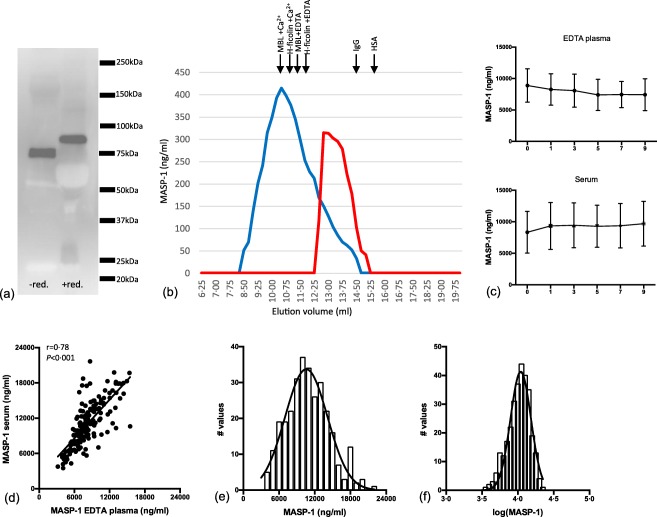
Characterization of the anti‐mannan‐binding lectin (MBL)‐associated serine protease 1 (MASP‐1) monoclonal antibody (mAb) and estimation of MASP‐1 in donor samples. (a) Western blot of 1 μl plasma under non‐reducing and reducing conditions developed with antibody against MASP‐1 (5A6B7). (b) Serum fractionated by gel‐permeation chromatography (GPC) in buffer with calcium (blue) or in buffer with high NaCl and ethylenediamine tetraacetic acid (EDTA) (red). Fractions were tested for MASP‐1 in the developed MASP‐1 assay. In the buffer with calcium, the main MASP‐1 signal is seen in volumes corresponding to where MBL and H‐ficolin elutes (given by arrows above the graph), whereas EDTA/high NaCl dissociates the MASPs from the pattern recognition molecules (PRMs) and the main signal is now seen in fractions eluting slightly earlier than immunoglobulin (Ig)G (150 kDa). (c) Serum and EDTA samples (n = 10) exposed to nine freeze/thaw cycles and measured for MASP‐1. Each time‐point represents the average of 10 different samples with a 95% confidence interval. (d) Correlation between measurements of MASP‐1 in serum and EDTA plasma. (e) Distribution of MASP‐1 concentrations in serum of 300 blood donors. (f) Distribution of MASP‐1 concentrations after log‐transformation. [Colour figure can be viewed at wileyonlinelibrary.com]
MASP‐1 assay
We have previously developed an assay for MASP‐1, but we needed a more effective alternative as the first assay was an inhibition assay based on the availability of recombinant MASP‐1, which is more laborious to produce and less stable to store than antibodies. We tested the new sandwich‐type assay in various ways. When separating the proteins in a serum pool by GPC analysed in the presence of calcium and testing the fractions in by the newly established MASP‐1 assay, MASP‐1 eluted at a position similar to the position where the PRMs (MBL and H‐ficolin indicated in Fig. 1b) elute. In contrast, the signal was seen at a position just slightly larger than IgG when the fractionation took place in EDTA/high NaCl buffer. We have reported this previously for the MASPs, i.e. the formation of PRM/MASP complexes at physiological conditions and disruption of the complexes in an EDTA/high NaCl buffer 19.
No signal was seen with plasma samples from patients with 3MC syndrome, i.e. genetically devoid of MASP‐1 (not shown).
MASP‐1 in plasma and serum proved stable to repeated freeze/thaw cycles (Fig. 1c). Coefficients of variation did not exceed 10% based on the three internal controls used in 20 separate assays. Values in serum and plasma showed correlation (r = 0·78) with a tendency to a higher concentration in serum than in plasma (Fig. 1d). The distribution of the concentrations determined approximated a Gaussian distribution, which improved when transformed logarithmically (Fig. 1e,f).
LP proteins in serum and plasma
No differences in the concentrations in serum and plasma were observed for MBL and MASP‐3 (Fig. 2). M‐ficolin, CL‐L1, MAp44, MASP‐2 and MAp19 showed higher concentrations in EDTA plasma than in serum. Conversely, H‐ficolin, CL‐K1 and MASP‐1 showed the highest concentrations in serum (Table 1). Approximation to normality could be assumed for CL‐L1, CL‐K1, MASP‐3, MAp44 and MAp19, whereas this was not the case for MBL, M‐ficolin, L‐ficolin, H‐ficolin, MASP‐1 and MASP‐2 (data not shown).
Figure 2.
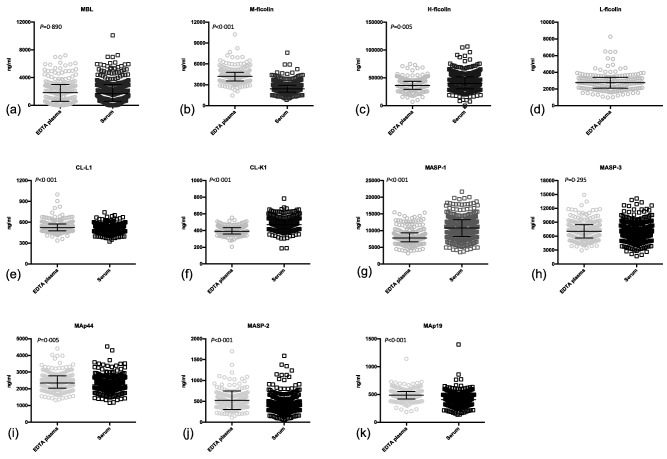
Concentration in plasma and serum of lectin pathway proteins. Bars indicate median and interquartile range. The actual values are listed in Table 1.
Table 1.
Concentrations of lectin pathway proteins in ethylenediamine tetraacetic acid (EDTA) plasma and serum and gender, age and diurnal variations
| EDTA plasma concentration µg/ml (median + IQR) | Serum concentration µg/ml (median + IQR) | Gender difference in plasma concentration yes = +, no = − | Age correlation (adult) yes = +, no = − | Diurnal variation yes = +, no = − | |
|---|---|---|---|---|---|
| MBL | 1·84 (0·57–3·01) | 1·69 (0·59–3·00) | − | + | − |
| M‐ficolin | 4·20 (3·54–4·81) | 2·42 (1·96–2·93) | + | − | + |
| L‐ficolin | 2·75 (2·10–3·39) | – | + | − | n.a. |
| H‐ficolin | 36·3 (29·4–43·7) | 39·9 (30·4–51·8) | + | − | + |
| CL‐L1 | 0·52 (0·48–0·58) | 0·49 (0·44–0·53) | − | − | − |
| CL‐K1 | 0·39 (0·36–0·43) | 0·47 (0·42–0·52) | − | − | n.a. |
| MASP‐1 | 7·79 (6·64–9·35) | 10·8 (8·27–13·3) | + | − | + |
| MASP‐3 | 7·04 (5·61–8·50) | 6·68 (5·56–8·30) | + | + | − |
| MAp44 | 2·35 (2·03–2·78) | 2·23 (1·98–2·52) | − | + | − |
| MASP‐2 | 0·49 (0·39–0·63) | 0·41 (0·29–0·53) | + | − | − |
| MAp19 | 0·49 (0·42–0·55) | 0·41 (0·34–0·49) | − | + | + |
IQR = interquartile range; MBL = mannan‐binding lectin; MASP = mannan‐binding lectin‐associated serine protease; CL‐L1 = collectin liver‐1; n.a. = not available.
LP proteins following freezing and thawing of plasma
We tested the stability of the LP proteins upon exposure to multiple freeze/thaw cycles. Although only relatively small differences were seen, the levels of CL‐L1, CL‐K1, L‐ficolin and H‐ficolin changed significantly after nine freeze/thaw cycles compared to before freezing (Supporting information, Fig. S1). H‐ficolin and CL‐L1 showed an average fall of 15 and 10%, respectively, whereas concentrations of CL‐K1 and L‐ficolin both increased 15% on average.
Gender and age differences
Concentrations of M‐, L‐ and H‐ ficolin and MASP‐1, ‐2 and ‐3 displayed significant gender differences (Fig. 3). L‐ficolin, H‐ficolin, MASP‐2 and MASP‐3 concentrations were lower in women than in men. M‐ficolin and MASP‐1 were higher in women than in men. The remaining proteins showed no concentration differences related to gender.
Figure 3.
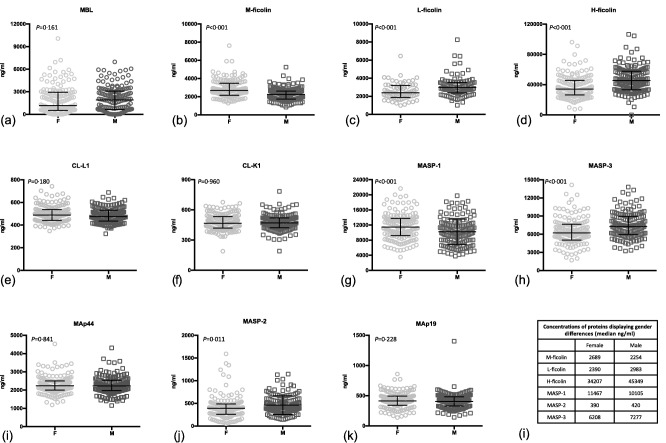
The concentrations of the lectin pathway proteins in serum [ethylenediamine tetraacetic acid (EDTA) plasma for L‐ficolin] from male (M) and female (F) blood donors (a–k). The same differences were observed for proteins in serum and EDTA plasma. Bars indicate median and interquartile range. Table indicates specific levels for proteins where a significant gender difference was observed (l).
The protein concentrations did not seem to change greatly with age in adults. For the proteins, where a significant correlation with age was observed (MBL, MASP‐3, MAp44 and MAp19) the r‐values were all within ± 0.2, with a wide range around the approximation line (Supporting information, Fig. S2).
Diurnal variation
A clear diurnal variation was observed for some of the LP proteins. This was most pronounced for H‐ficolin, M‐ficolin and MAp‐19 (Fig. 4). For H‐ficolin the concentration was halved remarkably from 10 a.m. [median 36·8 μg/ml, interquartile range (IQR) = 27·6–41·5] to 2 a.m. (median 18·9 μg/ml, IQR = 14·4–20·9). MAp19 displayed a drop in concentration in the afternoon, and when comparing 6 a.m. [0·64 μg/ml, standard deviation (s.d.) = 0·13] with 6 p.m. (0·53 μg/ml, s.d. = 0·17) a significant difference was observed. M‐ficolin showed a tendency to peak around 2 p.m. and 10 p.m. The difference between 2 p.m. (3·05 μg/ml, IQR = 2·57–4·20) and 2 a.m. (2·42 μg/ml, IQR = 2·17–3·35) was significant.
Figure 4.
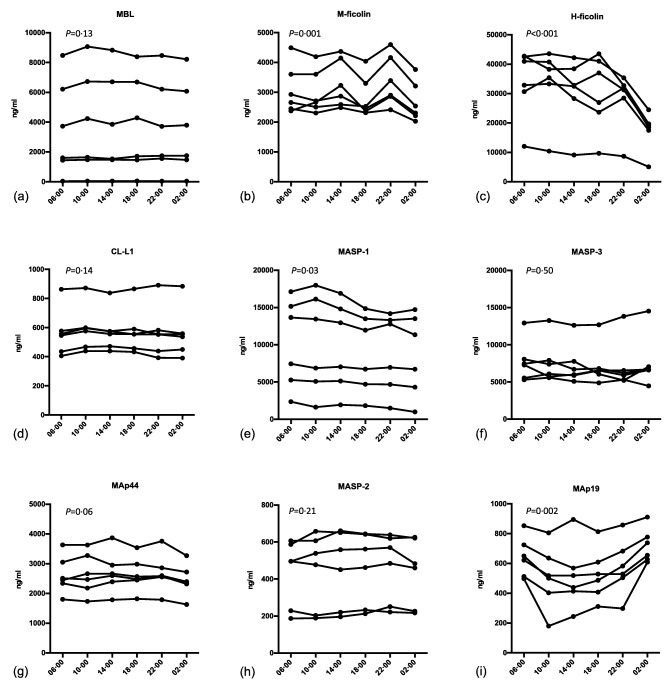
Diurnal variation of lectin pathway proteins in ethylenediamine tetraacetic acid (EDTA) plasma from six healthy individuals. Samples were taken at the time‐points given on the x‐axis. P‐values denote if there is an overall significant variation in concentration during 24 h.
Correlation between LP protein levels
Several protein concentrations correlated, as shown in Fig. 5a. H‐ficolin and MASP‐1 are the single proteins that correlated significantly with most of the other LP proteins. Concentrations of CL‐L1 and CL‐K1 correlated as expected (r = 0·81, P < 0·001) (Fig. 5b), as they are found predominantly as heterocomplexes of the two polypeptide chains 4. A strong correlation was also observed between H‐ficolin and L‐ficolin (Fig. 5c) (r = 0·61, P < 0·001). A strong correlation was determined as r above 0·60 or below −0·60, a moderate correlation at r = 0·30–0·60 or −0.60 to −0·30, and a weak correlation at r = 0·10–0·29 or −0·29 to −0.10.
Figure 5.
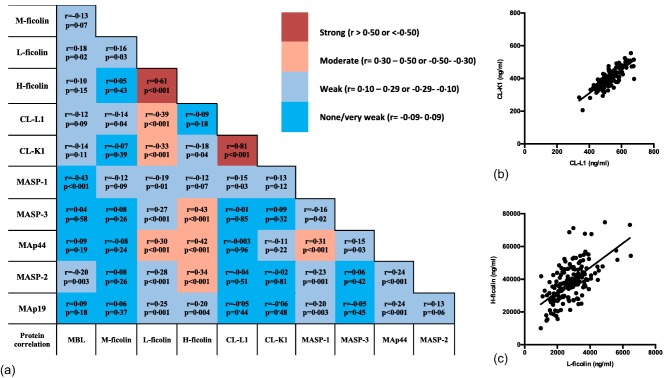
Plots and analysis of correlation between concentrations of lectin pathway (LP) proteins. Pairwise correlation between the concentrations of the proteins of the lectin pathway are given in (a). Grey tones indicate strong, moderate, weak and very weak correlations, as indicated on the bar next to (a). Spearman's rho and a P‐value are given for each correlation. Data points corresponding to the median values of the estimated concentrations are shown for the two strongest correlations observed, collectin liver‐1 (CL‐L1) versus CL‐K1 (b) and H‐ficolin versus L‐ficolin (c). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
The biological importance of the lectin pathway of complement activation is still little understood, and controversial findings abound. One way to approach an understanding of the physiological relevance of a substance is by studying the levels in various patient cohorts. In order to understand what is different or changed in the diseased state, a description of what can be considered ‘normal’ is critical. In the present study of a cohort of 300 adult Danish blood donors, we describe the concentrations of the lectin pathway proteins in plasma and serum, gender differences, age correlations, diurnal variations and the correlations between LP proteins.
Concentration of the LP proteins differ to some degree between serum and plasma. The causes for these differences are not obvious for all the proteins. It has been described that the concentrations of M‐ficolin are higher in EDTA plasma than in serum. This is explained by the release of calcium‐dependent surface‐bound M‐ficolin from granulocytes and monocytes 28. For L‐ficolin, we did not address this issue here as it has been established that the silica coagulation promotor now added adsorbs L‐ficolin. Several LP proteins, such as MASP‐1 and MASP‐2, have been implicated in the complement/coagulation cross‐talk 11, in which case concentrations in serum could be expected to change as coagulation runs its course. We see higher concentrations in serum for MASP‐1 and lower concentrations for MASP‐2 and MAp44 compared to plasma, and indeed most proteins display different concentrations in plasma than in serum (except MBL and MASP‐3), in line with coagulation possibly affecting complement proteins, although this is not conclusive.
We observed only an insignificant change in protein concentrations with age, which is in line with what has been described previously for some of the proteins; i.e. the most pronounced changes in concentrations occur during the first year of life 29.
M‐ficolin, H‐ficolin and MAp19 concentrations changed significantly during a 24‐h period. This was most pronounced for H‐ficolin, where a remarkable reduction of 50% was seen from morning to late night. This knowledge is pivotal in research where patient inclusion is carried out at all hours of the day, e.g. in acute medical research, where securing matched healthy controls would be complicated. As a minimum, listing of the time of day of the blood sampling should be performed and could, to some extent, alleviate the problem, allowing for adjustment of the diurnal variation in statistical models. Half‐lives of the lectin pathway proteins have been established only for MBL. When MBL purified from plasma was infused into MBL‐deficient volunteers the protein exhibited a half‐life of 69·5 h, with a wide range of 18–118 h 30. We found that H‐ficolin displays a remarkable diurnal variation, with a reduction of approximately 50% during the night. It could be speculated that if the half‐life of the molecule was in the range of 20 h, this would mean that H‐ficolin production follows an on/off rhythm.
Several of the LP protein concentrations showed gender differences. This might not be surprising, as there are tremendous gender differences in hormones; also, the distribution and amount of fatty tissue varies between men and women, and complement proteins have been implicated in regulation of adipose tissue 31. The most striking difference between genders was observed for the ficolins, where an average difference in concentration of approximately 20% was seen. To some extent it is remarkable that proteins encoded by the same gene do not display the same gender variation. MASP‐1, MASP‐3 and MAp44 are all splice variants from the MASP1 gene 17, and we found a 12% higher concentration of MASP‐1 and a 15% lower concentration of MASP‐3 in women compared to men, whereas MAp44 displayed no gender difference. For MASP‐2 and MAp19, both splice variants encoded by the MASP2 gene 26, we found MASP‐2 concentrations 7% lower in women and no difference regarding MAp19. Very little is known about the regulation of transcription of LP proteins; our results indicate that regulation of the serine proteases and the associated proteins are regulated by different mechanisms.
Hormonal influences on MBL have been described previously and both growth hormones and, in particular, thyroid hormones, increase the concentration of the protein in plasma 32, 33.
We observed a correlation between the levels of several of the LP proteins. This is not extraordinary in a system where most of the proteins exist in complexes. The LP proteins are synthesized predominantly by the liver 34; however, it has been described that H‐ficolin is abundant in lung tissue 35, that M‐ficolin is produced primarily by leucocytes 28 and that CL‐K1 is also produced by kidney cells 36. We confirmed that CL‐L1 and CL‐K1 concentrations correlate strongly, as we have reported previously 37. These proteins have been found to exist primarily as heteromeric complexes in the circulation 4. Another strong correlation was found between H‐ficolin and L‐ficolin, which has not been described previously and for which, to our knowledge, there is no good functional explanation. One might have expected indications for co‐ordinated synthesis of some of the PRMs and the associated serine proteases, as is seen for the synthesis of C1q and C1r and C1s, but such is clearly not the case.
This study lays the foundation for future investigation of the role of the lectin pathway proteins in diseases. All 11 proteins were quantified in a large cohort of blood donors on meticulously collected samples. A number of possible confounders were addressed, revealing several issues to consider when studying LP proteins in comparative studies. It is important to use the same material, i.e. either serum or plasma; gender‐matching is necessary, whereas age is not an issue in adulthood. Diurnal variations must be taken into account, particularly for H‐ficolin, M‐ficolin and MAp19.
Author contributions
A. T. and A. G. H. performed the laboratory experiments; A. T. was in charge of collecting blood samples and handling the blood samples after they were drawn; S. T., J. C. J and S. H. developed the assays used in the project and supervised laboratory procedures. A. T., S. T. and K. S. wrote the manuscript and all authors participated in the editing of the article.
Disclosure
The authors declare no disclosures.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Stability of the lectin pathway proteins in ethylenediamine tetraacetic acid (EDTA)‐plasma through nine freeze/thaw cycles.
Fig. S2. Correlation of lectin pathway proteins with age in adults (aged 18–70 years).
Acknowledgements
The authors would like to acknowledge the Danish Rheumatism Association, Aase and Ejnar Danielsens Fond and the Danish Council for Independent Research, Medical Sciences, who supported the project.
References
- 1. Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement‐related diseases. EMBO J 2015; 34:2735–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merle NS, Church SE, Fremeaux‐Bacchi V, Roumenina LT. Complement system part I – molecular mechanisms of activation and regulation. Front Immunol 2015; 6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kjaer TR, Thiel S, Andersen GR. Toward a structure‐based comprehension of the lectin pathway of complement. Mol Immunol 2013; 56:222–31. [DOI] [PubMed] [Google Scholar]
- 4. Henriksen ML, Brandt J, Andrieu J‐P et al Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system. J Immunol 2013; 191:6117–27. [DOI] [PubMed] [Google Scholar]
- 5. Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand J Immunol 2013; 78:181–93. [DOI] [PubMed] [Google Scholar]
- 6. Yongqing T, Drentin N, Duncan RC, Wijeyewickrema LC, Pike RN. Mannose‐binding lectin serine proteases and associated proteins of the lectin pathway of complement: two genes, five proteins and many functions? Biochim Biophys Acta 2012; 1824:253–62. [DOI] [PubMed] [Google Scholar]
- 7. Nonaka M, Kimura A. Genomic view of the evolution of the complement system. Immunogenetics 2006; 58:701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Degn SE, Jensenius JC, Thiel S. Disease‐causing mutations in genes of the complement system. Am J Hum Genet 2011; 88:689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rooryck C, Diaz‐Font A, Osborn DPS et al Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nat Genet 2011; 43:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenny L, Dobó J, Gál P, Schroeder V. MASP‐1 induced clotting – the first model of prothrombin activation by MASP‐1. PLOS ONE 2015; 10:e0144633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kozarcanin H, Lood C, Munthe‐Fog L et al The lectin complement pathway serine proteases (MASPs) represent a possible crossroad between the coagulation and complement systems in thromboinflammation. J Thromb Haemost 2016; 14:531–45. [DOI] [PubMed] [Google Scholar]
- 12. Dobó J, Szakács D, Oroszlán G et al MASP‐3 is the exclusive pro‐factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci Rep 2016; 6:31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horváth Z, Csuka D, Vargova K et al Association of low ficolin‐lectin pathway parameters with cardiac syndrome X. Scand J Immunol 2016; 84:174–81. [DOI] [PubMed] [Google Scholar]
- 14. Świerzko AS, Szala‐Poździej A, Kilpatrick DC et al Components of the lectin pathway of complement activation in paediatric patients of intensive care units. Immunobiology 2016; 221:657–69. [DOI] [PubMed] [Google Scholar]
- 15. Csuka D, Munthe‐Fog L, Hein E et al Activation of the ficolin‐lectin pathway during attacks of hereditary angioedema. J Allergy Clin Immunol 2014; 134:1388–93.e1. [DOI] [PubMed] [Google Scholar]
- 16. Bro‐Jeppesen J, Kjaergaard J, Thiel S et al Influence of mannan‐binding lectin and MAp44 on outcome in comatose survivors of out‐of‐hospital cardiac arrest. Resuscitation 2016; 101:27–34. [DOI] [PubMed] [Google Scholar]
- 17. Degn SE, Jensen L, Gál P et al Biological variations of MASP‐3 and MAp44, two splice products of the MASP1 gene involved in regulation of the complement system. J Immunol Methods 2010; 361:37–50. [DOI] [PubMed] [Google Scholar]
- 18. Mao S‐Y. Biotinylation of antibodies. Methods Mol Biol 2010; 588:49–52. [DOI] [PubMed] [Google Scholar]
- 19. Thiel S, Jensen L, Degn SE et al Mannan‐binding lectin (MBL)‐associated serine protease‐1 (MASP‐1), a serine protease associated with humoral pattern‐recognition molecules: normal and acute‐phase levels in serum and stoichiometry of lectin pathway components. Clin Exp Immunol 2012; 169:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wittenborn T, Thiel S, Jensen L, Nielsen HJ, Jensenius JC. Characteristics and biological variations of M‐ficolin, a pattern recognition molecule, in plasma. J Innate Immun 2010; 2:167–80. [DOI] [PubMed] [Google Scholar]
- 21. Thiel S, Møller‐Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan‐binding lectin pathway of complement activation. Immunobiology 2002; 205:446–54. [DOI] [PubMed] [Google Scholar]
- 22. Krarup A, Sørensen UBS, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan‐binding lectin, L‐ficolin, and H‐ficolin. Infect Immun 2005; 73:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Axelgaard E, Jensen L, Dyrlund TF et al Investigations on collectin liver 1. J Biol Chem 2013; 288:23407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Selman L, Henriksen ML, Brandt J et al An enzyme‐linked immunosorbent assay (ELISA) for quantification of human collectin 11 (CL‐11, CL‐K1). J Immunol Methods 2012; 375:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Møller‐Kristensen M, Jensenius JC, Jensen L et al Levels of mannan‐binding lectin‐associated serine protease‐2 in healthy individuals. J Immunol Methods 2003; 282:159–67. [DOI] [PubMed] [Google Scholar]
- 26. Degn SE, Thiel S, Nielsen O, Hansen AG, Steffensen R, Jensenius JC. MAp19, the alternative splice product of the MASP2 gene. J Immunol Methods 2011; 373:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hein E, Bay JT, Munthe‐Fog L, Garred P. Ficolin‐2 reveals different analytical and biological properties dependent on different sample handling procedures. Mol Immunol 2013; 56:406–12. [DOI] [PubMed] [Google Scholar]
- 28. Kjaer TR, Hansen AG, Sørensen UBS, Nielsen O, Thiel S, Jensenius JC. Investigations on the pattern recognition molecule M‐ficolin: quantitative aspects of bacterial binding and leukocyte association. J Leukoc Biol 2011; 90:425–37. [DOI] [PubMed] [Google Scholar]
- 29. Sallenbach S, Thiel S, Aebi C et al Serum concentrations of lectin‐pathway components in healthy neonates, children and adults: mannan‐binding lectin (MBL), M‐, L‐, and H‐ficolin, and MBL‐associated serine protease‐2 (MASP‐2). Pediatr Allergy Immunol 2011; 22:424–30. [DOI] [PubMed] [Google Scholar]
- 30. Valdimarsson H, Vikingsdottir T, Bang P et al Human plasma‐derived mannose‐binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol 2004; 59:97–102. [DOI] [PubMed] [Google Scholar]
- 31. Vlaicu SI, Tatomir A, Boodhoo D, Vesa S, Mircea PA, Rus H. The role of complement system in adipose tissue‐related inflammation. Immunol Res 2016; 64:653–64. [DOI] [PubMed] [Google Scholar]
- 32. Sorensen CM, Hansen TK, Steffensen R, Jensenius JC, Thiel S. Hormonal regulation of mannan‐binding lectin synthesis in hepatocytes. Clin Exp Immunol 2006; 145:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalkjær Riis AL, Hansen TK, Thiel S et al Thyroid hormone increases mannan‐binding lectin levels. Eur J Endocrinol 2005; 153:643–9. [DOI] [PubMed] [Google Scholar]
- 34. Beltrame MH, Catarino SJ, Goeldner I, Boldt ABW, de Messias‐Reason IJ. The lectin pathway of complement and rheumatic heart disease. Front Pediatr 2014; 2:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akaiwa M, Yae Y, Sugimoto R et al Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J Histochem Cytochem 1999; 47:777–86. [DOI] [PubMed] [Google Scholar]
- 36. Keshi H, Sakamoto T, Kawai T et al Identification and characterization of a novel human collectin CL‐K1. Microbiol Immunol 2006; 50:1001–13. [DOI] [PubMed] [Google Scholar]
- 37. Troldborg A, Thiel S, Jensen L et al Collectin liver 1 and collectin kidney 1 and other complement‐associated pattern recognition molecules in systemic lupus erythematosus. Clin Exp Immunol 2015; 182:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Stability of the lectin pathway proteins in ethylenediamine tetraacetic acid (EDTA)‐plasma through nine freeze/thaw cycles.
Fig. S2. Correlation of lectin pathway proteins with age in adults (aged 18–70 years).


